ABSTRACT
Intestinal stem cells (ISCs) are critical for the development and rapid turnover of intestinal epithelium. The regulatory effects of gut microbiota and their metabolites on ISCs stemness remain elusive. Fucose has been demonstrated to mediate host–microbe interactions in the intestine. However, the association between fucose, gut bacteria, and ISCs stemness remains unclear. To investigate the effects of fucose on ISCs-mediated intestinal epithelial cells (IECs) development, we administered fucose to 4-week-old mice for 4 weeks. ISCs stemness, IECs proliferation, and differentiation were examined. Variations in gut microbes and metabolism were detected using 16S rDNA sequencing and metabolomic analysis. Fucose was added to the bacterial culture medium to further study its effects on metabolism. Crypts were isolated from the mouse ileum for organoids culture in vitro to evaluate the effects of metabolites and the underlying mechanism. The results showed that fucose accelerated ISCs proliferation and secretory lineage differentiation in mice, whereas antibiotics eliminated these effects. The composition and functions of gut bacteria were altered by fucose treatment, while significant increases in Akkermansia and propanoate metabolism were noted. Propionic acid and propionate have been shown to promote organoid development. Fucose fermentation increases the production of propionic acid in Akkermansia muciniphila and enhances its ability to increase the stemness of ISCs. Moreover, ileal contents from fucose-treated mice promoted organoid development in a Gpr41/Gpr43-dependent manner. Fucose administration activates the Wnt signaling pathway in ISCs, and Wnt inhibitors suppress the effects of fucose. We conclude that fucose accelerates ISC-mediated intestinal epithelial development by promoting Akkermansia-related propanoate metabolism. These findings provide new insights into the promotion of gut homeostasis and the application potential of fucose as a prebiotic.
Introduction
The crypt-villus structure and rapid turnover of the intestinal epithelium maintain its absorption and barrier functions. This is dependent on intestinal stem cells (ISCs) residing in the intestinal crypts.Citation1,Citation2 When generating ISCs daughters, some ISCs divide into transit-amplifying (TA) cells and differentiate into secretory or absorptive cell lineages.Citation3,Citation4 These differentiated cells migrated upward along the villi. In addition, ISCs are activated to support intestinal regeneration and epithelial functions when stimulated by damage factors. Therefore, regulation of ISC stemness is a core factor in intestinal development and homeostasis maintenance.
Fucose is involved in constituting glycans expressed on the apices of intestinal epithelial cells (IECs) and mediates many biological processes in the gut, especially host–microbe interactions.Citation5,Citation6 Our previous studies showed that exogenous fucose administration protects mice from DSS-induced colitis by increasing intestinal epithelial fucosylation, regulating immune functions, and modulating the crosstalk between bile acids and gut microbiota.Citation7–9 However, the effects of fucose on ISCs stemness and ISC-mediated intestinal epithelial development remain unknown.
Gut microbiota interacts with IECs and maintains gut homeostasis. Accumulating evidence suggests a significant role for gut microbiota and microbiota-derived metabolites in directly or indirectly regulating ISCs functions.Citation10 Recent studies have found that Lactobacillus rhamnosus GG-derived lipoteichoic acid sustains the stem cell niche and maintains ISCs functions.Citation11 Administration of lactic-acid-producing bacteria (LAB) increased ISCs proliferation in homeostasis and regeneration in intestinal damage by producing lactate and triggering Paneth cell-mediated Wnt3 release.Citation12 It has been reported that fucose participates in the establishment of healthy microbiota and maintaining gut homeostasis. Commensal bacteria such as Bifidobacteria utilize fucosidases to cleave fucose residues not only to obtain nutrition but also to provide nutrients for other microbes to establish a harmonious microenvironment.Citation13 Moreover, fucose can be utilized by some gut bacteria and alter their metabolism, such as Akkermansia muciniphila (Akk) and Escherichia coli.Citation14,Citation15
Considering the multiple effects of fucose on maintaining intestinal homeostasis and gut microbiota, we investigated the application potential of fucose in ISC-mediated intestinal epithelium development in this study. Fucose was administrated to 4 weeks old mice to investigate its effect on ISCs in vivo. 16S rDNA sequencing and metabolomics were used to analyze alterations in gut bacteria and metabolism. In vitro organoid models were used to further verify the underlying mechanisms. Collectively, our findings suggest that fucose enhances the stemness of ISCs and promotes ISCs-mediated intestinal epithelial development by regulating gut bacteria and metabolism.
Results
Fucose administration promotes intestinal epithelial differentiation in vivo
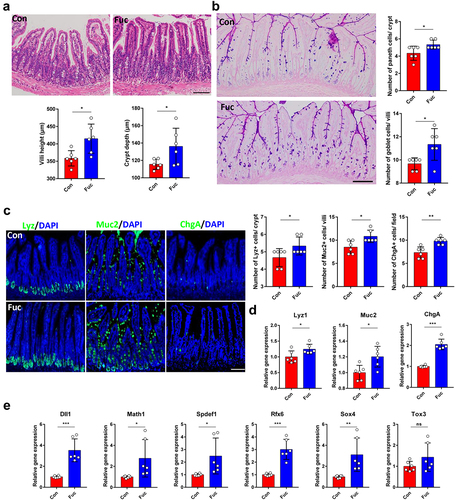
To evaluate the effects of fucose on intestinal development, fucose was given orally to 4 weeks old mice for 4 weeks. The conditions of the villi and crypts were assessed first. Histological examination showed that fucose treatment increased villus height and crypt depth in the ileum, indicating increased absorptive capacity of IECs (). We then analyzed whether there were changes in the number of differentiated intestinal cells following fucose treatment. Periodic acid-Schiff (PAS) staining showed that both goblet cells in the villi and Paneth cells in the crypts were significantly increased in fucose-treated mice (). These results were further confirmed by lysozyme (Lyz) and mucin 2 (Muc2) staining and mRNA expression analysis, which are markers of Paneth cells and goblet cells, respectively (). In addition, Chromogranin A (ChgA)-stained enteroendocrine cells also increased after fucose administration (). Consistent with these findings, the expression of the transcription factors Dll1, Math1, Spdef1, Rfx6, and Sox4, which regulate the differentiation of secretory lineage cellsCitation16,Citation17 were significantly increased in the fucose group. These results indicate that fucose enhances the differentiation of intestinal epithelial cells.
Figure 1. Fucose administration promotes intestinal epithelial differentiation in vivo. (a) HE staining of ileum sections from control and fucose-treated mice and statistical analysis of villi height and crypts depth (Scale bar, 100 μm). (b) PAS staining of sections of ileum tissues from control and fucose-treated mice and statistical analysis of number of goblet cells and Paneth cells (Scale bar, 100 μm). (c) if analysis of lysozyme, Muc2 and ChgA on ileum sections from control and fucose-treated mice (Scale bar, 100 μm). (d) Relative gene expression of Lyz1, Muc2 and ChgA in ileum tissues of control and fucose-treated mice. (e) Relative gene expression of Dll1, Math1, Spdef, Rfx6, Sox4, and Tox3 in ileum tissues of control and fucose-treated mice.
