ABSTRACT
Introduction
DBV712 250 µg (also referred to as Viaskin Peanut or peanut patch; Viaskin is a trademark of DBV Technologies) is an innovative approach to epicutaneous immunotherapy (EPIT). The patch-based technology system facilitates peanut protein (allergen) absorption into the intact non-vascularized epidermis to promote desensitization to peanut while limiting systemic allergen exposure.
Areas Covered
Efficacy and safety in children have been evaluated in four completed phase 3 studies. Overall, the results from these studies have demonstrated the peanut patch to be superior in desensitization compared with placebo and safe for daily use over multiple years.
Expert Opinion
These findings, as well as supportive evidence from phase 2 studies, confirm the potential for an effective treatment of peanut allergy in children. The purpose of this review is to summarize the safety and efficacy of the peanut patch in the treatment of peanut allergy.
1. Introduction
Peanut allergy is one of the most common food allergies with an overall prevalence of approximately 1% across all age groups, and occurs in 2% of the pediatric population [Citation1,Citation2]. In the United States (US) alone, approximately 1.6 million children are allergic to peanuts [Citation3]. Several epidemiologic studies have demonstrated an increase in peanut allergy prevalence, including a cross-sectional survey-based study in the US which showed that between 1997 and 2008 the prevalence of peanut allergy more than tripled, from 0.4% to 1.4% [Citation4]. Peanut allergy is typically considered a lifelong affliction as studies indicate that most children do not outgrow their peanut allergy, with resolution occurring in only about 25% of children [Citation5,Citation6].
Allergen immunotherapy (AIT) aims at desensitizing, i.e. reducing the reactivity to an allergen, through the repeated and prolonged contact of the immune system with the allergen, and based on current data, seems to offer the most potential for disease modification among current management strategies for food allergies. Various administration routes are being studied: the mouth/digestive tract (oral immunotherapy, OIT), the sublingual mucosa (sublingual immunotherapy, SLIT), and the skin (epicutaneous immunotherapy, EPIT) [Citation7].
DBV712 250 µg (also referred to as Viaskin Peanut or peanut patch; Viaskin is a trademark of DBV Technologies), developed by DBV Technologies is an innovative approach to EPIT and a patch-based technology system that uses the skin route and facilitates peanut protein (allergen) absorption into the intact skin. EPIT with the peanut patch has been tested in numerous clinical trials, for which a synthesis is provided in this review.
1.1. The peanut patch
The peanut patch consists of a film disc electrosprayed with peanut protein that is placed on top of a doubled-sided adhesive foam ring (). As a result, a condensation chamber is formed in which the peanut allergen is suspended above the skin. Natural water loss from the skin leads to solubilization and diffusion of the peanut allergen into the superficial layers of the non-vascularized epidermis, which is then transported via antigen presenting cells (APCs) to regional lymph nodes. Preclinical studies have demonstrated that these regional lymph node interactions promote a unique allergen-specific T-regulatory cell desensitization process [Citation8–10]. The mechanistic pathway of the peanut patch limits allergen (peanut protein) uptake into the bloodstream and thus minimizes risk of systemic allergen exposure. The amount of allergen used in the peanut patch, 250 µg of peanut protein, is approximately 1000 times lower than the amount used in the only FDA and EMA approved form of peanut OIT [Citation7,Citation8].
Figure 1. Overview of mechanism of action with the peanut patch.
The peanut patch contains 250 µg of dried peanut protein on a film disc that is placed on top of a doubled-sided adhesive foam ring. When placed on the skin the film disc and foam ring, which are held in place by an adhesive overlay, form a condensation chamber. Natural water loss from the skin leads to solubilization of the peanut allergen which is taken up by antigen-presenting cells, such as Langerhans cells, in the superficial layers of the non-vascularized epidermis.

The peanut patch is intended to be worn for a full day and replaced daily. The patch is applied on the interscapular area of the back, with the placement changing daily, rotating between 6 different zones [Citation11]. There is no up-dosing with the peanut patch; however, during the first few weeks of treatment, the duration of time the patch is worn is gradually increased to reach a full day (i.e. 24 hours). Post hoc analysis of the patch application data from a phase 3 study (PEPITES) showed that participants attained a medium duration of wear time of 21.1 h [Citation11]. No restrictions on the activities of daily living are required while wearing the peanut patch, including bathing or swimming, as the patch was designed to be a convenient and easy-to-use therapy.
1.2. Clinical trials
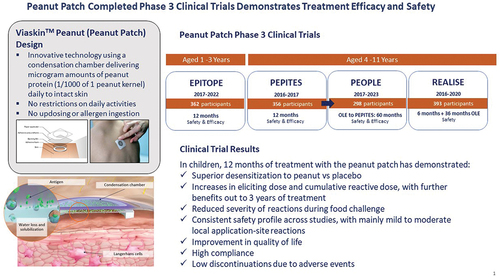
The clinical profile of the peanut patch has been evaluated in three phase-1 studies (PEP01.09, SOLAR, and EVOLVE) in participants aged 4 through 50 years and in participants aged 6 through 55 years in supportive phase 2 studies (ARACHILD, VIPES, OLFUS, and CoFAR6), which identified the age group (children) and dose (250 µg peanut protein) to study in the phase 3 trials. Safety and efficacy were assessed in three completed phase 3 studies (PEPITES and the open-label extension [OLE], PEOPLE, in children aged 4–11 years, and EPITOPE, in children aged 1–3 years) and safety alone in a phase 3 study, REALISE, in children aged 4–11 years. The phase 3 studies are briefly summarized below and in [Citation12–16]. Overall, since the start of the clinical development program, a total of 1307 peanut-allergic children aged 1–11 years have been exposed to the peanut patch for a total of 3264.1 subject years.
Table 1. Completed peanut patch phase 3 trials.
