ABSTRACT
The CdS/M/TiO2 (M = Ag, Ru, Au, Pd, and Pt) three-component nanojunction systems were constructed using a two-step photodeposition method, and evaluated for their photocatalytic activities through the degradation of methylene blue in aqueous solution under visible light irradiation. The authors found that the photocatalytic activity of CdS/M/TiO2 (M = Ag, Ru, Au, Pd, Pt) three-component nanojunctions was superior to that of CdS/TiO2 two-component system. Moreover, the photocatalytic activity of the three-component nanojunction system was found to be dependent significantly on the type of the noble metals. The results can be explained by the influence of charge transfer on the basis of the work functions of different noble metals.
INTRODUCTION
Since the discovery of photo-induced splitting of water on TiO2 electrodes,[ Citation 1 ] water splitting and environmental clean-up become two active fields supported by heterogeneous photocatalysis.[ Citation 2 , Citation 3 ] Environmental applications of heterogeneous photocatalysis have been considered among the most effective methods for elimination of many hazardous organic pollutants from the environment, particularly from the wastewater. Heterogeneous photocatalysis offers a “green” technology for completely decomposing such contaminants in the presence of semiconducting catalyst particles.
Among numerous semiconducting photocatalysts developed so far, TiO2 is regarded to be the most efficient and environmentally benign, which has been most widely used for photodegradation of various pollutants.[ Citation 4 ] However, its large band gap along with a fast recombination rate of photogenerated electron-hole pairs in TiO2 hinders its photocatalytic activity, limiting further commercialization and industrial applications. One effective approach to overcome such a hurdle involves coupling of different semiconductors with appropriate energy levels. For instance, CdS/TiO2 heterojunction has been widely studied for effective decomposition of organic compounds[ Citation 5 , Citation 6 , Citation 7 ] and for photocatalytic water splitting.[ Citation 8 , Citation 9 , Citation 10 ] In CdS/TiO2 heterojunction, CdS with narrow band gap acts as a visible-light sensitizer; combined with TiO2, CdS is also responsible for effective charge separation that enables suppression of the recombination process.[ Citation 11 , Citation 12 ]
In the past decade, Z-scheme photocatalytic systems (two-step photoexcitation) mimicking photosynthesis in a green plant have been investigated to realize a system for overall water splitting that are composed of H2- and O2-photocatalysts and a suitable electron mediator.[ Citation 13 , Citation 14 , Citation 15 , Citation 16 , Citation 17 ] The electron mediator in solution plays an indispensable role in shuttling the photo-generated carriers between the H2- and O2-photocatalysts. Recently, Kudo and co-workers succeeded in constructing a simple Z-scheme photocatalytic system driven by interparticle electron transfer without an electron mediator under visible light irradiation.18 In this (Ru/SrTiO3:Rh)-(BiVO4) system, the excited electrons in SrTiO3:Rh reduce water to form H2 on Ru co-catalyst, the holes in BiVO4 oxidize water to form O2 to accomplish overall water splitting, and the reversible Rh species at the surface of photocatalyst plays a pivotal role for electron transfer between particles. On the other hand, all solid-state Z-scheme CdS/Au/TiO2 three-component nanojunction systems have been developed to show much higher photocatalytic activity than single- and two-component systems for decomposition of organic compounds.[ Citation 19 , Citation 20 ] In such a three-component system, photo-induced electrons achieved a vectorial transfer of TiO2 → Au → CdS through a two-step excitation of CdS, TiO2, and Au as a mediator. Similarly, metal Ag species in the AgBr-Ag-Bi2WO[ Citation 21 ] 6 and AgBr-Ag-TiO22 2 Z-schematic nanojunction systems also acted as the electron transfer mediator, contributing to the enhancement of electron-hole separation and interfacial charge transfer. However, to the best of our knowledge, there have been few reports on the effects of different noble metals (such as Au, Ag, etc.) on the photocatalytic activity of such three-component Z-scheme systems.
In the present study, we constructed CdS/M/TiO2 (M=Ag, Ru, Au, Pd, Pt) three-component Z-scheme nanojunction systems with different noble metals as the electron transfer mediator. These CdS/M/TiO2 nanojunctions prepared by a two-step photodeposition method were used to carry out photocatalytic degradation of methylene blue. In particular, we investigated the effect of noble metals (Ag, Ru, Au, Pd, and Pt) on the photocatalytic activity of such Z-scheme systems.
