ABSTRACT
The nuclear lamina (NL) changes composition for regulation of nuclear events. We investigated changes that occur in Drosophila oogenesis, revealing switches in NL composition during germ cell differentiation. Germline stem cells (GSCs) express only LamB and predominantly emerin, whereas differentiating nurse cells predominantly express LamC and emerin2. A change in LamC-specific localization also occurs, wherein phosphorylated LamC redistributes to the nuclear interior only in the oocyte, prior to transcriptional reactivation of the meiotic genome. These changes support existing concepts that LamC promotes differentiation, a premise that was tested. Remarkably ectopic LamC production in GSCs did not promote premature differentiation. Increased LamC levels in differentiating germ cells altered internal nuclear structure, increased RNA production, and reduced female fertility due to defects in eggshell formation. These studies suggest differences between Drosophila lamins are regulatory, not functional, and reveal an unexpected robustness to level changes of a major scaffolding component of the NL.
Introduction
Nuclear architecture depends on the nuclear lamina (NL), an extensive protein network that underlies the inner nuclear membrane [Citation1,Citation2]. The major scaffolding components of the NL are A- and B-type lamins that assemble into distinct interacting networks [Citation3]. B-type lamins are widely expressed and tightly associated with the nuclear membrane due to permanent farnesylation, whereas A-type lamins are largely expressed in differentiated cells and are localized to the nuclear periphery, and in some cases, in the nuclear interior [Citation1,Citation2,Citation4]. A- and B-type lamins are associated with different chromatin types. A-type lamins are preferentially associated with gene-rich active chromatin, whereas B-type lamins are preferentially associated with gene-poor repressive chromatin [Citation5,Citation6]. These observations suggest that differences in the developmental expression of lamins have functional consequences, with A-type lamins playing a major role in the regulation of cell-type-specific gene expression coincident with differentiation.
The organization of the NL is complex. Lamins interact with hundreds of proteins [Citation7]. Among the first identified lamin-interacting proteins are the Lap2, emerin, MAN1-domain (LEM-D) proteins, named for the three founding family members [Citation8]. These proteins share a LEM-D that interacts with Barrier-to-autointegration factor (BAF, sometimes referred to as BANF1), a small protein that binds histones, lamins, and double-strand DNA in a sequence-independent manner [Citation9,Citation10]. Interactions between lamins, LEM-D proteins, and BAF build the nuclear architecture by anchoring chromatin at the nuclear periphery, thereby contributing to nuclear organization that is important for transcriptional regulation, cell signaling, mechanotransduction, DNA replication, and repair [Citation11–13]. Strikingly, deformation of the NL is a prominent feature of cancer and premature aging diseases, suggesting that maintenance of the nuclear architecture is important for cellular function and fitness [Citation14–18].
Normal development is accompanied by dramatic morphological changes in nuclear structure. The Drosophila ovary serves as an excellent system for studying these changes. The ovary is divided into 16 to 20 ovarioles (), each of which carries an assembly line of advancing stages of oocyte maturation [Citation19,Citation20]. Oogenesis begins in the germarium, a specialized structure that anchors two to three undifferentiated germline stem cells (GSCs) to the somatic cells of the niche. GSCs are noted for their large nuclear size and the presence of unpaired chromosomes that are organized around the NL, an arrangement not found in other cell types [Citation21,Citation22]. Asymmetric GSC divisions produce one germ cell that self-renews and one cystoblast (CB) that commits to differentiation. CBs enter four incomplete, synchronous mitotic divisions to form an interconnected 16-cell syncytium, with these germ cells carrying small, similarly sized nuclei. Within this syncytium, meiosis initiates, ultimately producing one oocyte arrested in meiotic prophase and 15 nurse cells (NCs [Citation23]). Somatic follicle cells surround the differentiating germ cells to form egg chambers (ECs) that leave the germarium. Fourteen distinct EC stages were characterized based on morphology, including prominent changes in nuclear structure (). In Stage 2 (S2) ECs, chromosomes in the oocyte nucleus begin to condense to form a karyosome, a dense chromatin aggregate that protects the meiotic chromosomes, confers transcriptional silencing, and ensures faithful chromosome segregation [Citation24,Citation25]. This nuclear structure remains until S9, when oocyte chromosomes slightly decondense, to permit a brief period of transcriptional reactivation [Citation26]. In contrast to oocyte nuclei, the NC nuclei in S2 ECs enter a developmental endocycle program, in which genome replication occurs without cytokinesis [Citation19,Citation27]. As a result, NC nuclei become polyploid and grow large to increase their synthetic capacity. Indeed, after S10, the nuclear content of NCs is transferred to the oocyte to provide the oocyte with the RNA and proteins needed for embryogenesis. Taken together, these observations highlight the fact that germ cell nuclei undergo extensive physical changes during differentiation.
Figure 1. Nuclear structure and nuclear lamina change during oogenesis. a. Shown is a schematic of an ovariole that carries advancing stages of oocyte maturation. At the anterior tip ovariole (left) is a germarium that contains the stem cell niche. Within the germarium, germline stem cells (GSCs) undergo asymmetric mitotic divisions producing one renewing and one differentiating daughter. Differentiation begins with four synchronous mitotic divisions that lead to the formation of a sixteen-cell cyst, comprised of fifteen nurse cells and one oocyte. In the posterior end of the germarium, the sixteen-cell cyst becomes encased by a monolayer of somatic follicle cells to form a Stage1 (S1) egg chamber (EC) that buds off from the germarium as a S2 EC. Continued differentiation is divided into morphologically distinct stages (up to S9 are shown), distinguished by the size and organization of NC (white circles) and oocyte nuclei (blue circles). The growing oocyte (black) is at the posterior of each EC. b. Confocal images of germaria, S6 and S9 ECs stained with antibodies against the B-type lamin (LamB) and the A-type lamin (LamC). White and yellow arrowheads indicate GSCs and NCs, respectively. White arrows indicate the position of the oocyte nucleus. Stages of EC development were based on morphology as described in [Citation64]. Scale bars, 5 µm. c., d. Shown are maximal projection confocal images of oocyte nuclei in a S6 and S9 ECs stained with antibodies N-terminal phospho-serine antibody (NT pSer, green) merged with a single Z-section image stained with antibodies against either LamB (white, c) or LamC (white, d). Scale bars, 5 µm.
![Figure 1. Nuclear structure and nuclear lamina change during oogenesis. a. Shown is a schematic of an ovariole that carries advancing stages of oocyte maturation. At the anterior tip ovariole (left) is a germarium that contains the stem cell niche. Within the germarium, germline stem cells (GSCs) undergo asymmetric mitotic divisions producing one renewing and one differentiating daughter. Differentiation begins with four synchronous mitotic divisions that lead to the formation of a sixteen-cell cyst, comprised of fifteen nurse cells and one oocyte. In the posterior end of the germarium, the sixteen-cell cyst becomes encased by a monolayer of somatic follicle cells to form a Stage1 (S1) egg chamber (EC) that buds off from the germarium as a S2 EC. Continued differentiation is divided into morphologically distinct stages (up to S9 are shown), distinguished by the size and organization of NC (white circles) and oocyte nuclei (blue circles). The growing oocyte (black) is at the posterior of each EC. b. Confocal images of germaria, S6 and S9 ECs stained with antibodies against the B-type lamin (LamB) and the A-type lamin (LamC). White and yellow arrowheads indicate GSCs and NCs, respectively. White arrows indicate the position of the oocyte nucleus. Stages of EC development were based on morphology as described in [Citation64]. Scale bars, 5 µm. c., d. Shown are maximal projection confocal images of oocyte nuclei in a S6 and S9 ECs stained with antibodies N-terminal phospho-serine antibody (NT pSer, green) merged with a single Z-section image stained with antibodies against either LamB (white, c) or LamC (white, d). Scale bars, 5 µm.](/cms/asset/3d112e07-38bc-44d9-88aa-53f6653de77d/kncl_a_2339214_f0001_oc.jpg)
Although extensive nuclear remodeling occurs during oogenesis, the mechanism by which the NL influences these processes is poorly understood. To address this deficiency, we defined the dynamic expression and localization patterns of lamin and the NL LEM-D proteins during oogenesis. Drosophila encodes two lamins (Table S1): the widely expressed B-type lamin (Lamin B also known as Lamin Dm0) that carries a C-terminal CaaX motif for farnesylation that confers integration into the inner nuclear membrane, and the differentiation-associated A-type lamin (Lamin C, LamC) that lacks the C-terminal CaaX motif [Citation28]. Genes encoding for both lamins are essential [Citation29,Citation30]. Hypomorphic lam (lamB) alleles have been identified, but none have been reported for lamC. The few surviving lamB mutant females are sterile [Citation31–33], demonstrating that LamB is required for oogenesis. The Drosophila genome encodes three NL LEM-D proteins that display different requirements during oogenesis (Table S1 [Citation34–37]). These include two emerin orthologs (emerin also known as Otefin and emerin2 also known as Bocksbeutel) and MAN1 [Citation38]. Whereas emerin loss causes GSC death linked to the non-canonical activation of the DNA damage response pathway [Citation39], emerin2 loss does not affect fertility [Citation37]. Strikingly, MAN1 loss reduces fertility owing to increased EC death [Citation34,Citation37]. Although all three Drosophila LEM-D proteins bind BAF [Citation34], emerin serves as the major regulator of BAF localization to the NL [Citation40] and similar to emerin, survival of GSCs requires BAF [Citation40]. These data indicate that NL proteins have distinct functional contributions during oogenesis.