PEPITES: randomized double-blind, placebo-controlled trial in 356 peanut-allergic children aged 4–11 years without a history of a very severe, life-threatening anaphylactic reaction with an entry eliciting dose (ED) ≤300 mg of peanut protein; 12 months of daily active treatment or placebo, randomization of 2:1 [Citation12,Citation13].
PEOPLE: OLE of PEPITES to evaluate 5 years of active treatment (efficacy and safety) with 24 months for participants in the initial PEPITES active group and 36 months for the initial PEPITES placebo group. Both arms then had the option of a further 24 months of treatment [Citation14].
REALISE: randomized, double-blind, placebo-controlled trial period followed by an OLE active treatment in 393 children aged 4–11 with a physician-diagnosed peanut allergy; 6 months of daily active treatment or placebo, randomization of 3:1, followed by open-label period of active treatment for up to 3 years in all participants [Citation15].
EPITOPE: randomized, double-blind, placebo-controlled trial in 362 peanut-allergic children aged 1–3 years without a history of a very severe, life-threatening anaphylactic reaction, with an entry ED ≤300 mg peanut protein; 12 months of daily active treatment or placebo, randomization of 2:1 [Citation16].
2. Treatment efficacy
2.1. Percentage of treatment responders
The phase 3 clinical studies showed a significantly higher percentage of treatment responders with active treatment compared with placebo at Month 12. In participants aged 1–3 years the responder rates were 67% vs 33.5%, respectively, with a difference of 33.4%, p < 0.001 (95% confidence interval [CI]: 22.4, 44.5) () [Citation16]. In participants aged 4–11 years, responder rates were 35.3% vs 13.6%, respectively, a difference of 21.7%, p < 0.001 (95% CI: 12.4%, 29.8%). Despite this statistically significant difference, the lower bound of the 95% CI did not meet the prespecified lower bound threshold of 15%, the clinical significance of which is unknown. At Year 3, in participants aged 4–11 years, the rate of treatment responders increased to 55.3% () [Citation12–14].
Figure 2. Proportion of treatment responders between active and placebo groups in children aged 1–3 years (a) and aged 4–11 years (b, c).
The primary measure of treatment effect compared the percentages of treatment responders between the active and placebo groups after 12 months of treatment among all randomized participants aged 1–3 years (a) and 4–11 years (b). Treatment responder defined as Month 12 ED ≥ 1000 mg (if baseline ED >10 mg) or ≥ 300 mg (if baseline ED ≤ 10 mg).
Proportion of participants aged 4–11 years meeting prespecified primary outcome at Months 12 and 36 in PEOPLE PP set according to primary outcome criteria in PEPITES. The PP set was defined as those participants who completed all treatment according to the study protocol without major deviations that could affect the assessment of the treatment effect, which included an evaluable DBPCFC performed as required by the protocol at Months 12 and 36 and compliance of ≥ 80% (c). CI, confidence interval; DBPCFC, double-blind, placebo-controlled food challenge; ED, eliciting dose; PP, per protocol.

2.2. Changes in eliciting dose (ED)
An ED is defined as the discrete dose of allergen protein ingested that triggers an allergic reaction with sufficient objective signs/symptoms to fulfill prespecified ‘stopping’ criteria during a food challenge.
Overall, nearly two-thirds (62.6%) of children aged 4–11 years on active treatment had an increased ED at Month 12, whereas a decrease in the ED was observed for one-third (33.9%) of the placebo participants. In other words, the active treatment provided a >4-fold odds of improving ED compared to a >7-fold odds of worsening with no treatment. At Year 3, 75.9% of participants increased their ED compared with Month 0, and 92.2% either maintained or increased it from Month 0 [Citation12–14].
2.2.1. Participants reaching an ED ≥1000 mg (ED1000), (~ 3 to 4 peanut kernels)
This endpoint represents the ability to ingest 3 to 4 peanuts before an allergic reaction is triggered. The proportion of children reaching an ED1000 at Month 12 was assessed in two pivotal phase 3 studies. Among the peanut patch-treated children aged 1–3 years, nearly two-thirds (64.2%) reached this endpoint, vs 29.6% with placebo () [Citation16]. In peanut patch-treated children aged 4–11 years, 31.5% reached this endpoint after 1 year, vs 10.2% with placebo; after 3 years, 51.8% reached this endpoint () [Citation12–14].
Figure 3. Proportion of participants achieving an eliciting dose of ≥ 1000 mg at month 12 with the peanut patch.
A key efficacy endpoint measured the proportion of participants achieving an ED ≥ 1000 mg after 12 months of active treatment in children aged 1–3 years in EPITOPE (a) and 4–11 years in PEPITES (b). The change in the proportion of participants aged 4–11 years achieving an ED ≥ 1000 mg between Month 12 and Month 36 was assessed in the open-label extension PEOPLE study. CI, confidence interval; ED, eliciting dose.

2.3. Changes in peanut protein cumulative reactive dose
The cumulative reactive dose (CRD) is defined as the sum of all doses (including partial doses) up to and including the ED. The CRD increased significantly at Month 12 in participants aged 1–3 and 4–11 years who received active treatment vs placebo, with median changes of 1300 mg and 280 mg, respectively (p < 0.001) [Citation12–14,Citation16]. No increases in median CRD were observed in placebo participants for either age group.
2.3.1. Participants reaching a CRD ≥3444 mg (CRD3444), (~12–14 peanut kernels)
This endpoint represents the ability to ingest a maximum of 12–14 peanuts during the double-blind, placebo-controlled food challenge (DBPCFC) before pre-defined objective symptoms of an allergic reaction to peanut are triggered. Thirty-seven percent of participants aged 1–3 years on active treatment vs 10% on placebo reached a CRD3444 [Citation16]. Longer-term active treatment in participants 4–11 years demonstrated continued increases in CRD, with median CRD reaching 944 mg by Year 3. Additionally, at Year 3, the percentage of participants who reached a CRD3444 increased from 15.6% at Month 12 to 24.1% [Citation12–14].
