Abstract
It has been shown that the jejunum represents the most important site for the nutrient’s absorption in several species. However, in pigs, this information seems to be controversial and limited information are available about differences in intestinal physiology between female and castrated male pigs. The trans-epithelial electrical resistance (TEER) and the active uptake of L-glutamate (L-Glu), L-arginine (L-Arg), L-methionine (L-Met) and D-glucose (D-Glu) in the jejunum and ileum of female (n = 5) and castrated male (n = 7) pigs fed a protein-restricted grower and finisher diet (128 and 112 g of crude protein/kg dry matter) were investigated. The intestine segments were collected at slaughter and mounted in Ussing chambers. Results were further investigated by protein expression analysis of the D-Glu transporter Sodium-dependent Glucose Transporter 1 (SGLT1) and using serum creatinine, non-esterified fatty acids (NEFAs) and serum glucose concentrations measurements as indicators of muscle mass deposition and metabolic status of the animals. A linear mixed-effects regression (Lme4) model was used for data analysis. Independent of sex, the uptake of both L-Met and D-Glu was higher (p < 0.001) in the ileum than in the jejunum (2.1- and 3.6-fold increases, respectively). The L-Arg uptake was higher (p = 0.001) in females compared to castrates (1.9-fold increase). No significant differences were observed between the segments in the SGLT1 protein expression, regardless of sex. Serum measurements were also not significantly different between the female and castrates. This study suggests that the ileum has an important role in the active uptake of amino acids and D-Glu uptake and differences exist between female and castrated finishing pigs.
HIGHLIGHTS
The L-Arginine uptake is higher in female compared to castrated pigs.
The uptake of L-methionine and D-glucose is higher in the ileum than in the jejunum.
The ileum has an important role in the active uptake of amino acids and D-glucose.
Introduction
The absorption of amino acids (AAs) and glucose is modulated by the luminal nutrient concentration in the intestine. The main site of nutrient’s uptake along the entire intestinal tract is the small intestine (duodenum, jejunum and ileum), where epithelial transporters and ion channels orchestrate the absorption processes. The AAs uptake is mediated by systems of transporters that are characterised by different preferences for anionic (system X-AG), cationic (system y+) and neutral (system B0) AAs. Specifically, the absorption of cationic AAs, such as L-arginine (L-Arg), can be both Na+-dependent or Na+-independent, and the transporters can be distributed both at the apical and basolateral membranes of the enterocytes. The uptake of anionic AAs such as L-glutamate (L-Glu) is strictly Na+-dependent while the transport of neutral AAs depends almost entirely on Na+-dependent transporters, with L-methionine (L-Met) being one of the most affine neutral substrates for the B0AT1 transporter system, located in the apical membrane of the enterocytes (Bröer Citation2008). The uptake of glucose from the gut lumen into the bloodstream is mainly mediated by sodium-dependent glucose co-transporter 1 (SGLT1), which is located at the apical side of the enterocytes (Lehmann and Hornby Citation2016). Part of the glucose is metabolised by the intestinal epithelial cells, but most is released into the bloodstream via the basolateral glucose transporter 2 (GLUT2) (Takata Citation1996). The expression of SGLT1 increases with increasing glucose concentrations in the intestinal lumen and its abundance varies along the small intestine (Röder et al. Citation2014). Differences in SGLT1 expression exist between the jejunum and ileum in different species, such as rats (Balen et al. Citation2008; Yoshikawa et al. Citation2011), horses (Dyer et al. Citation2009), beef cattle (Liao et al. Citation2010) and post-weaning piglets (Moran et al. Citation2010; Herrmann et al. Citation2012), with higher expression in the jejunum independently by the sex. Consequently, the jejunum is considered the most involved site for glucose uptake, and most studies have focused on this intestinal segment when investigating the modulation of glucose uptake. However, few studies have investigated AAs and glucose uptake in both the jejunum and ileum of finishing pigs. In a study comparing glucose uptake in different breeds of post-weaning piglets, the active electrogenic transport of glucose was found to be higher in the ileum, compared to the jejunum, independent of the breed (Herrmann et al. Citation2012), suggesting that the ileum could also play a key role in glucose uptake. Unfortunately, no information about sex-related differences were provided. The expression of AA transporters in the different segments of the small intestine was evaluated also in growing pigs fed a low-protein diet supplemented with AAs and compared with pigs fed intermediate and high protein content (Morales et al. Citation2015). The study showed that the dietary protein content did not affect the jejunal and ileal expression of the AA transporters investigated, namely b0+, y + L and B0 (Morales et al. Citation2015). However, a comparison between the intestinal segment was not considered. When investigating intestinal physiology, it is important to consider the sex of the animal as a significant factor. A previous study by Ruiz-Ascacibar et al. (Citation2017) highlighted significant differences among entire male, castrated and female pigs. Specifically, females exhibited a lower average daily gain (ADG), protein deposition and fat deposition rate at 100 kg body weight (BW) compared to castrates. These differences between females and castrates may be intricately linked to variations in gut physiology, particularly in the uptake of AAs and other essential nutrients such as glucose.
Therefore, the objective of this study was to compare different physiological traits of the mid-jejunum and ileum such as epithelial integrity, AAs and D-Glu uptake in female and castrated finishing pigs. To support the results obtained by the electrophysiological measurements, the intestinal protein expression of the SGLT1 transporter and the serum glucose concentration were quantified. Because of their association with muscle mass deposition (Hari et al. Citation2007) and the metabolic status of the animal (Hari et al. Citation2007; Tor et al. Citation2021), the serum creatinine and non-esterified fatty acid (NEFA) levels were also quantified, respectively.
Materials and methods
Animal ethics statement
The Swiss cantonal committee for animal care and use approved all procedures involving animals (2018_30_FR).
