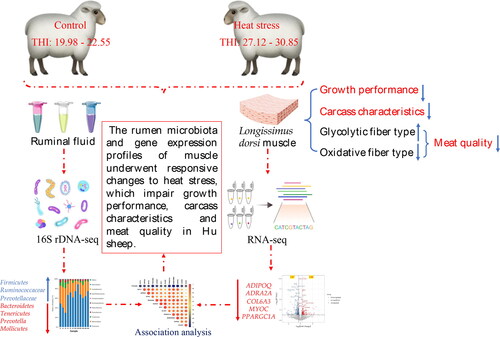
Abstract
This study aimed to investigate the effects of heat stress (HS) on growth performance, carcase characteristics and rumen-muscle axis of Hu sheep and to explore the underlying molecular mechanisms of the actions by establishing a heat-stressed Hu sheep. The results showed that HS significantly decreased growth performance and carcase characteristics, altered metabolic enzyme activities, and induced a shift in muscle energy metabolism towards a more glycolytic and less oxidative fibre type in the longissimus dorsi (LD) muscle (p < 0.05), thereby affecting the meat quality of Hu sheep. Furthermore, RNA-seq analysis of LD muscle found that HS down-regulated the expression of genes (ADIPOQ, ADRA2A, COL6A3, MYOC, PPARGC1A, etc.) involved in fat deposition and muscle development. 16S rDNA analyses of ruminal microbiome revealed that resist adverse environments-related Firmicutes, fatty acid synthesis-related Ruminococcaceae, Prevotellaceae, etc. were increased in the heat-stressed Hu sheep, and negatively correlated with genes involved in muscle development and lipid metabolism. However, energy metabolism-related Bacteroidetes and Tenericutes, and protein and carbohydrate cleavage-related Prevotella and Mollicutes were decreased in the heat-stressed Hu sheep and positively correlated with genes involved in muscle development and lipid metabolism. All together, these findings indicated that the rumen microbiota and gene expression profiles of muscle underwent responsive changes to HS, which impaired growth performance, carcase characteristics and meat quality in Hu sheep. Our findings provide valuable insights into the mechanisms underlying heat stress-induced effects in sheep, suggesting potential strategies for the prevention and control of HS as well as the development of new heat-resistant varieties in Hu sheep using molecular design breeding.
HIGHLIGHTS
HS decreased growth performance and carcase characteristics and meat quality of Hu sheep.
HS down-regulated the expression of genes involved in fat deposition and muscle development.
HS could adversely affect material metabolism, growth performance, and meat quality by altering the diversity of the ruminal microbiota.
Introduction
Global warming of the earth is a universally accepted reality (Zhu et al. Citation2020). The Intergovernmental Panel on Climate Change predicts that the average global surface temperature will increase by approximately 0.3–4.8 °C by 2100 (Angel et al. Citation2018). The occurrence of heat stress (HS) in domestic animals due to climate change has become a critical issue in the livestock industry (Lee et al. Citation2019). Normally, the seriousness of HS occurred in livestock is associated with temperature, humidity, wind speed and water condition (Shi et al. Citation2015). The temperature and humidity index (THI) is the sum of the effect of two variables, ambient temperature and humidity and is primarily used to evaluate the severity of HS in livestock (Zhong et al. Citation2019). Above a certain THI threshold (THI > 23.4), HS occurs, which increases the body temperature and induces physiological and behavioural responses, resulting in serious risks to livestock welfare and health (Leliveld et al. Citation2022).
HS, caused by climate change, negatively affects animal performance, particularly during the hot-humid summer season in subtropical regions such as South China (Kang et al. Citation2019; Zeng et al. Citation2020). In recent years, the majority of scholarly investigations pertaining to HS have primarily concentrated on the examination of n chickens, pigs and cattle. For example, HS has been observed to result in a decrease in growth rate, feed efficiency, eggshell quality, and survivability in chickens (Vinoth et al. Citation2018). Similarly, in cattle, HS could reduce daily weight gain, feed efficiency (Estrada-Angulo et al. Citation2016), reproduction, immunity (Rong et al. Citation2019), milk production, and increase mortality rates (Li et al. Citation2019). Furthermore, the presence of HS has been found to result in elevated mortality rates and reduced growth, market weight, and carcase value in pigs (Mendoza et al. Citation2017; Seibert et al. Citation2018). Additionally, it has been observed that heat stress in sheep leads to a decrease in the efficiency of dry matter intake, resulting in a subsequent manifestation of inappetence and anorexia (Karthik et al. Citation2021). Hu sheep are recognised as an ideal meat breed in Southwest China, where the scale and breeding density of domestic farming for their high-quality lean meat has increased yearly. With the intensive development of the Hu sheep industry and increasing global temperatures, HS in Hu sheep has become an increasingly severe and important issue in recent years. However, little attention has been paid to the effects of HS on Hu sheep.
Feed efficiency in ruminants is primarily determined by feed quality, ruminal fermentation, and microbiome dynamics (Hassan et al. Citation2020). The rumen is the principal nutrient-degrading organ responsible for ruminant growth and normal physiological functions through the balanced microbial utilisation of dietary carbon and nitrogen sources (Ma et al. Citation2022). HS has been shown to influence feeding behaviour, daily dry matter intake (DMI), and ruminal disorders (Crossland et al. Citation2018) and frequently linked to alterations in microbial physiological processes and proliferation (Chen et al. Citation2017). Moreover, HS can alter ruminal microbial populations and, consequently, the energy substrates available to the animal (Duffy et al. Citation2019). These shifts in ruminal microbiota can affect muscle mass, quality, and function via the gut-muscle axis (Chew et al. Citation2022).
Skeletal muscle growth and development are driven by the programmed expression of related genes (Xie et al. Citation2018). Although the impact of HS on livestock growth performance has been sufficiently established based on phenotypic trait alterations, there are few reports on the genotypic traits that are altered when livestock are exposed to HS. In addition, the relationship between HS and the regulatory network of the rumen-muscle axis in ruminants has received even less attention. In this study, by establishing a heat-stressed Hu sheep, we investigated the potential mechanism by which HS affects growth performance and meat quality in Hu sheep.
Materials and methods
The animal procedures were approved by the Ethics Committee on the Use of Animals in Experimentation at the Guangxi university (protocol no. GXU-2023-0135). The study was conducted in an experimental farm belonging to Guangxi university.
Animals and experimental diets
A total of 12 (aged 12 months, 47.23 ± 0.66 kg) healthy male Hu Sheep were randomly divided into two groups and housed in manually controlled greenhouses A and B (the greenhouses were established in Meat Sheep Farm of Agricultural Science New Town of Guangxi University, Chongzuo city, Guangxi, China) with 6 replicates in every group. The sheep houses were cleaned and disinfected regularly to keep the paddock ventilated, sanitary and dry. Ingredient compositions and nutrient contents of the basal diets for sheep are presented in Table . All the sheep received freely available water and were then fed with the basal diets at 7:00 every morning (purchased from Nanning Xinxin Zhuangde Agriculture and Animal Husbandry Technology Company Limited) throughout the experiment. Sheep also had free access to a salt block during the trial period. Sheep from both greenhouses initially received an appropriate temperature (T) of 23 °C for 7 days to adapt to the greenhouse environment. Later, six sheep lived in greenhouse A (23 °C) were as the control group (Con), the other six sheep lived in the greenhouse B (36 °C) were as heat stress group (HS). The feeding experiment lasted for 50 days, and the daily feed intake of each sheep was recorded to calculate average daily feed intake. Individual body weight was determined before and after the trial to calculate the average daily gain.