2.4. Reduction in reaction severity at double-blind, placebo-controlled food challenge (DBPCFC)
Another important goal of treatment was to reduce the severity of an allergic reaction, should it occur (for example, to accidental ingestion). Severity was assessed in two pivotal phase 3 studies by comparing DBPCFC data at entry and after treatment. At entry, no difference in the severity of symptoms on DBPCFC was observed between groups (active or placebo) for participants aged 1–3 and 4–11 years. At Month 12 in both age groups, the maximum objective symptom severity was significantly lower for the active treatment group vs placebo (p < 0.001). Additionally, nearly twice as many participants on active treatment had either no or mild symptoms during the Month 12 DBPCFC compared with placebo () [Citation12–14,Citation16].
Figure 4. Changes in reaction severity during DBPCFC between month 0 and month 12.
The severity of reactions during DBPCFC was graded by the investigator according to PRACTALL scoring as absent, mild, moderate, or severe in children aged 1–3 years in EPITOPE (a) and aged 4–11 years in PEPITES (b) [Citation52].
Reaction definitions: Grade 0: negative; Grade 1: only erythema, or erythema + infiltration; Grade 2: erythema, few papules; Grade 3: erythema, many or spreading papules; Grade 4: erythema, vesicles.
DBPCFC, double-blind, placebo-controlled food challenge.
![Figure 4. Changes in reaction severity during DBPCFC between month 0 and month 12.The severity of reactions during DBPCFC was graded by the investigator according to PRACTALL scoring as absent, mild, moderate, or severe in children aged 1–3 years in EPITOPE (a) and aged 4–11 years in PEPITES (b) [Citation52].Reaction definitions: Grade 0: negative; Grade 1: only erythema, or erythema + infiltration; Grade 2: erythema, few papules; Grade 3: erythema, many or spreading papules; Grade 4: erythema, vesicles.DBPCFC, double-blind, placebo-controlled food challenge.](/cms/asset/9e8a6061-394f-49ea-bd74-21631f4175a3/ierm_a_2315221_f0004_oc.jpg)
2.5. Predicted reduction in risk of allergic reactions to accidental ingestions
Meals prepared outside the home and packaged goods may pose a serious risk of peanut exposure, as peanut protein may be encountered (e.g. through cross-contact) at levels that may elicit a reaction in peanut-allergic individuals. Quantitative risk assessment (QRA) modeling is useful in predicting the likelihood that an allergic reaction will occur to such exposures and to assess any change in this risk associated with ED changes. Using consumption data of four commonly eaten packaged food products known to have high risk for unintended allergen presence and a high rate of product recall (cookies, doughnuts, ice cream, and salty snacks), QRA analysis determined that increasing an ED from ≤100 mg to 300 mg or 1000 mg translates into a reduced risk of experiencing an allergic reaction to one of these exposures by >95% and >99.7%, respectively [Citation17]. These results support the clinical significance of these thresholds as endpoints in food allergy studies. When QRA modeling was applied to the PEPITES population, a 73% to 78% reduction in risk of reaction per eating occasion for these foods was predicted for children actively treated for 12 months, with no reduction predicted in the placebo group [Citation18–20]. Restaurants are also a common setting where accidental peanut exposures occur through cross-contact. In kitchen simulation experiments with a professional chef designed to replicate real-world situations, similar risk reductions were noted when the PEPITES database was used in a QRA modeling approach, adding further support to the clinical relevance of the effect seen after 12 months of treatment [Citation19,Citation20].
Another way to measure the effect size of a treatment is through the number needed to treat (NNT), defined as the required number of patients treated to prevent 1 undesired outcome. In children aged 4–11 years, the NNTs for preventing an objective allergic reaction associated with consuming a peanut-contaminated food product and preventing a moderate-to-severe allergic reaction were 5.5 and 9.4, respectively [Citation21].
2.6. Sustained desensitization
Sustained desensitization (also referred to as sustained unresponsiveness [SU] or remission) in food allergy treatment refers to ongoing desensitization that remains even after regular (typically daily in the case of food AIT) treatment stops. SU is considered to signify that a long-lasting, disease modifying treatment effect has been achieved.
In participants aged 4–11 years, SU was evaluated by DBPCFC performed prior to and after 2 months of treatment cessation in the open-label PEOPLE study in a subgroup of eligible participants [Citation14,Citation22]. Within this subgroup, at the end of Year 3, approximately 78% of participants maintained an ED1000, in agreement with supportive phase 2 data, which demonstrated SU to CRD of ≥1444 mg peanut protein in 77.3% participants [Citation14,Citation23].
3. Safety
3.1. Overall safety
A consistent safety profile was observed across all phase 3 studies (). Among children aged 1–11 years in the active group, a majority (>88%) experienced at least 1 treatment-emergent adverse event (TEAE); most were mild-to-moderate in severity and consisted primarily of local skin reactions that decreased in frequency and severity over time (). Additionally, participant treatment compliance rates were consistently high (>96%) while treatment discontinuations due to adverse events (AEs) were considerably low (27 participants [2.8%]) across the phase 3 clinical studies [Citation12–16].
Figure 5. Changes in frequency and severity of Treatment-emergent Adverse Events Over Time in the PEOPLE (a) and REALISE (b) trials.
Treatment-relatedness and severity were determined by the investigator.
TEAE, treatment-emergent adverse event.

Table 2. Treatment-related adverse events in participants treated with the peanut patch: overview of the peanut patch safety profile in completed phase 3 studies.