In this study, we used immunohistochemical and genetic analyzes to define properties of the NL during oogenesis. We showed that in GSCs, the NL contains only LamB and predominantly emerin, whereas in differentiating NCs, the NL switches to one comprised predominantly of LamC and emerin2. Furthermore, we revealed a phosphorylation-associated change in the nuclear localization of LamC during EC development. In the early EC stages, LamC is restricted to the NL in NC and oocyte nuclei; however, in the later stages, LamC moves into the nuclear interior only in the oocyte nucleus, coinciding with its phosphorylation. These changes support the long-standing perception that Drosophila LamC plays a critical role in differentiation [Citation41]. To test this hypothesis, we used the GAL4-UASp system to ectopically express LamC during oocyte development. Surprisingly, GSCs did not prematurely differentiate, despite changing their internal nuclear structure, demonstrating that LamC expression is insufficient to promote germ cell differentiation. We conclude that wild type exclusion of LamC from GSCs reflects a transcriptional, not functional, difference from LamB in germ cell development. In later stages of oogenesis, LamC overproduction caused modest changes in nuclear size and structure, increased NC transcription, and reduced female fertility. Strikingly, we found that reduced hatching was caused by defects in eggshell formation, as evidenced by the restoration of hatching upon eggshell removal. Offspring produced from nos>lamC eggs developed into morphologically normal adults, indicating that overexpression of LamC throughout oogenesis did not affect offspring development. Together, these studies advance an understanding of how LamC contributes to nuclear functions during Drosophila oogenesis and reveal unexpected robustness in EC development to changes in the levels of this major NL scaffolding protein.
Materials and methods
Drosophila stocks and culture conditions
All Drosophila stocks were raised at room temperature (22°C) on cornmeal/agar food that contained p-hydroxybenzoic acid methyl ester as a mold inhibitor. In this study y1, w67c23 corresponds to the wild type reference control. A transgenic responder line was generated, wherein the A-type lamin, lamin C (RA) cDNA was inserted downstream of the UASp promoter in a P element vector that carries the mini-white transformation reporter and an attB site. Transgenic flies were generated by Bestgene, using the PhiC31 integration system to insert transgenes into the attP2 site on chromosome 3. The germline driver used in these studies was P[GAL4:VP16-nos] inserted on the third chromosome (obtained from Bloomington # 4937). Crosses between driver and responder animals were conducted at room temperature (22°C). Three CRISPR-generated endogenously tagged gfp-NL genes were used, lines that were made for the Geyer laboratory: gfp-baf [Citation40] was generated by Bestgene, and gfp-emerin2 and gfp-dman1 were generated by Fungene [Citation42].
Western analysis
LamC levels were assessed using proteins extracted from ovaries dissected from newly enclosed or 1- to 3-day-old females (Fig. S1C). Proteins were electrophoresed on a 4–12% gradient Tris gel and blotted onto a nitrocellulose membrane. Membranes were cut in between the 50- and 75-kD bands of the protein ladder. The top part of the membrane was probed with primary antibodies against LamC (mouse anti-LamC at 1:1000, DSHB LC28.26) or rabbit monoclonal anti-phospho-lamin A/C Ser22 at 1:1000 (Cell Signaling 13448T), referred to as N-terminal (NT) pSer, which recognizes the phosphorylation of Ser37 in Drosophila LamC [Fig. S1A [Citation43]]. The bottom part of the membrane was probed with α-Tubulin (mouse 1:2000, Sigma, T5168). Primary antibodies were detected using fluor-conjugated secondary antibodies (donkey anti-mouse 680, 1:10,000 LI-COR; donkey anti-rabbit 800 1:10,000 Invitrogen; donkey anti-mouse 800, 1:10,000 LI-COR). Western blots were imaged using LI-COR Odyssey CLx and quantified using ImageStudioLite (v5.2.5, LI-COR software, Lincoln, NE, USA). LamC protein levels were normalized to α-Tubulin levels and made relative to the level of LamC in the newly eclosed wild type sample.
Immunohistochemical analysis
Expression of multiple NL proteins were defined during oogenesis (Table S1). In these studies, ovaries were dissected from newly eclosed (<1-day) females in cold phosphate-buffered saline (PBS) solution, as described in [Citation42]. Briefly, dissected ovaries were fixed at room temperature in 4% EM Grade paraformaldehyde (Electron Microscopy Sciences no. 15710), washed in PBST (PBS with 0.3% Triton100) and blocked in 5% w/v BSA in PBST at room temperature for 1 h. Primary antibodies were incubated overnight at 4°C. Subsequently, tissues were washed in PBST three times each for 10 min and incubated with Alexa Fluor-conjugated secondary antibodies (Molecular Probes) at room temperature for 2 h. After additional washes in PBST, tissues were mounted in SlowFade (Thermo Fisher). Confocal images were collected with a Zeiss 710 or Leica SP8 confocal microscope and processed using the ImageJ imaging software (v1.54f; National Institutes of Health). The primary antibodies included 1) mouse anti-Lamin B at 1:300 (DSHB ADL84.12); 2) mouse anti-Lamin C at 1:200 (DSHB LC28.26); 3) rabbit monoclonal anti-phospho-lamin A/C Ser22 at 1:200 (Cell Signaling 13448T); 4) goat anti-emerin/Otefin at 1:300 [Citation37]; 5) goat anti-GFP at 1:2000 (Abcam 6673); 6) rat anti-Vasa at 1:50 (DSHB); and 7) rabbit anti-Fibrillarin at 1:500 (Abcam 5821). In some experiments, the NL was stained with Alexa Fluor Wheat Germ Agglutinin (WGA) at 1:500 (Thermo Fisher W32466). For each immunohistochemical analysis, germaria were imaged from at least five ovaries obtained from at least two biological replicates.
Image quantification
The impact of altered NL structures on the nuclear architecture was measured using two methods. First, the nucleolar structure was analyzed by staining for Fibrillarin and measuring the area of the signal using ImageJ software. In this analysis, a maximum projection tool was used to collapse ~five Z-stacks. Marked foci were traced along their surfaces and areas of the foci were recorded. Second, NT pSer levels were quantified from a summed Z-projection with the background subtracted using ImageJ (50 pixel rolling ball radius).
Quantification of nascent RNA
Five to ten ovaries were dissected in Grace’s medium from control or nos>lamC females, transferred to fresh medium and incubated for 10 min prior to the addition of 2 mM 5-ethynyl uridine (EU). For experiments testing the specificity of EU incorporation, the RNA polymerase inhibitor Actinomycin D (final concentration 20 μg/ml) was incubated with ovaries for a period of 60 min, after which ovaries were washed twice with Grace’s medium and fixed for 15 min in 4% paraformaldehyde diluted in Grace’s medium. After fixation, ovaries were washed six times for 10 min each with a Triton Wash (1X PBS, 0.5% Triton X-100), rinsed three times with 1X PBS, and incubated for 30 min in the Click-iT reaction cocktail, prepared according to manufacturer’s specifications (Thermo Fisher Scientific, Click-iT™ RNA Alexa Fluor™ 594 Imaging Kit, C10330). Ovaries were rinsed twice with Click-iT™ Reaction Rinse Buffer and twice with 1X PBS, and then washed for 30 min in 1X PBS. Ovaries were stained with Hoechst 33,342 (1:1000 in PBS) for 15 min to label DNA, and then rinsed three times in 1X PBS before mounting.
Fluorescence images were obtained using a Zeiss 710 confocal microscope. Images were processed using the ImageJ software. An image of an entire S9 EC was acquired. The stages of ECs development were defined using standard criteria [Citation44,Citation45]. Slices corresponding to individual oocytes or mid-chamber NCs were stacked into a single maximum-intensity Z-projection plane using ImageJ software. Data from three NCs were averaged for each EC. The chromatin in each cell type was outlined, and the mean fluorescence value was recorded in arbitrary units (A.U.). A background reading was obtained by determining the fluorescence of a similarly sized cytoplasmic region. For each cell, the relative signals were calculated as the fluorescence intensity of the nuclear signal minus the fluorescence intensity of its corresponding background. The chromatin size of NC and oocyte nuclei was also measured using ImageJ. Four independent experiments were conducted.