Fucose administration increases Lgr5+ ISCs and promotes ISCs proliferation
Since fucose treatment increased the number of differentiated cells in the intestine, we investigated whether fucose administration may enhance the functions of ISCs. Using Lgr5-EGFP-IRES-CreERT2 mice, we quantified the Lgr5-EGFP+ ISCs number in each crypt and found that there were more Lgr5+ ISCs in fucose-treated mice than in the controls (). Immunohistochemistry (IHC) analysis indicated that Olfm4 (another ISCs marker) stained cells showed a similar increase (). Furthermore, the protein expression of Lgr5 and Olfm4, as well as the mRNA expression of Lgr5, Olfm4, and Ascl2, in the ileum crypts of fucose-treated mice was higher than that in the controls ().
Figure 2. Fucose administration increases Lgr5+ ISCs and promote ISCs proliferation. (a) if analysis of Lgr5-EGFP on ileum sections from control and fucose-treated mice (Scale bar, 100 μm). (b) IHC analysis of Olfm4 on ileum sections from control and fucose-treated mice (Scale bar, 100 μm). (c) Western blot analysis of Lgr5 and Olfm4 in ileum crypts of control and fucose-treated mice. (d) Relative gene expression of Lgr5, Olfm4 and Ascl2 in ileum crypts of control and fucose-treated mice. (e) if analysis of Lgr5-EGFP and Ki67 in ileum crypts of control and fucose-treated mice (Scale bar, 50 μm). (f) EdU staining on ileum sections from control and fucose-treated mice at different timing (Scale bar, 100 μm). (g) Images of organoids derived from control and fucose-treated mice and statistical analysis of surface area and budding number (Scale bar, 50 μm).
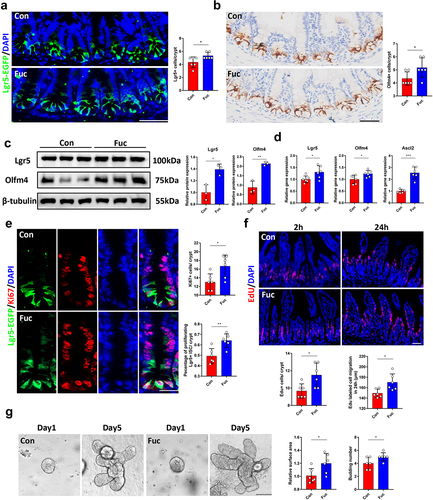
To investigate whether fucose treatment influenced ISCs proliferation, we stained the intestinal tissues with Ki67, a widely used marker for cell proliferation. As a result, more Ki67+ proliferating cells were found in crypts of fucose-treated mice. We further counted the number of Ki67+ Lgr5+ cells because Ki67+ cells include other cell types, such as transit amplifying (TA) cells. The number of proliferated ISCs increased in fucose-treated mice (). To further assess the ISC-mediated renewal of the intestinal epithelium, we labeled proliferating intestinal cells with EdU (5-ethynyl-2’-deoxyuridine). After 2 h of EdU labeling, EdU+ cells were detected in crypts, and we found more proliferating cells in fucose-treated mice, which corresponded with Ki67 staining. Twenty-four hours later, the migration of EdU+ cells was faster in the fucose group than in the control group (). In addition, we derived organoids from the ileal crypts of control and fucose-treated mice to further evaluate the functions of ISCs. At day 5 of culture, the surface area and budding number of organoids derived from fucose-treated mice were larger than those of the controls, which suggested stronger stemness of ISCs ().
Moreover, we examined the apoptosis in the two group and found no significant differences (Figure S1A).
Gut microbe participates in the effects of fucose on ISCs
There are direct effects of fucose such as facilitating fucosylation and indirect effects such as regulating bacteria. To investigate the underlying mechanism by which fucose promotes the stemness of ISCs, we treated organoids derived from untreated mice with fucose directly. However, no significant differences were found between the control and the focus-treated groups (Figure S1B). We further examined the gene expression of ISCs and differentiated cell markers and obtained similar results (Figure S1C). It seems that fucose does not act directly on ISCs. A previous study reported that the fucosylation level of Paneth cells is important for ISCs.Citation18 However, we found that fucose treatment did not significantly affect the fucosylation level per Paneth cells in the ileum and fucosylation of organoids, as shown by Ulex Europaeus Agglutinin I (UEA I) staining (Figure S1D and E). Cytokines such as IL-17a and IL-22 are also related to the stemness of ISCs and probably regulated by fucose.Citation18,Citation19 However, the expression of IL-17a, IL-22, IL-17 R, and IL-22 R did not increase significantly in the fucose group (Figure S1F and G).
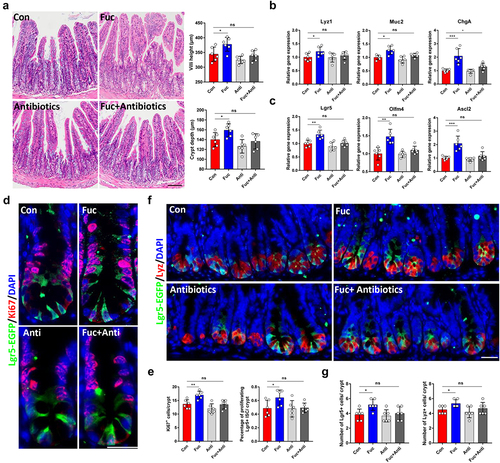
Since fucose was found to play a protective role in DSS-induced colitis in mice by regulating gut microbe and metabolism,Citation9,Citation20 we speculated that fucose may improve the functions of ISCs by regulating gut bacteria. To verify this assumption, we administered mice an antibiotic cocktail to eliminate gut bacteria prior to fucose treatment. The results showed that fucose failed to promote the development of the intestinal epithelium in the absence of gut bacteria (). No significant differences were noted in the expression of ISCs and differentiated cell markers between the control and fucose+ antibiotic groups (). In addition, when gut bacteria were eliminated, the proliferation of ISCs, indicated by Ki67 staining, showed little change under fucose treatment (), while the number of ISCs and Paneth cells in crypts was not altered (). In summary, these results demonstrate that fucose administration promotes ISCs-mediated intestinal epithelial development in the presence of gut bacteria.
Figure 3. Gut microbe participates in the effects of fucose on ISCs. (a) HE staining of ileum sections from control and fucose-treated mice with or without antibiotic treatment (Scale bar, 100 μm). (b) Relative gene expression of Lyz1, Muc2 and ChgA in ileum crypts of control and fucose-treated mice with or without antibiotic treatment. (c) Relative gene expression of Lgr5, Olfm4 and Ascl2 in ileum crypts of control and fucose-treated mice with or without antibiotic treatment. (d,e) if analysis of Lgr5-EGFP and Ki67 in ileum crypts of control and fucose-treated mice with or without antibiotic treatment and statistical analysis of proliferation cells (Scale bar, 50 μm). (f,g) if analysis of Lgr5-EGFP and Ki67 in ileum crypts of control and fucose-treated mice with or without antibiotic treatment and statistical analysis of Lgr5+ cells and Lyz+ cells.
