Abstract
Animal scientists actively seek strategies for ensuring the sustainable viability of animal ecosystems, with a focus on exploring alternative feed raw materials to reduce reliance on traditional ones. So, this study aims to analyse the impact of river-sourced Cladophora glomerata macroalgal biomass on the growth, slaughter performance, and physiological development of rabbits. Thirty weaned (52 days of age) Californian rabbits were assigned to three dietary treatments: standard compound diet (SCD), SCD enriched with 4% C. glomerata (CG4), and SCD enriched with 8% C. glomerata (CG8). Growth performance was recorded throughout the feeding trial, and at its conclusion (122 days of age), rabbits were euthanized, slaughtered, and subjected to intestinal analysis. Incorporation of CG diets showed no significant impact on body weight or average daily gain (p > 0.05); however, CG8 significantly lowered daily feed intake and feed conversion ratio (p < 0.05). The SCD resulted in a significantly higher lung percentage (p < 0.05), while CG diets had minimal effects on remaining slaughter performance traits. In duodenal content, CG-enriched diets increased acetic and propionic acid levels but reduced lactic (p < 0.05). Duodenal villus height remained stable, while in the ileum, CG4 inclusion resulted in a significantly higher villus (p < 0.05). Duodenal crypt depth increased with biomass supplementation; conversely, in the ileum, increased biomass led to decreased crypt depth (p < 0.05). In general, recent research suggests that adding C. glomerata to rabbit diets can be an effective alternative without adverse effects on growth, slaughter performance, or physiologial development.
HIGHLIGHTS
C. glomerata macroalgal biomass is usually considered waste.
Collecting C. glomerata macroalgal biomass from natural water bodies not only enhances their biodiversity and recreational value but also yields valuable raw material for diverse applications in biotechnology.
C. glomerata macroalgal biomass, with its high fibre content, aligns with the unique digestive physiology of rabbits, making it a suitable nutritional component for their dietary requirements.
Introduction
Optimising growth performance in production animals is crucial for the economic viability and sustainability of livestock enterprises. Efficient development trajectories lead to faster weight gain, increased meat, milk, or egg production, and financial benefits (Fernandes et al. Citation2021). So, producers strive to enhance feed conversion ratios, improving the transformation of ingested grain into valuable products (Mayerfeld Citation2023). Enchanted growth rates directly boost livestock production, enabling a response approach to market demands. Furthermore, improved growth is also linked to better animal health, reducing susceptibility to illnesses, and minimising the need for veterinary interventions (Charlier et al. Citation2022). Strategic prioritisation and enhancement of animal growth not only increase farmers’ profitability but also confer competitive advantages (Pomar et al. Citation2019), contributing to a more sustainable and resilient livestock sector.
Today, production-animal growers face a pressing challenge as they recognise the limitations of resources and grapple with the environmental impacts associated with intensive agricultural practices. Achieving a more sustainable livestock sector requires judicious management of natural resources, minimising ecological footprints, and addressing issues such as greenhouse gas emissions, biodiversity, and humane animal treatment (Banerjee et al. Citation2020). Growers are increasingly adopting circular economy principles, waste reduction strategies, and environmentally friendly technologies to enhance sustainable animal farming (Batra Citation2023). Striking a balance between meeting current production demands and ensuring long-term ecosystem prosperity is crucial for sustainability in the animal sector. As global concerns about climate change and resource depletion escalate, the imperative for sustainable practices in animal farming becomes more pronounced (Campos Citation2021).
Daily, animal scientists strive to develop strategies ensuring the sustainable viability of animal ecosystems. Exploring alternative feed additives to reduce dependency on traditional raw materials is crucial to this challenge. For instance, our prior research highlighted the significant potential of utilising freshwater Cladophora glomerata (C. glomerata) macroalgae biomass in rabbit nutrition, resulting in improved meat quality and consumer-accepted sensory properties (Nutautaitė et al. Citation2023; Nutautaitė et al. Citation2023). A comprehensive analysis of C. glomerata’s chemical composition supports its viability as a protein and amino acid source in animal nutrition (Nutautaitė et al. Citation2021), substantiating its categorisation as functional (Nutautaitė et al. Citation2021; Nutautaitė et al. Citation2022a). Previous scientific investigations confirm that freshwater macroalgae offer elevated nutrient content and beneficial effects on animal health (Al-Soufi et al. Citation2022; Garcia-Vaquero and Hayes Citation2016; Silva et al. Citation2020; Wan et al. Citation2019). Notably, the abundance of this biomass in Lithuanian rivers, currently considered waste, presents an opportunity for waste-reduction strategies aligned with sustainable practices in animal husbandry and enhancing the biodiversity of local water bodies. However, despite obtaining a functional rabbit meat using C. glomerata macroalgal biomass, it is essential to delve into key indicators of animal husbandry. Therefore, the aim of this study was to analyse the impact of river-sourced C. glomerata macroalgal biomass on the growth, slaughter performance, and physiological development of rabbits.
