ABSTRACT
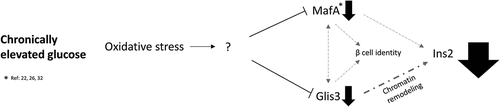
Chronically elevated levels of glucose are deleterious to pancreatic β cells and contribute to β cell dysfunction, which is characterized by decreased insulin production and a loss of β cell identity. The Krüppel-like transcription factor, Glis3 has previously been shown to positively regulate insulin transcription and mutations within the Glis3 locus have been associated with the development of several pathologies including type 2 diabetes mellitus. In this report, we show that Glis3 is significantly downregulated at the transcriptional level in INS1 832/13 cells within hours of being subjected to high glucose concentrations and that diminished expression of Glis3 is at least partly attributable to increased oxidative stress. CRISPR/Cas9-mediated knockdown of Glis3 indicated that the transcription factor was required to maintain normal levels of both insulin and MafA expression and reduced Glis3 expression was concomitant with an upregulation of β cell disallowed genes. We provide evidence that Glis3 acts similarly to a pioneer factor at the insulin promoter where it permissively remodels the chromatin to allow access to a transcriptional regulatory complex including Pdx1 and MafA. Finally, evidence is presented that Glis3 can positively regulate MafA transcription through its pancreas-specific promoter and that MafA reciprocally regulates Glis3 expression. Collectively, these results suggest that decreased Glis3 expression in β cells exposed to chronic hyperglycemia may contribute significantly to reduced insulin transcription and a loss of β cell identity.
Introduction
Insulin, a hormone produced and secreted by pancreatic β cells, is critical for the proper regulation of blood glucose homeostasis. Dysfunction of the β cells or resistance to insulin within the peripheral tissues results in the development of diabetes, which manifests as chronic hyperglycemia. Most cases of diabetes are classified as either Type 1 diabetes (T1DM), distinguished by autoimmune destruction of the pancreatic β cells or Type 2 diabetes (T2DM), characterized by peripheral insulin resistance and β cell dysfunction. T2DM currently accounts for >90% of all diabetes diagnoses.Citation1 A growing body of evidence suggests that chronic hyperglycemia can exert toxic effects on pancreatic β cells that contributes to β cell dysfunction in the form of decreased insulin production and secretion, apoptosis, or loss of β cell character.Citation2–5 Thus, the toxic effects of elevated blood glucose levels on pancreatic β cells, termed glucotoxicity, may precipitate the development of T2DM.
Transcriptional regulation of insulin is under complex controls and is mediated by a host of transcription factors that form a transcriptional regulatory complex through DNA-protein and protein–protein interactions.Citation6-8 Among these is the Krüppel-like transcription factor, Gli-similar 3 (Glis3), which positively regulates insulin transcription by binding Glis-responsive enhancer elements (GlisBS) within the proximal insulin promoter where it associates with a complex of other transcription factors including pancreatic and duodenal homeobox protein 1 (Pdx1) and v-Maf musculoaponeurotic fibrosarcoma oncogene homolog A (MafA).Citation9,Citation10 In humans, GLIS3 deficiency results in the development of a syndrome characterized by neonatal diabetes and congenital hypothyroidism.Citation11–13 Numerous genome-wide association studies have further implicated GLIS3 as a risk locus for the development of both Type 1 and Type 2 diabetes.Citation14–21
Previous studies have shown that oxidative stress resulting from chronically elevated glucose concentrations decreased the expression or DNA binding activities of Pdx1 and MafA within pancreatic β cells but little is known about the effects of glucotoxicity on Glis3 expression and function.Citation22–26 In this report, Glis3 transcription was shown to be dramatically decreased in INS1 (832/13) cells maintained under conditions of chronically elevated glucose. Glis3 expression was found to be instrumental for insulin expression due to its ability to behave similarly to a pioneer factor at the insulin promoter where it remodels chromatin to permit the binding of additional transcription factors such as Pdx1 and MafA. Additional evidence suggested that Glis3 directly regulates transcriptional expression of MafA through binding to its pancreas-specific enhancer while MafA may reciprocally influence Glis3 transcription. Finally, CRISPR/Cas9-mediated knockdown of Glis3 in INS1 cells resulted in a significant upregulation of β cell disallowed genes that was further exacerbated by growth in conditions of chronically elevated glucose. These findings identify Glis3 as a critical factor involved in the β cell response to glucotoxicity and the maintenance of β cell identity and may thus provide insight toward the development of therapeutic targets for the treatment of diabetes or other metabolic diseases.
Materials and methods
Cells and growth conditions
Rat insulinoma INS1 832/13 cells, kindly provided by Anton Jetten (National Institute of Environmental Health Sciences), were maintained in RPMI 1640 supplemented with 10% fetal bovine serum, 10 mM HEPES, 2 mM glutamine, 1 mM sodium pyruvate, and 50 µM β-mercaptoethanol. D-glucose was added to the media at the indicated concentrations. INS1 cells were maintained in media containing 11.1 mM D-glucose unless otherwise stated. For all experiments, INS1 cells were used between passages 25–50.
Generation of plasmids and expression constructs
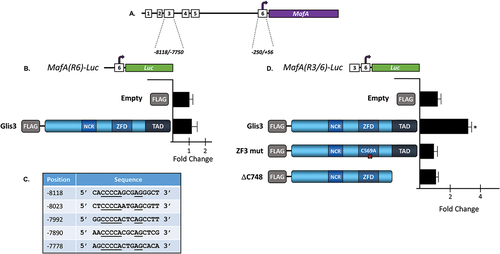
The generation of p3xFLAG-CMV10-Glis3 and Glis3 ZFD and TAD mutants was described previously.Citation9,Citation27 MafA (NM_194350) contains the Myc-DDK-tagged mouse MafA ORF within the pCMV6-Entry vector and was purchased from OriGene (Rockville, MD). The MafA promoter is comprised of six regions termed R1-R6 that span the region −8118 to +56 relative to the transcriptional start site.Citation28,Citation29 pMafA(R6)-Luc was generated by cloning the rat MafA promoter region, −250/+56, upstream of the luciferase cassette in pGL4.10 using the NheI and HindIII restriction sites. pMafA(R3/R6)-Luc was generated by cloning the rat MafA promoter region, −8118/−7750 upstream on the R6 region of pMafA(R6)-Luc using the KpnI and NheI restriction sites.
Quantitative reverse transcriptase real-time PCR analysis
RNA was isolated from INS1 832/13 cells at the indicated time point using a Direct-zol RNA miniprep kit (Zymo Research) following the manufacturer’s specifications including optional on-column DNAse digestion. Equal amounts of total RNA were used to generate cDNA using a high capacity cDNA synthesis kit (Applied Biosystems). cDNA was analyzed by qRT-PCR using PowerTrack SYBR green master mix (Applied Biosystems) and primers listed in with the exception of MafA, which was measured using TaqMan Universal PCR master mix (Applied Biosystems) and a predesigned rMafA Taqman Gene Expression Assay (ThermoFisher Scientific). All qRT-PCR was performed in triplicate using an Applied Biosystems 7500 RT-PCR system, and all experiments were performed a minimum of three times. The average Ct from triplicate samples was normalized against the average Ct of 18s rRNA or RPL13a using the 2−ΔΔCt method.
Table 1. List of primers used.