Fucose administration alters gut microbiota composition, increases abundance of Akkermansia, and facilitates propanoate metabolism
We next explored whether fucose altered the gut microbiota composition using 16S rDNA sequencing. Alpha diversity analysis showed that there were no significant differences between groups in the indices of microbial richness (including ACE, Chao1, and Observed species) and diversity (Shannon and Simpson) (). However, PCoA analysis of beta diversity, which reflects the microbial community structures, showed a significant separation between the microbiota of the two groups (), indicating that fucose may not influence microbial diversity, but may influence its composition. As shown in , the relative abundance of the Bacteroidetes phylum decreased and that of the Verrucomicrobia phylum increased. Consistent with this, the relative abundance of the Muribaculaceae family decreased, and that of the Akkermansiaceae family increased. At the genus level, the relative abundance of Muribaculaceae decreased, and Akkermansia increased (). Although the Firmicutes, Lactobacillaceae, and Lactobacillus genera appeared to have an increasing tendency, they were not statistically significant (Figure S2A). Furthermore, we performed LEfSe analysis to identify significantly different species between the control and fucose-treated groups. The linear discriminant analysis (LDA) score and cladogram showed that Akkermansia was the microbiota with the most significant influence and was a biomarker of the fucose group (). Since the composition of the gut microbiota is closely related to its functions, we next addressed alterations in the functions of the gut microbiota. KEGG analysis showed that the propanoate metabolism pathway was significantly enhanced in the fucose-treated group (). Interestingly, Akkermansia is an important gut microbiota that participates in gut propanoate metabolism and the production of propionic acid and propionate.Citation14,Citation16,Citation21
Figure 4. Fucose administration alters gut microbiota composition, increases abundance of Akkermansia and facilitates propanoate metabolism. (a) Alpha diversity analysis of gut bacteria from control and fucose-treated mice. (b) Beta diversity analysis of gut bacteria from control and fucose-treated mice. (c) Relative abundance of bacteria on the level of phylum, family and genus. (d,e) LEfSe analysis (LDA score and cladogram) of gut bacteria from control and fucose-treated mice. (f) KEGG analysis of gut bacteria from control and fucose-treated mice. (g) Relative abundance of Akkermansia and propionic acid in ileum contents and correlation analysis of them.
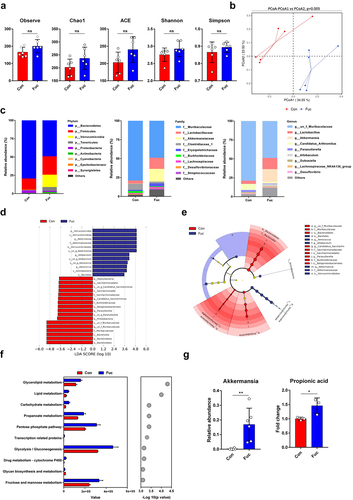
Thus, we analyzed the relative concentration of propionic acid in the ileum content by metabolomic analysis and found elevated levels of propionic acid in fucose-treated mice. The relative abundance of other types of bacteria that may participate in SCFAs metabolisms, such as Bifidobacterium, Dubosiella, and Faecalibacterium, showed no significant alteration (Figure S2B). The relative abundance of Akkermansia and the fold change of propionic acid are shown in , and the correlation analysis revealed a positive correlation between the relative abundance of Akkermansia and propionic acid (R = 0.886, P = 0.019).
Propionic acid and propionate promote organoid formation and ISCs proliferation
Although a significant role of SCFAs in maintaining gut homeostasis and preventing intestinal inflammation and carcinogenesis has been demonstrated,Citation22,Citation23 few studies have focused on the effects of propionic acid and propionate on the functions of ISCs. We found that both propionic acid (PA) and sodium propionate (Pro) treatment facilitated the formation of organoids in vitro at a concentration of 1 mM ( and Figure S2C). Correspondingly, the number of Lgr5+ ISCs and the level of ISCs marker expression increased in the PA and Pro groups (). EdU assays and Ki67 staining revealed that PA and Pro treatment promoted crypt cell proliferation in organoids (). However, we only detected increased expression of Lyz1, but not Muc2 and ChgA, in organoids treated with PA and Pro (), which may indicate that there were other metabolites participating in the fucose-mediated improvement of ISCs functions.
Figure 5. Propionic acid and propionate promote organoid formation and ISCs proliferation. (a) Images of organoids that were treated with propionic acid or propionate (Scale bar, 50 μm). (b) if analysis of Lgr5-EGFP in organoids that were treated with propionic acid or propionate (Scale bar, 50 μm). (c) Relative gene expression of Lgr5, Olfm4, and Ascl2 of organoids that were treated with propionic acid or propionate. (d) EdU and Ki67 staining of organoids that were treated with propionic acid or propionate. (e) Relative gene expression of Lyz1, Muc2, and ChgA of organoids that were treated with propionic acid or propionate.
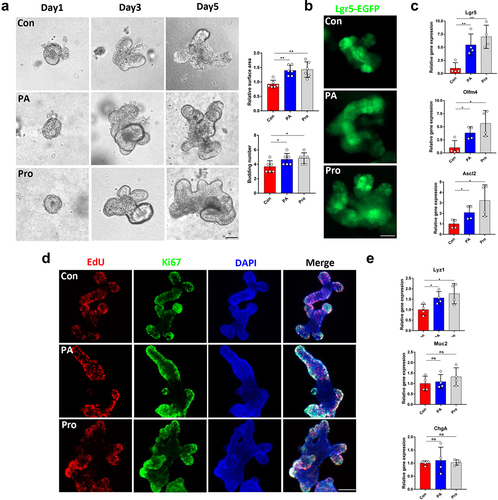
Fucose enhanced the property of Akk to promote ISCs stemness via increasing its propionic acid production
We further investigated whether fucose treatment could promote the function of Akk in promoting the stemness of ISCs. As shown in , the surface area and budding number of organoids in fucose-treated Akk group, especially in 5 mM fucose group (Akk+Fuc5), were increased compared to Akk group. Proliferated cells with indicated by EdU staining showed a similar tendency. In addition, the expression of Lgr5, Olfm4, Ascl2, Lyz1, Muc2, and ChgA was higher in the Akk+Fuc5 group than in the Akk group (). The results of the SCFA-targeted metabolomics analysis showed that propionic acid and acetic acid were the main SCFAs in the Akk culture supernatant, and the concentrations of propionic acid and acetic acid increased after fucose treatment, especially propionic acid (). Both these two kinds of SCFAs are involved in propanoate metabolism.
Figure 6. Fucose enhanced the property of Akk to promote ISCs stemness via increasing its propionic acid production. (a) Images of organoids that were treated with supernatant of Akk or fucose-treated Akk and EdU staining (Scale bar, 50 μm). Akk+Fuc1: Akk was pretreated with 1 mM fucose, Fuc5: Akk was pretreated with 5 mM fucose. (b) Relative gene expression of Lgr5, Olfm4, Ascl2 Lyz1, Muc2 and ChgA of organoids that were treated with supernatant of Akk or fucose-treated Akk. (c,d) the concentration of SCFAs in the supernatant of Akk and 5 mM fucose-treated Akk.
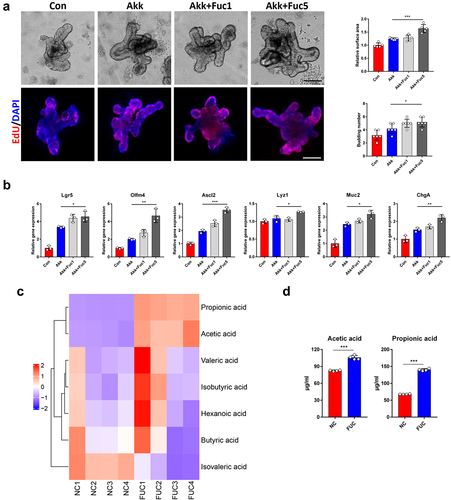
Ileal contents from fucose-administrated mice promote organoids development in a Gpr41/Gpr43-dependent manner
To further demonstrate the role of gut microbiota-related metabolism in the fucose-promoting functions of ISCs, we treated organoids with ileal contents. Organoids showed larger surface areas and more budding crypts after treatment with ileal feces (Fec group, ). Increased Lgr5-EGFP+ ISCs and ISCs marker gene expression was noted at the same time (). EdU and Ki67 staining revealed more proliferating cells in the ileal content group than in the control group (). We also stained organoids with Lyz, Muc2, and ChgA to detect differentiated Paneth, goblet, and enteroendocrine cells. Along with the increase in Lyz1, Muc2, and ChgA gene expression (), the ileal contents of fucose-treated mice were proven to promote the development of organoids.