Materials and methods
Animals and feeding trial scheme
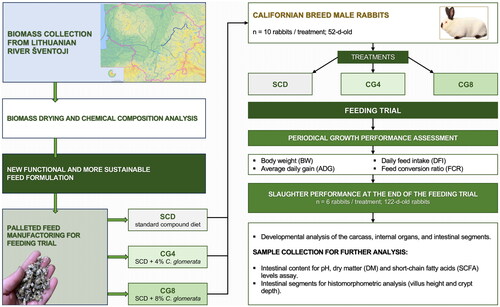
The feeding trial involved thirty male Californian-breed rabbits that were weaned at 52 days of age. The rabbits for the study were selected based on comparable weights and then randomly allocated to one of three different dietary regimens (n = 10 rabbits/diet). The research was carried out at a local rabbit breeding facility, where the animals were housed in individual cages measuring 34 × 34 × 61 cm, allowing for the housing of one rabbit per cage. Each rabbit was provided unrestricted access to dedicated nipple drinkers dispensing clean water and individual feed bowls to ensure optimal health conditions and performance. The facility was equipped with a heating system that maintained a temperature of 19 ± 2 °C. Throughout the trial, the photoperiod regimen consisted of 16 h of illumination followed by an 8-h period of darkness. The housing protocols conformed to the specifications delineated in Council Directive 98/58/EC of July 20, 1998, focusing on the welfare standards for animals in farming practices. The protocol structure of the rabbit feeding trail is displayed in Figure .
Rabbits were supplied with three distinct diets: a standard compound diet (SCD); SCD enriched with 4% biomass of freshwater C. glomerata (CG4); and SCD enriched with 8% biomass of C. glomerata (CG8). The feeding regimen was distributed twice daily, ad libitum. The formulation of the standard compound diet was meticulously designed to meet the nutritional requirements of growing rabbits by incorporating essential vitamins and minerals. The nutrient composition of the diet adhered to the recommendations provided by the National Research Council (NRC) (Arrington et al. Citation1977). Detailed information regarding the feed ingredients and their respective chemical compositions is presented in Table .
Table 1. Ingredients in rabbit feed and chemical composition of a standard compound diet and diets supplemented with river-sourced C. glomerata biomass (52–122 days old).
The biomass of C. glomerata utilised for feed formulation was manually collected from the Šventoji River in Lithuania, subjected to meticulous cleaning and drying processes, and subsequently incorporated into the production of CG-enriched feed. The chemical composition of the collected biomass was 22.36% crude protein, 2.35% crude fat, 20.80% crude ash, and 22.45% crude fibre. In previously reported research, biomass has been scrutinised for its more detailed chemical composition, including antioxidant properties (Nutautaitė et al. Citation2021; Nutautaitė et al. Citation2022a).
Growth and slaughter performance evaluation, sample collection
Over the period of the feeding trial (52–122 days) rabbits’ (n = 10 rabbits/diet) individual weights including body weight gain (BWG), average daily gain (ADG), daily feed intake (DFI), and feed conversion ratio (FCR) were systematically recorded and documented.
At the end of the feeding trial when the rabbits reached 122 days of age, a total of 18 rabbits (n = 6 rabbits/diet) were randomly selected, weighed, and subjected to an overnight fast. Subsequently, rabbits were humanely euthanized in accordance with established farming practices. The slaughter procedures were conducted at a rabbit breeding farm slaughterhouse, adhering to established protocols that align with the laws of the Republic of Lithuania, as specified in Order No. B1-866 of October 31, 2012, by the Director of the State Food and Veterinary Service. This order delineates the requirements for the care, keeping, and utilisation of animals for scientific and educational purposes.
The rabbit carcases underwent preparation as per the methods reported by Blasco and Ouhayoun (Citation2010) and were then chilled at 4 °C for 24 h in a well-ventilated room. Dissection procedures of carcases followed the recommendations set forth by the World Rabbit Science Association (WRSA) (Blasco and Ouhayoun Citation2010). A comprehensive examination of post-mortem carcase attributes, muscle, organ, and gut development was carried out. For further analysis, duodenum and ileum segments from the middle part were specifically collected for histomorphometric assay, while the content of distinct intestinal segments was collected for the determination of pH, dry matter (DM), and short-chain fatty acid (SCFA) profiles.
Intestinal content features
pH and dry matter assay
All intestinal content samples were collected post-mortem (n = 6 samples/diet). The pH of duodenum, small intestine, caecum, ileum, colon, and stomach contents chyme was determined using a pH metre InoLab pH 730 (WTW Electronic GmbH, Ihlow, Germany).
The dry matter (DM) content of the intestinal segment contents (n = 6 samples/diet) was assessed by subjecting them to drying in the Memmert UFE 400 drying cabinet (Memmert GmbH + Co, Schwabach, Germany), maintained at 105 °C. The samples underwent drying until reaching a consistent weight.
Short-chain fatty acid (SCFA) profile determination
The compositional analysis of short-chain fatty acids (SCFA) within duodenal content (n = 6 samples/diet) was conducted employing a gas chromatography system (Shimadzu GC-2010 Plus; Shimadzu Corp., Kyoto, Japan). A 2.5 mm x 2.6 mm glass tube filled with 10% SP-1200/1% HPO on 80/100 Chromosorb W AW was used, with the tube temperature set at 110 °C, the flame ionisation detector (FID) temperature at 108 °C, and the injector’s temperature at 195 °C. SCFA accumulation values were derived by determining the concentration of individual SCFA within the digestive content, expressed as a percentage of the total SCFA, following the analytical procedures outlined by Zduńczyk et al. (Citation2004).
Histomorphometrical analysis
For the assessment of histomorphometric properties, duodenum, and ileum samples from the middle part of the rabbit intestinal segment (n = 6 samples/diet) were fixed using a 10% neutral formalin solution. Subsequently, employing standard histologic procedures, the tissues were embedded in paraffin and sliced into 4 μm-thick tissue sections using a rotary microtome (Leica RM 2235; Leica Microsystems, Nussloch, Germany). These sections were stained with haematoxylin and eosin. Morphometric and microscopic measurements of villus heights and crypt depths for all diet samples were conducted. The prepared histological samples underwent examination using an Olympus BX63 microscope (Olympus Corp., Tokyo, Japan), an Olympus DP72 digital camera (Olympus Corp., Tokyo, Japan), and the Image-Pro Plus programme system for Windows, version 7.0 (Media Cybernetics, Inc., Bethesda, MD, USA, 2009).