CM-H2DCFDA assay
INS1 832/13 cells were grown on glass bottom dishes in media containing the indicated concentration of glucose for 48 h with complete media change every 24 h. After 48 h, culture media was replaced with HBSS (0.137 M NaCl, 5.4 mM KCl, 0.25 mM Na2HPO4, 0.44 mM KH2PO4, 1.3 mM CaCl2, 1.0 mM MgSO4, 4.2 mM NaHCO3) containing the indicated concentration of glucose and 10 µM CM-H2DCFDA in DMSO and incubated at 37°C for 30 min. Following incubation, for imaging, cells were washed 3× with PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.2 mM KH2PO4) containing 300 nM DAPI and 3 additional times with PBS alone. Fluorescence was detected using a Leica DMi8 fluorescence microscope, and images were captured using a DFC7000T cooled fluorescence camera and LAS X Expert software (Leica Microsystems). For fluorescence quantification, cells were trypsinized, pelleted, and washed with PBS before being resuspended in PBS and loaded into a 96-well plate. Each of two replicates were loaded in triplicate and fluorescence was measured using a SprectraMax i3X plate reader (Molecular Devices).
Luciferase reporter assays
Cells were plated in 12-well dishes at 1 × 105 cells/well and incubated for 24 h at 37°C. Cells were subsequently transfected with the indicated reporter, pCMV-β-galactosidase, and the indicated expression vector in serum-free medium without antibiotic using Lipofectamine 3000 (Invitrogen) per the manufacturer’s instructions. Each transfection was carried out in triplicate. Cells were harvested after 48 h by scraping them directly into 100 ul of reporter lysis buffer, and luciferase activity was measured using a luciferase assay kit (Promega). β-galactosidase levels were measured using the Tropix Galacto-Star β-galactosidase detection kit (Applied Biosystems) following the manufacturer’s protocol. Normalized relative luciferase activity was calculated by dividing each RLU by the corresponding β-galactosidase value and the average nRLU was calculated for triplicate samples ±SD. Each data point was assayed in triplicate and p-values were calculated using a student’s t-test. Each experiment was performed at least twice. Data from representative experiments are presented as mean nRLU ± SD.
Western blot analysis
Proteins were resolved by SDS-PAGE and then transferred to PVDF membrane by electrophoresis. Immunostaining was performed with the indicated antibody at 4°C for 18 h in SuperBlock reagent (ThermoFisher). Blots were subjected to three 10-min washes in TTBS (50 mM Tris, 0.2% Tween 20, and 150 mM NaCl), and bands were detected by enhanced chemiluminescence following the manufacturer’s protocol (Thermo Scientific).
Chromatin immunoprecipitation (ChIP) assays
INS1 832/13 cells were grown in 150 mm dishes and transfected with the indicated plasmids using Lipofectamine 3000 (Invitrogen) per the manufacturer’s instructions. 48 h later, the samples were subjected to ChIP assays using the MagnaChip A/G kit (EMD Millipore) following the manufacturer’s protocol. Briefly, the samples were crosslinked in 1% formaldehyde at room temperature for 10 min, the reactions were quenched with glycine, and the cells were lysed in lysis buffer containing protease inhibitor cocktail. Lysates were sonicated using four 10s pulses (40W) with 30s rests between pulses. Sheared lysates were incubated with a rabbit anti-Pdx1 monoclonal antibody (AbCam) overnight at 4°C. Antibody/chromatin complexes were recovered using a magnetic separator, and cross-linking was reversed by incubating samples at 62°C for 2 h in the presence of proteinase K followed by a 10 min incubation at 95°C. DNA was subsequently purified using spin columns and analyzed by RT-PCR using primers specific to the rIns2 proximal promoter ().
Formaldehyde assisted isolation of regulatory elements (FAIRE) analysis
INS1 832/13 cells were maintained in either 3 mM or 25 mM glucose in 150 mm dishes and transfected with either empty vector or p3xFLAG-CMV10-Glis3 using Lipofectamine 3000 (Invitrogen) per the manufacturer’s instructions. 48 h later, INS-1 cells were crosslinked in 1% formaldehyde at room temperature for 10 min, quenched with 0.125 M glycine, and lysed as described above for the ChIP assay. Lysate was sonicated using four 10 s pulses (40 W) with 30 s rests between pulses. Sheared DNA was subjected to three consecutive extractions in phenol-chloroform-isoamyl alcohol (25:24:1; pH 7.8), precipitated with ethanol, and treated with RNAse A. Cross-linking was reversed by incubating samples at 62°C for 2 h in the presence of proteinase K followed by a 10 min incubation at 95°C. DNA was finally purified using spin columns and analyzed by RT-PCR using primers specific to the rIns2 proximal promoter (). Since histone-bound DNA is more likely to move into the organic phase, relaxed DNA is expected to be more represented in the collected aqueous phase. Data is presented as average percentage of DNA recovered relative to input from triplicate samples ±SD.
Generation of Glis3 knockdown cell line using CRISPR/Cas9
Two sgRNA were generated that target exon 3 of the rGlis3 locus by cloning two 20 bp fragments corresponding to the targeted regions into pDR274 using BsaI to generate pDR274-EX3A and pDR274-EX3B. pDR274 was a gift from Keith Joung (Addgene plasmid # 42250; http://n2t.net/addgene: 42250; RRID: Addgene_42250).Citation30 The resulting constructs were then linearized using DraI and sgRNAs were produced in vitro using a MEGAshortscript T7 kit and 75 µM of linearized template. The efficiency of each sgRNA was optimized by cleaving a PCR amplicon encoding the full length 2.8 kb rGlis3 cDNA with a 10:10:1 molar ratio of sgRNA:Cas9:target DNA to determine maximal cleavage for both sgRNA.
To mutate the rGlis3 locus in INS1 832/13 cells, 3.75 µg of TrueCut Cas9 v2 (ThermoFisher Scientific) was combined with 22.5 pmol of each sgRNA and incubated at RT for 10 min to generate RNP complexes. Cas9/sgRNA complexes were then combined with 2 × 10Citation5 INS1 832/13 cells resuspended in Buffer R (ThermoFisher Scientific) and electroporated at 1650 V/10 ms/3 pulses using a NEON transfection system (ThermoFisher Scientific). Control cells were prepared following the same protocol but were electroporated using sgRNA without Cas9 protein. The electroporated cells were grown in media lacking antibiotic overnight at 37°C. The next day, genomic DNA was harvested from an aliquot of cells and CRISPR efficiency was determined using a GeneArt Genomic Cleavage Detection Kit (Invitrogen), which indicated cleavage efficiency > 80% (1 – [(1 – fraction cleaved)1/2]). After passaging, the electroporated cells were diluted and re-plated into 12-well dishes at a density of ~50 cells/well and grown to ~ 50% confluency. Total RNA was harvested from an aliquot of cells in each well, converted to cDNA, and a 219 bp fragment of Glis3 exon 3 was amplified using primers that flank the region targeted by sgRNA. Successful cleavage by both sgRNA would be expected to delete a 45–47 bp fragment from exon3 resulting in an amplicon 174–176 bp in length. The PCR products were visualized on an agarose gel and the subpopulation exhibiting the greatest proportion of lower molecular weight amplicon was selected for further propagation to generate the INS1-Glis3EX3del cell line. Control cells exclusively produced a 219 bp amplicon. PCR genotyping was routinely performed every 3–5 passages without any detectable change in the results over time.