Figure 7. Ileal contents from fucose administrated mice promotes organoids development in a Gpr41/Gpr43 dependent manner. (a) Images of organoids that were treated with ileal contents from control and fucose-treated mice and statistical analysis of surface area and budding number (Scale bar, 50 μm). (b) if analysis of Lgr5-EGFP in organoids that were treated with ileal contents from control or fucose-treated mice (Scale bar, 50 μm). (c) Relative gene expression of Lgr5, Olfm4 and Ascl2 of organoids that were treated with ileal contents from control or fucose-treated mice. (d) EdU and Ki67 staining of organoids that were treated with ileal contents from control or fucose-treated mice (Scale bar, 50 μm). (e) if analysis of lysozyme, Muc2 and ChgA on organoids that were treated with ileal contents from control or fucose-treated mice (Scale bar, 50 μm). (f) Relative gene expression of Lyz1, Muc2 and ChgA of organoids that were treated with ileal contents from control or fucose-treated mice. (g) Images of organoids that were treated with ileal contents from control and fucose-treated mice when there were Gpr41/Gpr43 antagonist (Scale bar, 50 μm). (h) EdU staining on organoids that treated with ileal contents from control and fucose-treated mice when there was Wnt-c59 (Scale bar, 50 μm).
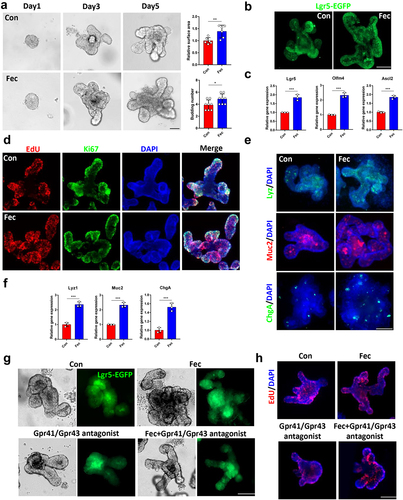
To investigate whether these effects of ileal contents are related to propionic acid and propionate, we examined the protein expression of Gpr41/Gpr43, which are SCFAs receptors, and found an elevated expression level of Gpr41/Gpr43 after ileal content treatment (Figure S3A). In addition, treatment with the Gpr41/Gpr43 antagonist β-hydroxybutyrate and GLPG0974 reversed the effect of ileal content on organoid size and Lgr5-EGFP expression (). In addition, the expression of Lgr5, Olfm4, Ascl2, Lyz1, Muc2, and ChgA did not increase after treatment with ileal contents in the presence of Gpr41/43 antagonists and the number of EdU-stained proliferating cells showed the same results (Figure S3B and ).
Fucose administration promotes ISCs functions through Wnt signaling pathway
As Wnt signaling is the primary factor involved in ISCs stemness maintenance and intestinal epithelium development,Citation4 we next studied whether fucose promotes ISCs function through this pathway. Mice treated with fucose had more β-catenin+ cells in the ileal crypts, indicating activation of the Wnt pathway (). This was further confirmed by IHC experiments for Wnt3 and Axin2 (Figure. S4A). Moreover, Wnt signaling genes Wnt3, Axin2, and Ctnnb1 expression in crypts increased in fucose-treated mice, which was consistent with the results of western blot analysis (). When organoids were treated with ileal contents of fucose-administered mice, the results were similar to those of the in vivo experiments, including increased immunofluorescence staining of β-catenin and expression of Wnt pathway-related genes (, S4B, and ). Furthermore, we examined the expression of Wnt ligands and receptor complex genes Wnt2b, Wnt4, Wnt5a, Wnt5b, Wnt6, Wnt9b, Lrp5, Lrp6, and Frizzled5–7 and regulated R-spondin1–3. As a result, Lrp5 and Frizzled5–7 had higher expression levels after ileal content treatment (Figure S4D and E), indicating that fucose-mediated metabolism alteration may promote Wnt/β-catenin signaling by increasing Wnt activation in ISCs but not by increasing Wnt sensitivity by R-spondin ligands. We further examined the role of gut microbiota in the activation of Wnt signaling and found that after the microbiota was eliminated by antibiotics, fucose failed to activate the Wnt/β-catenin pathway in mouse ileal crypts (Figure S4F and G). Furthermore, propionic acid and sodium propionate also increased Wnt signaling gene expression (Figure S4H).
Figure 8. Fucose administration promotes ISCs functions through Wnt signaling pathway. (a) if analysis of β-catenin in ileum crypts of control and fucose-treated mice (Scale bar, 100 μm). (b) Relative gene expression of Wnt3, Axin2 and Ctnnb1 in ileum crypts of control and fucose-treated mice. (c) Western blot analysis of Wnt3, Axin2 and β-catenin in ileum crypts of control and fucose-treated mice. (d) if analysis of β-catenin in organoids that were treated with ileal contents from control or fucose-treated mice. (e) Images of organoids that were treated with ileal contents from control and fucose-treated mice when there were Wnt signaling inhibitor Wnt-c59 (Scale bar, 50 μm). (f,g) Lgr5-EGFP and EdU staining of organoids that were treated with ileal contents from control and fucose-treated mice when there were Wnt signaling inhibitor Wnt-c59 (Scale bar, 50 μm).
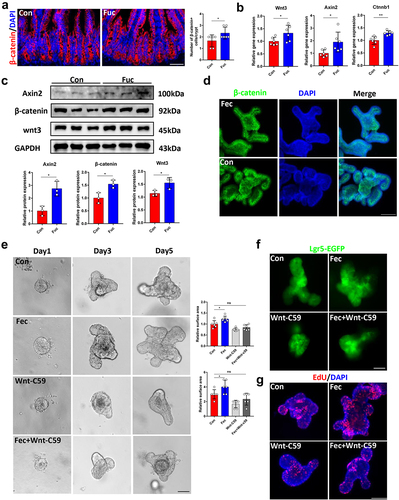
In addition, fucose administration did not increase villus height and crypt depth when mice received Wnt inhibitor Wnt-c59 treatment (Figure S5A). Consistently, the number of Lgr5+ ISCs was similar in the crypts of the Fucose+Wnt-c59 group and controls, as well as Ki67-stained proliferated cells (Figure S5B and C). In vitro, Wnt-c59 inhibited the effects of ileal contents from fucose-treated mice on the development of organoids and proliferation of ISCs () as well as the increased expression of ISCs markers (Figure S5D). These results were further confirmed by EdU assay (). The variation in the expression of the secretory lineage cell markers Lyz1, Muc2, and ChgA was also restrained by Wnt-c59 (Figure S5D). Taken together, fucose-mediated Akkermansia-related propanoate metabolism promotes ISCs function through the Wnt signaling pathway.