Statistical analysis
The study utilised samples from three distinct dietary treatments of rabbits, with each treatment comprising ten duplicate rabbits for growth performance (n = 10 rabbits/diet) and six duplicate rabbits for slaughter and remained analysis (n = 6 rabbits/diet). Six samples per dietary treatment were collected for each intestinal segment assay (n = 6 samples/diet). The data analysis was conducted using SPSS for Windows, version 25.0 (IBM Corp., Released 2017, Armonk, NY, USA). A one-way analysis of variance (ANOVA) test, followed by post-hoc analysis using Fisher’s least significant difference test, was performed to identify differences among the diets. A calculated p-value of less than 0.05 (p < 0.05) was reconned statistically significant.
Results
Growth and slaughter performance
Growth performance features for the whole feeding trial period (52–122 days of age) are presented in Table . Rabbit feed enrichment with C. glomerata biomass did not yield a statistically significant effect on the body weight gain (BWG) or average daily gain (ADG) over the whole feeding trial period (p > 0.05). However, significant differences were discerned between the CG4 and CG8 dietary treatments upon examination of rabbits' daily feed intake (DFI). Specifically, a 3.48 g significant elevation in DFI was noted in CG4 in comparison to CG8 (p < 0.05). This trend persisted when evaluating the feed conversion ratio (FCR), with CG4, exhibiting a significantly higher FCR (4.23 kg/kg), followed by a notably lower FCR in the SCD group (3.72 kg/kg), and the lowest FCR recorded in rabbits treated with CG8 (2.93 kg/kg) (p < 0.05).
Table 2. Impact of river-sourced C. glomerata macroalgal biomass on rabbit growth performance.
At the end of the feeding trial, the slaughter performance was evaluated (Table ). All anatomical components of rabbit carcases, inclusive of the intestinal organs and muscles, were extracted in percentage based on pre-slaughter weights. Subsequent analysis of these measurements revealed no statistically significant differences among the examined diets, with one exception: the lungs. A significantly higher percentage of lung persistence was discerned in rabbits treated with the SCD diet (0.57%) (p < 0.05), while under both CG4 and CG8 treatments, the corresponding lung percentages were 0.43%, exhibiting no significant disparity between the CG treatments (p < 0.05). Therefore, the examination of other distinct slaughter performance parameters resulted in no statistically significant differences among the diets in the assessment of drip loss, dressing-out percentage, carcase, leg muscle, and longissimus dorsi muscle yields (p > 0.05).
Table 3. Impact of river-sourced C. glomerata macroalgal biomass on rabbit slaughter performance.
Features of intestinal segments
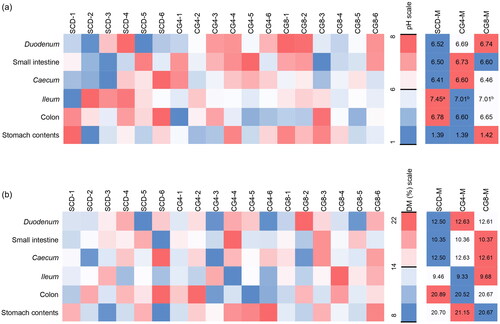
To assess the impact of C. glomerata-enriched feed on rabbit digestive processes, the pH and dry matter (DM) of the contents of distinct intestinal segments were analysed (Figure ). Among all the analysed segments, significant differences were observed solely in the assessment of ileum content pH values. Rabbits treated with SCD exhibited a significantly elevated pH value in the ileum content, surpassing those of biomass-enriched diets CG4 and CG8 by 0.44 and 0.38 units, respectively (p < 0.05; Figure ). Nonetheless, the mechanistic underpinnings of C. glomerata biomass supplementation in rabbit feed and its impact on pH and DM values in other intestinal segments remain indeterminate.
Figure 2. Impact of river-sourced C. glomerata macroalgal biomass on distinct intestinal segments content: (a) pH; (b) dry matter (DM). Diets: SCD, standard compound diet; CG4, SCD enriched with 4% macroalgal biomass; CG8, SCD enriched with 8% macroalgal biomass. Diet-number, means diet sample replicate number; Diet-M, represents the means of replicate samples. a,bMeans with different superscript letters (a,b) in a row were significantly different (p < 0.05).
The short-chain fatty acid profile of duodenum content is presented in Table . In general, acetic acid predominated across all diets, followed by propionic and lactic acids, with butyric acid notably absent in the duodenal contents of rabbits treated with CG8 diet. Excluding specific and significant differences between diets, SCD displayed the lowest acetic acid content (83.23%), with higher levels observed in CG8 (87.53%), and the highest content determined in CG4 (89.04%) (p < 0.05). After assessing propionic acid, CG8-treated rabbits exhibited 1.75 times more of this SCFA compared to those on the SCD diet (p < 0.05). On the contrary, no significant differences were noted in butyric acid levels, and it was entirely absent in CG8-treated rabbits’ duodenal content (p > 0.05). Nevertheless, C. glomerata supplementation resulted in a notable reduction in lactic acid: the greater the biomass inclusion, the lower the lactic acid levels. In this context, compared to SCD, the lactic acid content in CG4 and CG8 was, respectively, lower by 6.06% and 7.16%, demonstrating a significant decrease (p < 0.05).
Table 4. Impact of river-sourced C. glomerata macroalgal biomass short-chain fatty acids (SCFA) contents in rabbits’ duodenum.