Table 1. Dietary ingredients and nutrient content of basal diets in fattening sheep.
The respiratory rate and rectal temperature of Hu sheep in two groups were measured at 14:30 on the 7th day, 14th day, 21st day, 28th day of the trial period, respectively. Respiration rate (RR) and rectal temperature (RT) were determined by referring to Johnson et al. (Citation2012). The number of waist undulation in one minute were observed when the sheep were quiet. Each sheep was measured 3 times, and the average value was taken as the RR of the sheep. RT were measured using a rectal clinical veterinary thermometer (Vicks Speed Read digital thermometer), with the glass part being disinfected with an alcohol swab and inserted into the rectum to a depth of approximately two-thirds of it. Each temperature reading was repeated 3 times.
Slaughter surveys and sampling
At the end of the trial, the sheep were fasted for 24 h. Before slaughtering, blood samples were collected from the jugular veins of each sheep using empty collection tubes. The serum samples (supernatant) were collected immediately after the blood samples had been centrifuged at 3000× g for 15 min at 4 °C and were then stored at −80 °C for the determination of HSP70 concentration. HSP70 was determined according to the instructions of the Elisa kit (1.0 pg/mL, Hyperheal, Shanghai, China). Each sample was repeated three times, and the results were averaged.
All sheep were slaughtered in accordance with the agricultural industry standard of the People’s Republic of China (NY/T3469-2019). 12 sheep were stunned, bled, skinned, gutted and then split along the midline. Hot carcase weight and net meat weight were recorded, used to calculate dressing percentage and meat percentage. The dressing percentage refers to the percentage of carcase weight to pre-slaughter body weight, which is calculated as dressing percentage (%) = (hot carcase weight/pre-slaughter body weight) × 100. The net meat percentage refers to net meat weight as a percentage of hot carcase weight, i.e. (%) = (net meat weight/hot carcase weight) × 100(Zhang et al. Citation2015).
Rumen fluid samples were collected from the rumen of each sheep immediately after slaughter. The rumen contents were filtered through four layers of cheesecloth and collected into cryopreservation tubes, which were immediately frozen in liquid nitrogen and then stored at −80 °C for subsequent analysis.
Meat quality and enzyme activity
The meat quality of 12 sheep were tested and analysed according to the agricultural industry standard of the People’s Republic of China (NY/T1333-2007). A sample of LD muscle from the same area of each right carcase was collected to determine pH value, colour, drip loss rate, shear force and intramuscular fat content.
The pH value in the LD muscle was determined at 45 min and 24 h post-mortem using a pH metre (pH-STAR, Matthäus GmbH & Co. KG, Germany) equipped with a temperature compensation and calibrated using two buffers (pH 4.64 and 7.0). The meat colour was detected at 45 min post-mortem using a colorimeter (OPTO-Star, Matthäus GmbH & Co. KG, Germany) based on the specific parameters: lightness, redness and yellowness. The colorimeter was calibrated using a white tile before measurements were taken. After removing other connective tissue, the surface of the LD muscle was exposed to air for 20 min of blooming, and then measured for meat colour. To evaluate drip loss, muscle was cut into strips (1 cm × 1 cm × 5 cm) and weighed (W1) immediately after slaughter. Every muscle strip was wrapped in an airless bag and hung in a 4 °C refrigeration house for 24 h. The entire muscle strip was suspended in the airless bag and did not touch the sides of the bag. After 24 h, the muscle strip weight was recorded as W2. The drip loss was calculated as follows: W2/W1 × 100% (22). The pH, meat colour and drip loss rate were measured repeatedly a total of 6 times, and the average values were used for further data analysis. Warner–Bratzler shear force (WBSF) was measured using a Shear Force Instrument (Mecmesin Ltd., West Sussex, UK). Briefly, the LD muscle samples were placed in a water bath at 80 °C. After the central temperature reached 70 °C, the samples were removed and cooled to room temperature. Six cylindrical cores with a diameter of 1.27 cm were taken from each sample parallel to the direction of the muscle fibres to measure the shear force. The IMF content of the LD muscle was determined using the Soxhlet extraction method with petroleum ether extraction according to AOAC (1995) methods, and the results were expressed as percentages of the weight of wet muscle.
Enzyme activity of succinate dehydrogenase (1 U/L, SDH), malate dehydrogenase (0.01 U/ml, MDH) and lactate dehydrogenase (9.0 U/L, LDH) of LD muscle samples were determined using Enzymatic activity assay kits (purchased from Nanjing Jiancheng Bioengineering Co., LTD., China).
Analysis of muscle gene expression profiles using RNA-seq and RT-qPCR
Total RNA was randomly extracted from the LD muscle tissues of three sheep per treatment and sent to Shanghai Ouyi Biotechnology Co., Ltd. for high-throughput sequencing. After purification, the integrity and concentration of the total RNA were determined, and the qualified samples were purified to construct mRNA libraries. After quality checking, the libraries were sequenced using the Illumina HiSeq sequencing platform, and the sequencing data were recovered. Using FastQC software for quality control, clean reads were obtained and aligned with the reference genome. FPKM standardisation was used to analyse gene expression in the samples.
A t-test was performed to compare gene expression between two sets of samples, and an analysis of the enrichment of differentially expressed genes using the BLAST ratio in the GO and KEGG databases was performed to obtain annotation information. Through these processes, combined with criteria such as log2 |FoldChange| > 1, p < 0.05, and gene functions in signalling pathways, differentially expressed genes were identified in the muscle tissue of Hu sheep in both groups.
Quantitative real-time RT-PCR gene expression analysis relative gene expression was determined using two-step quantitative RT-qPCR. Briefly, total RNA was extracted from the LD muscle of three sheep per treatment using the TRIzol method, maintaining equal total RNA concentrations in all samples. Reverse transcription was performed using the TAKARA PrimeScript RT Reagent Kit with a gDNA Eraser. After the reaction, the product was stored in a refrigerator at −40 °C. RT-qPCR was used to determine the expression levels of key heat shock proteins present in the Hu sheep muscle. Specific primers were designed using Primer 5.0 software based on GenBank-published sheep gene sequences (Table S3; Nanning Genis Biotechnology Co., Ltd., synthesised all primers). The program for RT-qPCR was as follows: initial denaturation at 95 °C for 15 min, followed by 40 cycles of 98 °C for 10 s, annealing at 60 °C for 32 s, followed by 95 °C for 15 s, 55 °C for 15 s, and a final extension at 95 °C for 15 s. RT-qPCR reactions were conducted using a 20 µL reaction mix containing 10 µL of 2 × SuperReal PreMix Plus, 0.6 µL of each primer (10 µM), <100 ng cDNA template, followed by the addition of RNase-free ddH2O. The relative quantification of the expression of each gene was normalised to the relative quantification of the expression of GAPDH mRNA and calculated by the 2 − ΔΔ Ct equation.