Discussion
In the present study, we found that fucose administration promoted ISCs function and facilitated intestinal epithelial development. The role of the gut microbiota in this process was verified. We further observed an alteration in gut microbiota composition and a significant increase in probiotic Akkermansia in fucose-treated mice. This variation in microbiota composition was accompanied by the regulation of propanoate metabolism and an increase in propionic acid in ileal contents. Propionic acid and propionate have been shown to enhance the stemness of ISCs in organoids. Moreover, fucose fermentation increased propionic acid production by Akk. Finally, fucose was demonstrated to promote the function of ISCs in a Wnt signaling-dependent manner.
Although previous studies have demonstrated multiple effects of fucose in maintaining gut homeostasis and ameliorating intestinal diseases,Citation24 few studies have examined ISCs. Our recent study found that fucose could promote intestinal lamina propria monocytes secreting IL22 to facilitate ISCs regeneration in DSS-induced colitis,Citation19 indicating a potentially effective role of fucose in ISCs homeostasis. In the current study, we discovered the effects of fucose on ISC-mediated IECs development and found that fucose-feeding promotes the stemness of ISCs in 4 weeks old mice. Since there are direct effects of fucose, such as facilitating fucosylation and indirect effects, such as regulating gut bacteria, and the effects of fucose may be different in different circumstances, we evaluated the direct and indirect effects of fucose here. The fucosylation of ileum and organoids showed no significant difference after fucose treatment, while eliminating gut bacteria almost inhibits the effects of fucose, we concluded that regulating the gut microbe and metabolism is the main mechanism in the study herein. Fucose is a mediator of host-microbe symbiosis, and liberated fucose is available to microbes for use as dietary glycans, energy sources, or synthetic structural proteins.Citation6,Citation25 The alteration of fucose in the gut influences the colonization of the microbiota. Exogenous fucose administration may be a practicable way.Citation7 As expected, fucose treatment altered the composition and functions of the gut microbiota. A significant increase in the abundance of Akkermansia and propanoate metabolism in the ileum was observed, which is related to the increased stemness of ISCs, which is manifested by the proliferation rate and number of differentiated cells. A previous study found that core fucosylation of maternal milk N-glycan promotes the colonization of Bifidobacterium spp. and Lactobacillus spp., which provides evidence for the critical role of fucosylated glycans in shaping gut microbiota.Citation26 Several studies have shown that exogenous fucose regulates gut microbiota composition and related metabolic functions in DSS-induced colitis. For example, fucose supplementation reversed the ratio of Firmicutes to Bacteroidetes and increased the abundance of Lactobacillus in DSS mice, which was negatively correlated with the bile acid taurine-β-muricholic acid.Citation9 In addition, serum tryptophan levels in DSS-induced chronic colitis were decreased, accompanied by the depletion of tryptophan-producing E. coli and Bifidobacterium, while fucose addition reversed this alteration.Citation20 Another study showed that dietary fucose could regulate macrophage polarization by affecting the intestinal microflora in Muc2−/− mice.Citation27 Therefore, the application of fucose under different conditions should be further studied in the future.
As a next-generation probiotic, Akk has drawn increasing attention from researchers since it was first identified by Derrien et al.Citation28 Akk produces acetate and propionate in the gut via mucin fermentation. Importantly, Akk was demonstrated to be able to utilize mucin-derived fucose and prioritize this kind of non-mucin sugar over GalNAc and GlcNAc.Citation29,Citation30 Another study showed that Akk levels are correlated with FUT2-mediated fucosylation status.Citation31 Moreover, Akk abundance is closely related to dietary nutrient composition. Wang et al. revealed that colostrum fed mice exhibited elevated Akk levels and were more resistant to high fat-induced obesity that was associated with intestinal permeability regulation.Citation32 Similarly, polyphenols, unsaturated fatty acids, and fiber-containing foods showed positive effects on Akk abundance and alleviated high-fat induced obesity.Citation29 Based on these facts, it is reasonable that exogenous fucose could have increased the abundance of Akk in the present study. Previous studies on Akk-host cell interactions have mainly focused on IECs and immune cells in obesity or inflammatory bowel disease, but little is known about the association between Akk and ISCs. A recent study showed that 4 weeks of Akk administration significantly accelerated ISCs proliferation and differentiation, and pretreatment with Akk increased the resistance of the gut to radiation and methotrexate damage.Citation16 We suggest that fucose increases Akk abundance to enhance the stemness of ISCs, and this advantage vanishes when antibiotics are used to eliminate the bacteria. Moreover, fucose treatment elevated Akk-related propionic acid metabolism, which promoted the development of organoids in vitro in a Gpr41/43-dependent manner. Consistently, a previous study found that Akk and its metabolite propionate could regulate metabolic pathway-related genes and activate Gpr43 in intestinal organoids.Citation33
Accumulating evidence has revealed the roles of propionic acid and propionate in maintaining gut homeostasis. SCFAs, including propionate, are also the key energy source for ileum cells.Citation34 For instance, propionic acid stimulates Muc2 expression in IECs and enhances mucin secretion.Citation35 Oral propionic acid administration enhances intestinal barrier functions during lactation.Citation36 Propionate increases IECs spreading and polarization, thereby promoting cell migration along the crypt-villus axis.Citation37 Additionally, gut microbiota-derived propionate protects the intestinal mucosa in DSS-induced colitis.Citation38 Combined with our results that propionic acid and sodium propionate promoted organoid development and ISCs proliferation, we conclude that propionic acid and propionate play important roles in modulating ISCs-mediated IECs homeostasis and intestinal epithelial development. Interestingly, when fucose was added to the culture medium of Akk, the concentration of propionic acid in the supernatant was increased. This result further indicated the positive role of fucose in promoting gut microbe-related propanoate metabolism.
Wnt signaling is pivotal for continuous self-renewal of the intestinal epithelium.Citation39 We observed that fucose treatment in mice increased the expression of Wnt3 and Wnt target genes in the crypts. Furthermore, antibiotic treatment inhibited the activation effects of fucose on Wnt signaling, which further demonstrated the significant role of the gut microbiota in mediating the positive effects of fucose. Wnt signaling is also a mediator of secretory lineage cell differentiation.Citation39 In this study, a Wnt inhibitor effectively suppressed fucose-induced increase in proliferation of ISCs and differentiation of secretory cells, indicating that fucose accelerates ISCs-mediated intestinal epithelial development in a Wnt signal-dependent manner.
There are still limitations in the current study. On the one hand, we mainly focused on the effects of metabolites on ISCs. The interaction of fucose, Akkermansia muciniphila, ISCs, and intestinal epithelium was worthy of further study using organoid models combined with microinjection. On the other hand, further studies on the colon could perfect this study on the overall gut.
In conclusion, our results indicate a positive role of fucose in ISCs-mediated intestinal epithelial development by regulating gut microbes and metabolism, especially Akkermansia and propanoate metabolism. This study may provide new insights into the promotion of gut homeostasis and the application potential of fucose as a prebiotic.
Materials and methods
Animal experiments
Mice
C57BL/6 mice were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd., and Lgr5-EGFP-IRES-CreERT2 mice were purchased from The Jackson Laboratory and housed in a specific pathogen-free (SPF) grade facility of Huazhong University of Science and Technology. Mice had free access to food and water and were maintained under 12 h light/dark cycles. The animal experiments in this study were approved by the Animal Experimentation Ethics Committee of Huazhong University of Science and Technology (Approval ID 2020–2529).