Histomorphometrical measurements of duodenal and ileum
Rabbit duodenum and ileum segments underwent histomorphometric evaluation (Table ). However, no significant impact was observed on duodenum villus height, while in the ileum, the highest villus was discovered under CG4 treatment (1526.09 µm) and the lowest under CG8 (1079.12 µm). When comparing both C. glomerata-enriched diets, a significantly higher 446.97 µm villus was observed in the CG4 ileum compared to CG8; furthermore, in comparison to SCD, CG4 exhibited a significantly greater villus by 310.73 µm (p < 0.05).
Table 5. Impact of river-sourced C. glomerata macroalgal biomass on rabbits’ duodenum and ileum histomorphometric measurements.
The results of the crypt depth were distributed as follows: in the duodenum, the greater the biomass supplementation, the deeper the crypts (CG8 > CG4 > SCD); in the ileum, conversely, the greater the biomass supplementation, the lower the crypt depth (SCD > CG4 > CG8). CG8-treated rabbits exhibited 74.40 µm significantly greater duodenal crypt depth compared to SCD (p < 0.05). In contrast, CG8 ileum segment crypts were significantly shorter 2.39 times compared to SCD-treated rabbits (p < 0.05).
The villus-to-crypt ratio (V/C) exhibited a contrasting distribution: in the duodenum, the order was SCD > CG4 > CG8, while in the ileum, it was CG8 > CG4 > SCD (Table ). In SCD-treated rabbits’ duodenum, the V/C ratio was respectively higher by 1.57 and 2.36 units compared to both C. glomerata diets (CG4 and CG8, respectively) (p < 0.05). Conversely, biomass supplementation resulted in a higher V/C ratio in the ileum; it was significantly higher by 4.71 and 5.55 units in CG4 and CG8, respectively, compared to SCD (p < 0.05).
Discussion
Growth and slaughter performance
Rabbit growth performance significantly influences production efficiency and overall enterprise profitability (Mukaila Citation2023). Meeting the nutritional requirements is crucial for optimal growth, and certain Cladophora species, with comparable compositions to traditional plants used in feed production, simplify this principle (Heiba et al. Citation1997). Studies on C. glomerata biomass highlight its rich profile in essential fats and amino acids (Messyasz et al. Citation2015; Nutautaitė et al. Citation2021; Nutautaitė et al. Citation2022b). Notably, exogenous amino acids, known for enhancing nutrient digestibility and elevating quality, hold promise for more efficient growth performance in animals (Konkol et al. Citation2018). Despite macroalgal prevalent use in supplementing aquaculture and poultry feed, there is limited research on the application of C. glomerata in rabbit nutrition. Scientific evidence suggests that an optimal dosage ranging from 10% to 20% of C. glomerata can optimise productivity traits, including feed conversion ratio, in tiger shrimp (Anh et al. Citation2018). Similarly, in research on broiler chickens, a 15% supplementation of C. glomerata biomass in their feed demonstrated enhanced growth rates compared to standard feed (Abid and Abid Citation2006). Recent research into the growth performance of rabbits, incorporating river-sourced C. glomerata biomass throughout the entire feeding trial (52–122 days of age), indicates a lack of statistically significant impact on BWG and ADG across dietary treatments. However, notable differentials in DFI and FCR prompt a nuanced exploration of potential underlying mechanisms.
Daily feed intake, a crucial indicator of growth performance, signifies the essential quantity of feed required for the nutritional needs and well-being of animals (Kumar et al. Citation2022). So, achieving optimal growth entails a delicate balance, as insufficient intake may lead to malnutrition and poor growth, while excessive consumption can result in obesity and other health issues. In a recent study, a significant increase in DFI observed in CG4 compared to CG8 implies a potential impact on the palatability or satiety response to a diet enriched with 4% macroalgal biomass. This aligns with prior studies highlighting the palatability of diets incorporating macroalgae across various animal species (Øverland et al. Citation2019). The enhanced palatability may be attributed to bioactive compounds in C. glomerata, such as polyphenols and secondary metabolites, known to influence the taste and acceptability of feed ingredients (Bruneel et al. Citation2013; Nutautaitė et al. Citation2022a; Ramesh Kumar et al. Citation2019). Conversely, optimal biomass inclusion was determined to be 4%, as higher amounts led to reduced DFI in an 8%-enriched diet, indicating a potential upper limit for acceptable inclusion.
A lower FCR in rabbits indicates more efficient conversion of feed into body weight, directly affecting production costs and overall profitability in commercial rabbit farming operations (Exequiel et al. Citation2021). Furthermore, the distinct FCR patterns observed among the dietary regimens may indicate differences in feed utilisation efficiency. The notably lower FCR in CG8 observed in a recent study could potentially be linked to altered digestive processes or nutrient absorption influenced by the components of C. glomerata. For example, macroalgae are recognised reservoirs of bioactive compounds, including polysaccharides and peptides, which may modulate nutrient utilisation and absorption in the gastrointestinal tract (Beaulieu et al. Citation2015; Harnedy and FitzGerald Citation2011; O' Brien et al. Citation2022; Pimentel et al. Citation2019). In our case, the higher dosage of river-sourced biomass reduces FCR, while the lower dosage, on the contrary, increases this indicator. This means that feed enriched with 8% biomass could lead to more economical feed conversion into rabbit body weight.