Microbiota analysis using 16S rDNA sequencing
Six sheep microbial sample DNA per treatment was extracted using the PowerSoil® DNA Isolation Kit, following the manufacturer’s instructions. The full-length universal primers for microbial 16S rDNA were 27 F: AGRGTTTGATYNTGGCTCAG and 1492 R: TASGGHTACCTTGTTASGACTT. PCR reactions were performed using a 20 µL reaction mix containing 50 ng of extracted genomic DNA, 1 µL of each primer (10 µM), 10 µL of KOD One TM PCR Master Mix (Toyobo Co., Ltd., Osaka, Japan), 4 µL of 2.5 mmol/L dNTPs, and nuclease-free water to complete the system. The thermocycling program was as follows: initial denaturation at 95 °C for 15 min, followed by 25 cycles at 98 °C for 30 s, annealing at 50 °C for 30 s, 72 °C for 2 min, and a final extension at 72 °C for 7 min. The PCR results were validated using 1.5% agarose gel electrophoresis and then sent to Baimax Biotechnology Co., Ltd. for sequencing.
Sequences with an N-base ratio greater than 10% and low-quality reads were removed from the sequencing results. Flash 1.2.11 was used for read stitching, whereas Qiime (1.9.1200) was used for quality filtering of tags. The UCHIME algorithm from the Gold Database (R20110519) was used to remove chimaeras. The sample corresponding to the barcode was identified, and the barcode and primer sequences were removed. The Mothur V1.39.1 software was used for processing and removal of redundancy and tag abundance calculations. Sequences were clustered into operational taxonomic units (OTUs) according to a similarity > 97% using Uparse (USearch V9.2.645). Species were annotated in the SILVA taxonomic database using the RDP Classifier 2.2 to obtain taxonomic information on the OTUs. Functional prediction of the KEGG microbial community was performed using the PICRUSt2 software.
Statistical analysis
The temperature and humidity index data were tabulated using Excel 2016 software, and data from different treatments were compared using SPSS 20.0 software (version 17.0), with a student’s t-test determining statistical significance. Correlation between significant differentially expressed genes in the LD muscle and ruminal microbes were analysed using Spearman’s with p < 0.05. All the significantly correlated differentially expressed genes in the LD muscle and ruminal microbes were visualised by heatmap graphs. The test results were expressed as ‘mean ± standard error,’ and p < 0.05 was considered as the criterion for a significant difference. Data were plotted and analysed using GraphPad Prism 9.4.
Results
Establishment of a heat-stressed Hu sheep and effects of heat stress on the vital signs of Hu sheep
We attempted to establish a heat-stressed Hu sheep to investigate the effects of HS on the growth performance of Hu sheep. According to the temperature-humidity index (THI) calculations based on Marai (Marai et al. Citation2007), values between 22.2 and 23.3 indicate mild HS, values between 23.4 and 25.6 indicate moderate HS, and values above 25.6 indicate severe HS. The mean THI values for both the control and heat-stressed groups during the study period are shown in Figure . The THI values of the control and heat-stressed Hu sheep were 19.98 − 22.55, and 27.12 − 30.85, respectively. At all times of the day, the THI of the heat-stressed Hu sheep was higher than that of the control group Hu sheep. In addition, the relative expression patterns of HSP genes, particularly HSP60 and HSP110, were upregulated in the muscle tissue of heat-stressed Hu sheep (p < 0.05; Figure ). Furthermore, the serum concentration of HSP70 in heat-stressed Hu sheep was significantly higher than that in the control group Hu sheep (p < 0.05; Figure ). These results suggested that Hu sheep in the experimental group experienced severe HS, whereas Hu sheep in the control group Hu sheep were completely comfortable or experienced only mild HS. Further, the vital signs of Hu sheep were examined. The respiratory rate of heat-stressed Hu sheep was significantly higher than that of the control group Hu sheep (p < 0.01; Figure ). The rectal temperatures of heat-stressed Hu sheep were marginally higher than those of non-heat-stressed sheep; however, this difference was not statistically significant (p > 0.05; Figure ). Additionally, HS did not significantly alter any blood physiological indices in Hu sheep, indicating that heat-stressed Hu sheep flocks were in physiologically normal conditions (Table S1). In summary, a heat-stressed Hu sheep was successfully established and showed no other comorbidities.
Figure 1. Establishment of a heat-stressed model and effects of heat stress on the vital signs of Hu sheep. (A) Average THI of environment for the study period between control and heat-stressed Hu sheep. (B) The relative expression pattern of the HSP genes in the muscle tissue of Hu sheep between Con and HS group Hu sheep. (C) Serum HSP70 concentration of Hu sheep between Con and HS. (D) The mean respiratory rate of Hu sheep between Con and HS group Hu sheep. (E) The mean rectal temperature of Hu sheep between Con and HS group Hu sheep. The data represent the mean ± SEM (n = 7). * the mean difference was significant (p < 0.05), ** mean difference were extremely significant (p < 0.01) and *** mean difference were extremely significant (p < 0.001), compared with control group Hu sheep. Con: control group Hu sheep; HS: heat-stressed Hu sheep; THI: temperature and humidity index.

Effect of heat stress on growth performance, carcass characteristics, and meat quality of Hu sheep
Next, we investigated the effect of HS on the growth performance of Hu sheep. As shown in Table , the final body weight, average daily feed intake, and average daily gain of heat-stressed Hu sheep were significantly lower than those of the control group Hu sheep (p < 0.05; Table ). Moreover, heat-stressed Hu sheep had a lower net meat weight and meat-to-bone ratio than the control group Hu sheep (p < 0.05; Table ). However, other growth performance and carcase characteristics did not differ significantly between the two groups (p > 0.05; Table ).
Table 2. Effect of heat stress on growth performance and carcase characteristics of Hu sheep (n = 6).
In addition, we assessed the carcase meat quality. HS increased the yellowness value, drip loss, and pH24 h index, but decreased the pH45 min index (p < 0.05; Table ). However, other meat quality indices (brightness, redness, shear force, and intramuscular fat content) did not differ significantly between the two groups (p > 0.05; Table ). These findings suggested that HS adversely affected growth performance, carcase characteristics and meat quality of Hu sheep.
Table 3. Effect of heat stress on meat quality of Hu sheep (n = 6).