Fecundity and hatching assays
To determine female fecundity, embryo collection bottles were established that contained five < 1-day-old females of each tested genotype and three wild type males. Bottles were covered with orange juice agar egg collection plates (90% orange juice, 3.6% agar, 1% ethyl acetate) that had a small stub of wet yeast paste placed at the center. Plates were changed every 24 h. Every 2- to 3-days, the males were replaced with new 2- to 3-day-old males. For counting, eggs were gently washed off plates and collected on a nylon mesh. Fecundity is reported as the number of eggs laid per female per day. If a female died during the assay, it was assumed that the female died immediately before changing the egg collection plate. Three replicates were performed.
Embryos were transferred from orange juice collection plates to glass slides, to determine the viability of the eggs produced by females. Slides were placed directly on wet paper towels and placed in a humidified 25°C incubator. Alternatively, the eggs were first dechorionated for three minutes in 50% bleach, washed well and then transferred to glass slides, and placed in a humidified 25°C incubator. At least 90 embryos were transferred for each experiment. After 30 to 40 h, the number of hatched larvae was counted and recorded. To define the sex of adults emerging from nos>lamC fertilized eggs, 50 larvae of each genotype were transferred into food vials and allowed to develop into adulthood at 25°C. The morphology and sex of the emerged adults were recorded. Experiments were repeated at least three times.
Assessment of embryonic development
To understand the effects on embryonic development, a 24-h collection of eggs laid on orange juice plates with 3% agar were obtained. Eggs were dechorionated in 50% bleach, rinsed with water, and moved to fixative (17.5% 1× PBS, 2.5% of 37% formaldehyde, and 80% heptane) for 20 min at room temperature in a scintillation vial. The formaldehyde layer was removed, and 10 mL of heptane was added. Each vial was shaken vigorously to remove the eggshells. The released embryos fell to the bottom of the vial. These embryos were transferred to a 2 mL Eppendorf tube and washed three times for ten min each in methanol (MeOH) at room temperature. Embryos were rehydrated with successive 5 min washes in 75%, 50%, and 25% MeOH diluted in PBST, then washed three times for 15 min each in PBST. During the second wash, DAPI (1:500 mg/ml) was added to the PBST. PBST was removed, and Vectashield mounting media was added and equilibrated overnight at 4C° before mounting. Confocal images were collected with a Zeiss 710 confocal microscope and processed using the ZEN or ImageJ imaging software.
Scanning electron microscopy
Embryos were collected on orange juice plates with 3% agar, washed with distilled water and fixed overnight in 2.5% glutaraldehyde, 2% paraformaldehyde in 0.1 M cacodylate buffer pH 7.2. Embryos were washed with 0.1 M Na cacodylate pH 7.2 three times for 5 min each. After staining, embryos were rinsed in ddH2O three times for 5 min, washed with 6% thiocarbohydrazide, filtered, and rinsed again with ddH2O for 5 min each. Again, embryos were stained with 1%OsO4 in 0.1 M cacodylate for 30 min before rinsing twice in ddH2O. Next, embryos were rinsed and dehydrated in a series of ethanol washes (20%, 50%, 75%, 94%, and 100%), followed by critical point drying. Embryos were mounted on stubs, coated with gold-palladium using a Polaron E5100 sputter coater and imaged using a Hitachi S-4800 scanning electron microscope.
Results
Drosophila lamins show different expression patterns during oogenesis
The structure of germ cell nuclei changes dramatically during oogenesis. As a first step in understanding the role of the NL in nuclear structure and organization, we studied the expression patterns of LamC and LamB, the Drosophila A- and B-type lamins, respectively. Our analyzes began in the germarium and ended in S9 ECs, a developmental stage selected because it occurs just prior to NC apoptosis [Citation46,Citation47]. Ovaries were dissected from ~ 1-day-old wild type females and stained with antibodies against LamB and LamC (). Confocal microscopy revealed that LamB levels were high in GSCs and throughout the germarium (). These levels declined as the EC matured. In S9 ECs, LamB levels were undetectable in NC nuclei and only faintly detected in oocyte nuclei (). In contrast, LamC levels were undetectable in GSCs and developing cysts and increased once ECs emerged from the germarium (). LamC remained in the NC and oocyte nuclei of maturing ECs and was still detectable in S9 ECs, although its levels appeared lower (). These data demonstrate that Drosophila lamins display distinct accumulation patterns within developing germ cells, with LamC expression correlated with differentiation.
Table 1. Germ cell localization pattern of Drosophila NL proteins during oogenesis.
The two Drosophila lamins displayed contrasting nuclear localization. LamB remained in the NL of all germ cells throughout oogenesis, whereas LamC only remained in the NL of NCs. Notably, in the oocyte nucleus, LamC re-localized to the nuclear interior as an EC matured from S6 to S9 (; ). The disassembly of lamin networks is linked to lamin phosphorylation [Citation48,Citation49]. For this reason, we examined the phosphorylation status of LamC, using a previously characterized phospho-specific antibody that recognizes the phosphorylation of a conserved amino-terminal serine (Fig. S1A [Citation43]). Western blot analysis of ovarian extracts using this antibody revealed a single band corresponding to LamC (Fig. S1B). Staining wild type ovaries with the phospho-serine antibody identified foci within the oocyte nuclear interior, which increased in level as nuclei advanced from S6 to S9 (). Phospho-serine staining was not observed in NC nuclei. ECs co-stained with antibodies against LamB and the phospho-specific antibody showed no overlap, in contrast to the strong overlap between LamC and the phospho-specific antibody in S9 ECs (Fig. S1C). These findings imply that although both lamins carry a conserved N-terminal phosphorylation site, phosphorylation is restricted to LamC. Based on these data, we suggest that LamC undergoes developmental phosphorylation, leading to its disassembly and redistribution into the nuclear interior of oocytes.
Expression patterns of LEM-D proteins are distinct
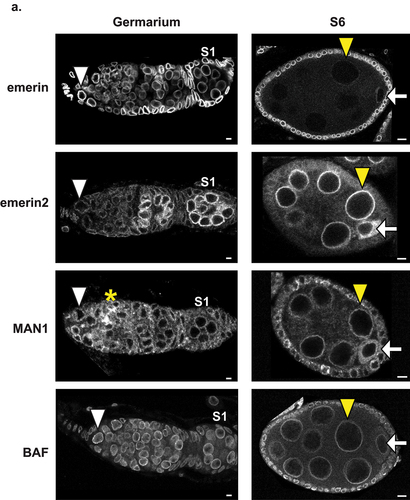
LEM-D protein expression was defined during oogenesis. For emerin, wild type ovaries were dissected and stained with antibodies against emerin. For emerin2 and MAN1, ovaries were dissected from females carrying endogenous alleles that encode N-terminally GFP-tagged proteins (gfp-emerin2 and gfp-man1) and stained with antibodies against GFP. Immunohistochemical analysis of wild type ovaries revealed that emerin levels were high in GSCs, decreased as ECs formed, and were undetectable in NCs of S6 and older ECs (, Fig.S2; ). In contrast, immunohistochemical analysis of gfp-emerin2 ovaries revealed that emerin2 expression was low in GSCs but increased during EC maturation. In early germ cells, emerin2 was predominantly localized to the NL in nuclei of NCs and the oocyte, whereas in S6, increased cytoplasmic staining was found in oocytes (, ). Observations of non-NL emerin2 suggest that there is a change in the emerin2 isoform that is expressed, with early stages expressing the isoform carrying the transmembrane domain (TMD) and later stages expressing the isoform lacking the TMD [Citation50]. Immunohistochemical analysis of gfp-man1 ovaries revealed consistent, but low levels of expression in all germ cells (, ). In the germarium, MAN1 was localized to the NL and appeared to accumulate in the fusome, a branched structure that extends between germ cells in the cyst. In ECs, MAN1 is localized to the NL in NCs and to the cytoplasm in oocytes. Finally, we performed immunohistochemical analysis of gfp-baf ovaries. This LEM-D partner protein was found in all germ cells of the germarium, persisted in S6 NCs and was weak in S6 oocytes (, ). In S9 ECs, BAF was nearly undetectable (, Fig. S3). Together, these observations demonstrate that LEM-D proteins show three distinct expression patterns during oogenesis, highlighting the independence of their regulation and localization. Furthermore, our immunohistochemical findings are consistent with functional data that established a critical requirement of emerin in GSC maintenance [Citation36]. Finally, the reduction of emerin levels in polyploid NCs mirrors the findings in polyploid somatic cells in other tissues [Citation40,Citation51], indicating that loss of emerin might be needed to allow nuclear expansion during genome amplification. High levels of BAF in GSCs and germaria are consistent with its functional requirement for GSC maintenance and mitosis [Citation37,Citation40].