The healthy growth and development of rabbits rely on the proper maturation of internal organs and intestines, crucial for effective nutrient absorption and maintaining a robust immune system (Koletzko et al. Citation1998). Following the feeding trial, slaughter performance was assessed by dissecting all anatomical components of rabbit carcases. No significant differences were found among treatments, except for lung percentage. Rabbits on the SCD showed a notable increase in lung percentage, while both C. glomerata-treated groups had comparable, smaller percentages. The observed rise in lung percentage in SCD-treated rabbits, despite the consistent impact of biomass on carcase composition, prompts further consideration and raises questions about the potential physiological mechanisms of biomass. One possible explanation for the impact on lung development and respiratory function is the presence of antioxidants, such as flavonoids and carotenoids, in C. glomerata biomass (Nutautaitė et al. Citation2022a). These antioxidants can reduce oxidative stress in the gut, potentially promoting intestinal health and suggesting a connection to the modulation of respiratory processes and lung development. Similar lung percentages in C. glomerata treatments imply that changes in biomass levels may not significantly affect lung percentage, suggesting no dose-dependent relationship with the observed physiological outcome.
Moreover, the assessment of additional slaughter performance parameters revealed no significant differences among dietary regimens, whether incorporating C. glomerata or not. Biomass in rabbit diets had a minimal impact on specific aspects of slaughter performance, such as meat water-holding capacity and the efficiency of converting live weight into carcase weight. The similar yields obtained clearly emphasise that the overall composition of these meat-associated anatomical structures remains largely unchanged with the dietary inclusion of C. glomerata.
Features of intestinal segments
In assessing the impact of C. glomerata-enriched feed on rabbit digestive processes, the focus was initially on pH and DM analysis. Significantly elevated pH values were observed in the ileum content of rabbits under the SCD compared to CG diets. However, the mechanistic underpinnings of biomass enrichment and its effects on pH and DM values in other intestinal segments remain uncertain. Variations in ileum pH may implement potential modulation of microbial ecology and fermentation processes, consistent with previous research indicating macroalgal prebiotic properties influencing gut microbiota composition (Michalak and Chojnacka Citation2015; O’Sullivan et al. Citation2010; Shannon et al. Citation2021). Therefore, the absence of significant variations in other intestinal segments among dietary treatments prompts exploration into nuanced interactions between C. glomerata supplementation and digestive processes throughout the gastrointestinal tract.
In scrutinising dietary impacts on rabbit physiology, duodenal content analysis reveals a consistent SCFA profile. Acetic acid predominates, followed by propionic and lactic acids, aligning with established SCFA patterns linked to gastrointestinal microbial fermentation. Significantly, the predominant acetic acid serves as a vital energy substrate in rabbit tissues, enhancing metabolic processes and influencing dietary fat utilisation (He et al. Citation2020). Additionally, it may regulate appetite and satiety, impacting overall dietary intake in rabbits. The elevated acetic acid content in CG4 and CG8 shows a potential enhancement of microbial fermentation processes, possibly influenced by C. glomerata’s prebiotic properties. This aligns with trends observed in prior studies on algae-based diets, promoting microbial activity and SCFA production in various animal species (Kulshreshtha et al. Citation2020; Patel et al. Citation2021). In contrast, following propionic acid in duodenal content, has been implicated in glucose metabolism (Lemosquet et al. Citation2004). It acts as a gluconeogenesis precursor, actively contributing to hepatic glucose production—a critical metabolic pathway for maintaining blood glucose levels and meeting energy requirements, especially during fasting or increased energy expenditure. The noteworthy decrease in lactic acid levels with increasing C. glomerata biomass highlights the potential prebiotic effects of specific macroalgal constituents, influencing gut microbiota composition. Moreover, the dose-dependent reduction in lactic acid content accentuates C. glomerata’s capacity to induce shifts in microbial populations along the gastrointestinal tract. In rabbits, lactic acid, which is consistently present, shows a dose-dependent decrease, typically associated with microbial fermentation processes in the caecum and colon (Davies and Rees Davies Citation2003). However, the reduction in lactic acid content in duodenum in response to CG diets suggests potential alterations in microbial populations, with implications for overall gut health and fermentation efficiency. Butyric acid, on the other hand, emerges from dietary fibre fermentation and has been linked to a variety of health advantages (Wang et al. Citation2019). Nevertheless, the lack of butyric acid in CG8-treated rabbits’ duodenum content demands a more in-depth investigation of the processes driving microbial metabolism and SCFA production in response to C. glomerata.
Histomorphomentrical properties of duodenum and ileum
Histomorphometric scrutiny is essential for comprehending the impact of alternative feed components on structural changes in the intestinal mucosa, including villus height and crypt depth, which directly influence nutrient absorption and overall gut health. Firstly, assessing the adaptability and response of intestinal tissue is crucial for optimising dietary formulations to enhance nutrient utilisation and promote animal well-being (Candebat et al. Citation2023). Secondly, histomorphometric assays are linked to the detection of possible issues or benefits connected with alternate feed components, directing towards the development of diets that improve digestive function and overall animal performance (der Poel et al. Citation2020). In a recent study, enriching rabbit diets with river-sourced C. glomerata significantly impacted mucosal architecture. While duodenal villus height remained stable, the ileum exhibited variation, being highest under CG4, lowest on CG8, and lower under SCD. In this case, increased villus height in the rabbit ileal segment indicates better mucosal morphogenesis, allowing for a larger surface area beneficial to improved nutrient absorption (Yu and Chiou Citation1997). In conclusion, the heightened villus in the rabbit ileum under a 4% biomass-enriched diet suggests improved digestion, better nutrient use, and potential benefits for optimal rabbit growth.