Heat stress affects mRNA expression of muscle fiber type-related genes, as well as MDH, SDH, and LDH activities in the longissimus dorsi muscle of Hu sheep
To investigate the effects of HS on muscle fibre type distribution, we analysed the expression of fibre-related genes in Hu sheep LD muscle by RT-qPCR. Compared to the control group Hu sheep, MyHC1, MyHC2a, and MyHC2x mRNA expression levels decreased in the heat-stressed Hu sheep, while MyH2b mRNA expression levels increased (p < 0.05; Figure ). Additionally, SDH and MDH activities decreased, while LDH activity increased in the LD muscle of the heat-stressed Hu sheep compared to the control group Hu sheep (p < 0.05; Figures ). And, the expression of antioxidant genes, including CAT, GPX, SOD2, and NRF2 decreased in the LD muscle following HS compared to that in the control group Hu sheep (p < 0.05; Figure ). These results indicated that heat-stressed Hu sheep had a lower oxidative capacity and higher glycolytic ability than the control group Hu sheep. Thus, HS could affect the activity of metabolic enzymes and induce a shift in muscle energy metabolism towards a more glycolytic and less oxidative fibre type in the LD muscle, thereby affecting meat quality in Hu sheep.
Figure 2. Heat stress affects mRNA expression of muscle fibre type-related genes, as well as MDH, SDH, and LDH activities of the longissimus dorsi muscle in Hu sheep. (A) Effect of heat stress on mRNA expression of MyHCs of the longissimus dorsi muscle in Hu sheep. (B–D) Effect of heat stress on energy metabolism enzyme activity of the longissimus dorsi muscle in Hu sheep. (E) Effect of heat stress on mRNA expression of antioxidant related genes of the longissimus dorsi muscle in Hu sheep. The data represent the mean ± SEM (n = 7). * the mean difference was significant (p < 0.05) and ** mean difference were extremely significant (p < 0.01), compared with control group Hu sheep. Con: control group Hu sheep; HS: heat-stressed Hu sheep.

Global mRNA expression pattern of the longissimus dorsi muscle in control and heat-stressed Hu sheep
Next, we investigated the molecular mechanisms why HS decreased the growth performance, carcase characteristics, and meat quality in Hu sheep. We used RNA-seq to investigate mRNA expression changes in the LD muscle between control and heat-stressed Hu sheep. Quality assessment of the sequencing data is summarised in Table S2. Clean reads were then mapped to the Ovis aries genome (assembly ARS-UI_Ramb_v2.0) (Table S2). Genes were considered differentially expressed if the P-value was <0.05, and |log2foldchange| was >1. As shown in Figure and Figure S1A, heat-stressed Hu sheep expressed 391 differentially expressed genes (DEGs) (144 up- and 247 down-regulated DEGs) compared to the control group Hu sheep (Tables S4–S6). For example, ACTC1, ADIPOQ, ADRA2A, CACNG7, COL6A3, ETV1, MYOC, PLCE1, PPARGC1A, SLC8A1 gene was significantly down-regulated in heat-stressed Hu sheep.
Figure 3. Global mRNA expression pattern of the longissimus dorsi muscle in control and heat-stressed Hu sheep. (A) Volcano plots of up-regulated and down-regulated differentially expressed genes of longissimus dorsi muscle between control and heat-stressed Hu sheep. The gray dots represent non-significantly differentially expressed genes, the 144 red dots represent significantly differentially up-regulated genes, and the 247 blue dots represent significantly differentially down-regulated genes. (B) Functional enrichment analysis of differentially expressed genes (DEGs) of longissimus dorsi muscle between control and heat-stressed Hu sheep. Light green represents terms of up-regulated genes; Light red represents terms of up-regulated genes. (C–D) The protein–protein-interaction (PPI) network of differentially down-regulated expressed genes involved in muscle development (C) and fat deposition (D); each of the nodes represents a protein. (E)RT-qPCR of DEGs compared with RNA-seq. Results of RT-qPCR analysis (alongside RNA-seq analysis) of DEGs fold-change in longissimus dorsi muscle between control and heat-stressed Hu sheep. The relative expression of each mRNA was calculated using the 2−ΔΔCt method.

The pathway and process enrichment analyses were carried out by using Metascape (http://metascape.org/gp/index.html#/main/step1) software. Energy metabolism-related terms, such as ‘Hyaluronan metabolic process,’ ‘Cellular carbohydrate catabolic process,’ ‘Cellular response to starvation,’ ‘Encoded amino acid transport,’ and ‘Lipid homeostasis’ were enriched for upregulated DEGs. In contrast, extracellular matrix-related terms, such as ‘Extracellular matrix organization,’ ‘Blood vessel development,’ ‘Extracellular matrix assembly,’ ‘Cell-substrate adhesion,’ ‘Positive regulation of cell migration,’ and ‘Elastic fiber formation’ were enriched for downregulated DEGs, which were critical for growth, wound healing, and fibrosis of muscle (Figure ). Then, protein–protein interactions of differentially down-regulated expressed genes were performed using the String online software (https://cn.string-db.org). The results indicating that these genes interact with each other to regulate muscle development (MYO7A, ITGBL1, ACTC1 and MYOC etc.) (Figure ) and fat deposition (IRS1, PPARGC1A, ADIPOQ and IGF1 etc.) (Figure ). We then randomly selected six DEGs and validated their expression patterns using RT-qPCR. The results showed that their expression patterns matched those from RNA-seq analysis (Figure ), validating the accuracy of the RNA-seq data. Altogether, HS impaired the expression of genes involved in fat deposition and muscle development, resulting in reduced growth performance and meat quality in Hu sheep.
Effects of heat stress on ruminal bacterial microbiome of Hu sheep
Ruminal microorganisms are indispensable for the feed conversion and energy efficiencies in ruminants. The ruminal fluid pH in the heat-stressed Hu sheep was significantly lower than that in the control group Hu sheep (p < 0.05; Figure ). Ruminal pH is one of the most significant determinants of ruminant performance because it affects fermentation patterns in the rumen (Krebs et al. Citation2021). Full-length 16S rDNA sequencing was performed on the ruminal fluid of control and heat-stressed Hu sheep to investigate the effects of HS on ruminal fermentation and microbiota. This sequencing yielded a total of 156039 raw CCS sequences (range = 12877–13057) from 12 samples (six control and six heat-stressed Hu sheep) using the PacBio platform. After read-quality filtering, 156,037 high-quality, effective CCS sequences were obtained, including 12995 ± 68 and 13011 ± 70 sequences in the control and heat-stressed Hu sheep, respectively. Based on 97% sequence similarity, the average number of OTUs among all the samples was 256 (range = 177–355, SEM = 65). The rarefaction curves for the number of OTUs indicated that the sequencing depth in this study was sufficient to accurately characterise the bacterial microbiota of ruminal fluid samples (Figure S1B). As shown in , 695 OTUs were shared between both groups. The number of OTUs exclusive to the ruminal fluid of the control and heat-stressed Hu sheep was 58 and 40, respectively, suggesting that they had different ruminal microbial species. Across all ruminal fluid samples, 19 phyla, 22 classes, 39 orders, 55 families, 133 genera, and 158 species were identified. The ten most abundant phyla and genera among the ruminal bacteria are shown in Figures . According to the α diversity indices (ACE, Chao1, and Simpson) and PCoA analysis (Figure S1C), there was no significant difference in the microbiota diversity values between the two groups Hu sheep. However, the α diversity indices of ruminal microbial flora were marginally lower in heat-stressed Hu sheep than in the control group Hu sheep (Figure ). We performed pairwise comparisons of the relative abundance of specific microbes between the two groups. At the phylum level (Figure ), the heat-stressed Hu sheep showed a lower relative abundance of Bacteroidetes and a higher relative abundance of Firmicutes and Verrucomicrobia than those in the control group Hu sheep (p < 0.05). Eleven bacterial genera were significantly affected by HS (p < 0.05; Figure ). Following LEfSe analysis (LDA = 4), significant differences in the microbial species were identified between the two groups. Specifically, LEfSe analysis identified four genera as potential biomarkers for distinguishing between the different treatment groups (Figure ). The heat-stressed Hu sheep was characterised by s_uncultured_bacterium_g_Quinella and g_Quinella. In addition, g__ Rikenellaceae_ RC9_ gut_ group and f_ Rikenellaceae characterised the control group Hu sheep. Alterations in the putative functions of the ruminal microbiota in heat-stressed Hu sheep were evaluated using the PICRUSt2 software. Interestingly, in the heat-stressed Hu sheep, the digestive system, biosynthesis of other secondary metabolites, transcription, and lipid metabolism were significantly decreased compared with control group Hu sheep (p < 0.05; Figure ). Thus, HS could adversely affect material metabolism, growth performance, and meat quality by altering the diversity of the ruminal microbiota.
