 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
Leg problems including lameness and tibial dyschondroplasia (TD) are one of the most important factors affecting health and animal welfare in meat-type poultry, which are considerably impacted by dietary vitamin regimens. The present study was conducted to investigate the effects of dietary vitamin levels on growth performance and tibia quality in 14-d-old meat ducks.
Methods
One-d-old Cherry Valley meat-type male ducks were allocated to two vitamin regimens for 14 d, which was formulated according to the recommendation of the National Research Council (NRC, 1994) or China Agricultural Industry Standards (NY/T 2122-2012). Each group included 8 replicates with 16 birds per replicate.
Results
There was no significant difference in the growth performance of ducks, whereas dietary NY/T vitamin supplementation decreased leg abnormalities as compared to the NRC group, along with similar tibia length, perimeter, and the relative weight of tibia. The tibia from the birds that received the NY/T vitamin diet exhibited markedly higher (p < 0.05) ash level, calcium (Ca) content, and bone strength when compared with the bone of NRC vitamin-treated birds. In the tibia proximal end, the increased trabecular area and a number of bone trabeculae were observed in the NY/T vitamin diet (p < 0.05). Moreover, a pronounced increase (p < 0.05) in the serum Ca, alkaline phosphatase activity, and C-terminal cross-linked telopeptide of type I collagen level as the indicators of bone formation were found in NT/T vitamin diet than them in NRC diet. Dietary supplementation with NY/T vitamin regimen also significantly upregulated the relative gene expression of phosphate regulating endopeptidase homolog x linked (Phex), an osteoblastic gene, as compared to the NRC vitamin diet (p < 0.05). However, the concentration of serum bone resorption markers and the transcription of osteoclast-related genes were comparable between the NRC and NY/T vitamin groups (p > 0.05).
Conclusion
NY/T vitamin diet improved tibia quality and subsequently decreased leg abnormalities in 14-d-old ducks, which was probably linked with the promoted bone formation.
HIGHLIGHTS
Dietary vitamin regimens treatment has not significant altered the growth performance and tibia growth of meat ducks
Diet with NY/T vitamin regimen decreased leg abnormalities
The supplementation of NY/T vitamin increased tibia ash content, density, and bone microstructure
The improved tibia quality was associated with the promoted bone formation in 14-d-old meat ducks.
Introduction
Progresses in genetic evolution, nutrition, and rearing conditions contribute to significant improvement in the growth rate and feed efficiency of commercial domestic birds, reflecting increased total meat yield, with little or no compensatory changes in their skeletal structure (Bello et al. Citation2014; Zhang et al. Citation2019). As a consequence, these birds often suffer inadvertently from leg disorders and bone deformations, which impair growth performance due to the difficulties in getting water and feed, and lead to high economic loss (Abbasi et al. Citation2017). In addition, leg weakness including lameness and tibia dyschondroplasia (TD) is also considered to be one of the most important factors affecting health and animal welfare suffering from pain, discomfort, and absent natural behaviours (Zhang et al. Citation2018). For commercial meat ducks, the prevalence of gait problems is about 14–21% (Jones and Dawkins Citation2010), and thus it is urgent to search for efficient strategies to improve tibia growth and mineralisation in meat ducks.
Nutritional manipulation is a common practice to improve the skeletal health of ducks such as using a low-nutrient density diet (Zhang et al. Citation2018), supplementation with calcium (Ca) (Zhang et al. Citation2018), vitamins (Likittrakulwong et al. Citation2021), resistance starch (Zhang et al. Citation2022), etc. For instance, dietary supplementation of vitamin D3 was confirmed to increase the tibial mass and mineralisation of meat ducks mainly through its suppressed roles in bone resorption (van Ballegooijen et al. Citation2015; Zhang et al. Citation2020). Vitamin D deficiency is usually characterised by inadequate bone disorders in humans (Whyte and Thakker Citation2013). Among the fat-soluble vitamins, vitamin K (Tsugawa and Shiraki Citation2020) and vitamin E (Chin and Ima-Nirwana Citation2015) were also linked with bone health via modifying the Gla‐protein family and exerting antioxidant activity, respectively. Ongoing evidence supplied by some excellent reviews also summarised the beneficial role of vitamin B on bone health (Dai and Koh Citation2015), in particular vitamin B12 (cobalamin) and B9 (folic acid), which could enhance the microarchitecture and decrease bone fragility. In this context, optimal vitamin level appropriate in meat ducks’ diets is significant to bone health and allows them to perform to their genetic potential. Although the vitamin levels were recommended by the National Research Council (NRC, 1994) (Dale Citation1994), China Agricultural Industry Standards (NY/T 2122-2012) (Standards. CAI Citation2012), DSM vitamin guideline (DSM, 2022) (Products DN Citation2022), and so on, the variations in each vitamin content of each other is very wide, especially the fat-soluble vitamins. Comparing the effects of different premix vitamin levels on bone development found that dietary supplementation of high‐vitamin level (DSM vitamin regimen) significantly improved sternum calcification than the low‐vitamin level diet (70% NRC vitamin regimen) in meat ducks (Zhang et al. Citation2019). It implies there might be different implications in tibia growth and mineralisation in commercial duck responses to various vitamin regimens from the recommended levels of NRC (1994) and NY/T (2122-2012).