Figure 2. Localization of LEM-D proteins and BAF during oogenesis. Confocal images of germaria and S6 ECs stained with antibodies that recognize LEM-D proteins (emerin, emerin2, and MAN1) or their protein partner, BAF. Stage 1 (S1) egg chambers are the first enveloped cysts within the germarium. White arrowheads mark the GSCs, yellow arrowheads mark the NCs, white arrows mark the oocyte and the yellow asterisk marks the fusome. Scale bars, 5 µm.
GSC maintenance is unchanged by ectopic expression of LamC
GSCs, CBs, and early mitotic germ cells did not express LamC (). Similar observations in other tissues have led to the prediction that LamC contributes to nuclear functions involved in differentiation [Citation41]. As such, we predicted that ectopic LamC production would lead to stem cell loss. To test this hypothesis, we generated flies carrying a UASp responder transgene encoding LamC (). These animals were crossed with animals carrying the nos-gal4vp16 driver. Ectopic expression was verified by dissecting ovaries from nos>lamC females and staining them with antibodies against LamC and the germ cell-specific helicase Vasa (). We found that LamC expression followed that of the (nos)-gal4vp16 driver, wherein LamC was present in the NL of GSCs, decreased in the mitotically active germ cells of the germarium, and increased upon formation of the 16-cell cyst. To estimate levels of LamC accumulation in the early stages of oogenesis in nos>lamC ovaries, western blot analysis was performed. Ovaries were dissected from newly eclosed females, as these ovaries are immature and carry ECs up to S5/6, optimizing the evaluation of LamC expression in early germ cells. The lamC gene encodes two protein isoforms with molecular weights of 69.9- and 72.1-kD. Western blot analysis showed that the 69.9-kD isoform predominates in the ovaries (Fig. S1B). The lamC responder transgene expresses only the 69.9-kD isoform, resulting in a ~ 1.7-fold increase relative to wild type controls (Fig. S1B, D). We next evaluated the effects of ectopic LamC on NL structure in GSCs by staining nos>lamC ovaries with antibodies against emerin and LamB. We found that GSCs with ectopic LamC maintained a smooth NL within their large nuclei, with unchanged emerin and LamB localization (). We conclude that the addition of LamC did not impact the overall NL structure.
Figure 3. Ectopic production of LamC alters internal nuclear structure without affecting GSC maintenance. a. Individuals carrying the P[GAL4:VP16-nos] driver transgene were crossed with individuals carrying the UASp-lamC responder transgene to generate nos>lamC females. b. Confocal images of wild type or nos>lamC ovaries stained with antibodies against LamC (white), Vasa (red) and emerin (cyan). Scale bars, 5 µm. c. Confocal images of wild type or nos>lamC ovaries stained with antibodies against Fibrillarin (FBL, green) and LamB (white). Scale bars, 5 µm. d., e. Quantification of the nuclear perimeter (d) or Fibrillarin area (e) in wild type (WT) or nos>lamC (LC) GSCs. Statistical analysis used the unpaired two sample t-test. Asterisks indicate significance ** < 0.01, **** <0.0001. f. Confocal images of germaria in ovaries dissected from < 1- or 15-day females of indicated genotypes. Shown are maximum projection images of ovaries were co-stained with antibodies against pMad (green) and LamB (white). Scale bars: 5 μm. g. Quantification of the number of pMad positive cells from < 1-day and 15-day ovaries of indicated genotypes. Statistical analysis used the unpaired two sample t-test, ns= not significant. For all graphs (d,e,g) shown in this figure, each box represents the 25th to 75th percentile interval, the line represents the median and the whisker represents the 5th to 95th percentile interval and non-outlier range. Total number of germaria analyzed is noted above each top whisker.
![Figure 3. Ectopic production of LamC alters internal nuclear structure without affecting GSC maintenance. a. Individuals carrying the P[GAL4:VP16-nos] driver transgene were crossed with individuals carrying the UASp-lamC responder transgene to generate nos>lamC females. b. Confocal images of wild type or nos>lamC ovaries stained with antibodies against LamC (white), Vasa (red) and emerin (cyan). Scale bars, 5 µm. c. Confocal images of wild type or nos>lamC ovaries stained with antibodies against Fibrillarin (FBL, green) and LamB (white). Scale bars, 5 µm. d., e. Quantification of the nuclear perimeter (d) or Fibrillarin area (e) in wild type (WT) or nos>lamC (LC) GSCs. Statistical analysis used the unpaired two sample t-test. Asterisks indicate significance ** < 0.01, **** <0.0001. f. Confocal images of germaria in ovaries dissected from < 1- or 15-day females of indicated genotypes. Shown are maximum projection images of ovaries were co-stained with antibodies against pMad (green) and LamB (white). Scale bars: 5 μm. g. Quantification of the number of pMad positive cells from < 1-day and 15-day ovaries of indicated genotypes. Statistical analysis used the unpaired two sample t-test, ns= not significant. For all graphs (d,e,g) shown in this figure, each box represents the 25th to 75th percentile interval, the line represents the median and the whisker represents the 5th to 95th percentile interval and non-outlier range. Total number of germaria analyzed is noted above each top whisker.](/cms/asset/30d409d7-139e-427b-a4b4-37306c5f6674/kncl_a_2339214_f0003_oc.jpg)
To understand whether LamC expression altered internal nuclear structures, we examined the structure of the nucleolus, the nuclear compartment that represents the site of rRNA transcription and ribosome biogenesis. Nucleolar size has been found to regulate the nuclear envelope shape in yeast [Citation52], and changes in nucleolar size and shape have been used as pathological markers of disease [Citation53]. To examine the nucleolar structure, nos>lamC ovaries were co-stained with antibodies against LamB and Fibrillarin, a nucleolar protein required for pre-rRNA processing [Citation54,Citation55]. Both wild type and nos>lamC GSCs showed a single focus of Fibrillarin adjacent to the NL (). In nos>lamC GSCs, nucleolar foci were larger than in wild type controls (), residing within nuclei of modestly decreased size (). Motivated by previous studies that found that increased rRNA transcription in GSCs delays differentiation and increases GSC number [Citation56], we examined GSC number. To this end, ovaries were dissected from < 1-day and 15-day wild type and nos>lamC females and co-stained with antibodies against LamB and phosphorylated Mad (pMad; ), a post-translationally modified transcription factor expressed exclusively in GSCs [Citation57]. Quantification of pMad positive cells in germaria of newly eclosed wild type and nos>lamC ovaries revealed germaria carrying a median of three or two GSCs, respectively (). These data indicate that the number of GSCs in nos>lamC ovaries lies within the expected wild type range [Citation58,Citation59]. Notably, GSC numbers were unchanged in 15-day-old wild type and nos>lamC ovaries. From these studies, we conclude that the introduction of LamC into the NL of GSCs does not affect GSC homeostasis.
Overexpression of LamC increases NC transcription in mid-oogenesis
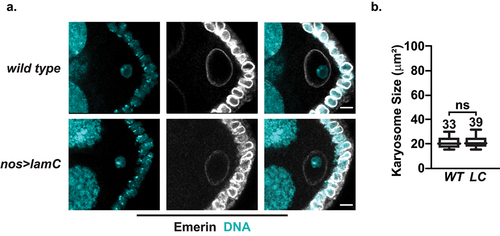
The nos-gal4vp16 driver directs LamC expression throughout oogenesis [Citation60]. Western blot analysis showed an ~ 5.1-fold increase in LamC levels in mature nos>lamC ovaries (Fig. S1B, D). We wondered whether increases in LamC levels outside of the germarium would affect developmental processes during oogenesis. To this end, we examined the structure of the karyosome, a structure that starts to form in S2 oocyte nuclei and represents the aggregation of prophase I arrested meiotic chromosomes [Citation24,Citation25]. Karyosome formation requires the regulation of multiple NL components, including the phosphorylation of BAF to weaken its binding to chromatin and LEM-D proteins [Citation61], disruption of interactions between nuclear pores and chromatin [Citation62], and maintenance of LamB [Citation63]. Karyosome size was measured in S6 ECs, a developmental stage where karyosome formation is complete [Citation61]. Ovaries were stained with emerin and DAPI (), and measurements of the collapsed confocal Z-sections of the DAPI signal were taken in S6 wild type and nos>lamC oocyte nuclei. We found that karyosome sizes were the same in ovaries of both genotypes (), indicating that chromatin compaction was not affected by increased LamC expression.
Figure 4. Karyosome formation is unaffected in nos>lamC ovaries. a. Left: Confocal image of wild type (top) or nos>lamC (bottom) S6 ECs stained with antibodies against emerin (white) and DAPI (cyan). Scale bars: 5 μm. b. Quantification of the size of the karyosome in S6 ECs. Each box represents the 25th to 75th percentile interval, the line represents the median and the whisker represents the 5th to 95th percentile interval and non-outlier range. Total number of S6 ECs analyzed is noted above each top whisker. Statistical analyzes were performed using the unpaired two-sample t-test. Asterisks indicate significance: ns= not significant.