Figure 4. Effects of heat stress on ruminal bacterial microbiome of Hu sheep. (A) Effect of heat stress on ruminal pH of Hu sheep. The data represent the mean ± SEM (n = 7). (B) The venn diagram of operational taxonomic unit (out) rumen bacterial between control and heat stress Hu sheep. The number within each differently coloured overlapping area is the number of OTUs shared by the overlapping groups. Non- overlapping areas indicate the number of OTUs unique to each group. (C) The top 10 phyla of the ruminal bacteria between control and heat stress Hu sheep. (those with a relative abundance of less than 2% were combined). (D) The top 10 genera of the ruminal bacteria between control and heat stress Hu sheep. (those with a relative abundance of less than 2% were combined). (E) Effect of bacterial alpha diversity indexes in rumen fluid between between control and heat-stressed Hu sheep. (F) Ruminal microbiota at the phylum level affected by heat stress in Hu sheep. (G) Ruminal microbiota at the genus level affected by heat stress in Hu sheep. (H) Cladogram displays significantly enriched bacterial taxa (from the class to the species level). (I) The significant different functions were predicted using PICRUSt2 software at level 2 between control and heat-stressed Hu sheep. The data represent the mean ± SEM (n = 6). * the mean difference was significant (p < 0.05), compared with control. Con: control group Hu sheep; HS: heat-stressed Hu sheep.

Analysis of Correlations between differentially expressed genes in the longissimus dorsi muscle and ruminal microbes of Hu sheep
Gut microbiota could influence animal growth through the microbiota-rumen-muscle axis (Meng et al. Citation2023). Correlations were established between DEGs involved in muscle development and lipid metabolism with three distinct genus-level ruminal floras and 11 distinct phylum-level floras to determine the relationship between differentially expressed genes in the LD muscle and ruminal microbiota in control and heat-stressed Hu sheep. Interestingly, Firmicutes, which can resist adverse environments, were increased in the heat-stressed Hu sheep and negatively correlated with genes involved in muscle development and lipid metabolism (Figure ). The energy metabolism-related Bacteroidetes and Tenericutes were decreased in control group Hu sheep and positively correlated with genes involved in muscle development and lipid metabolism (Figure ). Additionally, Rikenellaceae, Prevotella, and Mollicutes, associated with protein and carbohydrate cleavage, were decreased in the heat-stressed Hu sheep but positively correlated with genes involved in muscle development and lipid metabolism (Figure ). However, Ruminococcaceae, Prevotellaceae, et al. associated with fatty acid synthesis were significantly increased in the heat-stressed Hu sheep and negatively correlated with genes involved in muscle development and lipid metabolism (Figure ). These findings indicated that the rumen microbiota and gene expression profiles of muscle underwent responsive changes to HS, which impair growth performance, carcase characteristics and meat quality in Hu sheep.
Figure 5. Analysis of correlations between differentially expressed genes in the longissimus dorsi muscle and ruminal microbes of Hu sheep. (A) Heat map for Spearman correlation analysis between 3 difference flora at the genus and muscle development related genes. (B) Heat map for Spearman correlation analysis between 3 difference flora at the genus and lipid metabolism related genes. (C) Heat map for Spearman correlation analysis between 11 difference flora at phylum level and muscle development related genes. (D) Heat map for Spearman correlation analysis between 11 difference flora at phylum level and lipid metabolism related genes. Values range from −1 (negative correlation, bluish background) to +1 (positive correlation, reddish background).

Discussion
HS is one of the most significant environmental stressors of global warming, potentially affecting the welfare of livestock and even causing death (Tang et al. Citation2018). Therefore, HS has been a significant environmental constraint for almost all livestock sectors in hot climates (Liu et al. Citation2021) (Du et al. Citation2021), especially in the tropics and sub-tropical regions of the world (Uyanga et al. Citation2023). In this study, we established a heat-stressed Hu sheep to determine how HS affects growth performance and meat quality. Our data provide insights into the molecular mechanisms underlying the poor growth performance and meat quality in heat-stressed Hu sheep via the rumen-muscle axis.
As homeothermic animals, Hu sheep maintain a relatively constant body temperature using various autonomic and behavioural processes; however, this can vary depending on the environmental temperature and humidity. When the ambient temperature exceeds an animal’s capacity to dissipate heat, the animal experiences HS (Liu et al. Citation2022). Previous research has suggested that HS can cause severe stress-related inflammatory and metabolic changes, posing a threat to animal health and performance (Brownstein et al. Citation2017; Lyu et al. Citation2018; Li et al. Citation2019), such as decreased growth performance in pigs (Hou et al. Citation2017; Mendoza et al. Citation2017), reduced daily weight gain and feed efficiency and inhibit the growth in cattle (Estrada-Angulo et al. Citation2016; Russi et al. Citation2019; Chen et al. Citation2022). Our results are consistent with previous findings that HS decreases the growth performance of Hu sheep. Additionally, recent reports showed that the continuous exposure to heat stress has a significant impact on the quality of meat (alterations in flesh colour, flavours and water holding capacity) through the disruption of membrane integrity, impairment of neurological function, and the peroxidation of proteins and lipids in pig (Gao et al. Citation2020). This is consistent with our conclusion that HS can affect metabolic enzyme activities and induce a shift in muscle energy metabolism towards a more glycolytic and less oxidative fibre type in the LD muscle, which consequently affects the meat quality of Hu sheep. The expression patterns of four antioxidant genes (CAT, GPX, SOD2, and NRF2) were downregulated in the LD muscle of heat-stressed Hu sheep, indicating a muscle fibre-type transition towards less oxidative muscle fibres, such as MyHC1(Yamashita et al. Citation2018). This could potentially reduce meat tenderness because glycolytic muscle fibres are less tender (Kim et al. Citation2018).