Results
Glis3 is transcriptionally downregulated under conditions of chronically elevated glucose
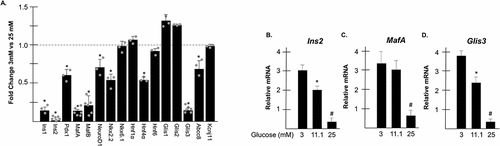
INS1 832/13 cells (hereafter referred to as INS1 cells) were cultured for 24 h in media supplemented with either 3 mM or 25 mM glucose. After 24 h, total RNA was harvested from the cells and RT-PCR was performed to measure relative transcriptional levels of candidate genes involved in β cell maintenance and glucose homeostasis. When maintained in conditions of chronically elevated glucose, levels of Ins1 and Ins2 mRNA were reduced >80% compared to cells maintained in 3 mM glucose () as previously reported.Citation22,Citation31,Citation32 Additionally, several transcription factors involved in the transcriptional regulation of insulin were also significantly downregulated including Pdx1, MafA, and NeuroD1 as well as transcription factors involved in β cell maintenance such as MafB, Hnf4α, and Nkx2.2 and genes involved in insulin secretion, such as Abcc8. Interestingly, expression of the transcription factor, Glis3 was reduced nearly 90% in INS1 cells maintained under glucotoxic conditions similarly to insulin and MafA. Given that INS1 cells are normally maintained in 11.1 mM glucose, expression of Ins2, MafA, and Glis3 was measured in INS1 cells cultured in 3 mM glucose (low glucose), 11.1 mM glucose, and 25 mM glucose (high glucose). Graded levels of expression were observed with expression at 11.1 mM glucose being more similar to low glucose conditions than chronically elevated glucose for all three genes ().
Figure 1. Glis3 mRNA is reduced under conditions of chronically elevated glucose. (a) INS1 832/13 cells were grown in media containing either 3 mM or 25 mM glucose. After 24 h, RNA was harvested, converted to cDNA, and gene expression was measured by qRT-PCR using the indicated primers. Relative mRNA expression for each gene was measured in triplicate and normalized to 18s rRNA. Bars represent average fold change of expression from cells grown in 25 mM glucose compared to cells grown in 3 mM glucose ±SD. * represents statistical difference from cells grown in 3 mM glucose (p < .05). Grey dots represent fold change from individual experiments. (b–d). INS1 832/13 cells were grown in media containing either 3 mM, 11.1 mM, or 25 mM glucose. After 24 h, RNA was harvested, converted to cDNA, and gene expression of the indicated target was measured by qRT-PCR as described in A. * represents statistical difference from cells grown in 3 mM glucose. # indicates statistical difference compared to cells maintained in both 3 mM and 11.1 mM glucose (p < .05).
Glis3 expression is reduced under conditions of oxidative stress
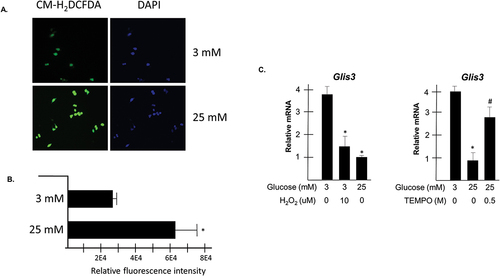
Previous studies have shown that dysregulation of β cells under glucotoxic conditions may be in part attributable to increased levels of oxidative stress.Citation2,Citation33 To confirm that oxidative stress was increased in INS1 cells cultured in 25 mM glucose for 24 h, the fluorescent oxidative stress indicator, CM-H2DCFDA was applied to the cells, which were subsequently imaged by fluorescence microscopy. As seen in , relative green fluorescence was increased in cells maintained in 25 mM glucose compared to those maintained in 3 mM without any detectable change in DAPI fluorescence signifying increased levels of intracellular reactive oxygen species. Quantification of fluorescence in the cells indicated a roughly twofold difference between cells maintained in 3 mM versus 25 mM glucose (). It was next of interest to evaluate whether increased levels of oxidative stress were involved in the observed downregulation of Glis3 mRNA under glucotoxic conditions. Accordingly, INS1 cells were grown in media containing either 3 mM or 25 mM glucose in the presence or absence of 10 µM H2O2 for 24 h and Glis3 expression was analyzed by RT-PCR. Glis3 transcripts were reduced in cells grown in 3 mM glucose with the addition of hydrogen peroxide, similar to levels observed when cells were maintained in 25 mM glucose (). Likewise, the addition of the antioxidant, 4-hydroxy-TEMPO to the culture media of cells grown in the presence of elevated glucose resulted in an approximately threefold increase in Glis3 expression relative to vehicle treated cells (). Collectively, these data suggest that transcriptional downregulation of Glis3 in INS1 cells maintained under conditions of chronically elevated glucose is coincident with increased oxidative stress.
Figure 2. Glis3 transcript levels are influenced by oxidative stress. A-B. INS1 832/13 cells were plated in glass bottom dishes in media containing either 3 mM or 25 mM glucose. After 48 h, cells were incubated in the presence of 10 µM CM-H2DCFDA for 30 min, washed in PBS, and imaged by fluorescence microscopy (a) or resuspended in PBS and the oxidized intracellular fluorescent reporter was quantified by a microplate reader (b). The mean arbitrary units of fluorescence measured in triplicate from two replicates for each condition are reported ± SD after subtracting background autofluorescence. * indicates statistical difference from vehicle treated cells maintained in 3 mM glucose (p < .05). (c) INS1 cells were grown in media containing either 3 mM or 25 mM glucose with the addition of the indicated concentration of H2O2 or 4-hydroxy-TEMPO. After 24 h, RNA was harvested and Glis3 expression was measured by qRT-PCR. Representative experiments are shown. Bars represent relative Glis3 expression from triplicate samples normalized to 18s rRNA ± SD. * indicates statistical difference from vehicle treated cells maintained in 3 mM glucose. # indicates statistical difference from vehicle treated cells maintained in both 3 mM and 25 mM glucose (p < .05).
Glis3 expression levels influence both insulin and MafA transcription
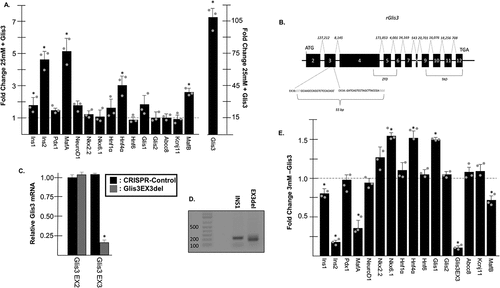
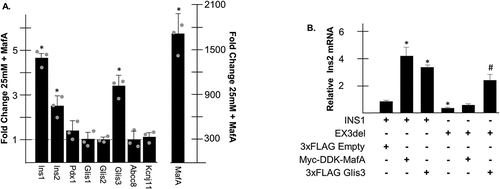
Given that Glis3 expression levels were dramatically reduced in cells maintained under glucotoxic conditions, it was of interest to determine how decreased Glis3 expression might contribute overall to β cell dysfunction. Therefore, INS1 cells maintained in 25 mM glucose were transfected with empty vector or p3×FLAG-CMV10-Glis3, which expresses murine Glis3 downstream of an N-terminal 3xFLAG epitope under the control of a CMV promoter, and after 24 h RNA was harvested for RT-PCR analysis. Glis3 overexpression resulted in a roughly fivefold increase in Ins2 and MafA expression relative to cells transfected with empty vector (). Lesser but statistically significant increases were also observed for Ins1, Hnf4α, and MafB when Glis3 was overexpressed. To determine the effect of Glis3 knockdown on gene expression, CRISPR/Cas9 technology was used to delete a 43 bp fragment of Glis3 exon 3, which would likely produce a nonfunctional protein (Glis3EX3del) attributable to a frameshift mutation following repair by NHEJ (). RT-PCR analysis using a Glis3 forward primer that targets bases within the 43 bp deleted region (Glis3 EX3) indicated that the targeted fragment of Glis3 exon 3 was reduced >80% in CRISPR transfected cells relative to controls (). These estimates were in agreement with the visualization of PCR products generated using INS1 cDNA as a template and primers that flank the targeted portion of Gils3 exon 3 on an agarose gel showing that the predominant band in the CRISPR mutated cells exhibited a reduced molecular weight (). Nonsense-mediated decay was not evident in the CRISPR-treated cells as there was no change in Glis3 expression detected by RT-PCR using primers that target Glis3 exon 2 (Glis3 EX2) (). Genomic cleavage detection assays additionally identified cells in which only one of the two gRNAs induced a successful cleavage, which also could have produced a frameshift mutation but would have been undetectable by PCR (data not shown). Although the zygosity of individual cells was unknown, Glis3 knockdown resulted in an ~75% decrease in Ins2 expression while MafA transcripts were reduced by ~60% (). Ins1 and MafB mRNA were both significantly reduced approximately 25% in the Glis3EX3del cell line and Hnf4α expression increased 1.5-fold. Taken together, these data suggest that decreased levels of Glis3 under conditions of chronically elevated glucose might contribute to a reduction in insulin and MafA expression.