Multiple nuclear changes occur during mid-oogenesis (S6 to S9). These include NC genome amplification, robust NC transcription, and transcriptional activation in oocytes. As the NL contributes to the nuclear regulation of DNA replication and transcription, we investigated the effects of increased LamC levels on these processes, focusing on S9 ECs. We chose this stage because NCs endocycles are complete, polytene chromosomes are dispersed, NCs are transcriptionally active, and oocyte transcription has initiated [Citation26,Citation64]. Staining nos>lamC ovaries with LamC antibodies revealed uneven levels in LamC within the NC NL. The anterior NCs had higher levels than the posterior NCs (). Nonetheless, increases in LamC were found throughout the EC, including in the oocyte nuclei, as indicated by measurements of the intensity of the NL signal (). Relocalization of LamC to the nuclear interior occurred in nos>lamC oocyte nuclei, even though the level of phosphorylated LamC did not increase (). We evaluated genome amplification in S9 NCs by measuring the perimeter of NC nuclei, as nuclear size correlates with the endocycle number [Citation65]. Middle NCs were chosen for this analysis because of their size consistency within wild type ECs. Our measurements established that the size of middle positioned nos>lamC NC nuclei was minimally increased (), implying that genome amplification was normal. Measurement of the perimeter of oocyte nuclei showed a reverse trend, with nos>lamC oocytes having modestly smaller nuclei ().
Figure 5. Ectopic production of LamC alters nuclear structure and function in S9 ECs. a. Confocal images of S9 wild type (top) or nos>lamC (bottom) ECs stained with antibodies against LamC (white) and N-terminal (NT) phosphoserine (green). Scale bars: 5 μm. Magnified images of boxed nuclei are shown below. Scale bars: 5 μm. b. Graph of the quantification of the intensity of the LamC staining in S9 oocyte nuclei in wild type (WT) or nos>lamC (LC) ovaries. c. Graph of the quantification of the intensity of the pSer staining in S9 oocyte nuclei of the indicated genotype. d. Graph of the quantification of perimeter of S9 oocyte nuclei of the indicated genotype. For NCs, the middle NC of the EC was chosen. e. Confocal image of wild type (top) or nos>lamC (bottom) ECs stained with antibodies against Fibrillarin (FBL, green) and Wheat Germ Agglutinin (WGA, Purple). Magnified images of boxed nuclei are shown below. Scale bars: 5 μm. f. Graph of the quantification of Fibrillarin intensity in middle NC of nuclei in S9 egg chambers of the indicated genotypes. g. Graph of the quantification of EU incorporation in NCs and oocytes of S9 ECs of the indicated genotypes. For each graph show in this figure (b, c, d, f, g), the box represents the 25th to 75th percentile interval, the line represents the median and the whisker represents the 5th to 95th percentile interval and non-outlier range. The total number of nuclei analyzed is noted above each top whisker. Asterisks indicate significance [Unpaired two sample t-test, *<0.05, ** <0.01, ***<0.0001, ns= not significant].
![Figure 5. Ectopic production of LamC alters nuclear structure and function in S9 ECs. a. Confocal images of S9 wild type (top) or nos>lamC (bottom) ECs stained with antibodies against LamC (white) and N-terminal (NT) phosphoserine (green). Scale bars: 5 μm. Magnified images of boxed nuclei are shown below. Scale bars: 5 μm. b. Graph of the quantification of the intensity of the LamC staining in S9 oocyte nuclei in wild type (WT) or nos>lamC (LC) ovaries. c. Graph of the quantification of the intensity of the pSer staining in S9 oocyte nuclei of the indicated genotype. d. Graph of the quantification of perimeter of S9 oocyte nuclei of the indicated genotype. For NCs, the middle NC of the EC was chosen. e. Confocal image of wild type (top) or nos>lamC (bottom) ECs stained with antibodies against Fibrillarin (FBL, green) and Wheat Germ Agglutinin (WGA, Purple). Magnified images of boxed nuclei are shown below. Scale bars: 5 μm. f. Graph of the quantification of Fibrillarin intensity in middle NC of nuclei in S9 egg chambers of the indicated genotypes. g. Graph of the quantification of EU incorporation in NCs and oocytes of S9 ECs of the indicated genotypes. For each graph show in this figure (b, c, d, f, g), the box represents the 25th to 75th percentile interval, the line represents the median and the whisker represents the 5th to 95th percentile interval and non-outlier range. The total number of nuclei analyzed is noted above each top whisker. Asterisks indicate significance [Unpaired two sample t-test, *<0.05, ** <0.01, ***<0.0001, ns= not significant].](/cms/asset/15361900-c163-4f27-a55d-1d60fef49a65/kncl_a_2339214_f0005_oc.jpg)
To define the effects of LamC overexpression on the internal nuclear structure of NCs, we examined the structure of the nucleolus. In NCs, high levels of rRNA transcription are accompanied by the formation of an irregular nucleolar structure consisting of a series of interconnected tubules positioned along the NL [Citation66]. Staining of nos>lamC S9 ECs revealed diffuse, not tubular, Fibrillarin localization, even though its levels were unchanged (). To determine whether this nucleolar morphology affected function, we assessed nascent RNA production in wild type and nos>lam S9 NCs. To this end, dissected ovaries were incubated in buffer containing the nucleotide analog EU, and then Click-it chemistry was used to detect synthesized RNA. Quantification of EU incorporation in middle position NC nuclei showed increased levels in the nos>lam NCs relative to wild type controls (, Fig.S3A). Taken together, our data reveal that overexpression of LamC affected both the structure of the nucleolus and total transcription in NCs. Recent studies have correlated increased EU incorporation with elevated nuclear actin levels [Citation67]. As vertebrate lamins bind actin in vitro [Citation68], we predict that increases in NL LamC levels might increase nuclear actin, thereby promoting transcription.
Phosphorylated lamin A/C binds to active enhancers in human fibroblasts [Citation69]. Although LamC levels increased in S9 oocyte nuclei, levels of N-terminal phospho-serine remained unchanged (), suggesting that only non-phosphorylated LamC increased in S9 oocyte nuclei. We wondered whether this change would affect oocyte transcription. Quantification of EU incorporation in oocytes demonstrated that the levels of transcription in nos>lamC oocyte nuclei matched those in wild type nuclei (). These observations demonstrate that regulated transcription of the oocyte nucleus was not perturbed. As nos>lamC S9 oocyte nuclei carry increased levels of non-phosphorylated LamC, these data are unable to resolve the contribution of LamC phosphorylation to oocyte transcription.
Egg production and quality are reduced by LamC overexpression
Overexpression of LamC changed the structure and function of NCs (). We wondered whether these functional consequences altered production or quality of eggs laid by nos>lamC females. To this end, we set up egg collection bottles that had wild type or nos>lamC females mated with wild type males. We quantified the number of eggs laid by these females over a 40-day period (). We found that nos>lamC females displayed strong egg laying for the first 10-days, but egg production declined at 15-days and continued at a low level for 35-days, matching the length of time of egg production in wild type females. This decline in egg laying was not accompanied by the loss of GSCs (). To understand whether egg quality was affected, we determined whether eggs collected from matings of 4- and 7-day-old wild type and nos>lamC females hatched into larvae. Strikingly, the hatching percentage of nos>lamC eggs was ~ 50% lower than wild type, regardless of nos>lamC female age (). These data suggest that elevated LamC expression reduces egg quality.
Figure 6. Effects of Lam C overexpression on fertility. a. Graphed is the fecundity (eggs per female per day) of wild type (solid) and nos>lamC (dashed) females over the course of forty days. All females were mated to wild type males. Asterisks indicate significance, determined for egg lays on every fifth day. [Unpaired two sample t-test, * < 0.05, ** < 0.01, ns= not significant] b. Graph of the percentage of eggs that hatched within 24 h following deposition by 4- and 7-day old females of indicated genotypes that were mated to wild type males. Bars represent the standard deviation from at least three independent experiments. The number of eggs analyzed is noted above each bar. Asterisks indicate significance [Unpaired two sample t-test ** < 0.01, *** < 0.001]. c. Scanning electron micrographs of eggs laid by wild type and nos>lamC females. The yellow arrowhead shows the micropyle and DA shows the location of the dorsal appendages. d. Graph of the percentage of developing embryos found in a 24-hr egg collection taken from females of the indicated genotypes [Unpaired two sample t-test, ns= not significant]. e. Graph of the percentage of dechorionated eggs that hatched into larvae that were laid by 4-day old wild type and nos>lamC females. [Unpaired two sample t-test, ns= not significant]. f. Graph of the percentage of female progeny eclosing from eggs laid by wild type and nos>lamC females [Unpaired two sample t-test, ns= not significant].