Therefore, the purpose of this study was to first explore the effects of vitamin regimen on performance, leg abnormalities, and tibia quality. To understand the potential mechanisms, the serum bone turnover markers and related gene expressions of bone metabolism were also involved in this study.
Materials and methods
Animal care
The study design and procedures involved in this study were approved by the Institutional Animal Care and Use Committee of Yibing College (Sichuan, China).
Study design and diets
A total of 256 male Cherry Valley meat-type ducks with an average body weight (BW) of 48.5 ± 0.36 g were collected from a commercial hatchery. Birds were placed randomly into NRC (1994) and NY/T (2012) vitamin regimen groups with 8 replicates (16 birds per pen) in a temperature- and humidity-controlled room. The temperature in the room was 32 °C for the first week and then gradually lowered to 26 °C by 14 d based on normal management practices. The relative humidity was maintained between 50% and 60%. Ducks were raised to 14 d under standard management conditions with ad libitum of feed and water. Basal feed was formulated to meet the nutrient requirements of NY/T (2122-2012) (Standards. CAI Citation2012, ). All vitamins used in this study were provided by DSM Ltd. (China). At 1 and 14 d of age, feed intake and BW were recorded, and body gain and feed conversion expressed as the ratio of food intake to gain (F: G) was calculated for each pen. In addition, the number of mortality and culled ducks suffered from leg deformations and fractures was counted to statistics the mortality due to leg deformations on a pen basis.
Table 1. Composition and nutrient levels in the basal diets.
Sampling
On d 14, 2 birds were selected based on the average BW of each replicate and sampled after fasting for 12 h. Blood was collected through the jugular vein with an 80 mm needle 22 G and centrifuged at 4000 × g/15 min at 4 °C for serum after complete coagulation. The left and right tibias were dissected for tibial histology, mRNA expression, bone growth, and bone mass determination.
TD score
After sampling, another three birds from each pen were randomly selected, and tibias were removed from both legs and the proximal end was subjected to TD score according to the previous description (Edwards and Veltmann Citation1983). When the tibia displayed no, slight, medium, or severe lesion, it would be scored as 0, 1, 2, or 3, respectively.
Tibia histological analysis
The fixed tibia samples were decalcified using 10% ethylene diamine tetraacetic acid (EDTA) solution, dehydrated, and embedded in paraffin wax. Then, the samples were cut into sagittal sections for alkaline phosphatase (ALP) and toluidine blue staining (Sigma-Aldrich, Inc., MO, USA), respectively. Histopathological images were collected using a microscope equipped (Nikon Corporation, Tokyo, Japan). Undergoing the greyscale transform, the toluidine blue slides were used for the histological analysis as previously described (Vidal et al. Citation2012), i.e. total area (T.Ar), calcified bone area (B.Ar), bone perimeter (B.Pm) can be obtained directly from images, and then the trabecular bone area (Tb.Ar), number (Tb.N), and thickness (Tb.Th) were calculated as the following equations:
Bone growth
Tibia growth was evaluated based on the fresh weight and dimensions. Tibial length and average diameter of the middle part of the tibia were measured by vernier calliper and flexible rule, respectively. These tibias were then weighed after being dried with filter paper, and the relative tibia fresh weights were calculated as the ratio of fresh weight to BW.
Tibia-breaking strength analysis
Biomechanical testing was performed by the 3-point bending method with a TA-XT Plus Texture Analyser (TA. XT. plus. Stable Micro Systems, Surrey, UK) at a constant 50 kg load cell. Loading proceeded at the mid-point of the tibia with a constant rate (5 mm/min) up to the breaking of the bone. The ultimate load was obtained from the force-displacement.
Tibia mineralisation
Tibial fat-free weight and density were determined according to previous methods (Zhang et al. Citation2017). Specifically, bone volume (cm3) was measured based on the quantum of water overflowing from a filled container, and subsequently, these tibias were defeated by immersing in ethyl ether, air-dried for 24 h at room temperature for fat-free weight determination. The density of tibia (g/cm3) was calculated as the ratio of fat-free weight to bone volume. Afterward, the tibia was ashed in a muffle furnace at 550 °C for 24 h. The ash content was calculated based on the percentage of dry-defatted weight. Ca and phosphorus (P) contents (% dry-defatted weight) in ash were determined through EDTA titration and ammonium metavanadate colorimetry, respectively.