Animals and diets
A total of 12 Swiss Large White pigs (5 females and 7 castrated males) housed at Agroscope animal facilities in Posieux, Switzerland, were used. The pigs were reared on a standard starter diet until an average BW of 22.4 kg (±1.8 kg SD) and subsequently allocated to a pen, where they remained until slaughter (BW of 106 ± 5 kg). During the grower (22.4 ± 1.8 kg to 65.9 ± 1.9 kg BW) and finisher phase (until 107.1 ± 4.1 kg BW), the pigs had ad libitum access to drinking water and a low-crude-protein diet, which was distributed by single-spaced automatic feeder stations with individual pig recognition systems (Schauer Maschinenfabrik GmbH & Co. KG, Prambachkirchen, Austria). Both the grower and finisher diets contained 80% of the crude protein and digestible essential AAs content of the Swiss feeding recommendations for swine and their chemical composition is reported in Table . Animals were slaughtered at an average age of 154.4 ± 9.0 days and a target slaughter weight of 100 kg at the research station abattoir after fasting for approximately 15 h.
Table 1. Dietary ingredients and analysed composition (g or MJ/kg as-fed) of the grower and finisher diets.Table Footnote1
Feed chemical analyses
Dry matter was determined gravimetrically after drying at 105 °C for 3 h. Ash content was determined after 3 h at 550 °C. CP content (total N × 6.25) was analysed with a LECO FP-2000 analyser (Leco, Mönchengladbach, Germany) (International Organisation for Standardisation (ISO 2008)). The AAs composition of the diets was determined after 24 h of acid hydrolysis (48 h for leucine, isoleucine and valine). Methionine and cystine were hydrolysed after peroxidation with formic acid. The AAs profile was determined by HPLC coupled with a fluorescence detector (Alliance 2695; Waters, Milford, MA) as described in the manual (Waters AccQ Tag Chemistry Package 052874 TP, rev. 1). In the diets, crude fibre and ether extract were determined according to methods 6.1.4 and 5.1.1, respectively (VDLUFA Citation2007). To determine the crude fat content in feed, samples were hydrolysed in 10% (v/v) HCl for 1 h. The hydrolysate was dried and then extracted with petroleum ether using the Büchi SpeedExtractor E 916 (Büchi Labortechnik AG, Flawil, Switzerland). The dry fat residue was determined by gravimetry. The AAs composition of the diets was determined after 24 h of acid hydrolysis (48 h for leucine, isoleucine and valine). Methionine and cystine were hydrolysed after peroxidation with formic acid. The AAs profile was determined by HPLC coupled with a fluorescence detector (Alliance 2695; Waters, Milford, MA) as described in the manual (Waters AccQ Tag Chemistry Package 052874 TP, rev. 1). Tryptophan content was quantified by HPLC (LC 1290 Infinity II LC System, Agilent Technologies, Santa Clara, CA) according to ISO 13904:2016. The digestible and net energy coefficients from each feed ingredient were obtained from the Swiss (Liebefeld-Posieux Citation2004) and French (Noblet et al. Citation2003) databases, respectively.
Production traits
The BW of all animals was monitored weekly. The total feed intake (FI), ADG, average daily feed intake (ADFI) and feed conversion ratio (FCR) were calculated for the overall growth period. Individual protein intake and carcase protein content determined at slaughter by dual energy X-ray absorptiometry (DXA) and estimated at the beginning of the experiment. The ADG was calculated as the weight gain during the experiment over the days on feed. Average values of feed intake, protein intake, ADG, FCR and carcase protein in female and castrated pigs are summarised in Table .
Table 2. Mean values with their standard error of the overall period feed intake, protein intake, carcase protein, average daily gain and feed conversion ratio in female and castrated pigs.
Blood collection and serum creatinine, NEFA and glucose quantification
Blood was sampled directly during bleeding after CO2 stunning using blood collection tubes with serum clot activator (Vacuette®; Greiner Bio-One GmbH, Kremsmuenster, Austria), which were stored upside down at room temperature for 1 h prior to processing. The Vacuette® serum tubes were then centrifuged for 15 min at 3000 g and subsequently for 2 min at 4000 g. Two aliquots of serum were stored at −20 °C in Eppendorf tubes. Levels of serum NEFAs (Randox laboratories; Crumlin, UK), glucose and creatinine (Biotecnica instruments Ltd., Roma, Italy) were measured in the serum following manufacturers’ procedure using an autoanalyser BT 1500 (Biotecnica instruments Ltd., Roma, Italy).
Ussing chamber evaluation of glucose and AA transport in pig jejunum and ileum samples
Samples of small intestine were collected from all the pigs (n = 12) approximately 15–20 min after slaughtering them. For each pig, two mid-jejunal samples were taken at the third metre distal to the pylorus and two distal-ileal samples were taken immediately before the ileocecal valve and transported to the lab immersed in ice-cold Krebs Ringer modified buffer (KRB) in a 50 ml Falcon. In parallel, one jejunal and one ileal sample per pig was immediately frozen in liquid nitrogen and stored at −80 °C for protein expression analysis. The jejunal and ileal samples were stripped of their outer muscle layers and mounted between the two halves of an Ussing chamber (Physiologic Instruments, San Diego, CA) within 10–15 min after tissue collection. Each sample was bathed on its mucosal and serosal surfaces (exposed area 1.0 cm2) with the corresponding KRB. After a 30–40 min equilibration period, baseline Isc (in mV) values were measured. The trans-epithelial electrical resistance (TEER) was also measured at 2-min intervals under current clamped conditions. Furthermore, AA and D-Glu uptake on tissues was performed according to the following protocol: after the stabilisation period (10–15 min), 10 mmol/l L-glutamate (L-Glu) was added to the mucosal buffer, followed by the addition of the same concentration of L-Arg, L-Met and D-Glu. The substrates were added in the aforementioned order at intervals of 15 min. Each AA or D-Glu addition was kept in an equilibrated osmotic condition by the addition of equimolar (10 mmol/L) mannitol on the serosal side. Forskolin (10 μmol/L) was added to the serosal compartment at the end of the experiment to test tissue viability, as forskolin stimulates adenosine 3′,5′-cyclic monophosphate (cAMP)-dependent protein kinase A that leads to a strong electrical change in the short circuit current (Isc) only in alive tissues. The total active transport through the tissue was verified by monitoring the change in Isc (ΔIsc), which was representative of ion flux, and thus active transport within the jejunal and ileal tissues.