Figure 3. Glis3 influences insulin and MafA expression. (a) INS1 832/13 cells were transfected with empty vector or pCMV10-3×FLAG-Glis3 and grown in media containing 25 mM. After 24 h, RNA was harvested, converted to cDNA, and gene expression was measured by qRT-PCR using the indicated primers. Relative mRNA expression for each gene was measured in triplicate and normalized to 18s rRNA. Bars represent average fold change of expression from cells transfected with 3×FLAG-Glis3 compared to empty vector ± SD. Grey dots represent fold change from individual experiments. * represents statistical difference from cells transfected with empty vector. (b) A map of the Glis3 gene locus in rat. Exons are numbered 2–12 along with the relative position of the start and stop codons. Size of introns in bp are shown above. The locations of the regions that encode for the DBD and TAD are indicated. The targets of the two gRNA within exon 3 are shown with the PAM sequences in blue font. (c) RNA was collected from INS1 832/13 or Glis3EX3del mutants as indicated and Glis3 expression was measured by qRT-PCR using primers that target either exon 2 or the gRNA-targeted region of exon 3. Bars represent relative Glis3 expression from triplicate samples normalized to 18s rRNA ± SD. (d) A representative 2% agarose gel is shown to the right containing a PCR amplicon from the indicated cell line using primers that flank the gRNA-targeted region of Glis3 exon 3. Expected size = 219 bp. Mutated size = 175 bp. E. INS1 832/13 or Glis3EX3del mutant cells were grown in media containing 3 mM glucose. After 24 h, RNA was harvested and RT-PCR was carried out as described in A. Bars represent average fold change of expression from Glis3EX3del cells compared to INS1 controls. Grey dots represent fold change from individual experiments. * represents statistical difference from INS1 control expression (p < .05).
Glis3 positively regulates MafA transcription through a pancreas-specific enhancer
Overexpression of Glis3 resulted in a ~5-fold increase in MafA transcription and Glis3 knockdown decreased MafA expression 60% in INS1 cells (). In order to gain more insight into Glis3-mediated transcriptional regulation of MafA, luciferase reporter assays were performed in INS1 cells. The MafA gene is controlled by a core promoter (R6) as well as several upstream tissue-specific enhancers including a pancreas-specific enhancer (R3)Citation28,Citation29 (). Overexpression of Glis3 did not significantly affect luciferase expression driven by the core promoter alone in INS1 cells (). Examination of the pancreas-specific MafA enhancer (R3) sequence revealed five putative Glis3 binding sites that are conserved in rat, mouse, and human (). Indeed, when pMafA(R3/6)-Luc, which contains the R3 enhancer immediately upstream of the R6 promoter was transfected into cells, Glis3 overexpression resulted in a roughly threefold increase in luciferase activity compared to empty vector (). Moreover, a mutation that disrupts the tetrahedral configuration of Glis3 zinc Finger 3 (C569A)Citation34 as well as deletion of the C-terminal transactivation domain (Glis3-ΔC748)Citation9 prevented Glis3-mediated activation of the luciferase reporter. Collectively, these results indicate that Glis3 may be capable of activating MafA transcription through its pancreas-specific enhancer.
Figure 4. Glis3 positively regulates MafA expression through its pancreas-specific enhancer. (a) Schematic diagram of the MafA upstream regulatory regions. The regions defining the core promoter (6) and the pancreas-specific enhancer (3) are shown in bp relative to the TSS. (b,d) INS1 832/13 cells were transfected with pCMV-β-Gal, the indicated luciferase reporter construct, and the specified Glis3 expression vector. After 48 h, cells were assayed for luciferase and β-galactosidase activity and the normalized relative luciferase activity (nRLU) was calculated and plotted. Representative experiments are shown. Each bar represents the mean ± S.D. * indicates statistically different than cells expressing empty vector (p < .05). NCR = N-terminal conserved region; ZFD = zinc finger domain; TAD = transactivation domain. (c) Five putative GlisBS were identified within the pancreas-specific R3 enhancer. Underlined bases indicate core GlisBS sites conserved between mouse, rat, and human. Position of each element is indicated in bp relative to the MafA TSS.
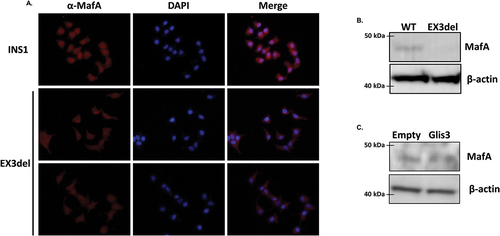
To determine whether knockdown of Glis3 influenced MafA expression at the protein level, immunocytochemistry was performed on WT INS1 or Glis3EX3del mutant cells using an antibody specific for MafA. Reduced Glis3 expression was coincident with decreased nuclear MafA expression () and Western blotting confirmed decreased MafA protein in INS1 cells with reduced Glis3 expression (). Overexpression of Glis3 similarly resulted in a marginal increase in MafA expression at the protein level ().
Figure 5. MafA expression is reduced in Glis3EX3del mutant cells. (a) INS1 or Glis3EX3del mutant cells were grown on glass bottom dishes and 24 h later cells were fixed and stained with rabbit anti-MafA primary antibody and an anti-rabbit AlexaFluor-594 secondary antibody. Nuclear staining was acquired using DAPI. (b) INS1 or Glis3EX3del mutants were grown for 24 h and proteins were separated by SDS-PAGE and analyzed by Western blotting using rabbit anti-MafA or mouse anti-β-actin primary antibodies and HRP-conjugated secondary antibodies. (c) INS1 cells were transfected with empty vector or pCMV10-3xFLAG-Glis3 and 24 h later proteins were separated by SDS-PAGE and analyzed by Western blotting as described in B.
MafA reciprocally regulates Glis3 transcription in INS1 cells
Since Glis3 was capable of activating MafA transcription in INS1 cells, it was of interest to determine whether any changes in gene expression observed following overexpression of Glis3 were attributable to increased MafA expression. INS1 cells maintained in 25 mM glucose were transfected with empty vector or pCMV6-Myc-DDK-MafA and after 24 h, RNA was harvested for RT-PCR analysis. Despite robust levels of MafA overexpression, few genes were significantly affected with the exception of Ins1 and Ins2, which increased ~ 5-fold and 2.5-fold, respectively (). Surprisingly, MafA overexpression also consistently induced an ~3-fold increase in Glis3 levels. It has been previously reported that Glis3 and MafA behave synergistically at the Ins2 promoter.Citation27 In order to determine how MafA-mediated activation of insulin is influenced by Glis3, WT INS1 or mutant Glis3EX3del cells were transfected with empty vector, p3xFLAG-CMV10-Glis3, or pCMV6-Myc-DDK-MafA and Ins2 expression was measured by RT-PCR. Although both Glis3 and MafA significantly upregulated Ins2 expression in INS1 cells, MafA overexpression failed to activate Ins2 in cells with reduced Glis3 expression (). These data suggest that Glis3 expression is required for maximal activation of insulin by MafA and that MafA and Glis3 may be involved in a self-regulatory feedback loop.