3.1.1. Local administration site reactions were the most frequently reported treatment-related TEAE
The majority of the participants experienced a treatment-related TEAE (i.e. any AE occurring after treatment initiation and considered by the investigator as related to treatment). The most frequently reported treatment-related TEAEs were local administration site reactions, and these were experienced with greater frequency in the active treatment group. These local site reactions occurred more frequently during Months 0–3 and decreased in frequency over time. Most application site reactions, which were graded by investigators on a 0 to 4 rating scale, were localized under the patch and graded as mild or moderate in severity. Local application site reactions most commonly self-resolved or resolved following treatment with topical corticosteroids (eFigure 1) [Citation12–16].
3.1.2. Summary of serious adverse events
Across the clinical studies, serious adverse events (SAEs), regardless of relatedness to treatment, occurred in <10% of participants aged 1–11 years in either treatment group, with an overall incidence rate comparable between the treatment groups. The most frequently reported SAEs included wheezing, bronchial hyperactivity, bronchospasm, asthma, and anaphylactic reactions, with participants responding to standard treatment. The incidence of SAEs decreased over time; in REALISE, 13 SAEs were reported during Year 1 vs four during Year 2, and one during Year 3. Five treatment-related SAEs were reported across the phase 3 studies, with only 1 event reported during the long-term study [Citation12–16].
3.1.3. Summary of anaphylactic reactions
To capture as many reactions as possible, investigators relied on the commonly used National Institute of Allergy and Infectious Diseases definition of anaphylaxis, which has been shown to be highly sensitive but moderately specific [Citation24,Citation25].
Rates of anaphylaxis were consistent across all phase 3 studies and more commonly reported in the active vs placebo groups. A total of 32 treatment-related episodes of anaphylaxis were reported in 29 participants aged 1–11 years across all phase 3 studies, all mild-to-moderate in severity. Of those, 16 patients reported an anaphylaxis reaction that required 1 dose of epinephrine. All but 1 of treatment-related events occurred in the active group and the frequency of anaphylactic reactions decreased over time [Citation13,Citation15].
3.1.4. Improvement of quality of life, assessed by FAQLQ and FAIM questionnaires
Peanut allergy quality of life (QoL) questionnaires assessed the patient’s burden of peanut allergy from the perspective of the patient (child form [CF]) and from the perspective of the parent/caregiver (parent form [PF]). Changes in the scores post-immunotherapy provide insight on the impact of treatment on the well-being of the patient and perceived well-being of the patient from the parents’ perspective.
An improved QoL was observed after long-term daily treatment with the peanut patch. In children aged 4–11 years, Food Allergy Quality of Life Questionnaire (FAQLQ) mean total scores improved from baseline to Month 24 significantly more in the active group vs placebo for PF and for CF (p = 0.008 and p = 0.023, respectively). As part of FAQLQ measurement, food-related anxiety data was collected and details of this assessment have been previously published [Citation26]. Food Allergy Independent Measure (FAIM) total scores exhibited a greater change from baseline to Month 24 in the active group vs placebo (for both CF and PF) [Citation12,Citation13,Citation15,Citation26]. Food allergy QoL improvements were largely driven by increases in ED, aligned with the goals of immunotherapy.
3.1.5. Changes in immunological markers (IgE, IgG4) and skin prick test
Skin prick test (SPT) wheal size and immunoglobulin E (IgE) concentration values are measured for the diagnosis of peanut allergy, with a larger wheal size and greater IgE concentration indicating a higher probability of allergy [Citation27]. In children aged 1–3 years, peanut-specific IgE (sIgE) decreased from baseline to Month 12 in the active group while it increased in the placebo group, and peanut-specific immunoglobulin G4 (sIgG4) increased from baseline to Month 12 in the active group with little change in placebo group [Citation16]. These sIgE and sIgG4 changes are consistent with what is seen in specific AIT for inhalant and venom allergies [Citation28]. Children aged 4–11 years experienced similar results (eFigure 2) as well as a median absolute change in SPT mean wheal diameter from baseline to Month 36 of 3.5 mm [Citation12,Citation13].
4. Discussion
The most common standard of care for peanut allergy management remains strict peanut avoidance and the use of epinephrine or other emergency medications in the event of an allergic reaction [Citation29,Citation30]. Peanut is commonly used in many food products, thus making strict avoidance difficult and accidental exposure to peanut remains a common issue, with more severe responses to peanut exposure compared to children with other food allergies. Moreover, peanut is one of the most common causes of fatal food-triggered anaphylaxis [Citation31]. Additionally, the constant vigilance to avoid peanut may result in limited social engagement and cause anxiety or fear of experiencing an allergic reaction because of unintentional exposure [Citation32–35]. The negative effects on the psychosocial well-being of peanut-allergic patients emphasize the need for safe and effective treatments that can reduce the risk of reaction due to accidental ingestion. When allergic patients demonstrate desensitization through allergen-specific OIT, SLIT, or EPIT, the amount of allergen that is required to elicit an allergic reaction (i.e. the patient’s threshold) increases. This increase in the patient’s threshold reduces their risk of experiencing an allergic reaction from accidental peanut exposure. All three immunotherapy techniques are based on frequent and regular exposure to the allergen, yet each uses a different route and exhibits distinguishing efficacy and safety profiles [Citation36].
OIT, which has one licensed product, consists of up-dosing and maintenance phases. Up-dosing at clinical visits consists of ingesting peanut protein at increasing doses, generally in milligrams, depending on the protocol. Each visit involving an increase in the daily ingested dose is usually carried out in a hospital/clinic setting due to the risk of allergic reactions (including anaphylaxis) with up-dosing. OIT is considered efficacious but exhibits a relatively high rate of systemic side effects including anaphylaxis and reactions leading to epinephrine use [Citation37]. OIT also requires limitations on activities of daily living and protection generally is lost if treatment is discontinued [Citation38,Citation39]. SLIT targets Langerhans cells (LCs) present in the oral mucosa and involves administration of doses up to 1000-fold less than OIT. Overall, treatment with SLIT has demonstrated a better safety profile, and while results in younger participants are more encouraging, a less robust desensitization to peanut compared with OIT [Citation40,Citation41].