![Figure 6. Effects of Lam C overexpression on fertility. a. Graphed is the fecundity (eggs per female per day) of wild type (solid) and nos>lamC (dashed) females over the course of forty days. All females were mated to wild type males. Asterisks indicate significance, determined for egg lays on every fifth day. [Unpaired two sample t-test, * < 0.05, ** < 0.01, ns= not significant] b. Graph of the percentage of eggs that hatched within 24 h following deposition by 4- and 7-day old females of indicated genotypes that were mated to wild type males. Bars represent the standard deviation from at least three independent experiments. The number of eggs analyzed is noted above each bar. Asterisks indicate significance [Unpaired two sample t-test ** < 0.01, *** < 0.001]. c. Scanning electron micrographs of eggs laid by wild type and nos>lamC females. The yellow arrowhead shows the micropyle and DA shows the location of the dorsal appendages. d. Graph of the percentage of developing embryos found in a 24-hr egg collection taken from females of the indicated genotypes [Unpaired two sample t-test, ns= not significant]. e. Graph of the percentage of dechorionated eggs that hatched into larvae that were laid by 4-day old wild type and nos>lamC females. [Unpaired two sample t-test, ns= not significant]. f. Graph of the percentage of female progeny eclosing from eggs laid by wild type and nos>lamC females [Unpaired two sample t-test, ns= not significant].](/cms/asset/e7e97a6a-7c1c-458a-b926-d08bd3c42324/kncl_a_2339214_f0006_oc.jpg)
To understand the possible reasons for the reduced egg quality, we analyzed eggshell patterning using scanning electronic microscopy, SEM (). Eggshells have several structures that are important for external embryogenesis. These include two dorsal appendages that mediate gas exchange for the developing embryo, a micropyle that serves as the sperm entry site, and an operculum that forms a trap door required for larval exit at the end of embryogenesis [Citation70]. Comparison of SEM images revealed that nos>lamC eggs had normal dorsal appendages but a disorganized eggshell that included a disfigured micropyle and a smooth, thick operculum (). As defects in the micropyle could reduce fertilization, and thereby hatching, we assessed whether nos>lamC eggs were engaged in embryogenesis. To this end, a 24-hr collection of eggs, laid by mated wild type and nos>lamC females, were dechorionated, DAPI stained, and imaged. Quantification of the number of developing embryos revealed that wild type and nos>lamC egg collections had indistinguishable levels of embryogenesis (). These data imply that, although disfigured, the nos>lamC micropyle was functional. Next, we tested whether the nos>lamC operculum was operational. As the operculum allows larvae to exit from the eggshell, we reasoned that defects in this structure might account for the lower hatching rate. To test this hypothesis, wild type and nos>lamC eggs were again dechorionated, and the well-washed embryos were transferred to glass slides and allowed to develop. Strikingly, eggshell removal rescued the hatching defect of nos>lamC eggs, with hatching returning to wild type levels (). Finally, we collected hatched larvae and allowed them to develop. Examination of phenotypes of resulting adults found that 50% of the progeny of nos>lamC eggs were female (), thereby eliminating sex determination as a contributor to decreased hatching. Adult progeny had a wild type morphology, indicating that LamC increases during oogenesis do not have a major impact on subsequent development. Taken together, we conclude that elevated LamC levels cause defects in eggshell patterning which reduce larval hatching and female fertility.
Discussion
Germ cells undergo dramatic changes in their nuclear structure during oogenesis. We show that these structural changes are accompanied by fluctuations in the levels of lamins, LEM-D proteins, and BAF (, S2). These changes predict that changes in NL composition are important for differentiation. For example, emerin and BAF levels decline as NCs enter the developmental endocycle program (). Notably, this decline correlates with observations that emerin and BAF repress endoreduplication in myofibers by sequestering a limiting endocycle factor at the nuclear periphery, E2F1 [Citation71]. These findings predict that NC endoreduplication requires the downregulation of emerin and BAF. We show that declines in emerin were accompanied by increased levels of emerin2 (). Although emerin and emerin2 are orthologs [Citation37], emerin, not emerin2, is the primary LEM-D protein responsible for BAF localization [Citation40]. As such, increases in emerin2 should not confer BAF NL localization. Taken together, these observations suggest that the switch from emerin to emerin2 enables genome amplification in NCs.
Developmental consequences of elevated LamC
As germ cells differentiate, there is a second switch that involves one of the major NL scaffolding components. Notably, LamC is absent in GSCs and increases as germ cells differentiate, whereas LamB is present in GSCs and declines as germ cells differentiate (). These observations align with the long-standing perception that Drosophila LamC is the differentiation lamin [Citation41]. However, when this developmental switch was disrupted by ectopic LamC expression, GSC homeostasis was not affected (). These surprising findings demonstrate that forced LamC production does not promote germ cell differentiation. This inability might reflect that GSCs express limiting levels of a LamC partner protein that is needed for LamC to direct differentiation. Alternatively, the absence of LamC in GSCs might reflect a regulatory, not a functional, difference between the Drosophila lamin genes, at least in the germline.
Our system produced increased LamC levels during EC development (). Despite changing the levels of this NL scaffolding protein, NC nuclei grew in size, increased their DNA content, and maintained active transcription. Indeed, levels of transcription were higher than in controls (), indicating that elevation of LamC did not repress the nuclear functions of replication or transcription. However, female fertility was impacted. First, egg production declined at 15-days, earlier than wild type controls (). This faster decline occurred without a loss of GSCs (). Lowered egg laying in aging nos>lamC females suggests that prolonged LamC overexpression might decrease GSC division or increase death of developing oocytes, thereby accelerating natural biological processes that reduce egg production during aging [Citation72]. Second, nos>lamC females produced low quality eggs that had reduced larval hatching due to eggshell defects, with our evidence implicating dysfunction of the operculum (). How LamC overexpression in germ cells affects eggshell formation is unknown. Eggshell production depends on regulated interactions between germ cells and overlying somatic follicle cells [Citation70,Citation73]. These interactions specify the fates of somatic follicle cells that surround the oocyte and direct cell-specific secretory programs, either of which could be affected by elevated LamC levels. Based on our observation that the form and/or function of the dorsal appendages and micropyle are unaffected (), we favor a model that suggests that the secretory process of cells responsible for operculum formation might be defective. This model builds from studies in vertebrates showing that lamins increase tissue stiffness [Citation74]. We predict that increased levels of LamC change the physical properties of NCs and interfere with the secretory function of the overlying somatic cells involved in operculum formation. Additional studies are required to test this model.
LamC undergoes developmental phosphorylation
Lamins are regulated by post-translational modifications [Citation48,Citation75]. Among these, phosphorylation plays a key role that is best known for its promotion of disassembly of the lamin network during mitosis. Indeed, ser22 is one of the two serine residues in Lamin A/C referred to as ‘mitotic sites’ [Citation48]. Nonetheless, phosphorylation of ser22 lamin A also occurs in interphase cells [Citation76]. We found that phosphorylation of the corresponding site in Drosophila LamC occurred as ECs developed (Fig. S1). Although this target serine is conserved in LamB, it is not phosphorylated (Fig. S1). We noted that LamC phosphorylation occurs only in the oocyte, and not in NC nuclei (), even though the oocyte nucleus resides in a syncytium with the larger polyploid NC nuclei. Based on these observations, we suggest that LamC phosphorylation is highly specific and localized during EC development.
Lamin A/C phosphorylation is predicted to have regulatory consequences [Citation48,Citation75]. Phosphorylation of the interphase Lamin A/C ser22 occurs upon cellular stress, facilitating its nucleoplasmic localization [Citation43]. A direct transcriptional role of nucleoplasmic phosphorylated ser22 of Lamin A/C has emerged from genome-wide mapping studies, wherein phospho-Lamin A/C binds active enhancers, a pattern that is altered in disease [Citation69]. These observations, coupled with the timing of phosphorylation of Drosophila LamC, suggest that phosphorylated LamC might contribute to the transcription of the oocyte genome. LamC overexpression did not affect oocyte transcription (). An alternative test of the role of phospho-LamC would be germ cell-specific depletion of LamC, as lamC mutants die during the prepupal stage of development [Citation30]. The extant RNAi responder line carries a transgene that lacks features needed for robust expression and mRNA reduction in the germline [Citation60,Citation77], requiring the generation of new tools to address this question. Nonetheless, oogenesis provides a developmental context for investigating the contribution of lamin phosphorylation to nuclear function.
In summary, these studies advance the understanding of changes in NL composition during Drosophila oogenesis and provide insight into how LamC contributes to these nuclear functions. Notably, we found that oogenesis is unexpectedly robust to changes in LamC levels, even though this is a major NL scaffolding protein. We suggest that the Drosophila ovary serves as an excellent platform for investigating how NL composition and nuclear function are coordinated for the execution of development.