Animal models
To investigate the effects of fucose on the development of intestinal epithelium, we administrated 4 weeks old mice were administered L-fucose (Sigma-Aldrich) daily for 4 weeks (250 mg/kg gavage). An antibiotic cocktail (200 mg/kg ampicillin, 200 mg/kg metronidazole, 200 mg/kg neomycin, and 100 mg/kg vancomycin) was used for 7 days to generate germ-free mice to evaluate the role of microbiota, as described previously.Citation40 Also, 5 mg/kg Wnt-c59 (MedChemExpress) was orally administered every day as a Wnt signaling pathway inhibitor from day 12 as described previously.Citation41 The mice were sacrificed on day 28, and tissues were collected for further experiments.
Histological examination
For histological examination, ileum specimens were fixed in 4% paraformaldehyde, embedded in paraffin, and stained with hematoxylin and eosin (H&E) after slicing. Villus height and crypt depth of the ileum were measured by ImageJ software from the villus tip to the crypt-villus junction and from the crypt-villus junction to the bottom of the crypt, respectively.
Paneth cell and goblet cell number count
Ileum specimens were fixed in Carnoy’s solution, embedded in paraffin, and stained using PAS staining methods. Paneth and goblet cell numbers were counted in a blinded manner.
Crypts isolation and organoids culture
The small intestines of the mice were isolated, washed with cold PBS, and cut into sections. To isolate crypts, the tissue sections were incubated with 2.5 mM EDTA at 4°C for 40 min. The supernatant was removed, and the tissues were suspended in cold PBS containing 0.1% BSA. The samples were then filtered using a 70 μm cell strainer and centrifuged at 290 g for 5 min. Then, the samples were washed with a cold PBS containing 0.1% BSA and centrifuged at 200 g for 5 min to wipe off single cells.
For organoid culture, 500 crypts per well were suspended in equal amounts of IntestiCult Organoid Growth Medium (Stemcell) and Matrigel (Corning), and 50 μL mixture was added to the center of 24-well plate. Extra medium was added after the domes solidified. The medium was replaced every 3 days. The surface areas and budding number of organoids were measured using ImageJ software (NIH) by analyzing several random field photos using an inverted microscope (Carl Zeiss).
Organoids treatment
To address whether fucose affects ISCs by regulating gut microbe-mediated metabolism, ileal contents were used to treat organoids as previously described.Citation16 Briefly, 100 mg of ileal contents was suspended in 1 ml serum-free DMEM/F12 medium (Gibco), vortexed for 1 h at 4°C, centrifuged at 4000 rpm for 10 min, and filtered using a 0.22 μm filter (Millipore). The organoids were treated with 0.01% ileal content. 0.1 μM Gpr41 and 3 mM Gpr43 antagonist β-hydroxybutyrate (Sigma-Aldrich) and GLPG0974 (Sigma-Aldrich) were used to inhibit Gpr41/Gpr43. One millimolar propionic acid (Sigma-Aldrich) and sodium propionate (Sigma-Aldrich) was used to examine the role of propionate metabolism in fucose-induced ISCs promotion. One hundred nanomolar Wnt-c59 was used to inhibit Wnt signaling.
Immunofluorescence and immunohistochemistry
For tissue immunofluorescence staining, paraffin sections of the ileum were hydrated, treated with citrate buffer (pH 6.0) for antigen retrieval, permeabilized with 0.3% Triton, blocked with 10% donkey serum, and incubated with the following primary antibodies and corresponding secondary antibodies (all 1:200 dilution): anti-Lysozyme antibody (Abcam, ab108508), anti-Muc2 antibody (Genetex, GTX100664), anti-chromogranin A antibody (Abcam, ab45179), anti-Ki67 antibody (Servicebio, GB111141), anti-GFP antibody (Abcam, ab290), anti-β-catenin antibody (Abclonal, A19657), UEA-I Rhodamine (Vector, RL-1062-2), Alexa Fluor 488 conjugated donkey anti-rabbit IgG (Antgene, ANT024S), Alexa Fluor 488 conjugated donkey anti-goat IgG (Antgene, ANT025S), and Alexa Fluor 594 conjugated donkey anti-rabbit IgG (Antgene, ANT030S). Nuclei were stained with DAPI.
For organoid immunofluorescence staining, the culture medium was removed and the wells were washed with PBS. Organoids were fixed with 4% paraformaldehyde, permeabilized with 0.3% Triton, blocked with 10% donkey serum, and incubated with the corresponding primary and secondary antibodies.
IHC staining of the ileum tissue was performed using a SABC-AP IHC kit for rabbit IgG (Boster Biological Technology Co., Ltd.) according to the manufacturer’s instructions. The primary antibody used was the anti-Olfm4 antibody (Cell Signaling, 39141).
RNA extraction and real-time PCR
Total RNA from tissues, crypts, and organoids was extracted using TRIzol reagent (Invitrogen), according to the manufacturer’s protocol. Reverse transcription was conducted using a HiScript II 1st Strand cDNA Synthesis Kit (Vazyme). Real-time RCP was performed using the Power SYBR Green Master Mix (Thermo Fisher Scientific) on a Roche LightCycler R480 system (Roche). The relative fold change in gene expression was calculated using the 2−ΔCT method normalized to GAPDH. The primers used are listed in Supplementary Table S1.
Western blot analysis
Total proteins from tissues and organoids were harvested using RIPA Lysis Buffer (Beyotime) with phenylmethyl sulfonyl fluoride (PMSF) protease inhibitor and phosphatase inhibitor. Samples were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes, followed by blocking with 5% skimmed milk for 1 h. The membranes were then incubated with primary antibodies and the corresponding secondary antibodies (all 1:1000 dilutions): anti-Lgr5 antibody (Abcam, ab75850), anti-Olfm4 antibody (Cell Signaling, 39141), anti-GAPDH antibody (Abclonal, AC001), anti-GPR41 antibody (Abclonal, A12636), anti-GPR43 antibody (Proteintech, 19952-1-AP), anti-β-tubulin (Abclonal, AC015), anti-Axin2 antibody (Proteintech, 20540-1-AP), anti-β-catenin (Abclonal, A19657), anti-Wnt3 antibody (Abclonal, A9328), and HRP conjugated Goat anti-rabbit IgG (Antgene, 72-8067). The bands were detected using a chemiluminescence imaging system (UVP) with ECL chemiluminescence detection kit (Vazyme). The relative fold change in protein expression was normalized to that of GAPDH or β-tubulin.
EdU assays
For the mouse intestine proliferation assessment, 50 μg EdU (Beyotime) in PBS was injected intraperitoneally into mice for 2 h or 24 h prior to sacrifice. The click reaction was performed following the manufacturer’s instructions. The migration distance of the fastest EdU labeled cells was calculated and measured from the bottom of the crypts.Citation42
For organoid EdU assays, EdU was added to the culture medium at 10 μM concentration and incubated for 2 h at 37°C, fixed with 4% paraformaldehyde, permeabilized with 0.3% Triton, and click reactions were conducted following the manufacturer’s instructions.
16S rDNA sequencing
Microbial DNA was extracted from the ileal contents and tested for purity and integrity. The 341F and 806R primers with good specificity and high coverage were used to amplify the 16S rDNA regions (V3–V4). After quality control, the samples were subjected to Illumina high-throughput sequencing (Illumina) for 16S rDNA sequencing. Microbial diversity was reflected by α diversity (determined by observed species, Chao1, ACE, Shannon, Simpson, and J indices) and β diversity (visualized by PCoA analysis). The microbial composition was analyzed. Linear discriminant analysis and Effect Size (LEfSe) analysis were performed to detect significantly different species. KEGG metabolic pathway analysis was performed to explore the potential microbe-mediated metabolic alterations.