Senti and colleagues underlined in a review that administration of an allergen should occur where high numbers of APCs are present to improve efficacy and thus decrease treatment duration, thereby making the skin, which contains a high density of APCs, an attractive route for desensitization [Citation42,Citation43]. EPIT with an occlusion chamber applied on intact skin, first tested with milk allergy consists of repeated exposure of microgram amounts of allergen onto the skin, which can result in suppression of allergic symptoms or desensitization to an offending allergen [Citation44]. While the peanut patch is the most clinically advanced approach to EPIT with furthest development in peanut allergy, other desensitization techniques through the skin are being investigated: disrupting the skin barrier via coated microneedle patches and allergen application on skin previously tape-stripped or microperforated. Strategies such as tape stripping may serve to increase allergen diffusion into the epidermis, thereby potentially decreasing the dose requirement to achieve clinical benefit, but potentially increasing the risk of systemic exposure to allergen [Citation43,Citation45,Citation46]. Preclinical studies with the peanut patch have shown that exposure to APCs in the epidermis (e.g. LCs) allows the allergen to be captured and processed by these APCs in the superficial layers of the skin. APCs then migrate to the regional lymph nodes and trigger an altered allergen-specific T cell response (i.e. activate immune responses and specific regulatory cytokine production) [Citation8,Citation47]. Interestingly, EPIT induces desensitization by leveraging the unique properties of the skin, an organ equipped with the largest immune system in the body, but rarely investigated or used as a tolerogenic organ. A mouse model of peanut allergy revealed that T regulatory cells induced with the peanut patch had expressed a much broader repertoire of homing receptors than OIT and SLIT, thus allowing for EPIT-induced T regulatory cells to travel to other sites of allergic reactivity, such as the gut or lung [Citation8,Citation47,Citation48].
In clinical studies of the peanut patch in children, at Month 12, increases in both ED and CRD were observed for the active group with continued gains after long term daily use [Citation12–14,Citation16]. The QRA model shows that increasing an individual’s ED to 300 mg (1 peanut) or 1000 mg (3–4 peanuts) of peanut protein is clinically relevant and associated with a substantial risk reduction [Citation17]. Thus, increasing ED provides a level of protection against potential allergic reactions including anaphylaxis in the event of accidental peanut exposure, and demonstrates its real-world clinical relevance. Additionally, these benefits appeared long lasting in children aged 4–11 years; 78% of the participants sustained an ED of ≥1000 mg after discontinuing treatment for 2 months [Citation12,Citation13].
The safety of the desensitization technique largely relies on the use of microgram amounts of allergen, and the specific capture by the skin APCs, limiting systemic allergen exposure and distribution. Indeed, EPIT with the peanut patch has shown to be a potentially suitable option for the treatment of life-threatening allergies, due to minimal reports of AEs leading to anaphylaxis or epinephrine use, without affecting activities of daily living in the way that OIT does [Citation12–16,Citation49].
Efficacy and safety profiles also help explain the observed benefit on QoL, which improved after 24 months of active treatment. The significant difference observed in change from baseline to Month 24 in FAQLQ mean total scores in participants with active treatment reveals that, in addition to improving ED and minimizing the risk of unintentional allergic exposure, the treatment also improves the emotional well-being of the participants by decreasing food-related anxiety and social dietary restrictions [Citation14,Citation15,Citation26].
5. Conclusions
Several AITs are being investigated for treatment of peanut allergy. The skin, which is one route under investigation for AIT administration, is a highly specialized immune organ with a unique ability to promote immune tolerance. The ViaskinTM Peanut technology platform, the most clinically advanced approach to EPIT, uses an occlusion chamber containing peanut allergen to facilitate absorption into intact skin; and with frequent repeated exposures, promotes desensitization and potentially tolerance. Treatment with the peanut patch has demonstrated a high compliance rate, >96% in previous phase 3 studies, and low AE-related discontinuation rates. The most frequently reported TEAE was local administration site reactions. Both local and systemic reactions, including anaphylaxis, were mild-to-moderate, readily managed, and decreased in frequency over time. The peanut patch met the efficacy primary endpoint in children aged 1–3 years with a responder rate of 67% vs 33.5% with placebo, and CRD improvements at Month 12. Furthermore, the longer-term sustained desensitization to 1000 mg of peanut protein may suggest an ability to modify the disease and potential to improve its prognosis in children aged 4–11 years. This goes along with improved FAQLQ scores and a substantial risk reduction of allergic reactions during unintentional peanut exposure. Taken together, these data suggest that the patch may be an effective option for the treatment of peanut allergy.
6. Expert opinion
The numerous clinical studies conducted in children with peanut allergy suggest that the peanut patch has a clinical benefit: as demonstrated in clinical trials by superior desensitization compared to placebo, improvements in quality of life, and good tolerability over multiple years of exposure. This potential treatment option is designed to not require alterations to lifestyle, to be simple to use, and is intended to be adapted to the lives of patients: phase 2 and 3 trials show high treatment compliance rates among participants and a substantial majority (>60%) of participants reported the patch as not difficult to apply or painful to remove [Citation16]. The simplicity of the patch also allows physicians to easily incorporate it into practice: EPIT does not require physician-monitored dose escalation and doctor visits are fewer with shorter durations. EPIT aims to provide a more readily accessible and potentially less burdensome approach for patients and caregivers in managing peanut allergy [Citation12–16,Citation42,Citation49,Citation50].