Protein expression analysis
About 1 cm2 of tissues were lysed in CelLytic MT buffer (Merck Life Science, Milano, Italy) complemented with a Complete TM protease inhibitor cocktail (Roche, Basel, Switzerland). After centrifugation at 12,000 g at 4 °C for 10 min, the protein concentration in the supernatant was determined using Coomassie Plus Assay Reagent (Thermo Scientific, Waltham, MA). The absorbance was read on an Asys UVM 340 microplate reader (Biochrom, Cambridge, UK). Then, 15 µg of total protein extracts were denatured at 95 °C for 5 min, separated via 7% SDS-PAGE gel (polyacrylamide: 30% w/v, acrylamide: 0.8% w/v and bisacrylamide (37·5:1)); 1·5 M Tris HCl, pH 8.8; SDS 0.4%; ammonium persulfate 10% and tetramethylethylenediamine 0.01%. Proteins were then blotted on a Western Bright polyvinylidene difluoride membrane (Witec, Luzern, Switzerland) at 90 V for 90 min using a Trans-Blot Turbo Transfer System (Bio-Rad, Hercules, CA). The membranes were blocked for 60 min at room temperature with 5% bovine serum albumin (Sigma-Aldrich, St. Louis, MA) in 0.1% Tween 20 (Sigma-Aldrich, St. Louis, MA) in phosphate-buffered saline (PBS) and further incubated in the same buffer supplemented with the primary antibodies anti-SGLT1 (ab14686, Abcam, Cambridge, UK,) or anti-vinculin (V4505, Sigma-Aldrich, St. Louis, MA) at 4 °C overnight. Anti-SGLT1 was diluted 1:1000 and anti-vinculin 1:2000 in 0.1% Tween 20, in PBS containing 5% bovine serum albumin. Vinculin was shown not to vary during the entire duration of the experiment (data not shown) and therefore used as housekeeping protein to normalise SGLT-1 expression. After washing, membranes were incubated for 1 h at room temperature with goat-anti-rabbit IgG HRP-conjugated (A9169, Sigma-Aldrich, St. Louis, MA) for SGLT-1 and goat-anti-mouse IgG HRP-conjugated (Merck Life Science, Milano, Italy) for vinculin, diluted 1:1000 and 1:3000, respectively, in 0.1% Tween 20 in PBS supplemented with 5% milk powder. After washing, signals were detected using a Quantum Kit (Witec, Luzern, Switzerland) and a G:BOX (Syngene International Ltd., Bangalore, India). Signals were quantified using Genetools software (Syngene International Ltd., Bangalore, India). The ratio of the intensities of the protein bands of interest versus the housekeeping protein was calculated for each filter, and the ratios from different Western blot filters were used to determine protein abundance.
Data analysis and statistics
All statistical analyses were conducted with R software (version 4.2.1). The data were analysed by ANOVA using linear mixed-effects regression (Lme4) models (Bates et al. Citation2015). The models contained the tissue (jejunum vs. ileum), the sex (castrates vs. female) and the two-way interaction as fixed effects and the animal as a random effect. For pairwise comparisons, the Sidak function was performed for a modified Tukey test for multiple comparisons of means. Means and pooled SEM were calculated with the lsmeans function from the emmeans package (Lenth and Lenth Citation2018). The residuals of linear mixed-effects models were checked for normality and homoscedasticity.
Results
The restricted animal cohort utilised in our study was constrained by the inherent limitations associated with the Ussing chamber system. Additionally, the uneven distribution of subjects across sexes was due to constraints encountered during the slaughter process. In order to maintain methodological coherence and facilitate a comprehensive interpretation of the findings, only the results obtained from the same cohort of pigs utilised in the Ussing chamber experiments were used for additional parameters analysed, such as serum creatinine, NEFA and glucose.
Efficiency traits
Castrated pigs eat more feed (P < 0.001) and more protein (P < 0.001) compared to female. Accordingly, they grew faster (P = 0.02) than female pigs. By contrast, carcase protein and the FCR were similar. Those results are summarised in Table .
Mid-jejunal and distal-ileal physiological traits
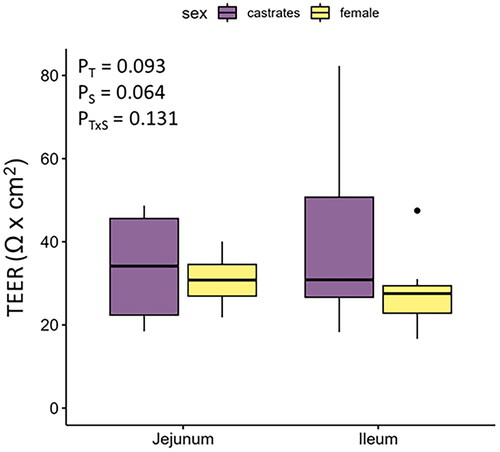
All the tissues were viable at the end of the Ussing chamber experiment. The TEER tended to be greater in the jejunum (Ptissue = 0.093) and in castrates (Psex = 0.064). Values are reported in Figure .