EXPERIMENTAL
Sample Preparation
TiO2 (P25) was acquired from Nippon Aerosil. Cd(NO3)2·4H2O, Na2S·9H2O and ethanol were purchased from Sigma-Aldrich and sulfur powder was purchased from Allied Chemical. Aqueous solutions of noble metal compound were prepared from H2PtCl6·H2O (Sigma-Aldrich), AgNO3 (J.T. Baker), Na2PdCl4 (Aldrich), RuCl3 (Strem), and HAuCl4·4H2O (Sigma-Aldrich). All chemicals were used as received.
CdS/M/TiO2 (M=Ag, Ru, Au, Pd, Pt) three-component photocatalysts were prepared by the two-step photodeposition approach,[ Citation 19 ]as described in the Supplementary Material, Scheme 1. In a typical synthesis process, 0.1 g P25 TiO2 was dispersed in 100 mL ethanol aqueous solution (v:v= 1:1). To this suspension, a 0.4 mg/mL (noble metal) aqueous solution (0.75 mL) of noble metal compound was added. Under stirring, the mixture was bubbled with argon for 30 min and then irradiated with a 150-W Xe lamp for 5 h to load noble metal on TiO2 (M [0.3 wt%]/TiO2). After the resulting M/TiO2 ethanol/water suspension, added with sulfur powder (0.011 g) and Cd(NO3)2·4H2O (0.154 g), had been bubbled with argon for 30 min, irradiation was carried out for a given period with a 150-W Xe lamp. Then the products CdS/M/TiO2 (M=Ag, Ru, Au, Pd, Pt) were centrifuged, washed with water and ethanol, and dried at 65°C in air. The CdS/TiO2 photocatalyst without noble metal loading was prepared by photodeposition of CdS on P25 TiO2 instead of M/TiO2.
CdS/TiO2 and CdS/Ag/TiO2 samples as reference (CdS/TiO2-R and CdS/Ag/TiO2-R) were prepared by the precipitation method. P25 TiO2 (or Ag [0.3 wt%]/TiO2 prepared by photodeposition), 0.1 g, was dispersed in an 100-mL ethanol aqueous solution (v:v=1:1) containing Cd(NO3)2·4H2O (0.154 g). Under stirring, 10 mL aqueous solution containing double excess of Na2S was added to the mixture, and the resulting yellow suspension was stirred overnight at room temperature. The products CdS/TiO2-R and CdS/Ag/TiO2-R were centrifuged, washed with water and ethanol, and dried at 65°C in air.
In all these photocatalyts, the mass contents of CdS and M (versus TiO2) were 72.2% and 0.3%, respectively.
Characterization
X-ray diffraction (XRD) patterns were obtained from a PANalytical X’pert diffractometer using Ni-filtered Cu Kα irradiation (wavelength 1.5406 Å) with the scanning step of 0.05°/s, the operation voltage and current were 45 kV and 40 mA, respectively. Ultraviolet-visible (UV-Vis) absorption spectra of the samples were determined on a Varian Cary 50 UV spectrophotometer with MgO as the reference, the scanning range was from 200 to 900 nm. Transmission electron microscopy (TEM) studies were carried out on a JEOL JEM 2010 instrument. Elemental analysis of the photocatalytst was conducted by an energy-dispersive X-ray spectrometer (EDS) attached to the transmission electron microscope.
Photocatalytic Degradation of Methylene Blue
Photocatalytic activity of the photocatalyts was determined by measuring the decomposition of methylene blue (MB) using a 150-W xenon lamp (Newport) as light source at ambient conditions.[ Citation 23 , Citation 24 ] Light was passed through a UV cut-off filter (λ> 400 nm; Newport FS-C S/N 147) (Supplementary Material, Figure S1), and guided onto the side window of an open cuvette filled with 3 mL of MB auqeous solution (optical density ≈1.0) and a given amount of photocatalyst (60 mg/L). The suspension was stirred continuously during the whole process. The concentration of methylene blue was estimated by measuring its maximum absorbance at 664 nm with a Varian Cary 50 UV spectrophotometer after irradiation for a period of time (Supplementary Material, Figure S2).