Crypt depth in the duodenum increased with increasing biomass supplementation (CG8 > CG4 > SCD), although in the ileum it decreased with increased biomass (SCD > CG4 > CG8). The importance of crypt depth stems from its ability to represent the structural dynamics of the intestinal mucosa. A deeper crypt in the duodenum signifies faster cell turnover and nutritional absorption capability, whereas a shallower crypt in the ileum indicates a more mature mucosal architecture (Modina et al. Citation2021). The observed differences highlight the segment-specific influence of C. glomerata on rabbit intestinal histomorphometry. Biomass impact on microbial fermentation, nutrient availability, and the modulation of specific signalling pathways involved in mucosal development are all potential mechanisms through which it may influence crypt depth. According to Al-Sagheer et al. (Citation2023) reduced duodenal crypt depth in rabbits suggests mature mucosal architecture, whereas increasing crypt depth demonstrates active mucosal development as well as potential nutritional absorption augmentation. Reduced crypt depth in the ileum indicates a stabilised mucosal architecture, whereas increasing crypt depth suggests active mucosal development as well as improved nutritional absorption.
Distinct V/C ratio patterns were observed in rabbit duodenum and ileum segments under C. glomerata treatments. However, SCD-treated rabbits exhibited a higher V/C ratio in the duodenum than both C. glomerata-enriched diets, suggesting increased absorptive capacity. Conversely, the ileum displayed an improved ratio with higher C. glomerata enrichment, indicating a positive impact on mucosal architecture and nutrient absorption. These findings underscore the segment-specific effects of C. glomerata on intestine histomorphometry, potentially influenced by biologically active substances in macroalgal biomass. The observed variations in V/C ratios highlight the intricate balance between villi and crypts, reflecting the structural dynamics of the intestinal mucosa and providing nuanced insights into nutrient absorption efficiency and overall gastrointestinal health in rabbits.
Conclusions
Incorporating river-sourced C. glomerata in rabbit diets had no significant impact on body weight gain or average daily gain. However, the 8% biomass-enriched diet resulted in lower daily feed intake and feed conversion ratio, suggesting potential economic benefits. Both C. glomerata-enriched diets reduced lung percentage without significantly affecting remaining carcase anatomical components or overall slaughter performance, suggesting minimal structural alterations due to dietary biomass inclusion. Biomass-enriched diets increased acetic and propionic acid levels but reduced lactic acid in duodenal content, showing potential improvement in microbial fermentation. From a histomorphometric point of view, duodenal villus height remained stable, while the ileum exhibited dose-dependent variations, with a 4%-enriched diet resulting in greater villus height. Duodenal crypt depth increased with biomass supplementation, indicating potential effects on cell turnover and nutrient absorption. Conversely, in the ileum, increased biomass led to decreased crypt depth, suggesting a more mature mucosal architecture. Overall, recent research suggests that incorporating C. glomerata into rabbit diets has the potential to be a viable alternative to traditional feed materials with no adverse impacts on growth, slaughter performance, or physiological development.
Ethical approval
The research adhered to the guidelines outlined in Directive 2010/63/EU of the European Parliament and the Council (22 September 2010) on the protection of animals used for scientific purposes. Additionally, it followed the Commission’s recommendation (18 June 2007) for the welfare of animals in farming. Ethical approval (Bioethical permit No. PK042495) was obtained from the Lithuanian University of Health Sciences Centre of Postgraduate Studies on 7 November 2022, sanctioned by the State Food and Veterinary Service through official letters (No. B6-(1.9)-2625 dated 16 October 2013 and updated on 28 March 2017, No. B6-(1.9)-852).
Authors’ contributions
Conceptualisation, MN, and VV; methodology, MN, SB, and AP; software, MN, and AP; data collection, MN, VV and ARS; investigation, MN, ARS, SB, AP and VV; resources, MN and VV; writing—original draft preparation, MN; writing—review and editing, VV and ARS; supervising, VV. All the authors have read and agreed to the published version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that supports the findings of this study are available on request from the corresponding author.
References
- Abid A, Abid R. 2006. Cladophora glomerata (L.) Kutzing as feed supplement to broiler chicks. Int J Biol Biotech. 3(2):423–427.
- Al-Sagheer AA, Alagawany M, Bassiony SS, Shehata AM, El-Metwally AE, El-Kholy MS. 2023. Inactivated Saccharomyces cerevisiae and selenium as alternatives to antibiotic in rabbits reared under summer conditions: effects on growth, nutrient utilization, cecal fermentation, blood components, and intestinal architecture. Anim Feed Sci Technol. 302:115688. doi: 10.1016/j.anifeedsci.2023.115688.
- Al-Soufi S, García J, Muíños A, López-Alonso M. 2022. Marine macroalgae in rabbit nutrition—a valuable feed in sustainable farming. Animals (Basel). 12(18):2346. doi: 10.3390/ani12182346.
- Anh N, Hai T, Hien T. 2018. Effects of partial replacement of fishmeal protein with green seaweed (Cladophora spp.) protein in practical diets for the black tiger shrimp (Penaeus monodon) postlarvae. J Appl Phycol. 30(4):2649–2658. doi: 10.1007/s10811-018-1457-7.
- Arrington LR, Cheeke PR, Lebas F, Lebas S. 1977. Nutrient Requirements of Rabbits (Anonymous Trans.) (Second revised edition ed.). Washington DC: National Reasearch Council (NRC).
- Banerjee A, Meena RS, Jhariya MK, Yadav DK. 2020. Natural resources intensification and footprints management for sustainable food system. In Anonymous Agroecological Footprints Management for Sustainable Food System. (Anonymous Trans), p. 25–68. Singapore: Springer.
- Batra G. 2023. Renewable energy economics: achieving Harmony between Environmental Protection and Economic Goals. SSC. 2(1):32. doi: 10.56106/ssc.2023.009.
- Beaulieu L, Bondu S, Doiron K, Rioux L, Turgeon SL. 2015. Characterization of antibacterial activity from protein hydrolysates of the macroalga Saccharina longicruris and identification of peptides implied in bioactivity. J Funct Foods. 17:685–697. doi: 10.1016/j.jff.2015.06.026.