Figure 1. Trans-epithelial electrical resistance (TEER; Ω × cm2) in the jejunum and ileum of female and castrated finishing pigs. T: tissue; S: sex.
The delta values of the short-circuit current (ΔIsc) reported in Table indicate that the absorption rate of L-Met (Ptissue < 0.001; +111.8%) was higher in the ileum than in the jejunum. The L-Glu had a tendence to the same trend (Ptissue = 0.061; +78%). Similarly, D-Glu was more efficiently absorbed in the ileum than in the jejunum (Ptissue < 0.001; +275%). Females absorbed L-Arg more efficiently (Psex = 0.001 + 105%) than castrated males, regardless of tissue (Table ).
Table 3. Change in short-circuit current induced by L-glutammate, L-arginine, L-methionine and D-glucose in the jejunum and ileum of female and castrated finishing pigs.
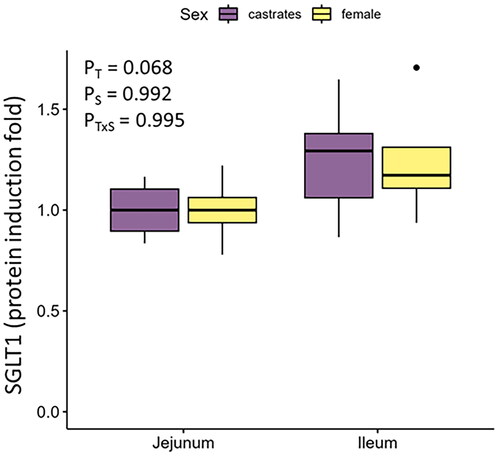
Despite the differences in active D-Glu uptake observed in the Ussing chamber experiments, the protein expression of the SGLT1 transporter tended to be similar (P = 0.068) between jejunal and ileal tissues (1 ± 0.14 and 1.25 ± 0.25 protein induction folds, respectively, Figure ). The expression of SGLT1 protein was similar (P > 0.05) also between female and castrated pigs in both jejunum and ileum (Figure ).
Serum metabolites
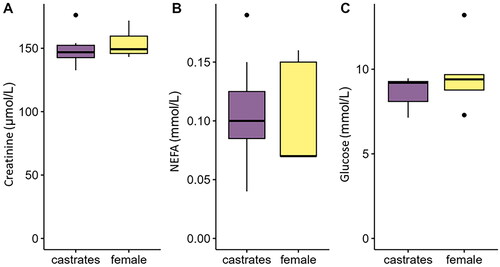
No differences between sexes were found in the quantified serum metabolites. Specifically, no differences between the two sexes were found for serum creatinine (P = 0.55, Figure ), NEFA (P = 0.91, Figure ) and glucose (P = 0.37, Figure ) concentration. The average values of the measured serum metabolites are reported in Figure .
Discussion
The main outcome of this study was to highlight the differences between the mid-jejunum and distal-ileum regarding glucose and AA uptake in female and castrated finishing pigs. Because of technical limitations, was not possible to extend such investigation to the remaining sites of the small intestine, that could be the target of future studies.
Jejunum and ileum comparison—AAs active uptake
The AAs tested in this study were chosen because of the different transporters involved in their active uptake along the gastrointestinal tract. Our results demonstrate that segmental differences in uptake of the neutral AAs, L-Met, exist in finishing pigs, with greater active uptake in the distal ileum than in the mid-jejunum. The essential AA, methionine, has several biological functions, serving as a methyl donor, antioxidant and precursor of bioactive compounds (Luo and Levine Citation2009), where the L form is the biologically active form of the methionine. Similar to the present results, Richards et al. (Citation2005) observed greater uptakes of DL-Methionine, a precursor of the L-Met, in the ileum than in the jejunum in broiler chickens. An Na + dependent active transport of methionine in the distal ileum has also been reported in other species, such as the rabbit (Munck, Citation1985). The main L-Met transporter in the pig intestine is the B0AT1 system (SLC6A19), located on the apical side of the enterocytes (Bröer Citation2008). This system carries all the neutral AAs and its expression has been found to increase from the duodenum to the ileum in humans (Terada et al. Citation2005). Similar results have been found in pigs. A study focused on the expression of putative Met transporters along the gastrointestinal tract of pigs and the Western blot analysis showed that the protein expression of B0AT1 was highest in the ileum compared to the other segments (Romanet et al. Citation2021). Higher B0AT1 expression in the ileum could explain the higher active uptake of L-Met observed in our study, compared to the jejunum. A recent study has investigated the role of the gut microbiota in regulating the Met cycle within the pig gut. The findings, outlined in Wu et al. (Citation2022), reveal a significant increase (almost 10-fold) in Met concentration within the contents of the caecum, colon, rectum and faeces of germ-free (GF) pigs compared to their counterparts in the control group. This notable increase underscores the profound influence of gut microbiota on Met metabolism, particularly within the large intestine. Interestingly, the presence of gut microbiota was also found to diminish Met concentration in the circulatory system. This phenomenon suggests that the gut microbiota could potentially modulate the expression and/or activity of Met transporters throughout the gastrointestinal tract (GIT), albeit not in the jejunum, where no significant differences in Met concentrations between GF and control animals were observed. This discrepancy might be attributed to two plausible reasons: first, the predominant role of jejunal microbiota in lipid and sugar degradation rather than AA metabolism, and second, the comparatively lower abundance and diversity of microbiota in the jejunal environment, as opposed to the ileum and large intestine. The involvement of gut bacteria in the modulation of the expression and/or activity of the AAs transporters is also suggested by other studies (Yin, Han, J et al. Citation2017; Yin, Menon, J et al. Citation2017; Yi et al. Citation2019). Consequently, it is plausible to hypothesise that the observed variations in active L-Met uptake between the jejunum and ileum could be mediated by the gut microbiota. Nonetheless, further comprehensive studies are needed to elucidate this intricate relationship.