RESULTS AND DISCUSSION
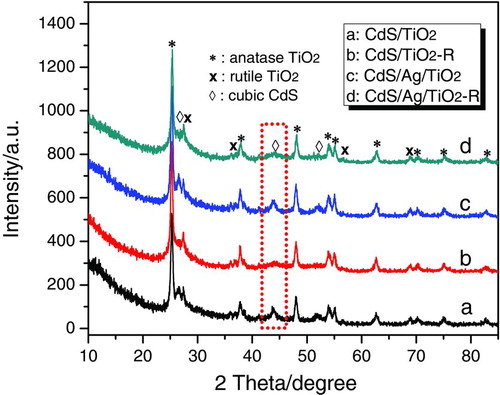
shows XRD patterns of CdS/M/TiO2 (M=Ag, Ru, Au, Pd, Pt) prepared by photodeposition method. Even though the noble metals (M) in CdS/M/TiO2 were different, these heterojunctions displayed quite similar crystal structures. All samples exhibited some diffraction peaks assigned to the anatase and rutile phases, which coexisted in P25 TiO2. In addition, there were three peaks with 2θ values of 26.5°, 44.0°, and 52.1°, corresponding to (111), (220), and (311) crystal planes of cubic CdS (space group F-43m; a= 5.818 Å; JSPDS Card No. 10-0454), respectively. The diffraction peaks assigned to noble metals could not be identified in these CdS/M/TiO2 heterojunctions because the mass content of noble metals (0.3%, versus TiO2) is under the detection limit for XRD analysis. The XRD patterns of CdS/TiO2 and CdS/Ag/TiO2 prepared by precipitation method as reference (CdS/TiO2-R and CdS/Ag/TiO2-R) are shown in . They also possessed cubic CdS, anatase and rutile TiO2 phases, similar to CdS/TiO2 and CdS/Ag/TiO2 prepared by the photodeposition method. However, as indicated by the weaker intensity of diffraction peaks assigned to cubic CdS phase in CdS/TiO2-R and CdS/Ag/TiO2-R, the cubic CdS in CdS/TiO2 and CdS/Ag/TiO2 prepared by the photodeposition method had better crystallinity than the samples prepared by precipitation.
FIGURE 1 XRD patterns of CdS/M/TiO2 (M=Ag, Ru, Au, Pd, Pt) prepared by photodeposition method. (Figure provided in color online.)
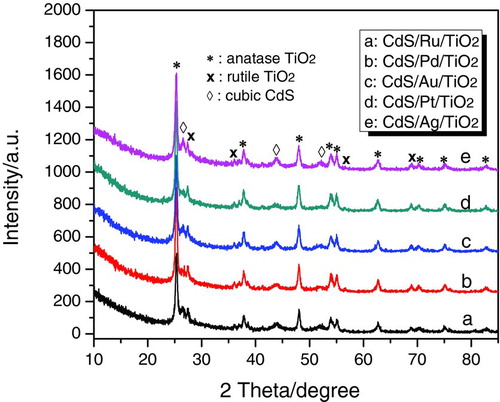
FIGURE 2 XRD patterns of CdS/TiO2 and CdS/Ag/TiO2 prepared by photodeposition method, and CdS/TiO2-R and CdS/Ag/TiO2-R prepared by precipitation method. (Figure provided in color online.)
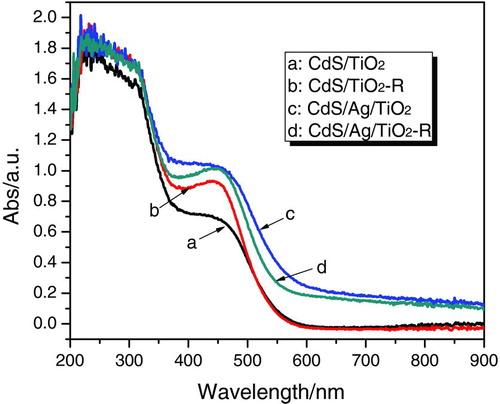
The UV-Vis diffuse reflectance spectra for CdS/M/TiO2 (M=Ag, Ru, Au, Pd, Pt) are shown in . The single TiO2 (P25) photocatalyst showed a sharp edge, whereas the CdS/TiO2 composite prepared by photodepostion method had two absorption edges; the main edge due to CdS is located at ∼550 nm and second one due to TiO2 at ∼400 nm. This is in agreement with the previous report by Jang et al. that the spectra of CdS/TiO2 composite photocatalysts prepared by precipitation method showed a combination of these two spectra.[ Citation 8 , Citation 25 ] When compared to CdS/TiO2, all the CdS/M/TiO2 nanojunctions had two similar absorption edges assigned to CdS and TiO2. However, additional absorption appeared in the range of 600–900 nm in the spectra of CdS/M/TiO2. This could be related to the existence of noble metal particles (M=Ag, Ru, Au, Pd, Pt) in these CdS/M/TiO2 nanojunctions, corresponding to the report by Doremus that small particles of noble metals showed an optical absorption band in visible light that resulted from collective oscillations of the free electrons in them.[ Citation 26 ] The UV-Vis diffuse reflectance spectra of CdS/TiO2-R and CdS/Ag/TiO2-R were also given in , which makes a comparison between optical property of the samples prepared by photodeposition method and precipitation method. CdS/TiO2 and CdS/Ag/TiO2 prepared by different methods had similar spectra with two absorption edges; the increased absorption in visible region (longer than 600 nm) of the CdS/Ag/TiO2 (or CdS/Ag/TiO2-R) spectrum was attributable to the presence of Ag, as discussed previously.