Currently, in food allergy, desensitization is being studied utilizing several routes of administration, mainly the digestive tract (OIT or SLIT) or the skin (EPIT). The skin route has multiple advantages. The amount of allergen exposure over the course of treatment is substantially lower with EPIT. The microgram amounts of allergen used with the peanut patch, which totals approximately 1 peanut kernel after 3 years of daily treatment, highlight the potency of the skin in inducing desensitization. In comparison, approximately 1000 peanut kernels are consumed after 3 years while on maintenance OIT. The mechanistic pathway of EPIT has been designed to limit allergen absorption into the bloodstream and thus reduces the risk of systemic allergen exposure and subsequently the likelihood of having an allergic response. For example, in contrast with OIT, evidence indicates that treatment with EPIT does not induce allergic reactions of the digestive mucosa, such as eosinophilic esophagitis. Moreover, utilizing the skin as the route for desensitization does not risk impacting the absorption capacities of the digestive tract at a period of life in children that constitutes a major window of opportunity for growth and proper development [Citation8,Citation37,Citation40,Citation41].
During food AIT children generally respond more readily to treatment than adolescents/adults. In phase 2 studies, the peanut patch had the greatest effect in children aged 6–11 years compared with adolescents/adults, aged 12–55 years (53.6% vs 46.4%, respectively) [Citation13,Citation16,Citation22]. The differences may be attributed to a greater immune plasticity in children as observed with other treatment and prevention studies. Other explanations for these observed differences in adolescents/adults might involve a lower permeability of the stratum corneum, a relatively lower dose on a per weight/surface area basis compared with younger children, a relatively smaller patch size relative to total body surface area, and the location where the patch is applied [Citation51]. In murine models, higher doses and exposure to larger surface areas increase efficacy. These aspects may be the focus of future studies investigating peanut EPIT.
The attributes of EPIT with the peanut patch point to potentially interesting outcomes. First, the patch could allow an instant start of desensitization in peanut-allergic children, a process that may also be further investigated with research of EPIT in other foods. Second, long-term surveillance studies might analyze whether EPIT treatment interferes with the allergic march. Third, all the current EPIT data illuminate the skin as an organ useful for allergy treatment instead of being regarded as a diseased, or potentially diseased organ. Overall, among the various AIT approaches for peanut allergy, EPIT with the peanut patch demonstrates efficacy of the skin route, particularly in the pediatric age range and the advantages over the oral/digestive route, and therefore may offer a safe, effective, and convenient treatment option for patients.
Article highlights
EPIT with the peanut patch demonstrated superior desensitization to peanut compared to placebo in peanut-allergic children.
Increases in eliciting dose and cumulative reactive dose to peanut after 12 months with further benefits out to 3 years of treatment in children.
Reduced severity of reactions to peanut during food challenges after 12 months of treatment with the peanut patch.
Consistent safety profile across studies, with mainly mild-to-moderate local application site reactions.
Participants reported high compliance with treatment and low discontinuations due to adverse events.
Declaration of interests
C Dupont reports being a co-founder and shareholder of DBV Technologies, receives consulting fees from Nestlé, Danone, BMS, and lecture fees from Novalac, Mead Johson. D Fleischer provides research support to institution from DBV Technologies and is a consultant for DBV Technologies. A Burks reports consulting fees from Allergy Therapeutics, Aimmune Therapeutics, Consortia TX, DBV Technologies, Ukko, grants to his institution from Burroughs Wellcome Fund, research grant from the National Institute of Health, and royalties from UpToDate. K Bee and S Chainani are employees of DBV Technologies. H Sampson reports consulting fees from N-Fold, LLC, DBV Technologies, Abbvie, and Siolta Therapeutics, grants to his institution from the National Institute of Allergy and Infectious Diseases and from Allergenis, and royalties from Elsevier. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
One reviewer is a co-founder of Saiba AG. The remaining reviewers have no other relevant financial relationships or otherwise to disclose.
Supplemental Material
Download MS Word (306.8 KB)Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/1744666X.2024.2315221
Additional information
Funding
References
- Bunyavanich S, Rifas-Shiman SL, Platts-Mills TA, et al. Peanut allergy prevalence among school-age children in a US cohort not selected for any disease. J Allergy Clin Immunol. 2014;134(3):753–755.
- Lieberman JA. Severity of peanut allergy and the unmet gaps in care: a call to action. Am J Manag Care. 2018;24(19 suppl):S412–S418.
- Gupta RS, Warren CM, Smith BM, et al. The public health impact of parent-reported childhood food allergies in the United States. Pediatrics. 2018;142(6):e20181235. doi: 10.1542/peds.2018-1235
- Sicherer SH, Muñoz-Furlong A, Godbold JH, et al. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010;125(6):1322–1326. doi: 10.1016/j.jaci.2010.03.029
- Peters RL, Allen KJ, Dharmage SC, et al. Natural history of peanut allergy and predictors of resolution in the first 4 years of life: a population-based assessment. J Allergy Clin Immunol. 2015;135(5):1257–1266.e1–2.