Author contributions
Conceptualization, I.E.P. and P.K.G.; methodology, I.E.P., S.D.J., and P.K.G.; investigation, I.E.P., S.D.J., K.M.P.; Data curation, I.E.P., S.D.J., K.M.P; original draft, P.K.G., review and editing I.E.P., S.D.J., K.M.P. and P.K.G.; funding acquisition, P.K.G.; supervision, P.K.G.
Supplemental Material
Download Zip (14.4 MB)Acknowledgments
We thank Nicholas Lyon for technical assistance in the production of the UASp-lamC transgene, Tingting Duan for both technical assistance and training laboratory members, and members of the Geyer laboratory and Lacy Barton (University of Texas, San Antonio) for helpful discussions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The Drosophila strains are available upon request. The data that support the findings of this study are openly available in https://doi.org/10.6084/m9.figshare.25250224. Supplemental files are published at figshare https://doi.org/10.6084/m9.figshare.25250224.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19491034.2024.2339214
Additional information
Funding
References
- Patil S, Sengupta K. Role of A- and B-type lamins in nuclear structure–function relationships. Biol Cell. 2021;113(7):295–19. doi: 10.1111/boc.202000160
- Wong X, Melendez-Perez AJ, Reddy KL. The nuclear Lamina. Cold Spring Harb Perspect Biol. 2022;14(2):14. doi: 10.1101/cshperspect.a040113
- Shimi T, Kittisopikul M, Tran J, et al. Structural organization of nuclear lamins A, C, B1, and B2 revealed by superresolution microscopy. Mol Biol Cell. 2015;26(22):4075–4086. doi: 10.1091/mbc.E15-07-0461
- Adam SA, Goldman RD. Insights into the differences between the A- and B-type nuclear lamins. Adv Biol Regul. 2012;52(1):108–113. doi: 10.1016/j.advenzreg.2011.11.001
- Guelen L, Pagie L, Brasset E, et al. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature. 2008;453(7197):948–951. doi: 10.1038/nature06947
- Shimi T, Pfleghaar K, Kojima S, et al. The A- and B-type nuclear lamin networks: microdomains involved in chromatin organization and transcription. Genes Dev. 2008;22(24):3409–3421. doi: 10.1101/gad.1735208
- de Las Heras JI, Zuleger N, Batrakou DG, et al. Tissue-specific NETs alter genome organization and regulation even in a heterologous system. Nucleus. 2017;8(1):81–97. doi: 10.1080/19491034.2016.1261230
- Lin F, Blake DL, Callebaut I, et al. MAN1, an inner nuclear membrane protein that shares the LEM domain with lamina-associated polypeptide 2 and emerin. J Biol Chem. 2000;275:4840–4847. doi: 10.1074/jbc.275.7.4840
- Furukawa K. LAP2 binding protein 1 (L2BP1/BAF) is a candidate mediator of LAP2-chromatin interaction. J Cell Sci. 1999;112(Pt 15):2485–2492. doi: 10.1242/jcs.112.15.2485
- Berk JM, Tifft KE, Wilson KL. The nuclear envelope LEM-domain protein emerin. Nucleus. 2013;4(4):298–314. doi: 10.4161/nucl.25751
- Goldman RD, Gruenbaum Y, Moir RD, et al. Nuclear lamins: building blocks of nuclear architecture. Genes Dev. 2002;16(5):533–547. doi: 10.1101/gad.960502
- Gonzalo S. DNA damage and lamins. Adv Exp Med Biol. 2014;773:377–399.
- Geyer PK, Vitalini MW, Wallrath LL. Nuclear organization: taking a position on gene expression. Curr Opin Cell Biol. 2011;23(3):354–359. doi: 10.1016/j.ceb.2011.03.002
- Davidson PM, Lammerding J. Broken nuclei–lamins, nuclear mechanics, and disease. Trends Cell Biol. 2014;24(4):247–256. doi: 10.1016/j.tcb.2013.11.004
- Gonzalo S, Kreienkamp R, Askjaer P. Hutchinson-gilford progeria syndrome: a premature aging disease caused by LMNA gene mutations. Ageing Res Rev. 2017;33:18–29. doi: 10.1016/j.arr.2016.06.007
- Lans H, Hoeijmakers JH. Cell biology: ageing nucleus gets out of shape. Nature. 2006;440(7080):32–34. doi: 10.1038/440032a
- Worman HJ, Ostlund C, Wang Y. Diseases of the nuclear envelope. Cold Spring Harb Perspect Biol. 2010;2(2):a000760. doi: 10.1101/cshperspect.a000760
- Pathak RU, Soujanya M, Mishra RK. Deterioration of nuclear morphology and architecture: a hallmark of senescence and aging. Ageing Res Rev. 2021;67:101264. doi: 10.1016/j.arr.2021.101264
- Spradling AC. Developmental genetics of oogenesis. (Bate M and Martinez-Aria A. New York: Cold Spring Harbor Laboratory Press, Cold Spring Harbor; 1993.
- Hinnant TD, Merkle JA, Ables ET. Coordinating proliferation, polarity, and cell fate in the drosophila female germline. Front Cell Dev Biol. 2020;8:19. doi: 10.3389/fcell.2020.00019
- Christophorou N, Rubin T, Huynh JR, et al. Synaptonemal complex components promote centromere pairing in pre-meiotic germ cells. PLoS Genet. 2013;9(12):e1004012. doi: 10.1371/journal.pgen.1004012
- Joyce EF, Apostolopoulos N, Beliveau BJ, et al. Germline progenitors escape the widespread phenomenon of homolog pairing during drosophila development. PLoS Genet. 2013;9(12):e1004013. doi: 10.1371/journal.pgen.1004013
- Hughes SE, Miller DE, Miller AL, et al. Female meiosis: synapsis, recombination, and segregation in drosophila melanogaster. Genetics. 2018;208(3):875–908. doi: 10.1534/genetics.117.300081
- Cullen CF, Brittle AL, Ito T, et al. The conserved kinase NHK-1 is essential for mitotic progression and unifying acentrosomal meiotic spindles in drosophila melanogaster. J Cell Bio. 2005;171(4):593–602. doi: 10.1083/jcb.200508127
- Bogolyubov DS. Karyosphere (Karyosome): a peculiar structure of the oocyte nucleus. Int Rev Cell Mol Biol. 2018;337:1–48.
- Navarro-Costa P, McCarthy A, Prudencio P, et al. Early programming of the oocyte epigenome temporally controls late prophase I transcription and chromatin remodelling. Nat Commun. 2016;7(1):12331. doi: 10.1038/ncomms12331
- Jacob J, Sirlin JL. Cell function in the ovary of Drosophila. I. DNA classes in nurse cell nuclei as determined by autoradiography. Chromosoma. 1959;10(1–6):210–228. doi: 10.1007/BF00396572
- Rzepecki R, Gruenbaum Y. Invertebrate models of lamin diseases. Nucleus. 2018;9(1):227–234. doi: 10.1080/19491034.2018.1454166
- Osouda S, Nakamura Y, de Saint Phalle B, et al. Null mutants of Drosophila B-type lamin Dm(0) show aberrant tissue differentiation rather than obvious nuclear shape distortion or specific defects during cell proliferation. Dev Biol. 2005;284(1):219–232. doi: 10.1016/j.ydbio.2005.05.022
- Schulze SR, Curio-Penny B, Li Y, et al. Molecular genetic analysis of the nested drosophila melanogaster lamin C gene. Genetics. 2005;171(1):185–196. doi: 10.1534/genetics.105.043208
- Guillemin K, Williams T, Krasnow MA. A nuclear lamin is required for cytoplasmic organization and egg polarity in Drosophila. Nat Cell Biol. 2001;3(9):848–851. doi: 10.1038/ncb0901-848
- Lenz-Bohme B, Wismar J, Fuchs S, et al. Insertional mutation of the drosophila nuclear lamin Dm0 gene results in defective nuclear envelopes, clustering of nuclear pore complexes, and accumulation of annulate lamellae. J Cell Bio. 1997;137(5):1001–1016. doi: 10.1083/jcb.137.5.1001
- Morgunova VV, Sokolova OA, Sizova TV, et al. Dysfunction of lamin B and physiological aging cause telomere instability in drosophila germline. Biochemistry (Mosc). 2022;87:1600–1610. doi: 10.1134/S000629792212015X
- Pinto BS, Wilmington SR, Hornick EE, et al. Tissue-specific defects are caused by loss of the drosophila MAN1 LEM domain protein. Genetics. 2008;180(1):133–145. doi: 10.1534/genetics.108.091371
- Wagner N, Kagermeier B, Loserth S, et al. The drosophila melanogaster LEM-domain protein MAN1. Eur J Cell Biol. 2006;85(2):91–105. doi: 10.1016/j.ejcb.2005.10.002
- Barton LJ, Pinto BS, Wallrath LL, et al. The drosophila nuclear lamina protein otefin is required for germline stem cell survival. Dev Cell. 2013;25(6):645–654. doi: 10.1016/j.devcel.2013.05.023
- Barton LJ, Wilmington SR, Martin MJ, et al. Unique and shared functions of nuclear lamina LEM domain proteins in drosophila. Genetics. 2014;197(2):653–665. doi: 10.1534/genetics.114.162941
- Wagner N, Krohne G. LEM-Domain proteins: new insights into lamin-interacting proteins. Int Rev Cytol. 2007;261:1–46.