Serum bone turnover markers
Commercial kits were applied for the concentrations of bone turnover markers in serum including ALP, procollagen type I N-terminal propeptide (P1NP), and C-terminal cross-linked telopeptide of type I collagen (CTx) were quantified with commercial assay kits. All kits were obtained from Meimian Industrial Co., Ltd. (Jiangsu, China). All samples were tested in triplicate within each assay by the manufacturer’s instructions. Serum Ca and P content were measured through the chromogenic complex with o-cresol phthalein and the molybdenum blue method using Bio-chemistry Analyser (Yellow Springs Instrument Co. Inc., Yellow Springs, OH), respectively.
Gene expression by real-time PCR
Total RNA was isolated and subjected to the check of quantity and quality by spectrometry and denaturing agarose gel electrophoresis, respectively. cDNA was synthesised by the cDNA reverse transcription kit (Takara, Dalian, China). The obtained cDNA was used for the gene expression analysis using SYBR green qPCR master mix (Takara) in an ABI 7500 RT-PCR detection system (Applied Biosystems). Target cDNA was amplified at 95 °C for 15 s followed by 40 cycles of 95 °C for 30 s and 60 °C for 34 s with a final melting curve analysis. Primers were designed using Primer 3 as shown in . Glyceraldehyde-3-phosphate dehydrogenase (Gapdh), and b-actin were selected as the housekeeping genes to normalise the relative abundances of RNA of interest.
Statistical analysis
The data obtained were expressed as mean ± standard deviation (SD) and subjected to the Shapiro-Wilk and Levene’s test for normal distribution and homogeneity of variances, respectively. Differences between NRC and NY/T groups were assessed using a two-tailed unpaired t-test (normal distribution) or the Mann-Whitney U test (non-normal distribution). When the p ≤ 0.05 and 0.5 < p < 0.1, the variation was considered statistically significant and of tendency, respectively.
Results
Effects of dietary vitamin levels on growth performance
As shown in , There was no significant difference in BW gain, feed intake, or feed conversion between NRC and NY/T vitamin groups (p > 0.05), whereas supplementation with NY/T vitamin level diet tended to increase (p = 0.082) the BW at 14 d when compared with the NRC vitamin regimen diet.
Table 2. Composition of the vitamin premixes for ducking (0–14 d)Table Footnotea.
Leg abnormalities and tibial growth response to dietary vitamin levels
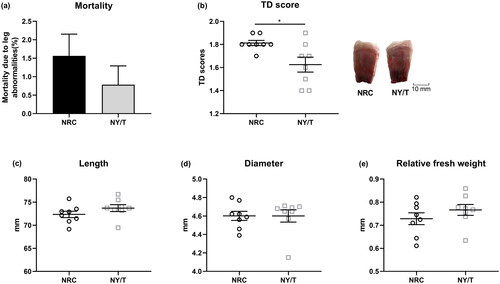
As compared to the NRC group, the mortality due to leg deformations of the NY/T vitamin diet-fed birds was slightly lower (), and NY/T vitamin supplementation significantly decreased (p < 0.05) the TD score (). No apparent differences (p > 0.05) were found for tibia growth, indicated by comparable tibia length, perimeter, and relative weight ().
Figure 1. Effect of dietary vitamin regimen on (a) the mortality due to leg abnormalities, (b) tibial dyschondroplasia (TD) score, (c) bone length, (d) diameter, and (e) relative fresh weight of 14-d-old ducks. Values are means ± standard deviation represented by vertical bars (n = 8). * Mean values are significantly different between the National Research Council (NRC) and China Agricultural Industry Standards (NY/T) group (p ≤ 0.05).
NY/T vitamin regimen increased tibia mass and strength
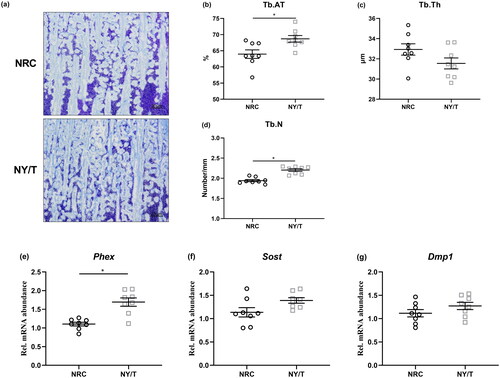
The influence of dietary vitamin regimen on tibia mass and breaking strength is summarised in . Birds that received the NY/T vitamin diet exhibited similar fat-free weight and higher density (p = 0.058) than those who consumed the NRC vitamin diet (). Ducks fed the NY/T vitamin diets also displayed markedly higher (p < 0.05) ash level, Ca content, and comparable P content in the tibia, as well as notably increased (p < 0.05) bone strength when compared with the NRC vitamin-treated birds (). The data of bone histomorphometry analysis also showed that the NY/T vitamin diet improved (p < 0.05) tibia microstructure of the tibia proximal end, evidenced by higher Tb.Ar and Tb.N in the NY/T group than that in NRC vitamin diets (). In terms of the osteocyte-specific marker genes expression, the diet with NY/T vitamin regimen upregulated the expressions of phosphate regulating endopeptidase homolog x-linked (Phex), sclerostin (Sost), and dentine matrix protein 1 (Dmp1) as compared to the NRC vitamin diet to varying degrees ().
