 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
Negative life events (NLEs), e.g., poor academic performance (controllable) or being the victim of a crime (uncontrollable), can profoundly affect the trajectory of one’s life. Yet, their impact on how the brain develops is still not well understood. This investigation examined the National Consortium on Alcohol and Neurodevelopment in Adolescence (NCANDA) dataset for the impact of NLEs on the initiation of alcohol and cannabis use, as well as underlying neural mechanisms.
Methods
This study evaluated the impact of controllable and uncontrollable NLEs on substance use initiation in 207 youth who initiated alcohol use, 168 who initiated cannabis use, and compared it to 128 youth who remained substance-naïve, using generalised linear regression models. Mediation analyses were conducted to determine neural pathways of NLE impacting substance use trajectories.
Results
Dose-response relationships between controllable NLEs and substance use initiation were observed. Having one controllable NLE increased the odds of alcohol initiation by 50% (95%CI [1.18, 1.93]) and cannabis initiation by 73% (95%CI [1.36, 2.24]), respectively. Greater cortical thickness in left banks of the superior temporal sulcus mediated effects of controllable NLEs on alcohol and cannabis initiations. Greater left caudate gray-matter volumes mediated effects of controllable NLEs on cannabis initiation.
Conclusions
Controllable but not uncontrollable NLEs increased the odds of alcohol and cannabis initiation. Moreover, those individuals with less mature brain structures at the time of the NLEs experienced a greater impact of NLEs on subsequent initiation of alcohol or cannabis use. Targeting youth experiencing controllable NLEs may help mitigate alcohol and cannabis initiation.
Introduction
Adolescence marks a critical period of increased vulnerability to addictions involving substances including alcohol and cannabis (Crews et al. Citation2007). Early initiation of substance use (SU) has been associated with the subsequent development of problematic SU (Crews et al. Citation2007; Jordan and Andersen Citation2017; Gray and Squeglia Citation2018) and other mental concerns (Swahn et al. Citation2012; Bagot et al. Citation2015). This study focused on initiating SU before age 21, i.e., before legal drinking age, which poses a significant risk factor for developing lifetime SU disorders (Feinstein et al. Citation2012; Jordan and Andersen Citation2017). Additionally, the likelihood of having lifetime SU disorders decreases by 4–5% for each year of delaying in SU initiation for adolescents aged 13–21 years (Jordan and Andersen Citation2017). Therefore, there is a need to identify modifiable risk factors for early initiation of SU that could be targeted in preventative interventions.
Among risk factors for early SU initiation, negative life events (NLEs) have been linked to SU and other mental problems (Veenstra et al. Citation2006; Hyman and Sinha Citation2009; Low et al. Citation2012; Christensen et al. Citation2022). NLEs include various stressful occurrences and can be categorised into uncontrollable and controllable events (Stern et al. Citation1982). Uncontrollable NLEs refer to events over which an individual has little agency to affect its occurrence, such as witnessing or being the victim of a crime or assault, parental divorce, or job loss. In contrast, controllable NLEs are events over which an individual has greater agency to affect its occurrence, such as poor academic performance, arguments with parents and/or siblings, and not being accepted by peers. Several studies have found associations between uncontrollable NLEs, either individual items or total NLEs, and heavy drinking and/or SU disorders in children and adolescents (Kilpatrick et al. Citation2000; Pilowsky et al. Citation2009; Enoch Citation2011; Fite et al. Citation2016; Zilberman et al. Citation2019). However, NLEs/SU-initiation associations are less well-understood and few studies investigated the impact of NLEs on alcohol initiation (Hyman et al. Citation2006; Rothman et al. Citation2008). Moreover, the impact of NLEs on cannabis initiation remains unknown, and no study has compared whether controllable and uncontrollable NLEs differentially impact SU initiation.
Greater susceptibility to initiating SU during adolescence has been linked to imbalanced development of the prefrontal cortex (PFC) and subcortical regions (Casey et al. Citation2008; Steinberg et al. Citation2008; Hardee et al. Citation2018). Specifically, maturational imbalance or dual-systems models posit that cortical and subcortical brain areas mature at different rates with differential impacts on socioemotional processing (mainly involving subcortical regions) relative to cognitive control processing (mainly involving the PFC) (Casey et al. Citation2008). Thus, relative to adults, adolescents are less likely to inhibit impulses related to seeking novelty or sensation, leading to increased risk-taking behaviours including SU initiation. Indeed, the PFC and some subcortical regions, such as the orbitofrontal cortex, caudate, nucleus accumbens, and amygdala, are defined as key brain structures in addiction circuits (Koob and Volkow Citation2016). In addition, alcohol and cannabis share some commonality in addiction circuits but also involve distinct neural alterations [see a recent meta-analysis (Klugah-Brown et al. Citation2020)]. Therefore, it is important to investigate whether overlapping and/or distinct brain structures may mediate NLEs/SU-initiation associations.