Untargeted metabolomics analysis
For untargeted metabolomics analysis, metabolites were extracted from ileal contents using equal amounts of methanol and acetonitrile with isotope labeling internal standard mixture. Untargeted metabolic profiling was performed by liquid chromatography-mass spectrometry (LC-MS) using a Vanquish ultra-performance liquid chromatograph (Thermo Fisher Scientific) and Thermo Q Exactive HFX mass spectrometer (Thermo Fisher Scientific). Different metabolites were screened by combining the fold changes and VIP values. Spearman’s correlation analysis was used to analyze the correlation between the bacteria and metabolites.
Bacteria culture
Akkermansia muciniphila (ATCC BAA-835) was purchased from Wuhan Research Institute of the First Light Industry (HBCICC59004). Bacteria were cultured in a Brain-Heart Infusion (BHI) culture medium under anaerobic conditions. To investigate the effects of fucose on SCFAs metabolism, 1 mM or 5 mM fucose was added to the medium. The bacteria and culture supernatants were collected by centrifugation at 6000 rpm for 15 min. To analyze the effects of Akk metabolites on organoids, the supernatant was centrifuged at 12,000 rpm for 10 min and filtered through 0.22 μm filters and bacterial culture supernatant was added to the medium at a final concentration of 0.1%.
SCFAs-targeted metabolomics analysis
Targeted metabolomic analysis was used to examine the concentration of SCFAs in the culture supernatant of Akk. Briefly, metabolites were extracted from the supernatant samples with 0.5% phosphoric acid, ethyl acetate, and 4-methylpentanoic acid, and tested by gas chromatography-mass spectrometry (GC-MS). Samples were separated using a DB-FFAP capillary column GC system (Agilent) and analyzed using a 5977 B MSD MS (Agilent). Data were analyzed using MSD ChemStation software.
Statistical analysis
Statistical analysis was performed using SPSS 25 and visualized using the GraphPad Prism software (version 7.0). Data are presented as the mean ± SEM. Differences between groups were analyzed using two-tailed t-test or one-way ANOVA followed by Tukey’s post hoc test. Statistical analysis of histology studies and immunostaining was using nested analysis. *P < 0.05, **P < 0.01 and ***P < 0.001 were considered statistically significant.
Author contribution
Caihan Duan and Junhao Wu contributed equally to this study. Caihan Duan performed the experiments, analyzed the data, and drafted the manuscript; Junhao Wu performed some of the experiments and analyzed the data. Zhe Wang and Chen Tan helped perform part of the experiments. Lingzhi Hou and Wei Qian supported the data evaluation; Chaoqun Han and Xiaohua Hou designed the study, revised the manuscript, provided funding, and obtained grants.
Supplemental Material
Download MS Word (12.4 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and analyzed during the current study are available in Science Data Bank (ScienceDB, https://www.scidb.cn/, DOI: 0.57760/sciencedb.07487) upon reasonable request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19490976.2023.2233149
Additional information
Funding
References
- Gehart H, Clevers H. Tales from the crypt: new insights into intestinal stem cells. Nat Rev Gastroenterol Hepatol. 2019;16(1):19–19. doi:10.1038/s41575-018-0081-y.
- Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154(2):274–284. doi:10.1016/j.cell.2013.07.004.
- Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15(1):19–33. doi:10.1038/nrm3721.
- van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71(1):241–260. doi:10.1146/annurev.physiol.010908.163145.
- Goto Y, Uematsu S, Kiyono H. Epithelial glycosylation in gut homeostasis and inflammation. Nat Immunol. 2016;17(11):1244–1251. doi:10.1038/ni.3587.
- Pickard JM, Chervonsky AV. Intestinal fucose as a mediator of host–microbe symbiosis. J Immunol. 2015;194(12):5588–5593. doi:10.4049/jimmunol.1500395.
- Li Y, Jiang Y, Zhang L, Qian W, Hou X, Lin R. Exogenous l-fucose protects the intestinal mucosal barrier depending on upregulation of FUT2-mediated fucosylation of intestinal epithelial cells. The FASEB J. 2021;35(7):e21699. doi:10.1096/fj.202002446RRRR.
- He R, Li Y, Han C, Lin R, Qian W, Hou X. L-Fucose ameliorates DSS-induced acute colitis via inhibiting macrophage M1 polarization and inhibiting NLRP3 inflammasome and NF-Kb activation. Int Immunopharmacol. 2019;73:379–388. doi:10.1016/j.intimp.2019.05.013.
- Ke J, Li Y, Han C, He R, Lin R, Qian W, Hou X. Fucose ameliorate intestinal inflammation through modulating the crosstalk between bile acids and gut microbiota in a chronic colitis murine model. Inflamm Bowel Dis. 2020;26(6):863–873. doi:10.1093/ibd/izaa007.
- Markandey M, Bajaj A, Ilott NE, Kedia S, Travis S, Powrie F, Ahuja V. Gut microbiota: sculptors of the intestinal stem cell niche in health and inflammatory bowel disease. Gut Microbes. 2021;13(1):1990827. doi:10.1080/19490976.2021.1990827.
- Riehl TE, Alvarado D, Ee X, Zuckerman A, Foster L, Kapoor V, Thotala D, Ciorba MA, Stenson WF. Lactobacillus rhamnosus GG protects the intestinal epithelium from radiation injury through release of lipoteichoic acid, macrophage activation and the migration of mesenchymal stem cells. Gut. 2019;68(6):1003–1013. doi:10.1136/gutjnl-2018-316226.
- Lee YS, Kim TY, Kim Y, Lee S-H, Kim S, Kang SW, Yang J-Y, Baek I-J, Sung YH, Park Y-Y, et al. Microbiota-derived lactate accelerates intestinal stem-cell-mediated epithelial development. Cell Host Microbe. 2018;24(6):833–846.e6. doi:10.1016/j.chom.2018.11.002.
- Turroni F, Milani C, Duranti S, Mahony J, van Sinderen D, Ventura M. Glycan utilization and cross-feeding activities by bifidobacteria. Trends Microbiol. 2018;26(4):339–350. doi:10.1016/j.tim.2017.10.001.
- Ottman N, Davids M, Suarez-Diez M, Boeren S, Schaap PJ, Martins dos Santos VAP, Smidt H, Belzer C, de Vos WM, et al. Genome-scale model and omics analysis of metabolic capacities of Akkermansia muciniphila reveal a preferential mucin-degrading lifestyle. Appl Environ Microbiol. 2017;83(18). doi:10.1128/AEM.01014-17.
- Kim J, Cheong YE, Jung I, Kim K. Metabolomic and transcriptomic analyses of Escherichia coli for efficient fermentation of L-Fucose. Mar Drugs. 2019;17(2):17. doi:10.3390/md17020082.
- Kim S, Shin YC, Kim TY, Kim Y, Lee Y-S, Lee S-H, Kim M-N, O E, Kim KS, Kweon M-N, et al. Mucin degrader Akkermansia muciniphila accelerates intestinal stem cell-mediated epithelial development. Gut Microbes. 2021;13(1):1–20. doi:10.1080/19490976.2021.1892441.