- Blasco A, Ouhayoun J. 2010. Harmonization of criteria and terminology in rabbit meat research. Revised proposal. World Rabbit Sci. 4(2):93–99.
- Bruneel C, Lemahieu C, Fraeye I, Ryckebosch E, Muylaert K, Buyse J, Foubert I. 2013. Impact of microalgal feed supplementation on omega-3 fatty acid enrichment of hen eggs. J Funct Foods. 5(2):897–904. doi: 10.1016/j.jff.2013.01.039.
- Campos H. 2021. Productivity in agriculture for a sustainable future. Innov Revol Agri. 1st ed. Cham: Springer International Publishing); p. 33–69. doi: 10.1007/978-3-030-50991-0_2#DOI.
- Candebat CL, Eddie T, Marc AF, Fernando F, Nankervis L. 2023. Exploring the physiological plasticity of giant grouper (Epinephelus lanceolatus) to dietary sulfur amino acids and taurine to measure dietary requirements and essentiality. Fish Physiol Biochem. 49(5):829–851. doi: 10.1007/s10695-023-01222-4.
- Charlier J, Barkema HW, Becher P, De Benedictis P, Hansson I, Hennig-Pauka I, La Ragione R, Larsen LE, Madoroba E, Maes D, et al. 2022. Disease control tools to secure animal and public health in a densely populated world. Lancet. Planetary Health. 6(10):e812–e824. doi: 10.1016/S2542-5196(22)00147-4.
- Davies RR, Rees Davies JAE. 2003. Rabbit gastrointestinal physiology. Vet Clin North Am Exot Anim Pract. 6(1):139–153. doi: 10.1016/S1094-9194(02)00024-5.
- der Poel A v, Abdollahi MR, Cheng H, Colovic R, den Hartog LA, Miladinovic D, Page G, Sijssens K, Smillie JF, Thomas M, et al. 2020. Future directions of animal feed technology research to meet the challenges of a changing world. Anim Feed Sci Technol. 270:114692. doi: 10.1016/j.anifeedsci.2020.114692.
- Exequiel S, Soledad P, Mariana R, Silvia B. 2021. Global feed conversion in semi-intensive rabbit production system of Argentina. Trop Anim Health Prod. 53(2):327. doi: 10.1007/s11250-021-02766-4.
- Fernandes JN, Hemsworth PH, Coleman GJ, Tilbrook AJ. 2021. Costs and benefits of improving farm animal welfare. Agriculture (Basel). 11(2):104. doi: 10.3390/agriculture11020104.
- Garcia-Vaquero M, Hayes M. 2016. Red and green macroalgae for fish and animal feed and human functional food development. Food Rev Int. 32(1):15–45. doi: 10.1080/87559129.2015.1041184.
- Harnedy PA, FitzGerald RJ. 2011. Bioactive proteins, peptides, and amino acids from macroalgae. J Phycol. 47(2):218–232. doi: 10.1111/j.1529-8817.2011.00969.x.
- He J, Zhang P, Shen L, Niu L, Tan Y, Chen L, Zhao Y, Bai L, Hao X, Li X, et al. 2020. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. IJMS. 21(17):6356. doi: 10.3390/ijms21176356.
- Heiba H, Al-Easa H, Rizk A. 1997. Fatty acid composition of twelve algae from the coastal zones of Qatar. Plant Foods Hum Nutr. 51(1):27–34. doi: 10.1023/A:1007980227542.
- Koletzko B, Aggett PJ, Bindels JG, Bung P, Ferré P, Gil A, Lentze MJ, Roberfroid M, Strobel S. 1998. Growth, development and differentiation: a functional food science approach. Br J Nutr. 80 Suppl 1(S1):S5–S45. doi: 10.1079/BJN19980104.
- Konkol D, Górniak W, Świniarska M, Korczyński M. 2018. Algae biomass in animal production. In Anonymous Algae Biomass: Characteristics and Applications. (Anonymous Trans.), p. 123–130. Cham: Springer International.
- Kulshreshtha G, Hincke MT, Prithiviraj B, Critchley A. 2020. A review of the varied uses of macroalgae as dietary supplements in selected poultry with special reference to laying hen and broiler chickens. JMSE. 8(7):536. doi: 10.3390/jmse8070536.
- Kumar Y, Soni A, Sahoo A. 2022. Dietary intervention and feeding regime for enhanced production in sheep and rabbit. Processing and Quality Evaluation of Postharvest products of Sheep and Rabbits [E-book], p. 24. Hyderabad: CSWRI.
- Lemosquet S, Delamaire E, Lapierre H, Blum JW, Peyraud JL. 2004. Glucose metabolism in lactating cows in response to isoenergetic infusions of propionic acid or duodenal glucose. J Dairy Sci. 92(7):3244–3257. doi: 10.3168/jds.S0022-0302(04)73332-9.
- Mayerfeld D. 2023. The Limits of Efficiency. In D. Mayerfeld (Ed.) Our Carbon Hoofprint. Food and Health (Anonymous Trans), p. 105–127. Cham: Springer.
- Messyasz B, Leska B, Fabrowska J, Pikosz M, Roj E, Cieslak A, Schroeder G. 2015. Biomass of freshwater Cladophora as a raw material for agriculture and the cosmetic industry. Open Chemistry. 13(1):1108–1118. doi: 10.1515/chem-2015-0124.
- Michalak I, Chojnacka K. 2015. Algae as production systems of bioactive compounds. Eng Life Sci. 15(2):160–176. doi: 10.1002/elsc.201400191.