Figure 2. Tibial quality responses to dietary vitamin regimen for meat ducks. (a) Fat-free weight, (b) density, (c) tibial ash, (d) calcium (Ca) and phosphorus (P) content, and (e) tibia-breaking strength. Values are means ± standard deviation represented by vertical bars (n = 8). * Mean values are significantly different between NRC and NY/T group (p ≤ 0.05).
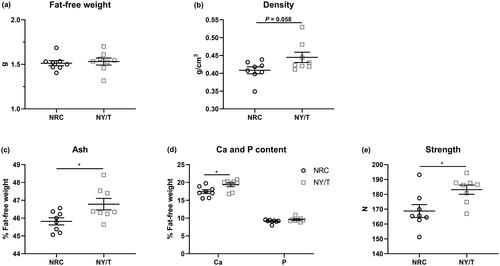
Figure 3. Effect of dietary vitamin regimen on tibial microstructure. (a) toluidine blue stained and the morphometric analysis for (b) the trabecular bone area (Tb.Ar), (c) trabecular thickness (Tb.Th), and (d) number (Tb.N) determined by histomorphometry. Real-time-PCR analysis of osteocyte marker genes including (e) phosphate regulating endopeptidase homolog x-linked (Phex), (f) sclerostin (Sost), and (g) dentine matrix protein 1 (Dmp1) in the tibia proximal end. Values are means ± standard deviation represented by vertical bars (n = 8). * Mean values are significantly different between NRC and NY/T group (p ≤ 0.05).
Dietary NY/T vitamin treatment promotes bone formation
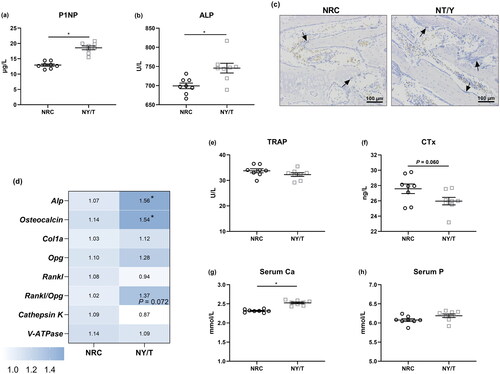
As illustrated in , a pronounced increase (p < 0.05) in the serum ALP activity and P1NP level as the indicators of bone formation were observed in the NT/T vitamin diet than in the NRC diet (), which was further confirmed by the ALP staining (). Dietary supplementation with NY/T vitamin regimen significantly increased (p < 0.05) the Mrna abundance of Alp and osteocalcin, along with parable collagen Ia (Col1a) expression, as compared to the NRC vitamin group ().
Figure 4. Impact of dietary vitamin regimen on bone turnover and calcium (Ca) and phosphorus (P) homeostasis. Serum bone formation markers, (a) alkaline phosphatase (ALP) and (b) procollagen type I N-terminal propeptide (P1NP) were determined, and (c) ALP was staining. (d) Bone turnover genes including alp, osteocalcin, collagen Ia (Col1a), steoprotegerin (Opg), receptor activator of nuclear factor kB ligand (Rankl), cathepsin K, and vacuolar-type ATPase (V-ATPase) in the tibia proximal end were determined. Serum bone resorption markers (e) (e) tartrate-resistant acid phosphatase (TRAP) and (f) C-terminal cross-linked telopeptide of type I collagen (CTx), as well as serum Ca and P were quantified. Values are means ± standard deviation represented by vertical bars (n = 8). * Mean values are significantly different between NRC and NY/T group (p ≤ 0.05).
Influence on bone resorption and serum Ca and P content
About the NRC vitamin group, the administration with NY/T vitamin did not notably change (p > 0.05) the transcription of osteoprotegerin (Opg), receptor activator of nuclear factor-κ B ligand (Rankl), vacuolar-type H+-ATPase (V-ATPase), and cathepsin K, whereas it tended to reduce the ratio of Rankl to Opg (p = 0.072). Reflecting on serum, NY/T vitamin manipulation failed to change the activity of TRAP as compared to the NRC vitamin diet (). Interestingly, feeding NY/T vitamin diet was noticed to decrease the content of CTx (p = 0.060, ). In addition, serum Ca but not P concentrations were significantly increased (p < 0.05) by the NY/T vitamin diet when compared to NRC diet in 14 d of age ducks ().