Figure 6. MafA positively influences Glis3 transcription. (a) INS1 832/13 cells were transfected with empty vector or pCMV6-Myc-DDK-MafA and grown in media containing 25 mM glucose. After 24 h, RNA was harvested, converted to cDNA, and gene expression was measured by qRT-PCR using the indicated primers. Relative mRNA expression for each gene was measured in triplicate and normalized to 18s rRNA. Bars represent average fold change of expression from cells transfected with Myc-DDK-MafA compared to empty vector ±SD. Grey dots represent fold change from individual experiments. * represents statistical difference from cells transfected with empty vector (p < .05). (b) INS1 or Glis3EX3del mutant cells were transfected with the indicated plasmids and maintained in media containing 25 mM glucose. After 24 h, RNA was harvested and Ins2 expression was measured by qRT-PCR. Bars represent relative Ins2 expression from triplicate samples normalized to 18s rRNA ± SD. * indicates statistical difference from INS1 cells transfected with empty vector. # indicates statistical difference from INS1 and Glis3EX3del mutant cells transfected with empty vector (p < .05).
Glis3 acts as a pioneer-like factor at the Ins2 promoter
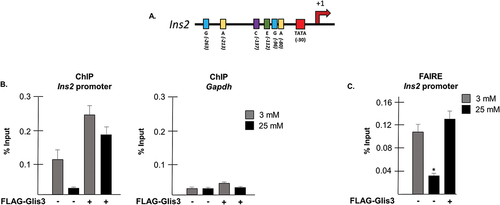
In light of the substantial decrease in Ins2 levels observed when Glis3 was knocked down and the diminished ability of MafA to activate Ins2 transcription, it was of interest to determine whether Glis3 binding might be obligatory for insulin activation by nearby transcription factors as suggested previously.Citation27 To gain further insight, a ChIP assay was performed on INS1 cells maintained in either 3 mM or 25 mM glucose using an antibody that targets rat Pdx1. Pdx1 was chosen due to the close proximity of its binding sites (A-boxes) to the GlisBS within the Ins2 promoter (), since its mRNA levels were not as severely affected by glucotoxicity as MafA (), its expression was not influenced by Glis3 (), and a reliable antibody was available. As expected, there was considerably less Pdx1 bound to the Ins2 promoter under conditions of chronically elevated glucose (). The amount of Pdx1 bound to the Ins2 promoter increased significantly however when Glis3 was overexpressed in the cells regardless of the glucose concentration. In order to determine whether Glis3 was associated with chromatin remodeling at the Ins2 promoter, a formaldehyde-assisted-isolation-of-regulatory-elements (FAIRE) assay was performed. Indeed, a significantly greater amount of the Ins2 promoter was recovered when cells were maintained at 3 mM compared to 25 mM glucose (). When cells overexpressing 3×FLAG-Glis3 were maintained in 25 mM glucose, however, a threefold increase in the amount of Ins2 promoter retrieved was observed, suggesting that Glis3 contributes to relaxation of the chromatin at the insulin promoter. Taken together, these data indicate that Glis3 has pioneer-factor-like qualities and its presence at the Ins2 promoter may be required for transcriptional activation of insulin by other factors including Pdx1 and MafA.
Figure 7. Glis3 behaves like a pioneer factor at the Ins2 promoter. (a) Schematic diagram of the mouse Ins2 promoter. TATA: TATA-box; A: Pdx1 binding site; E: NeuroD1/β2 binding site; C: MafA binding site; G: Glis3 binding site. Positions are indicated in bp relative to the Ins2 TSS. (b) INS1 832/13 cells were transfected with empty vector or pCMV10-3xFLAG-Glis3 as indicated and grown in media containing the specified concentration of glucose. After 48 h, a ChIP assay was performed and RT-PCR was used to determine the relative copies of the Ins2 or GAPDH promoter regions in the resulting purified DNA. Bars represent the percentage of each specified promoter relative to input ±SD. (c) INS1 832/13 cells were transfected with empty vector or pCMV10-3xFLAG-Glis3 as indicated and grown in media containing the specified concentration of glucose. After 48 h, cells were crosslinked in 1% formaldehyde, quenched with glycine, and sonicated. DNA was extracted in phenol-chloroform-isoamyl alcohol, ethanol precipitated, and purified using spin columns. Purified DNA was analyzed by RT-PCR to determine the relative amount of Ins2 promoter in each sample. Bars represent the average percentage of DNA recovered relative to input from triplicate samples ± SD. * indicates statistical difference from cells transfected with empty vector grown in 3 mM glucose (p < .05).
Downregulation of Glis3 expression promotes activation of β cell disallowed genes
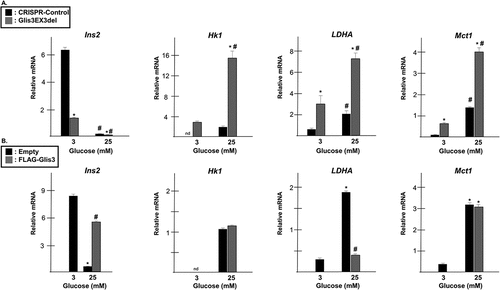
In addition to the downregulation of β cell-associated genes such as insulin and MafA, exposure to chronically elevated levels of glucose has previously been shown to result in the upregulation of ‘disallowed genes’ that are normally suppressed in healthy β cells.Citation35–38 Since loss of Glis3 resulted in decreased expression of insulin and MafA, it was of interest to determine whether decreased Glis3 levels affected the expression of β cell disallowed genes. Expression of hexokinase 1 (Hk1), lactate dehydrogenase A (LDHA), and Monocarboxylic Acid Transporter 1 (Mct1/SLC16A1) was found to be much higher in INS1 cells maintained under high glucose conditions (). Importantly, the expression of these disallowed genes was increased even further when Glis3 expression was reduced in the Glis3EX3del mutant cells suggesting that Glis3 influences the regulation of these genes independently of glucotoxicity. Under high glucose conditions, loss of Glis3 resulted in a roughly threefold increase in LDHA and Mct1 expression and a nearly 10-fold increase in Hk1 expression (). Moreover, exogenous expression of Glis3 in INS1 cells maintained in 25 mM glucose resulted in a fourfold decrease in LDHA expression while having little effect on Hk1 and Mct1 expression (). These data suggest that Glis3 expression may be important for repressing LDHA and maintaining β cell identity and loss of Glis3 under conditions of glucotoxicity may contribute to β cell dedifferentiation.
Figure 8. Expression of β cell “disallowed genes” increases in Glis3EX3del mutant cells. (a) INS1 or Glis3EX3del mutant cells were grown in media containing the indicated glucose concentration. After 24 h, RNA was harvested, converted to cDNA, and gene expression was measured by qRT-PCR using the indicated primers. Relative mRNA expression for each gene was measured in triplicate and normalized to 18s rRNA. Bars represent relative mRNA expression from triplicate samples normalized to 18s rRNA ± SD. * indicates statistical difference from INS1 control cells grown in media containing 3 mM glucose. # indicates statistical significance from paired cells maintained in 3 mM glucose (p < .05). (b) INS1 832/13 cells were transfected with empty vector or pCMV10-3xFLAG-Glis3 and grown in media containing the indicated glucose concentration. After 24 h, RNA was harvested, converted to cDNA, and gene expression was measured by qRT-PCR as described above. * indicates statistical difference from control cells maintained in 3 mM glucose. # indicates statistical difference from control cells maintained in 25 mM glucose (p < .05).