FIGURE 3 UV-Vis diffuse reflectance spectra of TiO2 and CdS/M/TiO2 (M=Ag, Ru, Au, Pd, Pt) prepared by photodeposition method. (Figure provided in color online.)
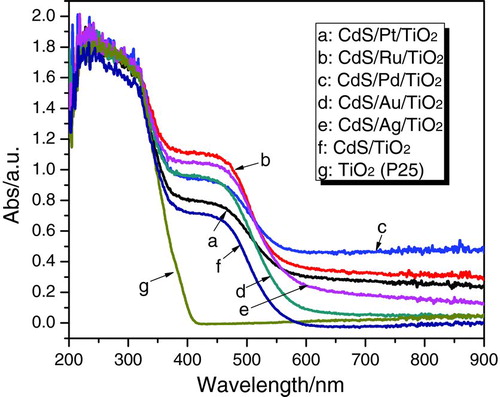
FIGURE 4 UV-Vis diffuse reflectance spectra of CdS/TiO2 and CdS/Ag/TiO2 prepared by photodeposition method, and CdS/TiO2-R and CdS/Ag/TiO2-R prepared by precipitation method. (Figure provided in color online.)
To visualize the hybridization of CdS and Ag/TiO2 in CdS/Ag/TiO2 prepared by the photodeposition and precipitation method, CdS/Ag/TiO2 and CdS/Ag/TiO2-R were investigated by TEM. The representative TEM image of the CdS/Ag/TiO2-R sample is shown in , displaying separated phases of TiO2 and CdS, as confirmed by EDS element analysis (Supplementary Material, Figure S3a, b). The Ag signal could not be identified in the EDS spectra, because of the very small amount of Ag in CdS/Ag/TiO2-R sample. In contrast, the obvious separation of CdS and TiO2 phases does not occur in CdS/Ag/TiO2 sample, as shown in . Moreover, the EDS spectra of CdS/Ag/TiO2 (Supplementary Material, Figure S3c) indicate homogenous dispersion of CdS. Thus, CdS is supposed to deposit on the surface of Ag/TiO2 particles and cover the Ag sites during the photodeposition process (the formation of CdS/Ag/TiO2 Z-scheme nanojunction), as deduced from the previous studies that Ag acted as the reduction sites for photocatalytic reduction of S to S2− ions.[ Citation 19 , Citation 20 ] Photocatalytic activity of the CdS/M/TiO2 (M=Ag, Ru, Au, Pd, Pt) nanojunctions was tested by visible-light photodecomposition of methylene blue. shows the normalized optical density change of methlyene blue at ∼664 nm under visible light irradiation (>400 nm) as catalyzed by CdS/M/TiO2 (M=Ag, Ru, Au, Pd, Pt) as a function of time. We found that the pure TiO2 (P25), which is a well-known UV-active photocatalyst, had a very low visible-light photocatalytic activity. This is because only a small part of light passed through the UV cut-off filter could be absorbed by TiO2 and utilized for photocatalytic decomposition of methylene blue (Supplementary Material, Figure S1). CdS/TiO2 two-component nanojunction showed higher photocatalytic activity than pure TiO2 (P25), due to the photosensitization of CdS in visible light region and higher charge separation in a CdS/TiO2 nanojunction system.27 Compared to pure TiO2 and the CdS/TiO2 two-component nanojunction, all the CdS/M/TiO2 (M=Ag, Ru, Au, Pd, Pt) three-component nanojunctions exhibited higher visible-light photocatalytic performance. The charge transfer mechanism, which is similar to Z-scheme in green plants’ photosynthesis, has been proposed by other researchers.[ Citation 19 , Citation 20 , Citation 21 ] The vectorial photo-generated electron transfer of TiO2→ M (noble metal) → CdS in three-component nanojunction systems greatly improves the separation rate of photo-induced charges, resulting in higher photocatalytic activity. On the other hand, the photocatalytic activity of the CdS/M/TiO2 (M=Ag, Ru, Au, Pd, Pt) three-component nanojunctions showed a significant dependence on the type of noble metal, and the photocatalytic activity decreased in the order of Ag > Ru ≈ Au > Pd > Pt.