Discussion
In the poultry industry, the recommended vitamin levels are usually referring to the standards from NRC (1994) (Dale Citation1994), NY/T (2122-2012) (Standards. CAI Citation2012), and DSM (2022) (Products DN Citation2022) with wide variations in each vitamin content of each other. Considering the comprehensive goal of vitamin utilisation in commercial meat ducks is to optimise animal health, welfare, and nutritional products rather than simple growth performance. The effects of dietary vitamin premixes on tibia quality, an important trait of bone health, were evaluated in the current study. The outcomes indicated that the supplementation of the NY/T vitamin diet could improve tibia mass and microstructure, and subsequently increase bone strength and decrease leg abnormalities in 14-d-old ducks, which might be attributed to its promoting effect on bone formation.
In this study, feeding NRC or NY/T vitamin diets to meat ducks did not significantly alter weight gain, feed intake, and feed conversion during 1 to 14 d, whereas supplementation with the NY/T vitamin level diet tended to increase the BW at 14 d when compared with the NRC vitamin regimen diet. Similarly, when comparing to the NRC (1994) vitamin diet, supplementation with the DSM (2011) vitamin regimen has a slightly higher BW of meat ducks at 14 d (Ren et al. Citation2017). Zhang et al. (Citation2019) pointed out that a diet with the DSM (2016) vitamin premix remarkably increased the BW of ducks at 49 d, whereas the dietary vitamin treatment did not change the growth performance of 14- and 35-d-old ducks (Zhang et al. Citation2019). Nevertheless, a slight effect on the growth performance of the broiler also was noticed by reducing the dietary vitamin levels from 28 to 49 d (Khajali et al. Citation2006). The withdrawal of vitamins from the broiler diet did not impact the BW at 42 d (Mirshekar et al. Citation2013). Study feeding the basal diet 0, 33%, 66%, 100%, and 133% vitamin premix further supported the insignificant influences of dietary vitamin levels on the growth performance of meat-type birds, indicating that the quantities of vitamins in NRC recommendation might be sufficient to the minimum recommended requirement for meat ducks (Moravej et al. Citation2013). These controversial results about the roles of vitamin levels in growth performance might partly derive from the age of meat-type birds or maternal nutrition for ducklings (Ren et al. Citation2016).
Leg health is closely associated with growth performance and carcase process in meat-type bird production because the birds with leg abnormality have difficulties in accessing the food and water, leading to slight BW and higher incidences of off-grade carcases. In the present study, although the vitamin levels failed to alter tibia length, perimeter, and relative weight, a beneficial impact of the NY/T vitamin diet on leg problems of meat ducks was noticed evidenced by the lower mortality due to leg abnormalities and TD score in the NY/T group. One of the potential reasons might be due to the increased vitamin D3 level in the NY/T vitamin regimen (400 vs. 2000 in the NRC and NY/T groups, respectively). Indeed, a diet with 1500 or 3,500 IU/kg of vitamin D3 notably reduced the mortality and TD score in broilers (Khan et al. Citation2010). Improved tibia strength could enhance the ability of the tibia to support the load from BW increase and external mechanical pressure, which might explain partly the positive roles of NY/T in the leg health of meat ducks in this study. Ducks fed the NY/T vitamin diets displayed markedly higher bone strength when compared with the NRC vitamin-treated birds. The increase in tibia density indicating more structurally solid is linked to the mineral constantly depositing in the periosteal surface during the period of bone mineralisation (Shim et al. Citation2012). Accordingly, the bone mass was further determined and showed that dietary inclusion of NY/T vitamin regimen improved the ash level and Ca content, as well as the bone microstructure through increasing Tb.Ar and Tb.N, and reducing Tb.Sp, suggesting that the NY/T vitamin level offered could promote tibia mineralisation and increase bone mass. Consistent with these findings, a low nutrient density diet supplemented by the NY/T vitamin diets was noticed to increase the mineral deposition and strength compared to the NRC vitamin diets (Zhang et al. Citation2020). The supplementation of high‐vitamin levels (DSM, 2016) in the diet al.so significantly promoted the sternum calcification of meat ducks, instructed by increased ash, Ca, and P content in 49-d-old meat ducks. Collectively, these results revealed that a dietary NY/T vitamin regimen exerts beneficial effects on tibia mass and mechanical properties of meat ducks from 1 to 14 d (Zhang et al. Citation2019).