The gut-muscle axis is a novel concept based on the correlation between gut microbiota composition and muscle function (Chen et al. Citation2019). The gut microbiome can contribute to increasing and maintaining muscle mass and quality by modulating the gut microbiome-muscle axis through the production of SCFAs (Daily and Park Citation2022). The rumen, an important organ of ruminants for nutrient digestion and absorption, harbours a complex microbial community of thousands of species that produce enzymes of various functional groups (Ma et al. Citation2020). Therefore, we focused on the rumen-muscle axis and identified genes and ruminal microflora that were regulated by HS. Genes related to muscle development (ITGA11, ACTC1, ADIPOQ PTGFR, MYOC, COL1A1, and others) and adipogenesis (ACOT11, ACOX2, APOLD1, LIPA, SYT3, SLC27Z6, and others) were down-regulated in heat-stressed Hu sheep. These genes exert a direct influence on the maturation of muscle tissue and the accumulation of intramuscular fat, consequently impeding the growth and development of Hu sheep and compromising the quality of their meat. These results are consistent with previous reports of downregulation of growth related genes in goats after heat stress (Angel et al. Citation2018). In addition, GO terms of ‘blood vessel development,’ ‘positive regulation of cell migration,’ and ‘elastic fibre formation extracellular matrix organisation were enriched for downregulated DEGs, which were critical for growth, wound healing, and fibrosis of muscle (Chen et al. Citation2020). Furthermore, prior studies utilising hypothalamus RNA-seq have demonstrated that HS has the potential to induce calcium dyshomeostasis, impede biogenesis, trigger apoptosis, thereby leading to reduced growth and development, as well as heightened organ damage in Hu sheep (Li et al. Citation2019). These findings offer additional substantiation that HS has the potential to diminish growth performance and meat quality through the modification of gene expression programs.
Volatile fatty acids are produced through the fermentation of dietary by the complex microbial ecosystem in the rumen and act as major sources of energy for ruminants (Maia et al. Citation2016). Furthermore, we have observed notable variations in the bacterial composition of the ruminal microbiota as a consequence of heat stress. Notably, we found that HS had no significant effect on the number of OTUs, whereas the abundance of certain genus and phyla was significantly affected. Firmicutes, which are upregulated in the ruminal fluid of heat-stressed Hu sheep, possess a cell wall and can produce endophytic spores, as well as withstand extreme environments (Liu et al. Citation2022). The phylum Bacteroidetes is considered the most stable component of the gastrointestinal microbiota of healthy adults, functioning as a symbiotic microorganism in the gastrointestinal tract and playing a role in protein metabolism (Hsieh et al. Citation2021). At the genus level, Rikenellaceae plays a role in the fermentation of carbohydrates and proteins (Yang et al. Citation2020), Prevotella possesses enzymes that can degrade cellulose and xylan (Song et al. Citation2019), and Christensenellaceae positively correlates with lipid metabolism (Lu et al. Citation2020). The results showed that HS changed the composition of rumen microbes and affected metabolic function. Furthermore, analysis of correlations between differentially expressed genes in the LD muscle and ruminal microbes highlight that HS could affect growth performance, carcase characteristics and meat quality of Hu sheep by regulating the rumen-muscle axis. The existence of a correlation between gut microbiota and muscle metabolites establishes a theoretical foundation for the concept of the gut-muscle axis. Altogether, these findings indicated that the rumen microbiota and gene expression profiles of muscle underwent responsive changes to HS, which impair growth performance, carcase characteristics and meat quality in Hu sheep.
Conclusions
In conclusion, HS has a negative impact on growth performance and meat quality in Hu sheep. The RNA-seq and 16S rDNA analyses indicated that the rumen microbiota and gene expression profiles of muscle underwent responsive changes to HS, which impair growth performance, carcase characteristics and meat quality in Hu sheep. Our findings provide valuable insights into the mechanisms underlying HS-induced effects in sheep, suggesting potential strategies for the prevention and control of HS as well as the development of new heat-resistant varieties in Hu sheep using molecular design breeding.
Ethical approval
The animal procedures were approved by the Ethics Committee on the Use of Animals in Experimentation at the Guangxi university (protocol no. GXU-2023-0135).
Supplemental Material
Download MS Word (251.3 KB)Acknowledgments
We thank colleagues in the laboratory and our collaborators for their useful suggestions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Additional information
Funding
References
- Angel SP, Bagath M, Sejian V, Krishnan G, Bhatta R. 2018. Expression patterns of candidate genes reflecting the growth performance of goats subjected to heat stress. Mol Biol Rep. 45(6):2847–2856. doi: 10.1007/s11033-018-4440-0.
- Brownstein AJ, Ganesan S, Summers CM, Pearce S, Hale BJ, Ross JW, Gabler N, Seibert JT, Rhoads RP, Baumgard LH, et al. 2017. Heat stress causes dysfunctional autophagy in oxidative skeletal muscle. Physiol Rep. 5(12):e13317. doi: 10.14814/phy2.13317.
- Chen C, Li Q, Wang QF, Lu DH, Zhang H, Wang J, Fu RT. 2017. Transcriptional profiling provides new insights into the role of nitric oxide in enhancing Ganoderma oregonense resistance to heat stress. Sci Rep. 7(1):15694. doi: 10.1038/s41598-017-15340-6.
- Chen H, Peng T, Shang HL, Shang XL, Zhao XH, Qu MR, Song XZ. 2022. RNA-seq analysis reveals the potential molecular mechanisms of Puerarin on intramuscular fat deposition in heat-stressed beef cattle. Front Nutr. 9:817557. doi: 10.3389/fnut.2022.817557.
- Chen LH, Huang SY, Huang KC, Hsu CC, Yang KC, Li LA, Chan CH, Huang HY. 2019. Lactobacillus paracasei PS23 decelerated age-related muscle loss by ensuring mitochondrial function in SAMP8 mice. Aging. 11(2):756–770. doi: 10.18632/aging.101782.
- Chen Z, Li L, Xu S, Liu Z, Zhou C, Li Z, Liu Y, Wu W, Huang Y, Kuang M, et al. 2020. A Cdh1-FoxM1-Apc axis controls muscle development and regeneration. Cell Death Dis. 11(3):180. doi: 10.1038/s41419-020-2375-6.
- Chew W, Lim YP, Lim WS, Chambers ES, Frost G, Wong SH, Ali Y. 2022. Gut-muscle crosstalk. A perspective on influence of microbes on muscle function. Front Med . 9:1065365. doi: 10.3389/fmed.2022.1065365.
- Crossland WL, Norris AB, Tedeschi LO, Callaway TR. 2018. Effects of active dry yeast on ruminal pH characteristics and energy partitioning of finishing steers under thermoneutral or heat-stressed environment. J Anim Sci. 96(7):2861–2876. doi: 10.1093/jas/sky165.