- Peters RL, Guarnieri I, Tang MLK, et al. The natural history of peanut and egg allergy in children up to age 6 years in the HealthNuts population-based longitudinal study. J Allergy Clin Immunol. 2022;150(3):657–665.e13. doi: 10.1016/j.jaci.2022.04.008
- Pavón-Romero GF, Parra-Vargas MI, Ramírez-Jiménez F, et al. Allergen immunotherapy: current and future trends. Cells. 2022;11(2):212. doi: 10.3390/cells11020212
- Dioszeghy V, Mondoulet L, Dhelft V, et al. Epicutaneous immunotherapy results in rapid allergen uptake by dendritic cells through intact skin and downregulates the allergen-specific response in sensitized mice. J Immunol. 2011;186(10):5629–5637. doi: 10.4049/jimmunol.1003134
- Hervé PL, Dioszeghy V, Matthews K, et al. Recent advances in epicutaneous immunotherapy and potential applications in food allergy. Front Allergy. 2023 Oct 27;4:1290003. PMID: 37965375; PMCID: PMC10641725. In-depth review of the immunologic properties of the skin and data supporting the mechanism of action of epicutaneous immunotherapy for food allergy. doi: 10.3389/falgy.2023.1290003
- Mondoulet L, Dioszeghy V, Puteaux E, et al. Intact skin and not stripped skin is crucial for the safety and efficacy of peanut epicutaneous immunotherapy (EPIT) in mice. Clin Transl Allergy. 2012;2(1):22. doi: 10.1186/2045-7022-2-22
- Fleischer DM, Spergel JM, Kim EH, et al. Evaluation of daily patch application duration for epicutaneous immunotherapy for peanut allergy. Allergy Asthma Proc. 2020;41(4):278–284. doi: 10.2500/aap.2020.41.200045
- Fleischer DM, Greenhawt M, Sussman G, et al. Effect of epicutaneous immunotherapy vs placebo on reaction to peanut protein ingestion among children with peanut allergy: the PEPITES randomized clinical trial. JAMA. 2019;321(10):946–955. doi: 10.1001/jama.2019.1113
- Greenhawt M, Kim EH, Campbell DE, et al. Improvements in eliciting dose across baseline sensitivities following 12 months of epicutaneous immunotherapy (EPIT) in peanut-allergic children aged 4 to 11 years. J Allergy Clin Immunol Pract. 2020;8(9):3219–3221. doi: 10.1016/j.jaip.2020.05.030
- Fleischer DM, Shreffler WG, Campbell DE, et al. Long-term, open-label extension study of the efficacy and safety of epicutaneous immunotherapy for peanut allergy in children: PEOPLE 3-year results. J Allergy Clin Immunol. 2020;146(4):863–874. doi: 10.1016/j.jaci.2020.06.028
- Pongracic JA, Gagnon R, Sussman G, et al. Safety of epicutaneous immunotherapy in peanut-allergic children: REALISE randomized clinical trial results. J Allergy Clin Immunol Pract. 2022;10(7):1864–1873.e10. doi: 10.1016/j.jaip.2021.11.017
- Greenhawt M, Sindher SB, Wang J, et al. Phase 3 trial of epicutaneous immunotherapy in toddlers with peanut allergy. N Engl J Med. 2023;388(19):1755–1766. doi: 10.1056/NEJMoa2212895
- Baumert JL, Taylor SL, Koppelman SJ. Quantitative assessment of the safety benefits associated with increasing clinical peanut thresholds through immunotherapy. J Allergy Clin Immunol Pract. 2018;6(2):457–465.e4. doi: 10.1016/j.jaip.2017.05.006
- Remington BC, Krone T, Kim EH, et al. Estimated risk reduction to packaged food reactions by epicutaneous immunotherapy (EPIT) for peanut allergy. Ann Allergy Asthma Immunol. 2019;123(5):488–493.e2. doi: 10.1016/j.anai.2019.08.007
- Remington BC, Blom WM, Bassa B, et al. Risk of shared equipment in restaurants for consumers with peanut allergy: a simulation for preparing Asian foods. Ann Allergy Asthma Immunol. 2020;125(5):543–551.e6. doi: 10.1016/j.anai.2020.07.030
- Remington BC, Campbell DE, Green TD, et al. Post hoc analysis of epicutaneous immunotherapy for peanut allergy phase 3 results: relevance for exposure through restaurant meals. Ann Allergy Asthma Immunol. 2021;126(2):208–209. doi: 10.1016/j.anai.2020.11.015
- Remington BC, Koppelman SJ, Green TD, et al. Predicted number of peanut-allergic patients needed to treat with epicutaneous immunotherapy (EPIT) to prevent one allergic reaction: a novel approach to assessing relevance. Allergy. 2021;76(10):3223–3226. doi: 10.1111/all.14973
- Brown-Whitehorn TF, de Blay F, Spergel JM, et al. Sustained unresponsiveness to peanut after long-term peanut epicutaneous immunotherapy. J Allergy Clin Immunol Pract. 2021;9(1):524–526. doi: 10.1016/j.jaip.2020.08.017
- Sampson HA, Shreffler WG, Yang WH, et al. Effect of varying doses of epicutaneous immunotherapy vs placebo on reaction to peanut protein exposure among patients with peanut sensitivity: a randomized clinical trial. JAMA. 2017;318(18):1798–1809. doi: 10.1001/jama.2017.16591
- Loprinzi Brauer CE, Motosue MS, Li JT, et al. Prospective validation of the NIAID/FAAN criteria for emergency department diagnosis of anaphylaxis. J Allergy Clin Immunol Pract. 2016;4(6):1220–1226. doi: 10.1016/j.jaip.2016.06.003
- Campbell RL, Hagan JB, Manivannan V, et al. Evaluation of national institute of allergy and infectious diseases/food allergy and anaphylaxis network criteria for the diagnosis of anaphylaxis in emergency department patients. J Allergy Clin Immunol. 2012;129(3):748–752. doi: 10.1016/j.jaci.2011.09.030
- DunnGalvin A, Fleischer DM, Campbell DE, et al. Improvements in quality of life in children following epicutaneous immunotherapy (EPIT) for peanut allergy in the PEPITES and PEOPLE studies. J Allergy Clin Immunol Pract. 2021;9(1):216–224.e1. doi: 10.1016/j.jaip.2020.08.015
- Togias A, Cooper SF, Acebal ML, et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of allergy and Infectious Diseases–sponsored expert panel. Ann Allergy Asthma Immunol. 2017;118(2):166–173.e7. doi: 10.1016/j.anai.2016.10.004
- Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(1 suppl):S1–S55.
- Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored expert panel report. J Allergy Clin Immunol. 2010;126(6):1105–1118. doi: 10.1016/j.jaci.2010.10.008
- Muraro A, Roberts G, Worm M, et al. Anaphylaxis: guidelines from the European Academy of Allergy and clinical immunology. Allergy. 2014;69(8):1026–1045. doi: 10.1111/all.12437
- Alshajarah HA, Alghamdi HA, Alberi ZA, et al. Peanut-induced anaphylaxis in children: a literature review. Cureus. 2022;14(12):e32946. doi: 10.7759/cureus.32946
- Avery NJ, King RM, Knight S, et al. Assessment of quality of life in children with peanut allergy. Pediatr Allergy Immunol. 2003;14(5):378–382. doi: 10.1034/j.1399-3038.2003.00072.x
- Bollinger ME, Dahlquist LM, Mudd K, et al. The impact of food allergy on the daily activities of children and their families. Ann Allergy Asthma Immunol. 2006;96(3):415–421. doi: 10.1016/S1081-1206(10)60908-8
- King RM, Knibb RC, Hourihane JO. Impact of peanut allergy on quality of life, stress and anxiety in the family. Allergy. 2009;64(3):461–468. doi: 10.1111/j.1398-9995.2008.01843.x
- Primeau MN, Kagan R, Joseph L, et al. The psychological burden of peanut allergy as perceived by adults with peanut allergy and the parents of peanut-allergic children. Clin Exp Allergy. 2000;30(8):1135–1143.
- Kim EH, Burks AW. Food allergy immunotherapy: oral immunotherapy and epicutaneous immunotherapy. Aller. 2020 Jun;75(6):1337–1346. doi: 10.1111/all.14220. Epub 2020 Feb 28. PMID: 32034781.
- Chu DK, Wood RA, French S, et al. Oral immunotherapy for peanut allergy (PACE): a systematic review and meta-analysis of efficacy and safety. Lancet. 2019;393(10187):2222–2232. doi: 10.1016/S0140-6736(19)30420-9
- Anagnostou A. Weighing the benefits and risks of oral immunotherapy in clinical practice. Allergy Asthma Proc. 2021;42(2):118–123. doi: 10.2500/aap.2021.42.200107
- European Medicines Agency. Palforzia: European public assessment report (EPAR): Authorisation Details; [first published 2020 Dec 21; updated 2022 Sep 19; cited 2022 Dec 2]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/palforzia#authorisation-details-section
- Kim EH, Bird JA, Kulis M, et al. Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization. J Allergy Clin Immunol. 2011;127(3):640–646.e1. doi: 10.1016/j.jaci.2010.12.1083
- Kim EH, Bird JA, Keet CA, et al. Desensitization and remission after peanut sublingual immunotherapy in 1- to 4-year-old peanut-allergic children: a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2023. S0091–S6749(23)01116–01118. doi: 10.1016/j.jaci.2023.08.032
- Pfaar O, Bousquet J, Durham SR, et al. One hundred and ten years of allergen immunotherapy: a journey from empiric observation to evidence. Allergy. 2022;77(2):454–468. doi: 10.1111/all.15023
- Senti G, Freiburghaus AU, Kundig TM. Epicutaneous/transcutaneous allergen-specific immunotherapy: rationale and clinical trials. Curr Opin Allergy Clin Immunol. 2010;10(6):582–586. doi: 10.1097/ACI.0b013e32833f1419
- Dupont C, Kalach N, Soulaines P, et al. Cow’s milk epicutaneous immunotherapy in children: a pilot trial of safety, acceptability, and impact on allergic reactivity. J Allergy Clin Immunol. 2010;125(5):1165–1167.
- Spina L, Weisskopf M, von Moos S, et al. Comparison of microneedles and adhesive-tape stripping in skin preparation for epicutaneous allergen delivery. Int Arch Allergy Immunol. 2015;167(2):103–109. doi: 10.1159/000434681
- Takeuchi A, Nomoto Y, Watanabe M, et al. Application of microneedles to skin induces activation of epidermal Langerhans cells and dermal dendritic cells in mice. Biol Pharm Bull. 2016;39(8):1309–1318. doi: 10.1248/bpb.b16-00113
- Clausen BE, Stoitzner P. Functional specialization of skin dendritic cell subsets in regulating T cell responses. Front Immunol. 2015;6:534. doi: 10.3389/fimmu.2015.00534
- Mondoulet L, Dioszeghy V, Ligouis M, et al. Epicutaneous immunotherapy on intact skin using a new delivery system in a murine model of allergy. Clin Exp Allergy. 2010;40(4):659–667. doi: 10.1111/j.1365-2222.2009.03430.x
- Yu Y, Kiran Kumar MN, Wu MX. Delivery of allergen powder for safe and effective epicutaneous immunotherapy. J Allergy Clin Immunol. 2020;145(2):597–609. doi: 10.1016/j.jaci.2019.11.022
- Shamji MH, Durham SR. Mechanisms of allergen immunotherapy for inhaled allergens and predictive biomarkers. J Allergy Clin Immunol. 2017;140(6):1485–1498. doi: 10.1016/j.jaci.2017.10.010
- Del Duca E, Pavel AB, Dubin C, et al. Major differences in expression of inflammatory pathways in skin from different body sites of healthy individuals. J Invest Dermatol. 2019;139(10):2228–2232.e10. doi: 10.1016/j.jid.2019.04.008
- Sampson HA, Gerth van Wijk R, Bindslev-Jensen C, et al. Standardizing double-blind, placebo-controlled oral food challenges: american academy of allergy, asthma and immunology-european academy of allergy and clinical immunology PRACTALL consensus report. J Allergy Clin Immunol. 2012;130(6):1260–1274.