Discussion
In this report, we demonstrate that expression of the transcription factor, Glis3, is dramatically downregulated at the transcriptional level under conditions of chronically elevated glucose in INS1 cells. β cells exposed to chronically elevated levels of glucose ultimately become dysfunctional and exhibit reduced insulin production and secretion and undergo a loss of β cell identity through diminished expression of β cell-specific genes and ectopic expression of so-called ‘β cell disallowed genes'.Citation2,Citation39 If glucotoxic conditions are prolonged, the resulting β cell impairment, which is characteristic of type 2 diabetes, can become irreversible.Citation40 Chronic hyperglycemia has resulted in decreased transcriptional levels of Pdx1 and MafA in rodent models of diabetes.Citation41-43 In addition, islets from type 2 diabetic human pancreata showed a marked decrease in Pdx1 and MafA expression.Citation44 It has previously been demonstrated that a reduction in Glis3 expression negatively impacts insulin transcription owing to its ability to bind enhancer elements within the insulin promoter and positively regulate insulin transcription.Citation9,Citation27,Citation45 Here, we propose that Glis3 acts similarly to a pioneer factor to relax the chromatin surrounding the insulin promoter and permit binding by other key transcription factors. In support of this, it was demonstrated both that exogenous MafA was unable to activate Ins2 expression and that Pdx1 binding at the Ins2 promoter was significantly reduced when Glis3 was reduced. FAIRE analysis further confirmed that Glis3 expression was coincident with a relaxed chromatin state at the Ins2 promoter. A recent report by Akerman, et al. also presented evidence that GLIS3 has pioneer-factor-like activity at the insulin promoter.Citation46 The results presented in this report are limited by the fact that they were obtained in a cell line that exhibits key physiological differences from beta cells in vivo. Other reports, however, have demonstrated that GLIS3 expression was significantly downregulated in human pancreatic islets from patients with T2DM compared to healthy individuals and importantly RNA-seq data indicated that Glis3 mRNA was downregulated 40% and 60% in rat islets exposed to mildly elevated levels of glucose for 4 and 10 weeks, respectively.Citation38,Citation47–50 Collectively, these data indicate that Glis3 may play a pivotal role in the regulation of insulin transcription and may explain why reduced Glis3 expression has such a profound effect on the levels of insulin transcription.
Additionally, in this report, it was shown that MafA transcription was regulated by Glis3. Glis3 knockdown resulted in a >50% reduction in MafA expression while MafA transcription increased ~5-fold upon Glis3 overexpression. Regulation of MafA transcription by Glis3 likely occurs through the pancreas-specific MafA enhancer since Glis3 was capable of activating a luciferase reporter construct under control of this region. Several putative GlisBS were identified within the MafA pancreas-specific enhancer that were conserved between rodents and humans although Glis3-mediated activation of MafA transcription has not yet been shown to require any of these elements. Interestingly, Scoville, et al. recently reported a ChIP-seq analysis that suggested Glis3 interacted near the MafA gene.Citation51 Future studies will analyze the specific contribution of Glis3 to MafA transcriptional regulation during pancreas development and in the mature islet.
MafA appears to have a reciprocal ability to regulate Glis3 expression since overexpression of MafA resulted in a >3-fold increase in Glis3 transcription. It is not clear whether the effect of MafA on Glis3 expression is direct or indirect at this time. It is interesting to speculate that Glis3 and MafA form a self-regulatory feedback loop given the critical importance of Glis3 in regulating insulin expression identified in this study and the contribution of MafA toward maintaining β cell identity.Citation52 Interestingly, a report by Dai et al. demonstrated that MAFB expression was decreased following exposure to chronic hyperglycemia in human islet grafts while another recent report provided evidence that rodent MafB was capable of maintaining β cell identity in the absence of MafA.Citation53,Citation54 The data presented in this report suggest that Glis3 influences the expression of MafB in addition to MafA, which may explain why reduced Glis3 expression resulted in such a substantial activation of β cell disallowed genes. Interestingly, Glis3 knockdown resulted in the activation of Hk1, LDHA, and Mct1 in INS1 cells maintained in low glucose conditions suggesting that Glis3 expression contributes to the repression of these “disallowed genes.” Moreover, exogenous Glis3 was capable of significantly reducing lactose dehydrogenase expression in INS1 cells maintained in high glucose in further support of the idea that Glis3 plays a role in maintaining β cell identity. It is interesting that Glis3 overexpression did not appear to affect the levels of Hk1 and Mct1 mRNA but this may be due to the fact that Glis3 was only transiently overexpressed and LDHA mRNA has a relatively very short half-life compared to Hk1 and Mct1Citation55,Citation56. It is additionally possible that supplementary high-glucose-induced mechanisms counteract the suppression of Hk1 and Mct1 by Glis3. Taken together, these data support a model that implicates decreased Glis3 expression as a key factor in mediating β cell dysfunction and loss of β cell identity in response to glucotoxicity.
In conclusion, a model is proposed whereby chronically elevated glucose levels produce ROS that through a mechanism that is not yet clear, result in PTMs to both Glis3Citation57 and MafACitation58 and reduce Glis3 transcription. Reduced levels of Glis3 negatively impact the expression of insulin due to the subsequent condensation of the chromatin surrounding the insulin promoter and by contributing to a reduction in MafA transcription. Reduced levels of MafA further diminish expression of Glis3 and the reduction in both factors (along with MafB) contribute to a loss of β cell identity and the expression of β cell disallowed genes. A better understanding of the mechanisms that lead to Glis3 downregulation in response to oxidative stress may help identify therapeutic targets for the treatment of type 2 diabetes.
Acknowledgments
The authors would like to thank Erin Clayton for technical assistance with molecular cloning and RT-PCR analyses.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(Supplement_1):S15–16. doi:10.2337/dc21-S002.
- Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and β-cell dysfunction. Endocr Rev. 2008;29(3):351–366. doi:10.1210/er.2007-0023.
- Swisa A, Glaser B, Dor Y. Metabolic stress and compromised identity of pancreatic beta cells. Front Genet. 2017;8:21. doi:10.3389/fgene.2017.00021.
- LeRoith D. Beta-cell dysfunction and insulin resistance in type 2 diabetes: role of metabolic and genetic abnormalities. Am J Med. 2002;113(Suppl 6A):3s–11s. doi:10.1016/s0002-9343(02)01276-7.
- Kawahito S, Kitahata H, Oshita S. Problems associated with glucose toxicity: role of hyperglycemia-induced oxidative stress. World J Gastroenterol. 2009;15(33):4137–4142. doi:10.3748/wjg.15.4137.
- Andrali SS, Sampley ML, Vanderford NL, Ozcan S. Glucose regulation of insulin gene expression in pancreatic β-cells. Biochem J. 2008;415(1):1–10. doi:10.1042/BJ20081029.
- Melloul D, Marshak S, Cerasi E. Regulation of insulin gene transcription. Diabetologia. 2002;45(3):309–326. doi:10.1007/s00125-001-0728-y.
- Ohneda K, Hooi E, German M. Regulation of insulin gene transcription. Semin Cell Dev Biol. 2000;11(4):227–233. doi:10.1006/scdb.2000.0171.