FIGURE 5 TEM images of (A) CdS/Ag/TiO2-R prepared by precipitation method, and (B) CdS/Ag/TiO2 prepared by photodeposition method.
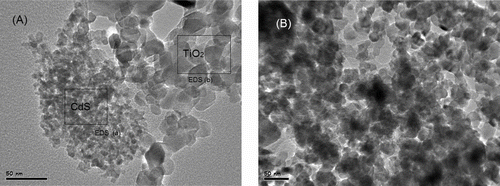
FIGURE 6 Photodecomposition of methylene blue catalyzed by CdS/M/TiO2 (M=Ag, Ru, Au, Pd, Pt) nanojunctions prepared by photodeposition method, under the irradiation of visible light (>400 nm) for up to 90 min. (Figure provided in color online.)
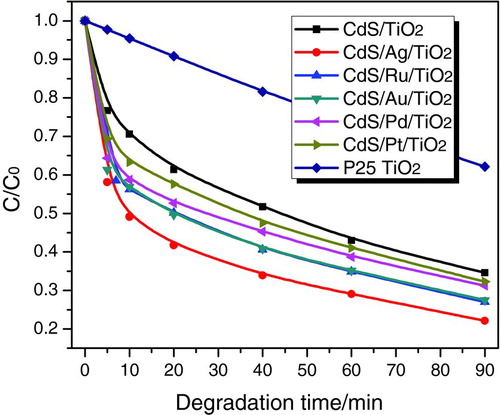
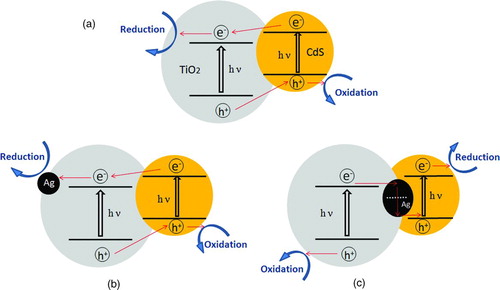
Based on the energy band diagram, the charge transfer process in CdS/M/TiO2 (M=Ag, Ru, Au, Pd, Pt) three-component nanojunction systems is illustrated in . Considering the noble metal acting as a mediator in the vectorial electron-transfer process, their Fermi energy levels are supposed to affect the interfacial charge transfer and thus the charge separation in the CdS/M/TiO2 (M=Ag, Ru, Au, Pd, Pt) three-component nanojunctions. In general, the minimum energy needed to move an electron from the Fermi energy level into vacuum is defined as the work function.28 According to the different work functions of these noble metals,29 we obtain the height of Fermi energy levels in the reducing order of Ag > Ru > Au > Pd > Pt, as depicted in . This trend is in good correlation with the photocatalytic activity of CdS/M/TiO2 (M=Ag, Ru, Au, Pd, Pt) nanojunctions depending on the type noble metals, i.e., the higher the Fermi level of the noble metal, the better the photocatalytic activity of CdS/M/TiO2.
As the charge transfer mechanism proposed in , photo-generated electrons in the conduction band of TiO2 easily flow into noble metal through the Schottky barrier (electron transfer I: TiO2→ M), and the holes left in the valence band of TiO2 are available for oxidation reaction. Simultaneously, the photo-generated holes in the valence band of CdS also easily flow into the noble metal to recombine with the stored electrons (electron transfer II: M → CdS), because of the higher Fermi energy levels of noble metal than the valence band level of CdS, and the electrons left in the conduction band of CdS are available for reduction reaction. Thus, the resulted vectorial photo-generated electrons transfer of TiO2→ M (noble metal) → CdS realizes the complete separation of photo-generated holes in the valence band of TiO2 (VB-hole [TiO2]) and electrons in the conduction band of CdS (CB-electron [CdS]). Therefore, we can deduce that the higher Fermi levels of noble metals in CdS/M/TiO2 nanojunctions make the electron transfer II (M → CdS) much quicker, because of the larger difference between the Fermi level of noble metal and the valence band level of CdS. This will lead to more efficient charge separation between VB-hole (TiO2) and CB-electron (CdS), which contributes to the better photocatalytic activity of CdS/M/TiO2, depending on the noble metals (Ag > Ru ≈ Au > Pd > Pt).