Differences in AAs absorption between the jejunum and ileum have also been investigated in vivo in female chickens (Mitchell and Levin Citation1981). In particular, they observed that ileal enterocytes had a greater absorptive capacity than jejunal enterocytes for glycine and valine, but not for Met. However, the authors also clarified that the whole jejunum has a greater number of enterocytes than the whole ileum. Therefore, considering the whole intestinal segment, the functional maximum absorption capacity for glycine and valine can be considered comparable in the two regions (Mitchell and Levin Citation1981). Future studies comparing the absorptive capacity of the jejunum and ileum should take into account other factors such as the number of enterocytes in a specific segment, but also the contact time of the nutrients in the respective regions.
Another aspect to take into account in our study is that the three different AAs were added to the same apical buffer at intervals of 15 min. This strategy allowed us to obtain results about the uptake of the different AAs on the same tissues, reducing the inter-individual variability and the number of animals to be used for the experiment. However, mutual inhibitory actions between different classes of AA have been observed (Christensen et al. Citation1969; Calvert and Shennan Citation1996; Bröer Citation2008). An example is the system B0+ which is a Na + dependent transporter with a high affinity for both neutral and cationic AAs (Bröer Citation2008). However, this transporter has not been found in swine (Bröer Citation2008). To limit any interference of the cationic AA, L-Arg, on the uptake of the neutral AA, L-Met was added to the apical side 15 min after adding L-Arg. Nevertheless, one cannot exclude the possibility that any interference occurred. The Na + independent counterpart (system b0+) has been found in pig small intestines and is also accessible to other AAs, such as the anionic AA, L-Glu (Munck LK et al. Citation2000). The potential effects of such interactions on the uptake of a specific AA need to be taken into account when interpreting the present results.
Jejunum and ileum comparison – D-glu active uptake
The uptake of D-Glu was greater in the ileum than in the jejunum of finishing pigs. Herrmann et al.’s (Citation2012) study on post-weaning piglets from different breeds and Von Heimendahl et al.’s (Citation2010) study on growing pigs at 14 weeks of age also observed greater D-Glu uptake in the ileum compared to the jejunum. These studies could suggest that the difference in D-Glu uptake is a general characteristic of post-weaning pigs, regardless of age. Interestingly, contrasting results were found in newborn and pre-weaned piglets (30-day-olds) (Puchal and Buddington Citation1992; Moran et al. Citation2010). This could indicate that the intestinal physiology of the D-Glu uptake is altered by the weaning process, during which adaptive and maturational processes, such as villus length, lactase activity and macromolecule fluxes across the jejunum are induced in the gut by weaning (Montagne et al. Citation2007). The importance of the weaning process in regulating the D-Glu uptake in the jejunum and ileum is also demonstrated by a study in which the D-Glu uptake was measured in the mid-jejunum and distal ileum of 90-day-old pigs previously exposed to early or late weaning (Li et al. Citation2022). The study showed that the intestinal segments of pigs previously exposed to early weaning had reduced SGLT1 activity (by approximately 30%) compared to late-weaned controls. Although jejunal and ileal D-Glu uptake were not statistically compared in this study, ΔIsc values were numerically higher in the ileum compared to the jejunum of both early and late weaned pigs (Li et al. Citation2022).
In line with the results of previous studies (Balen et al. Citation2008; Moran et al. Citation2010; Herrmann et al. Citation2012), the differences in uptake between the two segments was not reflected in differences in SGLT1 protein expression. The present data suggest that SGLT1 protein expression in the jejunum is similar to that of the ileum. However, more studies are needed to further investigate the activation state of the SGLT1 protein.
Regarding the higher ileal D-Glu uptake compared to the jejunum, despite non-significant differences in the SGLT1 protein expression, such a difference could be driven by a different status of the post-translational modification of the SGLT1 protein. However, the SGLT1 phosphorylation status in post-weaning piglets has already been found not to be associated with segment-specific glucose transport features (Klinger et al. Citation2018). The Ser418 phosphorylation status of SGLT1, indeed, was similar between the jejunum and ileum in pigs and cannot be considered the cause for the differences in glucose absorption between the two small intestinal segments (Klinger et al. Citation2018). Therefore, other post-translational modifications need to be investigated to explain such differences. An explanation for the results found in the present and other studies could be the involvement of other ion fluxes. For example, it has been observed that glucose and related SGLT1 activity may induce Ca2+ influx (Kellett Citation2011) and Ca2+ dependent Cl− secretion (Yin, Han, J et al. 2017; Yin, Menon, L et al. 2017). These ion fluxes may affect electrogenic glucose transport by modifying the apical membrane potential, resulting in stimulation or attenuation of SGLT1 activity by hyperpolarisation or depolarisation, respectively.