- Kang HS, Kim Y-S, ZeRuth G, Beak JY, Gerrish K, Kilic G, Sosa-Pineda B, Jensen J, Foley J, Jetten AM. et al. Transcription factor Glis3, a novel critical player in the regulation of pancreatic β-cell development and insulin gene expression. Mol Cell biol. 2009;29(24):6366–6379. doi:10.1128/MCB.01259-09.
- Yang Y, Chang BH, Samson SL, Li MV, Chan L. The Krüppel-like zinc finger protein Glis3 directly and indirectly activates insulin gene transcription. Nucleic Acids Res. 2009;37(8):2529–2538. doi:10.1093/nar/gkp122.
- Senee V, Chelala C, Duchatelet S, Feng D, Blanc H, Cossec J-C, Charon C, Nicolino M, Boileau P, Cavener DR. et al. Mutations in GLIS3 are responsible for a rare syndrome with neonatal diabetes mellitus and congenital hypothyroidism. Nat Genet. 2006;38(6):682–687. doi:10.1038/ng1802.
- Dimitri P, Warner JT, Minton JAL, Patch AM, Ellard S, Hattersley AT, Barr S, Hawkes D, Wales JK, Gregory JW. et al. Novel GLIS3 mutations demonstrate an extended multisystem phenotype. Eur J Endocrinol. 2011;164(3):437–443. doi:10.1530/EJE-10-0893.
- Sarıkaya E, Kendirci M, Demir M, Dündar M. Neonatal diabetes, congenital hypothyroidism, and congenital glaucoma coexistence: a case of GLIS3 mutation. J Clin Res Pediatr Endocrinol. 2022;15(4):426–430. doi:10.4274/jcrpe.galenos.2022.2021-12-19.
- Inshaw JRJ, Sidore C, Cucca F, Stefana MI, Crouch DJM, McCarthy MI, Mahajan A, Todd JA. Analysis of overlapping genetic association in type 1 and type 2 diabetes. Diabetologia. 2021;64(6):1342–1347. doi:10.1007/s00125-021-05428-0.
- Inshaw JRJ, Cutler AJ, Crouch DJM, Wicker LS, Todd JA. Genetic variants predisposing most strongly to type 1 diabetes diagnosed under age 7 years lie near candidate genes that function in the immune system and in pancreatic β-cells. Diabetes Care. 2020;43(1):169–177. doi:10.2337/dc19-0803.
- Boesgaard TW, Grarup N, Jørgensen T, Borch-Johnsen K, Hansen T, Pedersen O. Variants at DGKB/TMEM195, ADRA2A, GLIS3 and C2CD4B loci are associated with reduced glucose-stimulated beta cell function in middle-aged Danish people. Diabetologia. 2010;53(8):1647–1655. doi:10.1007/s00125-010-1753-5.
- Cho YS, Chen C-H, Hu C, Long J, Hee Ong RT, Sim X, Takeuchi F, Wu Y, Go MJ, Yamauchi T. et al. Meta-analysis of genome-wide association studies identifies eight new loci for type 2 diabetes in east Asians. Nat Genet. 2012;44(1):67–72. doi:10.1038/ng.1019.
- Hu C, Zhang R, Wang C, Wang J, Ma X, Hou X, Lu J, Yu W, Jiang F, Bao Y. et al. Variants from GIPR, TCF7L2, DGKB, MADD, CRY2, GLIS3, PROX1, SLC30A8 and IGF1 are associated with glucose metabolism in the Chinese. PLOS ONE. 2010;5(11):e15542. doi:10.1371/journal.pone.0015542.
- Liu C, Li H, Qi L, Loos RJF, Qi Q, Lu L, Gan W, Lin X. Variants in GLIS3 and CRY2 are associated with type 2 diabetes and impaired fasting glucose in Chinese Hans. PLOS ONE. 2011;6(6):e21464. doi:10.1371/journal.pone.0021464.
- Rees SD, Hydrie MZI, O’Hare JP, Kumar S, Shera AS, Basit A, Barnett AH, Kelly MA. Effects of 16 genetic variants on fasting glucose and type 2 diabetes in South Asians: ADCY5 and GLIS3 variants may predispose to type 2 diabetes. PLOS ONE. 2011;6(9):e24710. doi:10.1371/journal.pone.0024710.
- Santin I, Eizirik DL. Candidate genes for type 1 diabetes modulate pancreatic islet inflammation and beta-cell apoptosis. Diabetes Obes Metab. 2013;15(Suppl 3):71–81. doi:10.1111/dom.12162.
- Harmon JS, Stein R, Robertson RP. Oxidative stress-mediated, post-translational loss of MafA protein as a contributing mechanism to loss of insulin gene expression in glucotoxic beta cells. J Biol Chem. 2005;280(12):11107–11113. doi:10.1074/jbc.M410345200.
- Kawamori D, Kajimoto Y, Kaneto H, Umayahara Y, Fujitani Y, Miyatsuka T, Watada H, Leibiger IB, Yamasaki Y, Hori M. et al. Oxidative stress induces nucleo-cytoplasmic translocation of pancreatic transcription factor PDX-1 through activation of c-Jun NH(2)-terminal kinase. Diabetes. 2003;52(12):2896–2904. doi:10.2337/diabetes.52.12.2896.
- Kaneto H, Matsuoka TA. Involvement of oxidative stress in suppression of insulin biosynthesis under diabetic conditions. Int J Mol Sci. 2012;13(12):13680–13690. doi:10.3390/ijms131013680.
- Olson LK, Redmon JB, Towle HC, Robertson RP. Chronic exposure of HIT cells to high glucose concentrations paradoxically decreases insulin gene transcription and alters binding of insulin gene regulatory protein. J Clin Invest. 1993;92(1):514–519. doi:10.1172/jci116596.
- Sharma A, Olson LK, Robertson RP, Stein R. The reduction of insulin gene transcription in HIT-T15 beta cells chronically exposed to high glucose concentration is associated with the loss of RIPE3b1 and STF-1 transcription factor expression. Mol Endocrinol. 1995;9(9):1127–1134. doi:10.1210/mend.9.9.7491105.
- ZeRuth GT, Takeda Y, Jetten AM. The Krüppel-like protein gli-similar 3 (Glis3) functions as a key regulator of insulin transcription. Mol Endocrinol. 2013;27(10):1692–1705. doi:10.1210/me.2013-1117.
- Raum JC, Gerrish K, Artner I, Henderson E, Guo M, Sussel L, Schisler JC, Newgard CB, Stein R. FoxA2, Nkx2.2, and PDX-1 regulate Islet β-cell-specific mafA expression through conserved sequences located between base Pairs −8118 and −7750 upstream from the transcription start site. Mol Cell biol. 2006;26(15):5735–5743. doi:10.1128/mcb.00249-06.
- Raum JC, Hunter CS, Artner I, Henderson E, Guo M, Elghazi L, Sosa-Pineda B, Ogihara T, Mirmira RG, Sussel L. et al. Islet β-cell-specific MafA transcription requires the 5′-flanking conserved region 3 control domain. Mol Cell Biol. 2010;30(17):4234–4244. doi:10.1128/mcb.01396-09.
- Hwang WY, Fu Y, Reyon D, Maeder ML, Tsai SQ, Sander JD, Peterson RT, Yeh JRJ, Joung JK. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol. 2013;31(3):227–229. doi:10.1038/nbt.2501.
- Lee KM, Seo YJ, Kim M-K, Seo H-A, Jeong J-Y, Choi H-S, Lee I-K, Park K-G. Mediation of glucolipotoxicity in INS-1 rat insulinoma cells by small heterodimer partner interacting leucine zipper protein (SMILE). Biochem Bioph Res Co. 2012;419(4):768–773. doi:10.1016/j.bbrc.2012.02.098.