FIGURE 7 Energy band diagram and charge transfer mechanism in CdS/M/TiO2 (M=Ag, Ru, Au, Pd, Pt) three-component nanojunction systems. (Figure provided in color online.)
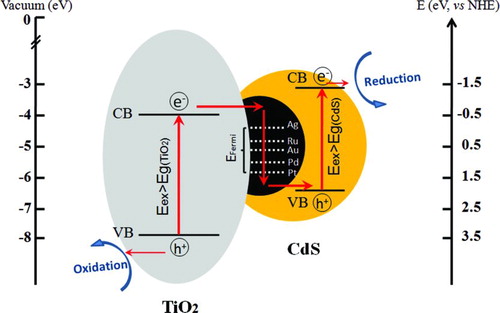
In order to illustrate the effect of vectorial electrons transfer of TiO2→ M → CdS on the improved photocatalytic activity of CdS/M/TiO2 three-component nanojunctions, CdS/TiO2 two-component nanojunction (CdS/TiO2-R) and CdS/Ag/TiO2 three-component nanojunction (CdS/Ag/TiO2-R) were also prepared for photodegradation of methylene blue by precipitation method. As shown in , CdS/TiO2 (photodeposition method) and CdS/TiO2-R (precipitation method) displayed almost the same photocatalytic efficiency for methylene blue degradation under visible light irradiation up to 90 min. This is due to the similar hybridization of CdS with TiO2 and charge transfer in the CdS/TiO2 two-component nanojucntions (), even though prepared by different methods. CdS/Ag/TiO2-R obtained via photodeposition of Ag on TiO2 and subsequent precipitation of CdS on TiO2 showed higher photoactivity than CdS/TiO2 two-component nanojunctions, as the Ag metal deposited on TiO2 would capture the electrons from the conduction band of TiO2 and accelerate charge separation (). Compared to CdS/Ag/TiO2-R, the CdS/Ag/TiO2 three-component nanojunction with different hybridization of CdS with Ag/TiO2, in which Ag nanoparticles deposited on TiO2 was covered by CdS via photodeposition (), showed much higher photocatalytic activity.
FIGURE 8 Photodecomposition of methylene blue catalyzed by photocatalysts prepared by photodeposition method (CdS/TiO2 and CdS/Ag/TiO2), and by precipitation method (CdS/TiO2-R and CdS/Ag/TiO2-R), under the irradiation of visible light (>400 nm) for up to 90 min. (Figure provided in color online.)
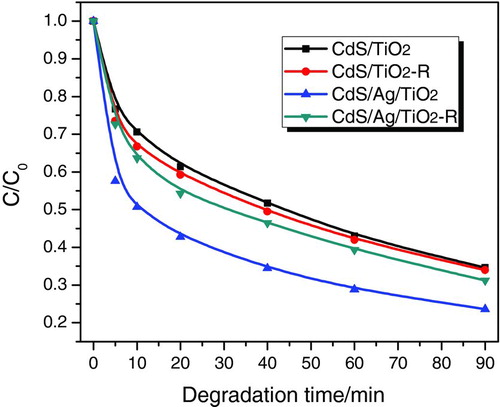
FIGURE 9 Schematic illustration of charge transfer in nanojunction systems. (a) CdS/TiO2 two-component nanojunctions prepared by photodeposition and precipitation (CdS/TiO2 and CdS/TiO2-R); (b) CdS/Ag/TiO2 three-component nanojunction prepared by precipitation method (CdS/Ag/TiO2-R); (c) CdS/Ag/TiO2 three-component nanojunction prepared by photodeposition method (CdS/Ag/TiO2). (Figure provided in color online.)
CONCLUSIONS
Z-scheme CdS/M/TiO2 (M=Ag, Ru, Au, Pd, Pt) three-component nanojunction systems have been successfully constructed via a two-step photodeposition method. Compared to CdS/TiO2 two-component nanojunction system, the CdS/M/TiO2 Z-scheme systems exhibited higher photocatalytic activity for methylene blue degradation under visible light irradiation. This enhancement is mainly due to the vectorial electrons transfer of TiO2→ M → CdS, which greatly improves the separation of photo-induced charges. Moreover, the noble metals (M=Ag, Ru, Au, Pd, Pt) make a great effect on the photocatalytic activities of CdS/M/TiO2 nanojunction systems, depending on the work functions of the noble metals. The photocatalytic activity of CdS/M/TiO2 (M=Ag, Ru, Au, Pd, Pt) nanojunction systems decreased in the order of Ag > Ru ≈ Au > Pd > Pt, corresponding to the height of Fermi energy levels in the reducing order of Ag > Ru > Au > Pd > Pt.