To understand the relationships between dietary vitamin regimen and bone remodelling, bone formation was evaluated histologically and biochemically in the present study. The increased concentration of circulating bone formation markers, P1NP and ALP, suggested that the promoting roles of NY/T on the tibia quality of meat ducks are likely mediated by increasing bone formation. As a pivotal important enzyme that hydrolyses pyrophosphate (PPi) to inorganic phosphate (Pi), in addition to clearing the inhibitor of mineralisation, the producing Pi also facilitates bone mineralisation during the stage of bone formation (Orimo Citation2010). Osteocalcin is primarily produced by osteoblasts and therefore its fluctuation in serum serves as a biochemical marker for bone formation (Mizokami et al. Citation2017). Serum higher ALP activity, ALP staining, upregulated mRNA level of Alp, osteocalcin, and the osteocytes gene (Phex, Sost, and Dmp1) implied that NY/T promoted the bone formation of tibia. From the composition perspective, the increased fold of vitamin D3, vitamin K3, vitamin B2, vitamin E, and vitamin B12 in NY/T vitamin levels were the top 5 higher than NRC vitamins (), which probably accelerated the tibia calcification. It is well-known that vitamin D plays key roles in bone mass by promoting Ca and P absorption and modifying bone turnover (Zhang et al. Citation2020). In this regard, the higher concentration of serum Ca in the NY/T vitamin regimen may be put down to higher vitamin D levels in this study. In addition, the improvement in tibia ash and microstructure might be due to the adequate vitamin K content and vitamin E in the NY/T vitamin diet. Vitamin K was proved as a key cofactor in the synthesis of the Gla‐protein to regulate bone turnover and sufficient vitamin K contents positively related to bone mineral density (BMD) (Tsugawa and Shiraki Citation2020). Research has also shown that vitamin E was also linked with bone health via depressing oxidative stress-inducing bone resorption (Chin and Ima-Nirwana Citation2015). Remarkably, in the current study, the increased bone ash in the NY/T vitamin diet may also be explained by higher B vitamin levels, including vitamin B2, vitamin B9, and vitamin B12 (Dai and Koh Citation2015). Emerging evidence showed that vitamin B2 exerted beneficial effects on osteoblastic proliferation and ALP activity (Chaves Neto et al. Citation2010), and its deficiency in maternal diet-induced abnormal foetal development, and skeletal malformation (Warkany and Nelson Citation1942). However, some studies also assumed that dietary vitamin A reduced osteoblast mineralisation (Lind et al. Citation2013), indicating that the improved role of NT/T vitamin diet in the tibia mass and strength of meat duck came from a comprehensive role of many vitamins.
Table 3. The performance of bird response to different vitamin regimen treatments.
Table 4. The primers for quantitative real-time PCR.
In addition, the dynamic change in bone mass also relies on bone resorption, which is involved in the proliferation and differentiation of osteoclasts. Rankl is expressed on the surface of osteoclast to induce osteoclast differentiation, which was blocked by Opg, a decoy receptor of Rank (Yamamoto et al. Citation2006). During bone resorption, V-ATPases and cathepsin K are secreted by osteoclasts to dissolve the organic and inorganic components of bone (Bi et al. Citation2017). Meanwhile, TRAP and CTx are also released into the circulation by osteoclasts and have served as a marker of bone resorption (Fardellone et al. Citation2014). In this study, dietary vitamin treatment did not discernibly alter the osteoclastic gene expression such as Opg, Rankl, cathepsin K, and V-ATPase, as well as the content of TRAP, suggesting that dietary vitamin regimen has no apparent effects on bone resorption, which was consistent with previous study about the effects of dietary vitamin level on sternum for meat ducks (Zhang et al. Citation2019). However, we also noticed that the NY/T vitamin diet tended to decrease the ratio of Rankl to Opg and CTx concentration. In this sense, supplementation of NY/T vitamin regimen in diet might inhibit bone resorption to increase the tibia ash content. Further studies are required to define the relationship between bone remodelling and vitamin levels.
Conclusion
In conclusion, dietary vitamin regimen treatment has no significant effect on the growth performance and tibia growth of meat ducks, evidenced by similar tibia length, perimeter, and relative weight. However, the supplementation of NY/T vitamin increased tibia ash and improved the bone microstructure of ducks, which might be associated with the promoted bone formation. Consequently, the diet with NY/T vitamin regimen increased tibial density and decreased leg abnormalities of 14-d-old meat ducks. Of note, a main limitation in this study is the utilisation of bentonite in the basal diet. there might be a potential interaction between bentonite and dietary vitamins, especially vitamin A, implied that it is possible that some of our conclusions may include overestimation or underestimation of the roles of vitamin regimens in growth performance and bone metabolism. Further experiments would be essential to exclude this possibility.
Ethical approval
The project is unanimously approved by the the Institutional Animal Care and Use Committee of Yibin University.