- Park KG, Lee K-M, Seo H-Y, Suh J-H, Kim H-S, Wang L, Won K-C, Lee H-W, Park J-Y, Lee K-U. et al. Glucotoxicity in the INS-1 rat insulinoma cell line is mediated by the orphan nuclear receptor small heterodimer partner. Diabetes. 2007;56(2):431–437. doi:10.2337/db06-0753.
- Dinić S, Arambašić Jovanović J, Uskoković A, Mihailović M, Grdović N, Tolić A, Rajić J, Đorđević M, Vidaković M. Oxidative stress-mediated beta cell death and dysfunction as a target for diabetes management. Front Endocrinol. 2022;13:1006376. doi:10.3389/fendo.2022.1006376.
- Beak JY, Kang HS, Kim YS, Jetten AM. Functional analysis of the zinc finger and activation domains of Glis3 and mutant Glis3(NDH1). Nucleic Acids Res. 2008;36(5):1690–1702. doi:10.1093/nar/gkn009.
- Pullen TJ, Rutter GA. When less is more: the forbidden fruits of gene repression in the adult β-cell. Diabetes Obes Metab. 2013;15(6):503–512. doi:10.1111/dom.12029.
- Pullen TJ, Khan AM, Barton G, Butcher SA, Sun G, Rutter GA. Identification of genes selectively disallowed in the pancreatic islet. Islets. 2010;2(2):89–95. doi:10.4161/isl.2.2.11025.
- Quintens R, Hendrickx N, Lemaire K, Schuit F. Why expression of some genes is disallowed in β-cells. Biochem Soc Trans. 2008;36(3):300–305. doi:10.1042/bst0360300.
- Ebrahimi AG, Hollister-Lock J, Sullivan BA, Tsuchida R, Bonner-Weir S, Weir GC. Beta cell identity changes with mild hyperglycemia: implications for function, growth, and vulnerability. Mol Metab. 2020;35:100959. doi:10.1016/j.molmet.2020.02.002.
- Bensellam M, Jonas JC, Laybutt DR. Mechanisms of β-cell dedifferentiation in diabetes: recent findings and future research directions. J Endocrinol. 2018;236(2):R109–r143. doi:10.1530/joe-17-0516.
- Remedi MS, Emfinger C. Pancreatic β-cell identity in diabetes. Diabetes Obes Metab. 2016;18(S1):110–116. doi:10.1111/dom.12727.
- Jonas JC, Sharma A, Hasenkamp W, Ilkova H, Patanè G, Laybutt R, Bonner-Weir S, Weir GC. Chronic hyperglycemia triggers loss of pancreatic β cell differentiation in an animal model of diabetes. J Biol Chem. 1999;274(20):14112–14121. doi:10.1074/jbc.274.20.14112.
- Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell. 2012;150(6):1223–1234. doi:10.1016/j.cell.2012.07.029.
- Brereton MF, Iberl M, Shimomura K, Zhang Q, Adriaenssens AE, Proks P, Spiliotis II, Dace W, Mattis KK, Ramracheya R. et al. Reversible changes in pancreatic islet structure and function produced by elevated blood glucose. Nat Commun. 2014;5(1):4639. doi:10.1038/ncomms5639.
- Cinti F, Bouchi R, Kim-Muller JY, Ohmura Y, Sandoval PR, Masini M, Marselli L, Suleiman M, Ratner LE, Marchetti P. et al. Evidence of β-cell dedifferentiation in human type 2 diabetes. J Clin Endocr Metab. 2016;101(3):1044–1054. doi:10.1210/jc.2015-2860.
- Yang Y, Chang BH, Chan L. Sustained expression of the transcription factor GLIS3 is required for normal beta cell function in adults. EMBO Mol Med. 2013;5(1):92–104. doi:10.1002/emmm.201201398.
- Akerman I, Maestro MA, De Franco E, Grau V, Flanagan S, García-Hurtado J, Mittler G, Ravassard P, Piemonti L, Ellard S. et al. Neonatal diabetes mutations disrupt a chromatin pioneering function that activates the human insulin gene. Cell Rep. 2021;35(2):108981. doi:10.1016/j.celrep.2021.108981.
- Dooley J, Tian L, Schonefeldt S, Delghingaro-Augusto V, Garcia-Perez JE, Pasciuto E, Di Marino D, Carr EJ, Oskolkov N, Lyssenko V. et al. Genetic predisposition for beta cell fragility underlies type 1 and type 2 diabetes. Nat Genet. 2016;48(5):519–527. doi:10.1038/ng.3531.
- Lu Y, Li Y, Li G, Lu H. Identification of potential markers for type 2 diabetes mellitus via bioinformatics analysis. Mol Med Rep. 2020;22(3):1868–1882. doi:10.3892/mmr.2020.11281.
- Marselli L, Thorne J, Dahiya S, Sgroi DC, Sharma A, Bonner-Weir S, Marchetti P, Weir GC. Gene expression profiles of beta-cell enriched tissue obtained by laser capture microdissection from subjects with type 2 diabetes. PLOS ONE. 2010;5(7):e11499. doi:10.1371/journal.pone.0011499.
- Taneera J, Lang S, Sharma A, Fadista J, Zhou Y, Ahlqvist E, Jonsson A, Lyssenko V, Vikman P, Hansson O. et al. A systems genetics approach identifies genes and pathways for type 2 diabetes in human islets. Cell Metab. 2012;16(1):122–134. doi:10.1016/j.cmet.2012.06.006.
- Scoville D, Lichti-Kaiser K, Grimm S, Jetten A. GLIS3 binds pancreatic beta cell regulatory regions alongside other islet transcription factors. J Endocrinol. 2019;243(1):1–14. doi:10.1530/joe-19-0182.
- Nishimura W, Takahashi S, Yasuda K. MafA is critical for maintenance of the mature beta cell phenotype in mice. Diabetologia. 2015;58(3):566–574. doi:10.1007/s00125-014-3464-9.
- Dai C, Kayton NS, Shostak A, Poffenberger G, Cyphert HA, Aramandla R, Thompson C, Papagiannis IG, Emfinger C, Shiota M. et al. Stress-impaired transcription factor expression and insulin secretion in transplanted human islets. J Clin Invest. 2016;126(5):1857–1870. doi:10.1172/jci83657.
- Deng Z, Kuno A, Ojima M, Takahashi S. MafB maintains β-cell identity under MafA-deficient conditions. Mol Cell Biol. 2022;42(8):e0054121. doi:10.1128/mcb.00541-21.
- Boidot R, Végran F, Meulle A, Le Breton A, Dessy C, Sonveaux P, Lizard-Nacol S, Feron O. Regulation of monocarboxylate transporter MCT1 expression by p53 mediates inward and outward lactate fluxes in tumors. Cancer Res. 2012;72(4):939–948. doi:10.1158/0008-5472.can-11-2474.
- Huang D, Hubbard CJ, Jungmann RA. Lactate dehydrogenase a subunit messenger RNA stability is synergistically regulated via the protein kinase a and C signal transduction pathways. Mol Endocrinol. 1995;9(8):994–1004. doi:10.1210/mend.9.8.7476996.
- Hoard TM, Yang XP, Jetten AM, ZeRuth GT. PIAS-family proteins negatively regulate Glis3 transactivation function through SUMO modification in pancreatic β cells. Heliyon. 2018;4(7):e00709. doi:10.1016/j.heliyon.2018.e00709.
- Liang J, Chirikjian M, Pajvani UB, Bartolomé A. MafA regulation in β-cells: from transcriptional to post-translational mechanisms. Biomolecules. 2022;12(4):535. doi:10.3390/biom12040535.