UGNM_A_469004_SUP_0001.zip
Download Zip (952.4 KB)Acknowledgments
The authors acknowledge the support by the National Basic Research Program of China (No. 2009CB220000), Natural Science Foundation of China (No. 50821064 and No. 90610022), and the U.S. Department of Energy. One of the authors (S.S.) also thanks the support from the China Scholarship Council.
[Supplementary materials are available for this article. Go to the publisher's online edition of the International Journal of Green Nanotechnology: Materials Science and Engineering to view the free supplementary file.]
REFERENCES
- Fujishima , A. and Honda , K. 1972 . Electrochemical photolysis of water at a semiconductor electrode . Nature , 238 : 37 – 38 .
- Fujishima , A. , Zhang , X. and Tryk , D. A. 2007 . Heterogeneous photocatalysis: Fromwater photolysis to applications in environmental cleanup . Int. J. Hydrogen Energy , 32 : 2664 – 2672 .
- Mao , S. S. and Chen , X. 2007 . Selected nanotechnologies for renewable energy applications . Int. J. Energy Res. , 31 : 619 – 636 .
- Chen , X. and Mao , S. S. 2007 . Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications . Chem. Rev. , 107 : 2891 – 2959 .
- Wu , L. , Yu , J. C. and Fu , X. 2006 . Characterization and photocatalytic mechanism of nanosized CdS coupled TiO2 nanocrystals under visible light irradiation . J. Mol. Catal. A Chem. , 244 : 25 – 32 .
- Bessekhouad , Y. , Robert , D. and Weber , J. V. 2004 . Bi2S3/TiO2 and CdS/TiO2 heterojunctions as an available configuration for photocatalytic degradation of organic pollutant . J. Photochem. Photobio. A Chem. , 163 : 569 – 580 .
- Zhu , J. H. , Yang , D. , Geng , J. Q. , Chen , D. M. and Jiang , Z. Y. 2008 . Synthesis and characterization of bamboo-like CdS/TiO2 nanotubes composites with enhanced visible-light photocatalytic activity . J. Nanoparticle Res. , 10 : 729 – 736 .
- Jang , J. S. , Ji , S. M. , Bae , S. W. , Son , H. C. and Lee , J. S. 2007 . Optimization of CdS/TiO2 nano-bulk composite photocatalysts for hydrogen production from Na2S/Na2SO3 aqueous electrolyte solution under visible light (λ ≥ 420 nm) . J. Photochem. Photobio. A Chem. , 188 : 112 – 119 .
- Park , H. , Choi , W. and Hoffmann , M. R. 2008 . Effects of the preparation method of the ternary CdS/TiO2/Pt hybrid photocatalysts on visible light-induced hydrogen production . J. Mater. Chem. , 18 : 2379 – 2385 .
- Ogisu , K. , Takanabe , K. , Lu , D. L. , Saruyama , M. , Ikeda , T. , Kanehara , M. , Teranishi , T. and Domen , K. 2009 . CdS nanoparticles exhibiting quantum size effect by dispersion on TiO2: photocatalytic H2 evolution and photoelectrochemical measurements . Bull. Chem. Soc. Jpn. , 82 : 528 – 535 .
- Kim , J. C. , Choi , J. , Lee , Y. B. , Hong , J. H. , Lee , J. I. , Yang , J. W. , Lee , W. I. and Hur , N. H. 2006 . Enhanced photocatalytic activity in composites of TiO2 nanotubes and CdS nanoparticles . Chem. Commun. , 48 : 5024 – 5026 .
- Sant , P. A. and Kamat , P. V. 2002 . Interparticle electron transfer between size-quantized CdS and TiO2 semiconductor nanoclusters . Phys. Chem. Chem. Phys. , 4 : 198 – 203 .
- Sayama , K. , Yoshida , R. , Kusama , H. , Okabe , K. , Abe , Y. and Arakawa , H. 1997 . Photocatalytic decomposition of water into H2 and O2 by a two-step photoexcitation reaction using a WO3 suspension catalyst and an Fe3 +/Fe2 + redox system . Chem. Phys. Lett. , 277 : 387 – 391 .