- Daily JW, Park S. 2022. Sarcopenia is a cause and consequence of metabolic dysregulation in aging humans: effects of gut dysbiosis, glucose dysregulation, diet and lifestyle. Cells-. 11(3):338. doi: 10.3390/cells11030338.
- Du D, Lv W, Su R, Yu C, Jing X, Bai N, Hasi S. 2021. Hydrolyzed camel whey protein alleviated heat stress-induced hepatocyte damage by activated Nrf2/HO-1 signaling pathway and inhibited NF-kappaB/NLRP3 axis. Cell Stress Chaperones. 26(2):387–401. doi: 10.1007/s12192-020-01184-z.
- Duffy EM, Wilson HC, Schmidt TB, Yates DT, Petersen JL. 2019. Effect of environmental temperature and beta-adrenergic agonist supplementation on rumen volatile fatty acid production in sheep. Transl Anim Sci. 3(Suppl 1):1744–1748. doi: 10.1093/tas/txz079.
- Estrada-Angulo A, Aguilar-Hernández A, Osuna-Pérez M, Núñez-Benítez VH, Castro-Pérez BI, Silva-Hidalgo G, Contreras-Pérez G, Barreras A, Plascencia A, Zinn RA. 2016. Influence of quaternary benzophenantridine and protopine alkaloids on growth performance, dietary energy, carcass traits, visceral mass, and rumen health in finishing ewes under conditions of severe temperature-humidity index. Asian-Australas J Anim Sci. 29(5):652–658. doi: 10.5713/ajas.15.0300.
- Gao J, Yang PG, Cui YJ, Meng QS, Feng YJ, Hao Y, Liu JR, Piao XS, Gu XH. 2020. Identification of metabonomics changes in Longissimus Dorsi muscle of finishing pigs following heat stress through LC-MS/MS-based metabonomics method. Animals-Basel. 10(1):129. doi: 10.3390/ani10010129.
- Hassan FU, Arshad MA, Ebeid HM, Rehman MSU, Khan MS, Shahid S, Yang CJ. 2020. Phytogenic additives can modulate Rumen microbiome to mediate fermentation kinetics and methanogenesis through exploiting diet-microbe interaction. Front Vet Sci. 7:575801. doi: 10.3389/fvets.2020.575801.
- Hou L, Xu J, Li H, Ou J, Jiao Y, Hu C, Wang C. 2017. MiR-34c represses muscle development by forming a regulatory loop with Notch1. Sci Rep. 7(1):9346. doi: 10.1038/s41598-017-09688-y.
- Hsieh SY, Lian YZ, Lin IH, Yang YC, Tinkov AA, Skalny AV, Chao JC. 2021. Combined Lycium babarum polysaccharides and C-phycocyanin increase gastric Bifidobacterium relative abundance and protect against gastric ulcer caused by aspirin in rats. Nutr Metab. 18(1):4. doi: 10.1186/s12986-020-00538-9.
- Johnson JS, Scharf B, Weaber RL, Eichen PA, Spiers DE. 2012. Patterns of heat response and adaptation on summer pasture: a comparison of heat-sensitive (Angus) and-tolerant (Romosinuano) cattle. J. Therm. Biol. 37(4):344–350.
- Kang D, Park J, Shim K. 2019. Heat treatment at an early age has effects on the resistance to chronic heat stress on broilers. Animals. 9(12):1022. doi: 10.3390/ani9121022.
- Karthik D, Suresh J, Reddy YR, Sharma GRK, Ramana JV, Gangaraju G, Pradeep Kumar Reddy Y, Yasaswini D, Adegbeye MJ, Reddy PRK. 2021. Farming systems in sheep rearing: impact on growth and reproductive performance, nutrient digestibility, disease incidence and heat stress indices. PLOS One. 16(1):e0244922. doi: 10.1371/journal.pone.0244922.
- Kim J, Wellmann KB, Smith ZK, Johnson BJ. 2018. All-trans retinoic acid increases the expression of oxidative myosin heavy chain through the PPAR delta pathway in bovine muscle cells derived from satellite cells. J Anim Sci. 96(7):2763–2776. doi: 10.1093/jas/sky155.
- Krebs GL, De Rosa DW, White DM, Blake BL, Dods KC, May CD, Tai ZX, Clayton EH, Lynch EE. 2021. Intake, nutrient digestibility, rumen parameters, growth rate, carcase characteristics and cannabinoid residues of sheep fed pelleted rations containing hemp (Cannabis sativa L.). Stubble. Transl Anim Sci. 5(4):txab213.
- Lee JS, Kacem N, Kim WS, Peng DQ, Kim YJ, Joung YG, Lee C, Lee HG. 2019. Effect of Saccharomyces boulardii supplementation on performance and physiological traits of holstein calves under heat stress conditions. Animals. 9(8):510. doi: 10.3390/ani9080510.
- Leliveld LMC, Riva E, Mattachini G, Finzi A, Lovarelli D, Provolo G. 2022. Dairy cow behavior is affected by period, time of day and housing. Animals. 12(4):512. doi: 10.3390/ani12040512.
- Li YK, Kong LX, Deng M, Lian ZQ, Han YR, Sun BL, Guo YQ, Liu GB, Liu DW. 2019. Heat Stress-Responsive Transcriptome Analysis in the Liver Tissue of Hu Sheep. Genes. 10(5):395. doi: 10.3390/genes10050395.
- Li YX, Feng XP, Wang HL, Meng CH, Zhang J, Qian Y, Zhong JF, Cao SX. 2019. Transcriptome analysis reveals corresponding genes and key pathways involved in heat stress in Hu sheep. Cell Stress Chaperones. 24(6):1045–1054. doi: 10.1007/s12192-019-01019-6.
- Liu Y, Sun M, Hou P, Wang W, Shen X, Zhang L, Han S, Pan C. 2022. Analysis of microbial community structure and volatile compounds in pit mud used for manufacturing Taorong-type Baijiu based on high-throughput sequencing. Sci Rep. 12(1):7347. doi: 10.1038/s41598-022-10412-8.
- Liu Y, Yin S, Tang J, Liu Y, Jia G, Liu G, Tian G, Chen X, Cai J, Kang B, et al. 2021. Hydroxy selenomethionine improves meat quality through optimal skeletal metabolism and functions of Selenoproteins of pigs under chronic heat stress. Antioxidants. 10(10):1558. doi: 10.3390/antiox10101558.
- Liu Z, Liu YS, Xing T, Li JL, Zhang L, Jiang Y, Gao F. 2022. Transcriptome analysis reveals the mechanism of chronic heat stress on meat quality of broilers. J Anim Sci Biotechno. 13(1):110.
- Lu J, Zhu M, Cao H, Zhang X, Wang Z, Zhang X, Li X, Hu J, Yang G, Shi X. 2020. Impact of fermented corn-soybean meal on gene expression of immunity in the blood, level of secretory immunoglobulin A, and mucosa-associated bacterial community in the intestine of grower-finisher pigs. Front Vet Sci. 7:246. doi: 10.3389/fvets.2020.00246.