Sex-related differences in electrophysiological measurements
Despite lower values of feed intake, protein intake and ADG of females compared to castrates, the carcase protein and the FCR were similar. This could suggest that females are more protein efficient than the castrated. The literature is inconsistent regarding the effect of sex on the protein efficiency. Ruiz-Ascacibar et al. (Citation2017) found no differences in protein efficiency between female and castrated pigs, but a more recent investigation found that Swiss Large White females were 1.3% more protein efficient than Swiss Large White castrated males (Ewaoluwagbemiga et al. Citation2023). In this study it has been observed that compared to the castrates, females had a higher L-Arg uptake, a tendency to a lower TEER through the electrophysiological measurements and a tendency to a lower small intestine TEER in females compared to castrates. Physiological concentrations of L-Arg are essential in maintaining normal cellular function (Tapiero et al. Citation2002). In pigs, starting from AAs such as L-Arg, the liver, kidneys and pancreas synthesise creatinine, which is mainly located in skeletal muscle as an energy source (Janicki and Buzała Citation2013). Therefore, higher L-Arg intake in females may have resulted in higher serum creatinine levels in female serum samples than in castrates’ serum samples. The lack of differences in serum creatinine levels between sexes could be explained by the lower protein intake in females compared to castrates throughout the growing and finishing periods. Despite the lower protein intake, the similar carcase protein content suggests an efficient utilisation and deposition of protein in females, and the higher L-arg uptake could be part of such efficient utilisation of nutrients in females pigs. Furthermore, AAs are converted to creatine in skeletal muscle and only 1–2% of the daily creatine and phosphocreatine stores are broken down to creatinine and excreted in the urine. The rate-limiting step in the endogenous synthesis of creatine takes place in the kidney, where arginine: glycine amidinotransferase enzymes convert arginine to the precursor of creatine (Janicki and Buzała Citation2013). The blood creatinine level therefore depends on a number of physiological processes that are not solely dependent on AA absorption.
A deeper understanding of the difference in L-Arg uptake between castrates and females may be elucidated by considering the role of L-Arg and its metabolic pathways in female reproductive physiology. Specifically, the catabolism of L-Arg through nitric oxide (NO) synthases results in the synthesis of NO and L-citrulline. The L-Arg-NO system has been implicated in the central regulation of reproductive processes in females, as well as in the modulation of oxidative stress during oocyte development. This phenomenon arises from the rapid reaction between oxygen-derived free radicals, particularly superoxide and NO, leading to the formation of highly reactive intermediates. Comprehensive insights into the involvement of L-Arg in NO production and its intricate interplay with the female reproductive cycle, even in non-pregnant states, are detailed in the comprehensive review of Bodis et al. (Citation2022).
It has been observed in rats that female had significantly higher levels of L-Arg in plasma compared to male rats (Zhang et al. Citation2021) and that plasma levels of AAs differ between female and male rats (Okame et al. Citation2015). The active uptake of L-Arg is mainly mediated by the cationic AA transporter CAT-1, but its expression between female and male resulted similar in rats, despite sex differences in L-Arg plasma concentration (Zhang et al. Citation2021). Thus, since CAT-1 can be modulated post-translationally and its level can also be regulated by the AAs availability (Hatzoglou et al. Citation2004), more research should be carried out in the future to fully understand how CAT-1 and other forms of arginine transporter function in the castrates and female intestine and if its activity correlates with protein efficiency in pigs. The role of sex steroids in the regulation of intestinal permeability has not been fully elucidated. Several variables need to take into account when considering sex-related differences in intestinal integrity. Several of such differences are very well summarised in the review of Pigrau et al. (Citation2016). According to the literature, sex hormones have a strong effect in modulating the gut microbiota, the brain-gut homeostasis, mucosal functioning the therefore the development of the intestinal barrier (Pigrau et al. Citation2016). The role of sex-hormones in the regulation of intestinal physiology is being increasingly recognised and also our results suggest that the sex factor need to be considered in pigs when studies on intestinal physiology are performed.
Conclusion
This study suggests that in addition to the jejunum, the ileum also plays an important role in the active uptake of selected AAs, particularly L-Met. This also suggests a higher electrogenic D-Glu uptake in the ileum compared to the middle jejunum in finishing pigs, as was already observed in post-weaning and growing pigs. However, the involvement of apical membrane depolarisation mediated by other ion fluxes in the regulation of SGLT1 activity and/or glucose uptake warrants further study. In addition, this study suggests that sex-related differences exist in the intestinal active uptake of L-Arg in pigs.
Acknowledgements
The authors acknowledge Guy Maïkoff and all the barn technicians from the Experimental Piggery for taking care of the animals and for their help in the sample collection. The authors also thank Carmen Vonnez for the serum metabolites analyses.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are available from the corresponding author, [T.M.], upon reasonable request.
Additional information
Funding
References
- Balen D, Ljubojevic M, Breljak D, Brzica H, Zlender V, Koepsell H, Sabolic I. 2008. Revised immunolocalization of the Na+-D-glucose cotransporter SGLT1 in rat organs with an improved antibody. Am J Physiol Cell Physiol. 295(2):C475–C489. doi: 10.1152/ajpcell.00180.2008.
- Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using Lme4. J Stat Soft. 67(1). doi: 10.18637/jss.v067.i01.
- Bodis J, Farkas B, Nagy B, Kovacs K, Sulyok E. 2022. The role of L-arginine-NO system in female reproduction: a narrative review. Int J Mol Sci. 23(23):14908. doi: 10.3390/ijms232314908.
- Bröer S. 2008. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev. 88(1):249–286. doi: 10.1152/physrev.00018.2006.
- Calvert D, Shennan D. 1996. Evidence for an interaction between cationic and neutral amino acids at the blood-facing aspect of the lactating rat mammary epithelium. J Dairy Res. 63(1):25–33. doi: 10.1017/s0022029900031514.
- Christensen HN, Handlogten ME, Thomas EL. 1969. Na+-facilitated reactions of neutral amino acids with a cationic amino acid transport system. Proc Natl Acad Sci USA. 63(3):948–955. doi: 10.1073/pnas.63.3.948.
- Dyer J, Al-Rammahi M, Waterfall L, Salmon KS, Geor RJ, Bouré L, Edwards GB, Proudman CJ, Shirazi-Beechey SP. 2009. Adaptive response of equine intestinal Na+/glucose co-transporter (SGLT1) to an increase in dietary soluble carbohydrate. Pflugers Arch. 458(2):419–430. doi: 10.1007/s00424-008-0620-4.