- Barton LJ, Duan T, Ke W, et al. Nuclear lamina dysfunction triggers a germline stem cell checkpoint. Nat Commun. 2018;9(1):3960. doi: 10.1038/s41467-018-06277-z
- Duan T, Kitzman SC, Geyer PK. Survival of Drosophila germline stem cells requires the chromatin-binding protein Barrier-to-autointegration factor. Development. 2020;147. doi: 10.1242/dev.186171
- Palka M, Tomczak A, Grabowska K, et al. Laminopathies: what can humans learn from fruit flies. Cell Mol Biol Lett. 2018;23(1):32. doi: 10.1186/s11658-018-0093-1
- Duan T, Rodriguez-Tirado F, Geyer PK. Immunohistochemical analysis of nuclear lamina structures in the drosophila ovary using CRISPR-tagged Genes. Methods Mol Biol. 2023;2626:109–134.
- Virtanen L, Holm E, Halme M, et al. Lamin A/C phosphorylation at serine 22 is a conserved heat shock response to regulate nuclear adaptation during stress. J Cell Sci. 2023;136(4):136. doi: 10.1242/jcs.259788
- King RC. Ovarian development in Drosophila melanogaster. New York: Academic Press; 1970.
- Mahowald AP, Kambysellis MP. Oogenesis.In: Ashburner M, Wright TRF, editors. Gen bio Droso. 1980;2c:141–224.
- Ali-Murthy Z, Fetter RD, Wang W, et al. Elimination of nurse cell nuclei that shuttle into oocytes during oogenesis. J Cell Bio. 2021;220(7):220. doi: 10.1083/jcb.202012101
- Peterson JS, Timmons AK, Mondragon AA, et al. The End of the beginning: cell Death in the Germline. Curr Top Dev Biol. 2015;114:93–119.
- Liu SY, Ikegami K. Nuclear lamin phosphorylation: an emerging role in gene regulation and pathogenesis of laminopathies. Nucleus. 2020;11(1):299–314. doi: 10.1080/19491034.2020.1832734
- Zaremba-Czogalla M, Piekarowicz K, Wachowicz K, et al. The different function of single phosphorylation sites of drosophila melanogaster lamin Dm and lamin C. PLOS ONE. 2012;7(2):e32649. doi: 10.1371/journal.pone.0032649
- Wagner N, Schmitt J, Krohne G. Two novel LEM-domain proteins are splice products of the annotated drosophila melanogaster gene CG9424 (Bocksbeutel). Eur J Cell Biol. 2004;82(12):605–616. doi: 10.1078/0171-9335-00350
- Ashery-Padan R, Ulitzur N, Arbel A, et al. Localization and posttranslational modifications of otefin, a protein required for vesicle attachment to chromatin, during drosophila melanogaster development. Mol Cell Biol. 1997;17(7):4114–4123. doi: 10.1128/MCB.17.7.4114
- Male G, Deolal P, Manda NK, et al. Nucleolar size regulates nuclear envelope shape in Saccharomyces cerevisiae. J Cell Sci. 2020;133. doi: 10.1242/jcs.242172
- Correll CC, Bartek J, Dundr M. The nucleolus: a multiphase condensate balancing ribosome synthesis and translational capacity in health, aging and ribosomopathies. Cells. 2019;8(8):8. doi: 10.3390/cells8080869
- Tollervey D, Lehtonen H, Jansen R, et al. Temperature-sensitive mutations demonstrate roles for yeast fibrillarin in pre-rRNA processing, pre-rRNA methylation, and ribosome assembly. Cell. 1993;72(3):443–457. doi: 10.1016/0092-8674(93)90120-F
- Wu P, Brockenbrough JS, Metcalfe AC, et al. Nop5p is a small nucleolar ribonucleoprotein component required for pre-18 S rRNA processing in yeast. J Biol Chem. 1998;273(26):16453–16463. doi: 10.1074/jbc.273.26.16453
- Zhang Q, Shalaby NA, Buszczak M. Changes in rRNA transcription influence proliferation and cell fate within a stem cell lineage. Science. 2014;343(6168):298–301. doi: 10.1126/science.1246384
- Chen D, McKearin D. Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Curr Biol. 2003;13(20):1786–1791. doi: 10.1016/j.cub.2003.09.033
- Xie T. Germline stem cell niches. Stem Book. Cambridge (MA): Harvard Stem Cell Institute; 2008. doi: 10.3824/stembook.1.23.1
- Spradling A, Fuller MT, Braun RE, et al. Germline stem cells. Cold Spring Harb Perspect Biol. 2011;3(11):a002642. doi: 10.1101/cshperspect.a002642
- Rorth P. Gal4 in the drosophila female germline. Mech Dev. 1998;78(1–2):113–118. doi: 10.1016/S0925-4773(98)00157-9
- Lancaster OM, Cullen CF, Ohkura H. NHK-1 phosphorylates BAF to allow karyosome formation in the drosophila oocyte nucleus. J Cell Bio. 2007;179(5):817–824. doi: 10.1083/jcb.200706067
- Breuer M, Ohkura H. A negative loop within the nuclear pore complex controls global chromatin organization. Genes Dev. 2015;29(17):1789–1794. doi: 10.1101/gad.264341.115
- Nieken KJ, O’Brien K, McDonnell A, et al. A large-scale RNAi screen reveals that mitochondrial function is important for meiotic chromosome organization in oocytes. Chromosoma. 2023;132(1):1–18. doi: 10.1007/s00412-023-00784-9
- Dej KJ, Spradling AC. The endocycle controls nurse cell polytene chromosome structure during drosophila oogenesis. Development. 1999;126(2):293–303. doi: 10.1242/dev.126.2.293
- Dapples CC, King RC. The development of the nucleolus of the ovarian nurse cell of drosophila melanogaster. Z Zellforsch Mikrosk Anat. 1970;103(1):34–47. doi: 10.1007/BF00335399
- Groen CM, Tootle TL. Visualization of actin cytoskeletal dynamics in fixed and live drosophila egg chambers. Methods Mol Biol. 2015;1328:113–124.
- Talbot DE, Vormezeele BJ, Kimble GC, et al. Prostaglandins limit nuclear actin to control nucleolar function during oogenesis. Front Cell Dev Biol. 2023;11:1072456. doi: 10.3389/fcell.2023.1072456
- Simon DN, Zastrow MS, Wilson KL. Direct actin binding to A- and B-type lamin tails and actin filament bundling by the lamin a tail. Nucleus. 2010;1(3):264–272. doi: 10.4161/nucl.11799
- Ikegami K, Secchia S, Almakki O, et al. Phosphorylated lamin A/C in the nuclear interior binds active enhancers associated with abnormal transcription in progeria. Dev Cell. 2020;52(6):699–713 e611. doi: 10.1016/j.devcel.2020.02.011
- Horne-Badovinac S. The drosophila micropyle as a system to study how epithelia build complex extracellular structures. Philos Trans R Soc Lond B Biol Sci. 2020;375:20190561. doi: 10.1098/rstb.2019.0561
- Wang S, Stoops E, Cp U, et al. Mechanotransduction via the LINC complex regulates DNA replication in myonuclei. J Cell Bio. 2018;217(6):2005–2018. doi: 10.1083/jcb.201708137
- Zhao R, Xuan Y, Li X, et al. Age-related changes of germline stem cell activity, niche signaling activity and egg production in Drosophila. Aging Cell. 2008;7(3):344–354. doi: 10.1111/j.1474-9726.2008.00379.x
- Cavaliere V, Bernardi F, Romani P, et al. Building up the Drosophila eggshell: first of all the eggshell genes must be transcribed. Dev Dyn. 2008;237(8):2061–2072. doi: 10.1002/dvdy.21625
- Swift J, Ivanovska IL, Buxboim A, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341(6149):1240104. doi: 10.1126/science.1240104
- Zheng M, Jin G, Zhou Z. Post-translational modification of lamins: mechanisms and functions. Front Cell Dev Biol. 2022;10:864191. doi: 10.3389/fcell.2022.864191
- Kochin V, Shimi T, Torvaldson E, et al. Interphase phosphorylation of lamin a. J Cell Sci. 2014;127:2683–2696. doi: 10.1242/jcs.141820
- Ni JQ, Zhou R, Czech B, et al. A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nat Methods. 2011;8(5):405–407. doi: 10.1038/nmeth.1592