- Lyu L, Wen H, Li Y, Li J, Zhao J, Zhang S, Song M, Wang X. 2018. Deep transcriptomic analysis of Black Rockfish (Sebastes schlegelii) provides new insights on responses to acute temperature stress. Sci Rep. 8(1):9113. doi: 10.1038/s41598-018-27013-z.
- Ma J, Shah AM, Shao Y, Wang Z, Zou H, Kang K. 2020. Dietary supplementation of yeast cell wall improves the gastrointestinal development of weaned calves. Anim Nutr. 6(4):507–512. doi: 10.1016/j.aninu.2020.06.003.
- Ma P, Hong YF, Liu CX, Sun YQ, Liu MZ, Yang ZA, Ma PY, Wu HX, Xue FG. 2022. Rumen microbiota responses to the enzymatic hydrolyzed cottonseed peptide supplement under high-concentrate diet feeding process. Front Vet Sci. 9:984634. doi: 10.3389/fvets.2022.984634.
- Maia MR, Fonseca AJ, Oliveira HM, Mendonca C, Cabrita AR. 2016. The potential role of seaweeds in the natural manipulation of rumen fermentation and methane production. Sci Rep. 6(1):32321. doi: 10.1038/srep32321.
- Marai IFM, El-Darawany AA, Fadiel A, Abdel-Hafez MAM. 2007. Physiological traits as affected by heat stress in sheep - A review. Small Ruminant Res. 71(1–3):1–12. doi: 10.1016/j.smallrumres.2006.10.003.
- Mendoza SM, Boyd RD, Ferket PR, van Heugten E. 2017. Effects of dietary supplementation of the osmolyte betaine on growing pig performance and serological and hematological indices during thermoneutral and heat-stressed conditions. J Anim Sci. 95(11):5040–5053. doi: 10.2527/jas2017.1905.
- Meng P, Zhang X, Li D, Yang H, Lin X, Zhao H, Li P, Wang Y, Wang X, Ge J. 2023. Leonurine regulates hippocampal nerve regeneration in rats with chronic and unpredictable mild stress by activating SHH/GLI signaling pathway and restoring gut microbiota and microbial metabolic homeostasis. Neural Plast. 2023:1455634–1455612. doi: 10.1155/2023/1455634.
- Rong Y, Zeng MF, Guan XW, Qu KX, Liu JY, Zhang JC, Chen H, Huang BZ, Lei CZ. 2019. Association of HSF1 genetic variation with heat tolerance in Chinese cattle. Animals.. 9(12):1027. doi: 10.3390/ani9121027.
- Russi JP, DiLorenzo N, Relling AE. 2019. Effects of rumen-protected carbohydrate supplementation on performance and blood metabolites in feedlot finishing steers during heat stress. Transl Anim Sci. 3(1):513–521. doi: 10.1093/tas/txy122.
- Seibert JT, Abuajamieh M, Sanz Fernandez MV, Johnson JS, Kvidera SK, Horst EA, Mayorga EJ, Lei S, Patience JF, Ross JW, et al. 2018. Effects of heat stress and insulin sensitizers on pig adipose tissue. J Anim Sci. 96(2):510–520. doi: 10.1093/jas/skx067.
- Shi P, Tang L, Wang L, Sun T, Liu L, Cao W, Zhu Y. 2015. Post-heading heat stress in rice of South China during 1981–2010. PLOS One. 10(6):e0130642. doi: 10.1371/journal.pone.0130642.
- Song XF, Zhong L, Lyu N, Liu F, Li BX, Hao YN, Xue Y, Li J, Feng YQ, Ma Y, et al. 2019. Inulin can alleviate metabolism disorders in ob/ob mice by partially restoring leptin-related pathways mediated by gut microbiota. GenomProteom Bioinform. 17(1):64–75. doi: 10.1016/j.gpb.2019.03.001.
- Tang S, Zhou S, Yin B, Xu J, Di L, Zhang J, Bao E. 2018. Heat stress-induced renal damage in poultry and the protective effects of HSP60 and HSP47. Cell Stress Chaperones. 23(5):1033–1040. doi: 10.1007/s12192-018-0912-3.
- Uyanga VA, Musa TH, Oke OE, Zhao J, Wang X, Jiao H, Onagbesan OM, Lin H. 2023. Global trends and research frontiers on heat stress in poultry from 2000 to 2021: a bibliometric analysis. Front Physiol. 14:1123582. doi: 10.3389/fphys.2023.1123582.
- Vinoth A, Thirunalasundari T, Shanmugam M, Uthrakumar A, Suji S, Rajkumar U. 2018. Evaluation of DNA methylation and mRNA expression of heat shock proteins in thermal manipulated chicken. Cell Stress Chaperones. 23(2):235–252. doi: 10.1007/s12192-017-0837-2.
- Xie Y, Xu L, Wang Y, Fan LX, Chen YL, Tang MJ, Luo XB, Liu LW. 2018. Comparative proteomic analysis provides insight into a complex regulatory network of taproot formation in radish (Raphanus sativus L.). Hortic Res. 5(1):51. doi: 10.1038/s41438-018-0057-7.
- Yamashita Y, Nakada S, Yoshihara T, Nara T, Furuya N, Miida T, Hattori N, Arikawa-Hirasawa E. 2018. Perlecan, a heparan sulfate proteoglycan, regulates systemic metabolism with dynamic changes in adipose tissue and skeletal muscle. Sci Rep. 8(1):7766. doi: 10.1038/s41598-018-25635-x.
- Yang CT, Tsedan G, Liu Y, Hou FJ. 2020. Shrub coverage alters the rumen bacterial community of yaks (Bos grunniens) grazing in alpine meadows. J Anim Sci Technol. 62(4):504–520. doi: 10.5187/jast.2020.62.4.504.
- Zeng HF, Xi YM, Li YQ, Wang ZD, Zhang L, Han ZY. 2020. Analysis of Astragalus Polysaccharide Intervention in heat-stressed dairy cows’ serum metabolomics. Animals. 10(4):574. doi: 10.3390/ani10040574.
- Zhang C, Luo JQ, Yu B, Zheng P, Huang ZQ, Mao XB, He J, Yu J, Chen JL, Chen DW. 2015. Dietary resveratrol supplementation improves meat quality of finishing pigs through changing muscle fiber characteristics and antioxidative status. Meat Sci. 102:15–21. doi: 10.1016/j.meatsci.2014.11.014.
- Zhong S, Ding Y, Wang YY, Zhou GC, Guo HR, Chen YL, Yang YX. 2019. Temperature and humidity index (THI)-induced rumen bacterial community changes in goats. Appl Microbiol Biotechnol. 103(7):3193–3203. doi: 10.1007/s00253-019-09673-7.
- Zhu JY, Zhu H, Cao YJ, Li JH, Zhu QY, Yao JM, Xu CY. 2020. Effect of simulated warming on leaf functional traits of urban greening plants. BMC Plant Biol. 20(1):139. doi: 10.1186/s12870-020-02359-7.