- Ewaoluwagbemiga EO, Bee G, Kasper C. 2023. Genetic analysis of protein efficiency and its association with performance and meat quality traits under a protein-restricted diet. Genet Sel Evol, 55(1): 35. doi: 10.1186/s12711-023-00812-3.
- Hari P, Bagga A, Mahajan P, Lakshmy R. 2007. Effect of malnutrition on serum creatinine and cystatin C levels. Pediatr Nephrol. 22(10):1757–1761. doi: 10.1007/s00467-007-0535-x.
- Hatzoglou M, Fernandez J, Yaman I, Closs E. 2004. Regulation of cationic amino acid transport: the story of the CAT-1 transporter. Annu Rev Nutr. 24(1):377–399. doi: 10.1146/annurev.nutr.23.011702.073120.
- Herrmann J, Schröder B, Klinger S, Thorenz A, Werner A-C, Abel H, Breves G. 2012. Segmental diversity of electrogenic glucose transport characteristics in the small intestines of weaned pigs. Comp Biochem Physiol A Mol Integr Physiol. 163(1):161–169. doi: 10.1016/j.cbpa.2012.05.204.
- ISO I. 2008. 16634-1: food products: determination of the total nitrogen content by combustion according to the dumas principle and calculation of the crude protein content—part 1: oilseeds and animal feeding stuffs. Geneva, Switzerland: ISO.
- Janicki B, Buzała M. 2013. The role of creatine in the organism of pigs and its effect on the quality of pork: a review/Rola kreatyny w organizmie świń i jej wpływ na jakość mięsa wieprzowego: artykuł przeglądowy. Annals of Animal Science. 13(2):207–215. doi: 10.2478/aoas-2013-0003.
- Kellett GL. 2011. Alternative perspective on intestinal calcium absorption: proposed complementary actions of Cav1. 3 and TRPV6. Nutr Rev. 69(7):347–370. doi: 10.1111/j.1753-4887.2011.00395.x.
- Klinger S, Lange P, Brandt E, Hustedt K, Schröder B, Breves G, Herrmann J. 2018. Degree of SGLT1 phosphorylation is associated with but does not determine segment‐specific glucose transport features in the porcine small intestines. Physiol Rep. 6(1):e13562. doi: 10.14814/phy2.13562.
- Lehmann A, Hornby PJ. 2016. Intestinal SGLT1 in metabolic health and disease. Am J Physiol Gastrointest Liver Physiol. 310(11):G887–G898. doi: 10.1152/ajpgi.00068.2016.
- Lenth R, Lenth MR. 2018. Package ‘lsmeans’. Am Stat. 34(4):216–221.
- Li Y, Thelen KM, Fernández KM, Nelli R, Fardisi M, Rajput M, Trottier NL, Contreras GA, Moeser AJ. 2022. Developmental alterations of intestinal SGLT1 and GLUT2 induced by early weaning coincides with persistent low-grade metabolic inflammation in female pigs. Am J Physiol Gastrointest Liver Physiol. 322(3):G346–G359. doi: 10.1152/ajpgi.00207.2021.
- Liao S, Harmon D, Vanzant E, McLeod K, Boling J, Matthews J. 2010. The small intestinal epithelia of beef steers differentially express sugar transporter messenger ribonucleic acid in response to abomasal versus ruminal infusion of starch hydrolysate. J Anim Sci. 88(1):306–314. doi: 10.2527/jas.2009-1992.
- Liebefeld-Posieux A. 2004. Fütterungsempfehlungen und Nährwerttabellen für Schweine. Zollikofen, Switzerland: LmZ, Zollikofen; p. 242.
- Luo S, Levine RL. 2009. Methionine in proteins defends against oxidative stress. FASEB J. 23(2):464–472. doi: 10.1096/fj.08-118414.
- Mitchell M, Levin R. 1981. Amino acid absorption in jejunum and ileum in vivo—a kinetic comparison of function on surface area and regional bases. Experientia. 37(3):265–266. doi: 10.1007/BF01991646.
- Montagne L, Boudry G, Favier C, Le Huërou-Luron I, Lallès JP, Sève B. 2007. Main intestinal markers associated with the changes in gut architecture and function in piglets after weaning. Br J Nutr. 97(1):45–57. doi: 10.1017/S000711450720580X.
- Morales A, Buenabad L, Castillo G, Arce N, Araiza B, Htoo J, Cervantes M. 2015. Low-protein amino acid–supplemented diets for growing pigs: effect on expression of amino acid transporters, serum concentration, performance, and carcass composition. J Anim Sci. 93(5):2154–2164. doi: 10.2527/jas.2014-8834.
- Moran AW, Al-Rammahi MA, Arora DK, Batchelor DJ, Coulter EA, Ionescu C, Bravo D, Shirazi-Beechey SP. 2010. Expression of Na+/glucose co-transporter 1 (SGLT1) in the intestine of piglets weaned to different concentrations of dietary carbohydrate. Br J Nutr. 104(5):647–655. doi: 10.1017/S0007114510000954.
- Munck BG. 1985. Transport of neutral and cationic amino acids across the brush-border membrane of the rabbit ileum. J Membr Biol. 83(1–2):1–13. doi: 10.1007/BF01868733.
- Munck LK, Grondahl ML, Thorboll JE, Skadhauge E, Munck BG. 2000. Transport of neutral, cationic and anionic amino acids by systems B, bo,+, XAG, and ASC in swine small intestine. Comp Biochem Physiol A Mol Integr Physiol. 126(4):527–537. doi: 10.1016/s1095-6433(00)00227-0.