Acknowledgment
The authors gratefully thank all the people involved in this work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abbasi T, Shakeri M, Zaghari M, Kohram H. 2017. Growth performance parameters, bone calcification and immune response of in ovo injection of 25-hydroxycholecalciferol and vitamin K(3) in male ross 308 broilers. Theriogenology. 90:260–265. doi: 10.1016/j.theriogenology.2016.12.016.
- Bello A, Bricka RM, Gerard PD, Peebles ED. 2014. Effects of commercial in ovo injection of 25-hydroxycholecalciferol on broiler bone development and mineralization on days 0 and 21 posthatch. Poult Sci. 93(5):1053–1058. doi: 10.3382/ps.2013-03608.
- Bi H, Chen X, Gao S, Yu X, Xiao J, Zhang B, Liu X, Dai M. 2017. Key triggers of osteoclast-related diseases and available strategies for targeted therapies: a review. Front Med . 4:234. doi: 10.3389/fmed.2017.00234.
- Chaves Neto AH, Yano CL, Paredes-Gamero EJ, Machado D, Justo GZ, Peppelenbosch MP, Ferreira CV. 2010. Riboflavin and photoproducts in MC3T3-E1 differentiation. Toxicol in Vitro. 24(7):1911–1919. doi: 10.1016/j.tiv.2010.07.026.
- Chin KY, Ima-Nirwana S. 2015. The biological effects of tocotrienol on bone: a review on evidence from rodent models. Drug Des Devel Ther. 9:2049–2061. doi: 10.2147/DDDT.S79660.
- Dai Z, Koh WP. 2015. B-vitamins and bone health–a review of the current evidence. Nutrients. 7(5):3322–3346. doi: 10.3390/nu7053322.
- Dale N. 1994. National research council nutrient requirements of poultry - Ninth revised edition (1994). J Appl Poult Res. 3(1):101–101. doi: 10.1093/japr/3.1.101.
- Edwards HM, Jr., Veltmann JR. Jr. 1983. The role of calcium and phosphorus in the etiology of tibial dyschondroplasia in young chicks. J Nutr. 113(8):1568–1575. doi: 10.1093/jn/113.8.1568.
- Fardellone P, Séjourné A, Paccou J, Goëb V. 2014. Bone remodelling markers in rheumatoid arthritis. Mediators Inflamm. 2014:484280–484285. doi: 10.1155/2014/484280.
- Jones TA, Dawkins MS. 2010. Environment and management factors affecting Pekin duck production and welfare on commercial farms in the UK. Br Poult Sci. 51(1):12–21. doi: 10.1080/00071660903421159.
- Khajali F, Khoshoei EA, Moghaddam AK. 2006. Effect of vitamin and trace mineral withdrawal from finisher diets on growth performance and immunocompetence of broiler chickens. Br Poult Sci. 47(2):159–162. doi: 10.1080/00071660600610732.
- Khan SH, Shahid R, Mian AA, Sardar R, Anjum MA. 2010. Effect of the level of cholecalciferol supplementation of broiler diets on the performance and tibial dyschondroplasia. J Anim Physiol Anim Nutr . 94(5):584–593. doi: 10.1111/j.1439-0396.2009.00943.x.
- Likittrakulwong W, Moonsatan S, Incharoen T. 2021. Enhancement of tibia bone and eggshell hardness through the supplementation of bio-calcium derived from fish bone mixed with chelated trace minerals and vitamin D3 in laying duck diet. Vet Anim Sci. 14:100204. doi: 10.1016/j.vas.2021.100204.
- Lind T, Sundqvist A, Hu L, Pejler G, Andersson G, Jacobson A, Melhus H. 2013. Vitamin a is a negative regulator of osteoblast mineralization. PLOS One. 8(12):e82388. doi: 10.1371/journal.pone.0082388.
- Mirshekar R, Dastar B, Shabanpour B, Hassani S. 2013. Effect of dietary nutrient density and vitamin premix withdrawal on performance and meat quality of broiler chickens. J Sci Food Agric. 93(12):2979–2985. doi: 10.1002/jsfa.6127.
- Mizokami A, Kawakubo-Yasukochi T, Hirata M. 2017. Osteocalcin and its endocrine functions. Biochem Pharmacol. 132:1–8. doi: 10.1016/j.bcp.2017.02.001.
- Moravej H, Alahyari-Shahrasb M, Kiani A, Bagherirad M, Shivazad M. 2013. Effects of different levels of vitamin premix in finisher diets on performance, immuno-competence and meat lipid oxidation of chickens fed on corn-soybean meal. Vet Res Forum. 4:13–18.
- Orimo H. 2010. The mechanism of mineralization and the role of alkaline phosphatase in health and disease. J Nippon Med Sch. 77(1):4–12. doi: 10.1272/jnms.77.4.
- Products DN. 2022. Vitamin supplementation guidelines, 12th ed. Heerlen Netherlands: DSM.