- Gehart H, van Es JH, Hamer K, Beumer J, Kretzschmar K, Dekkers JF, Rios A, Clevers H. Identification of enteroendocrine regulators by real-time single-cell differentiation mapping. Cell. 2019;176(5):1158–1173.e16. doi:10.1016/j.cell.2018.12.029.
- Kamioka M, Goto Y, Nakamura K, Yokoi Y, Sugimoto R, Ohira S, Kurashima Y, Umemoto S, Sato S, Kunisawa J, et al. Intestinal commensal microbiota and cytokines regulate Fut2 + Paneth cells for gut defense. Proc Natl Acad Sci USA. 2022;119(3):119. doi:10.1073/pnas.2115230119.
- Tan C, Hong G, Wang Z, Duan C, Hou L, Wu J, Qian W, Han C, Hou X. Promoting effect of L-Fucose on the regeneration of intestinal stem cells through AHR/IL-22 pathway of intestinal lamina propria monocytes. Nutrients. 2022;14(22):14. doi:10.3390/nu14224789.
- Borisova MA, Snytnikova OA, Litvinova EA, Achasova KM, Babochkina TI, Pindyurin AV, Tsentalovich YP, Kozhevnikova EN. Fucose ameliorates tryptophan metabolism and behavioral abnormalities in a mouse model of chronic colitis. Nutrients. 2020;12(2):12. doi:10.3390/nu12020445.
- Kirmiz N, Galindo K, Cross KL, Luna E, Rhoades N, Podar M, Flores GE. Comparative genomics guides elucidation of vitamin B 12 biosynthesis in novel human-associated Akkermansia strains. Appl Environ Microbiol. 2020;86(3). doi:10.1128/AEM.02117-19.
- Sivaprakasam S, Prasad PD, Singh N. Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacology Therapeutics. 2016;164:144–151. doi:10.1016/j.pharmthera.2016.04.007.
- Parada Venegas D, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, Harmsen HJ, Faber KN, Hermoso MA. Short Chain Fatty Acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019;10:277. doi:10.3389/fimmu.2019.00277.
- Goto Y, Obata T, Kunisawa J, Sato S, Ivanov II, Lamichhane A, Takeyama N, Kamioka M, Sakamoto M, Matsuki T, et al. Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science. 2014;345(6202):1254009. doi:10.1126/science.1254009.
- Garber JM, Hennet T, Szymanski CM. Significance of fucose in intestinal health and disease. Mol Microbiol. 2021;115(6):1086–1093. doi:10.1111/mmi.14681.
- Li M, Bai Y, Zhou J, Huang W, Yan J, Tao J, Fan Q, Liu Y, Mei D, Yan Q, et al. Core fucosylation of maternal milk N-Glycan Evokes B cell activation by selectively promoting the l-Fucose metabolism of gut bifidobacterium spp. and lactobacillus spp. mBio. 2019;10(2):10. doi:10.1128/mBio.00128-19.
- Litvinova EA, Bets VD, Feofanova NA, Gvozdeva OV, Achasova KM, Alperina EL, Kozhevnikova EN. Dietary fucose affects macrophage polarization and reproductive performance in mice. Nutrients. 2021;13(3):855. doi:10.3390/nu13030855.
- Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol. 2004;54(5):1469–1476. doi:10.1099/ijs.0.02873-0.
- Zhai Q, Feng S, Arjan N, Chen W. A next generation probiotic, Akkermansia muciniphila. Crit Rev Food Sci Nutr. 2019;59(19):3227–3236. doi:10.1080/10408398.2018.1517725.
- Ottman N, Geerlings SY, Aalvink S, de Vos WM, Belzer C. Action and function of Akkermansia muciniphila in microbiome ecology, health and disease. Best Pract Res Clin Gastroenterol. 2017;31(6):637–642. doi:10.1016/j.bpg.2017.10.001.
- Wacklin P, Tuimala J, Nikkilä J, Tims S, Mäkivuokko H, Alakulppi N, Laine P, Rajilic-Stojanovic M, Paulin L, de Vos WM, et al. Faecal microbiota composition in adults is associated with the FUT2 gene determining the secretor status. PLoS One. 2014;9(4):e94863. doi:10.1371/journal.pone.0094863.
- Wang JH, Bose S, Kim HG, Han K-S, Kim H. Fermented Rhizoma Atractylodis Macrocephalae alleviates high fat diet-induced obesity in association with regulation of intestinal permeability and microbiota in rats. Sci Rep. 2015;5(1):8391. doi:10.1038/srep08391.
- Lukovac S, Belzer C, Pellis L, Keijser BJ, de Vos WM, Montijn RC, Roeselers G. Differential modulation by Akkermansia muciniphila and Faecalibacterium prausnitzii of host peripheral lipid metabolism and histone acetylation in mouse gut organoids. mBio. 2014;5(4). doi:10.1128/mBio.01438-14.
- Yao Y, Cai X, Fei W, Ye Y, Zhao M, Zheng C. The role of short-chain fatty acids in immunity, inflammation and metabolism. Crit Rev Food Sci Nutr. 2022;62(1):1–12. doi:10.1080/10408398.2020.1854675.
- Willemsen LE, Koetsier MA, van Deventer SJ, Van Tol E.A. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts. Gut. 2003;52(10):1442–1447. doi:10.1136/gut.52.10.1442.
- Xia Z, Han Y, Wang K, Guo S, Wu D, Huang X, Li Z, Zhu L. Oral administration of propionic acid during lactation enhances the colonic barrier function. Lipids Health Dis. 2017;16(1):62. doi:10.1186/s12944-017-0452-3.
- Bilotta AJ, Ma C, Yang W, Yu Y, Yu Y, Zhao X, Zhou Z, Yao S, Dann SM, Cong Y, et al. Propionate enhances cell speed and persistence to promote intestinal epithelial turnover and repair. Cell Mol Gastroenterol Hepatol. 2021;11(4):1023–1044. doi:10.1016/j.jcmgh.2020.11.011.
- Bajic D, Niemann A, Hillmer AK, Mejias-Luque R, Bluemel S, Docampo M, Funk MC, Tonin E, Boutros M, Schnabl B, et al. Gut microbiota-derived propionate regulates the expression of Reg3 mucosal lectins and ameliorates experimental colitis in mice. J Crohns Colitis. 2020;14(10):1462–1472. doi:10.1093/ecco-jcc/jjaa065.
- Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17(14):1709–1713. doi:10.1101/gad.267103.
- Tang X, Wang W, Hong G, Duan C, Zhu S, Tian Y, Han C, Qian W, Lin R, Hou X, et al. Gut microbiota-mediated lysophosphatidylcholine generation promotes colitis in intestinal epithelium-specific Fut2 deficiency. J Biomed Sci. 2021;28(1):20. doi:10.1186/s12929-021-00711-z.
- Wu H, Xie S, Miao J, Li Y, Wang Z, Wang M, Yu Q. Lactobacillus reuteri maintains intestinal epithelial regeneration and repairs damaged intestinal mucosa. Gut Microbes. 2020;11(4):997–1014. doi:10.1080/19490976.2020.1734423.
- Yang L, Yang H, Chu Y, Song Y, Ding L, Zhu B, Zhai W, Wang X, Kuang Y, Ren F, et al. CREPT is required for murine stem cell maintenance during intestinal regeneration. Nat Commun. 2021;12(1):270. doi:10.1038/s41467-020-20636-9.