- Sayama , K. , Mukasa , K. , Abe , R. , Abe , Y. and Arakawa , H. 2002 . A new photocatalytic water splitting system under visible light irradiation mimicking a Z-scheme mechanism in photosynthesis . J. Photochem. Photobio. A: Chem. , 148 : 71 – 77 .
- Abe , R. , Sayama , K. and Sugihara , H. 2005 . Development of new photocatalytic water splitting into H2 and O2 using two different semiconductor photocatalysts and a shuttle redox mediator IO3 −/I− . J. Phys. Chem. B , 109 : 16052 – 16061 .
- Higashi , M. , Abe , R. , Takata , T. and Domen , K. 2009 . Photocatalytic overall water splitting under visible light using ATaO2N (A = Ca, Sr, Ba) and WO3 in a IO3 −/I− shuttle redox mediated system . Chem. Mater. , 21 : 1543 – 1549 .
- Sasaki , Y. , Iwase , A. , Kato , H. and Kudo , A. 2008 . The effect of co-catalyst for Z-scheme photocatalysis systems with an Fe3 +/Fe2 + electron mediator on overall water splitting under visible light irradiation . J. Catal. , 259 : 133 – 137 .
- Sasaki , Y. , Nemoto , H. , Saito , K. and Kudo , A. 2009 . Solar water splitting using powdered photocatalysts driven by Z-Schematic interparticle electron transfer without an electron mediator . J. Phys. Chem. C , 113 : 17536 – 17542 .
- Tada , H. , Mitsui , T. , Kiyonaga , T. , Akita , T. and Tanaka , K. 2006 . All-solid-state Z-scheme in CdS-Au-TiO2 three-component nanojunction system . Nat. Mater. , 5 : 782 – 786 .
- Zhu , H. , Yang , B. , Xu , J. , Fu , Z. , Wen , M. , Guo , T. , Fu , S. , Zuo , J. and Zhang , S. 2009 . Construction of Z-scheme type CdS-Au-TiO2 hollow nanorod arrays with enhanced photocatalytic activity . Appl. Catal. B Environ. , 90 : 463 – 469 .
- Zhang , L. , Wong , K.-H. , Chen , Z. , Yu , J. C. , Zhao , J. , Hu , C. , Chan , C.-Y. and Wong , P.-K. 2009 . AgBr-Ag-Bi2WO6 nanojunction system: a novel and efficient photocatalyst with double visible-light active components . Appl. Catal. A: Gen. , 363 : 221 – 229 .
- Hu , C. , Lan , Y. Q. , Qu , J. H. , Hu , X. X. and Wang , A. M. 2006 . Ag/AgBr/TiO2 visible light photocatalyst for destruction of azodyes and bacteria . J. Phys. Chem. B , 110 : 4066 – 4072 .
- Chen , X. B. , Lou , Y. B. , Samia , A. C. S. , Burda , C. and Gole , J. L. 2005 . Formation of oxynitride as the photocatalytic enhancing site in nitrogen-doped titania nanocatalysts: comparison to a commercial nanopowder . Adv. Funct. Mater. , 15 : 41 – 49 .
- Zhao , Y. , Qiu , X. and Burda , C. 2008 . The effects of sintering on the photocatalytic activity of N-doped TiO2 nanoparticles . Chem. Mater. , 20 : 2629 – 2636 .
- Jang , J. S. , Kim , H. G. , Borse , P. H. and Lee , J. S. 2007 . Simultaneous hydrogen production and decomposition of H2S dissolved in alkalinewater over CdS-TiO2 composite photocatalysts under visible light irradiation . Int. J. Hydrogen Energy , 32 : 4786 – 4791 .
- Doremus , R. 1998 . Optical absorption of island films of noble metals: wave length of the plasma absorption band . Thin Solid Films , 326 : 205 – 210 .
- Baker , D. R. and Kamat , P. V. 2009 . Photosensitization of TiO2 nanostructures with CdS quantum dots: particulate versus tubular support architectures . Adv. Funct. Mater. , 19 : 805 – 811 .
- Hudson , J. B. 1998 . Surface Science: An Introduction , New York : John Wiley & Sons .
- Halas , S. and Durakiewicz , T. 1998 . Work functions of elements expressed in terms of the Fermi energy and the density of free electrons . J. Phys. Condens. Matter. , 10 : 10815 – 10826 .