- Ren ZZ, Jiang SZ, Zeng QF, Ding XM, Bai SP, Wang JP, Luo YH, Su ZW, Xuan Y, Zhang KY, et al. 2017. Effect of maternal canthaxanthin and 25-hydroxycholecalciferol supplementation on the performance of ducklings under two different vitamin regimens. J Anim Physiol Anim Nutr. 101(2):359–368. doi: 10.1111/jpn.12453.
- Ren ZZ, Wang JP, Zeng QF, Ding XM, Bai SP, Luo YH, Su ZW, Xuan Y, Zhang KY. 2016. The effects of maternal dietary vitamin premixes, canthaxanthin, and 25-hydroxycholecalciferol on the performance of progeny ducklings. Poult Sci. 95(3):630–635. doi: 10.3382/ps/pev370.
- Shim MY, Karnuah AB, Mitchell AD, Anthony NB, Pesti GM, Aggrey SE. 2012. The effects of growth rate on leg morphology and tibia breaking strength, mineral density, mineral content, and bone ash in broilers. Poult Sci. 91(8):1790–1795. doi: 10.3382/ps.2011-01968.
- Standards. CAI. Nutrient requirement of meat-type ducks (NY/T 2122-2012). Beijing 2012.
- Tsugawa N, Shiraki M. 2020. Vitamin K nutrition and bone health. Nutrients. 12(7):12. doi: 10.3390/nu12071909.
- van Ballegooijen AJ, Robinson-Cohen C, Katz R, Criqui M, Budoff M, Li D, Siscovick D, Hoofnagle A, Shea SJ, Burke G, et al. 2015. Vitamin D metabolites and bone mineral density: the multi-ethnic study of atherosclerosis. Bone. 78:186–193. doi: 10.1016/j.bone.2015.05.008.
- Vidal B, Pinto A, Galvão MJ, Santos AR, Rodrigues A, Cascão R, et al. 2012. Bone histomorphometry revisited. Acta Reumatol Port. 37:294–300.
- Warkany J, Nelson RC. 1942. Congenital malformations induced in rats by maternal nutritional deficiency: one plate (Four Figures). J Nutr. 23(4):321–333. doi: 10.1093/jn/23.4.321.
- Whyte MP, Thakker RV. 2013. Rickets and osteomalacia. Medicine. 41(10):594–599. doi: 10.1016/j.mpmed.2013.07.012.
- Yamamoto Y, Udagawa N, Matsuura S, Nakamichi Y, Horiuchi H, Hosoya A, Nakamura M, Ozawa H, Takaoka K, Penninger JM, et al. 2006. Osteoblasts provide a suitable microenvironment for the action of receptor activator of nuclear factor-kappaB ligand. Endocrinology. 147(7):3366–3374. doi: 10.1210/en.2006-0216.
- Zhang H, Liao H, Zeng Q, Wang J, Ding X, Bai S, Zhang K. 2019. Effects of commercial premix vitamin level on sternum growth, calcification and carcass traits in meat duck. J Anim Physiol Anim Nutr (Berl). 103(1):53–63. doi: 10.1111/jpn.13001.
- Zhang H, Qin S, Zhu Y, Zhang X, Du P, Huang Y, Michiels J, Zeng Q, Chen W. 2022. Dietary resistant starch from potato regulates bone mass by modulating gut microbiota and concomitant short-chain fatty acids production in meat ducks. Front Nutr. 9:860086. doi: 10.3389/fnut.2022.860086.
- Zhang H, Zeng Q, Bai S, Wang J, Ding X, Xuan Y, Su Z, Fraley GS, Yao B, Zhang K, et al. 2020. Dietary supplementation of 25-hydroxycholecalciferol increases tibial mass by suppression bone resorption in meat ducks. Anim Nutr. 6(4):467–479. doi: 10.1016/j.aninu.2020.05.006.
- Zhang H, Zeng Q, Bai S, Wang J, Ding X, Xuan Y, Su Z, Zhang K. 2018. Effect of graded calcium supplementation in low-nutrient density feed on tibia composition and bone turnover in meat ducks. Br J Nutr. 120(11):1217–1229. doi: 10.1017/S0007114518002556.
- Zhang HY, Liao H, Zeng QF, Wang JP, Ding XM, Bai SP, Zhang KY. 2017. A study on the sternum growth and mineralization kinetic of meat duck from 35 to 63 days of age. Poult Sci. 96(11):4103–4115. doi: 10.3382/ps/pex223.
- Zhang HY, Zeng QF, Bai SP, Wang JP, Ding XM, Xuan Y, Su ZW, Fraley GS, Zhang KY. 2019. Study on the morphology and mineralization of the tibia in meat ducks from 1 to 56 d. Poult Sci. 98(9):3355–3364. doi: 10.3382/ps/pez121.