- Noblet J, Bontems V, Tran G. 2003. Estimation de la valeur énergétique des aliments pour le porc. INRA Prod Anim. 16(3):197–210. doi: 10.20870/productions-animales.2003.16.3.3660.
- Okame R, Nakahara K, Murakami N. 2015. Plasma amino acid profiles at various reproductive stages in female rats. J Vet Med Sci. 77(7):815–821. doi: 10.1292/jvms.15-0095.
- Pigrau M, Rodiño‐Janeiro B, Casado‐Bedmar M, Lobo B, Vicario M, Santos J, Alonso‐Cotoner C. 2016. The joint power of sex and stress to modulate brain–gut–microbiota axis and intestinal barrier homeostasis: implications for irritable bowel syndrome. Neurogastroenterol Motil. 28(4):463–486. doi: 10.1111/nmo.12717.
- Puchal AA, Buddington RK. 1992. Postnatal development of monosaccharide transport in pig intestine. Am J Physiol. 262(5 Pt 1):G895–G902. doi: 10.1152/ajpgi.1992.262.5.G895.
- Richards JD, Atwell CA, Vázquez-Añón M, Dibner JJ. 2005. Comparative in vitro and in vivo absorption of 2-hydroxy-4 (methylthio) butanoic acid and methionine in the broiler chicken. Poult Sci. 84(9):1397–1405. doi: 10.1093/ps/84.9.1397.
- Röder PV, Geillinger KE, Zietek TS, Thorens B, Koepsell H, Daniel H. 2014. The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing. PLoS One. 9(2):e89977. doi: 10.1371/journal.pone.0089977.
- Romanet S, Aschenbach JR, Pieper R, Zentek J, Htoo JK, Whelan RA, Mastrototaro L. 2021. Expression of proposed methionine transporters along the gastrointestinal tract of pigs and their regulation by dietary methionine sources. Genes Nutr. 16(1):14. doi: 10.1186/s12263-021-00694-4.
- Ruiz-Ascacibar I, Stoll P, Kreuzer M, Boillat V, Spring P, Bee G. 2017. Impact of amino acid and CP restriction from 20 to 140 kg BW on performance and dynamics in empty body protein and lipid deposition of entire male, castrated and female pigs. Animal. 11(3):394–404. doi: 10.1017/S1751731116001634.
- Takata K. 1996. Glucose transporters in the transepithelial transport of glucose. J Electron Microsc (Tokyo). 45(4):275–284.
- Tapiero H, Mathé G, Couvreur P, Tew KD. 2002. I. arginine. Biomed Pharmacother. 56(9):439–445. doi: 10.1016/s0753-3322(02)00284-6.
- Terada T, Shimada Y, Pan X, Kishimoto K, Sakurai T, Doi R, Onodera H, Katsura T, Imamura M, Inui KI. 2005. Expression profiles of various transporters for oligopeptides, amino acids and organic ions along the human digestive tract. Biochem Pharmacol. 70(12):1756–1763. doi: 10.1016/j.bcp.2005.09.027.
- Tor M, Vilaró F, Ros-Freixedes R, Álvarez-Rodríguez J, Bosch L, Gol S, Pena RN, Reixach J, Estany J. 2021. Circulating non-esterified fatty acids as biomarkers for fat content and composition in pigs. Animals. 11(2):386. doi: 10.3390/ani11020386.
- VDLUFA. 2007. Verband deutscher landwirtschaftlicher untersuchungs‐und forschungsanstalten. Handbuch der landwirtschaftlichen versuchs‐und untersuchungsmethodik (VDLUFA‐methodenbuch), Bd. III. Die Chemische Untersuchung von Futtermitteln. Speyer, Germany.
- Von Heimendahl E, Breves G, Abel H. 2010. Fiber-related digestive processes in three different breeds of pigs. J Anim Sci. 88(3):972–981. doi: 10.2527/jas.2009-2370.
- Wu X, Han Z, Liu B, Yu D, Sun J, Ge L, Tang W, Liu S. 2022. Gut microbiota contributes to the methionine metabolism in host. Front Microbiol. 13:1065668. doi: 10.3389/fmicb.2022.1065668.
- Yi H, Yang G, Xiong Y, Wu Q, Xiao H, Wen X, Yang X, Wang L, Jiang Z. 2019. Integrated metabolomic and proteomics profiling reveals the promotion of Lactobacillus reuteri LR1 on amino acid metabolism in the gut–liver axis of weaned pigs. Food Funct. 10(11):7387–7396. doi: 10.1039/c9fo01781j.
- Yin J, Han H, Li Y, Liu Z, Zhao Y, Fang R, Huang X, Zheng J, Ren W, Wu F, et al. 2017. Lysine restriction affects feed intake and amino acid metabolism via gut microbiome in piglets. Cell Physiol Biochem. 44(5):1749–1761. doi: 10.1159/000485782.
- Yin L, Menon R, Gupta R, Vaught L, Okunieff P, Vidyasagar S. 2017. Glucose enhances rotavirus enterotoxin-induced intestinal chloride secretion. Pflugers Arch. 469(9):1093–1105. doi: 10.1007/s00424-017-1987-x.
- Yoshikawa T, Inoue R, Matsumoto M, Yajima T, Ushida K, Iwanaga T. 2011. Comparative expression of hexose transporters (SGLT1, GLUT1, GLUT2 and GLUT5) throughout the mouse gastrointestinal tract. Histochem Cell Biol. 135(2):183–194. doi: 10.1007/s00418-011-0779-1.
- Zhang J, Jing Y, Zhang H, Liu P. 2021. Effects of sex and estrous cycle on the brain and plasma arginine metabolic profile in rats. Amino Acids. 53(9):1441–1454. doi: 10.1007/s00726-021-03040-5.