- Modina SC, Aidos L, Rossi R, Pocar P, Corino C, Di Giancamillo A. 2021. Stages of gut development as a useful tool to prevent gut alterations in piglets. Animals (Basel). 11(5):1412. doi: 10.3390/ani11051412.
- Mukaila R. 2023. Measuring the economic performance of small-scale rabbit production agribusiness enterprises. World Rabbit Sci. 31(1):35–46. doi: 10.4995/wrs.2023.18660.
- Nutautaitė M, Racevičiūtė-Stupelienė A, Bliznikas S, Jonuškienė I, Karosienė J, Koreivienė J, Vilienė V. 2022a. Evaluation of phenolic compounds and pigments in freshwater Cladophora glomerata biomass from various lithuanian rivers as a potential future raw material for biotechnology. Water (Basel). 14(7):1138. doi: 10.3390/w14071138.
- Nutautaitė M, Racevičiūtė-Stupelienė A, Bliznikas S, Vilienė V. 2023. Enhancement of rabbit meat functionality by replacing traditional feed raw materials with alternative and more sustainable freshwater Cladophora glomerata macroalgal biomass in their diets. Foods. 12(4):744. doi: 10.3390/foods12040744.
- Nutautaitė M, Racevičiūtė-Stupelienė A, Pockevičius A, Vilienė V. 2023. Sensory evaluation of rabbit meat from individuals fed functional and more sustainable diets enriched with freshwater Cladophora glomerata macroalgal biomass. Animals (Basel). 13(13):2179. doi: 10.3390/ani13132179.
- Nutautaitė M, Vilienė V, Racevičiūtė-Stupelienė A, Bliznikas S, Karosienė J, Koreivienė J. 2021. Freshwater Cladophora glomerata biomass as promising protein and other essential nutrients source for high quality and more sustainable feed production. Agriculture (Basel). 11(7):582. doi: 10.3390/agriculture11070582.
- Nutautaitė M, Vilienė V, Racevičiūtė-Stupelienė A, Bliznikas S, Karosienė J, Koreivienė J. 2022, April 26. Cladophora glomerata as a potential nutrient source in animal nutrition Paper presented at the meeting of 1st International PhD Student’s Conference at the University of Life Sciences in Lublin, Poland: ENVIRONMENT – PLANT – ANIMAL – PRODUCT.
- O' Brien R, Hayes M, Sheldrake G, Tiwari B, Walsh P. 2022. Macroalgal proteins: a review. Foods. 11(4):571. doi: 10.3390/foods11040571.
- O’Sullivan L, Murphy B, McLoughlin P, Duggan P, Lawlor PG, Hughes H, Gardiner GE. 2010. Prebiotics from marine macroalgae for human and animal health applications. Mar Drugs. 8(7):2038–2064. doi: 10.3390/md8072038.
- Øverland M, Mydland LT, Skrede A. 2019. Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. J Sci Food Agric. 99(1):13–24. doi: 10.1002/jsfa.9143.
- Patel AK, Singhania RR, Awasthi MK, Varjani S, Bhatia SK, Tsai M-L, Hsieh S-L, Chen C-W, Dong C-D. 2021. Emerging prospects of macro- and microalgae as prebiotic. Microb Cell Fact. 20(1):1–112. doi: 10.1186/s12934-021-01601-7.
- Pimentel FB, Alves RC, Harnedy PA, FitzGerald RJ, Oliveira MBPP. 2019. Macroalgal-derived protein hydrolysates and bioactive peptides: Enzymatic release and potential health enhancing properties. Trends Food Sci Technol. 93:106–124. doi: 10.1016/j.tifs.2019.09.006.
- Pomar C, van Milgen J, Remus A. 2019. 18: precision livestock feeding, principle and practice. Poultry Pig Nutri. Wageningen Academic Publishers. 397–418. doi: 10.3920/978-90-8686-884-1.
- Ramesh Kumar B, Deviram G, Mathimani T, Duc PA, Pugazhendhi A. 2019. Microalgae as rich source of polyunsaturated fatty acids. Biocatal Agric Biotechnol. 17:583–588. doi: 10.1016/j.bcab.2019.01.017.
- Shannon E, Conlon M, Hayes M. 2021. Seaweed components as potential modulators of the gut microbiota. Mar Drugs. 19(7):358. doi: 10.3390/md19070358.
- Silva A, Silva SA, Carpena M, Garcia-Oliveira P, Gullón P, Barroso MF, Prieto MA, Simal-Gandara J. 2020. Macroalgae as a source of valuable antimicrobial compounds: extraction and applications. Antibiotics (Basel). 9(10):642. doi: 10.3390/antibiotics9100642.
- Wan AHL, Davies SJ, Soler‐Vila A, Fitzgerald R, Johnson MP. 2019. Macroalgae as a sustainable aquafeed ingredient. Rev Aquacult. 11(3):458–492. doi: 10.1111/raq.12241.
- Wang M, Wichienchot S, He X, Fu X, Huang Q, Zhang B. 2019. In vitro colonic fermentation of dietary fibers: fermentation rate, short-chain fatty acid production and changes in microbiota. Trends Food Sci Technol. 88:1–9. doi: 10.1016/j.tifs.2019.03.005.
- Yu B, Chiou WS. 1997. The morphological changes of intestinal mucosa in growing rabbits. Lab Anim. 31(3):254–263. doi: 10.1258/002367797780596301.
- Zduńczyk Z, Juśkiewicz J, Jankowski J, Koncicki A. 2004. Performance and caecal adaptation of turkeys to diets without or with antibiotic and with different levels of mannan-oligosaccharide. Arch Anim Nutr. 58(5):367–378. doi: 10.1080/00039420400005042.