The investigation quantified the associations between types of NLEs and initiation of alcohol use (AUI) and cannabis use (CUI), and explored brain mediators of such associations. Analyses used data from the National Consortium on Alcohol and Neurodevelopment in Adolescence (NCANDA) (Brown et al. Citation2015). We hypothesised that both controllable and uncontrollable NLEs would relate to AUI and CUI and that overlapping and unique brain regions would partially mediate these relationships.
Methods
Study design
The longitudinal NCANDA cohort (Brown et al. Citation2015) consists of 831 participants aged 12–21 years at baseline, with a range of measures including self-reported use patterns, types of life events, and neuroimaging data at baseline and annual follow-ups. Participants with any use of alcohol or cannabis at baseline, initiation of any illicit substance, or initiation of regular use of any substance (legal or illegal) before their alcohol or cannabis first use were excluded from analyses. Throughout this paper, SU refers to the use of alcohol or cannabis. Participants who never initiated alcohol or cannabis use up through follow-up year four were classified as non-using (NU) participants.
A prospective study design was employed. Visit time points at which self-reported alcohol or cannabis initiation occurred, along with the number of NLEs in the past 12 months, were identified for each participant. Neuroimaging data at the visit before the onset of SU were extracted. For NU participants, NLEs were extracted after the visit at which their last neuroimaging data were collected. Participants with missing data on analysis variables were excluded. Final analyses included data from 207 AUI, 168 CUI, and 128 NU participants.
MRI preprocessing
The structural imaging data extracted from the NCANDA_PUBLIC_3Y_STRUCTURAL_V01 release (Pohl et al. Citation2023a) were pre-processed and quality-controlled by the NCANDA team (Pfefferbaum et al. Citation2018). Structural brain features included surface area, thickness, and gray-matter volume (GMV) of 68 cortical regions from the Desikan-Killiany atlas (Desikan et al. Citation2006) and 17 subcortical GMVs (Zhao et al. Citation2021, Citation2022). Eight participants with estimated intracranial volumes smaller than 100 mm3 were excluded from analyses.
Onset of alcohol and cannabis initiation
SU and other behavioural measures were extracted from the NCANDA_PUBLIC_4Y_REDCAP_V01 release (Pohl et al. Citation2023b). Participants were defined as having AUI if they had a full drink of alcohol for the first time. Having a taste or sip of alcohol was not counted as having initiated alcohol use. Participants were defined as having CUI if they had ever tried a puff or more of cannabis for the first time. Among 207 AUI and 168 CUI participants, 100 initiated both alcohol and cannabis after their baseline visit. Thirty-six initiated alcohol first, 16 cannabis first, and 48 both in the same year. On average, the second substance was initiated 8.56 () months after the first.
Controllable and uncontrollable NLEs
The 61-item Life Events Questionnaire (LEQ) (Masten et al. Citation1994), collected at each annual visit, measured negative, positive, and ambiguous life events. To explore dose-response relationships, this study focused on the number of endorsed controllable and uncontrollable NLEs in the past 12 months (eFigure 1). Briefly, controllable NLEs included 13 events (e.g., breaking up with boyfriend/girlfriend, arguments with parents and/or siblings, running away from home, failing a grade, poor academic performance, peer non-acceptance, legal trouble, and suicidal thoughts), while uncontrollable events consisted of 22 items (e.g., death and/or illness of family members, familial financial problems, parental separation and divorce, parental job loss, arguments between family members, and family members developing severe emotional problems). The LEQ has good predictive validity (Segrin Citation2001).
Statistical analysis
Generalised linear regression models were fitted to assess relationships between types of NLEs and substance initiation. As an exploratory analysis, a two-step discovery approach (Zhao and Castellanos Citation2016) identified brain mediators of controllable NLEs on SU initiation. In Step 1, an iterative Random Forest (RF) algorithm (Basu et al. Citation2018) was used to select the most important brain features for predicting AUI and CUI, respectively. The number of brain features to retain for Step 2 was determined empirically by identifying the cut-off after which there was a significant drop in variable importance scores. RF analyses suggested thickness of the left bank of the superior temporal sulcus (STS) as a common candidate brain mediator for AUI and CUI, with left thalamus proper GMV as a unique potential mediator for AUI, and left caudate GMV for CUI (eFigure 1). In Step 2, causal mediation analyses determined the potential mediating roles of identified brain features in Step 1. Mediation analyses decomposed the total effects of NLEs on the outcomes into direct and indirect (i.e., mediation) effects with their confidence intervals (95%CIs) estimated using the quasi-Bayesian approximation approach (Imai et al. Citation2010).
We controlled for age, gender, race/ethnicity, parental highest education level, pubertal stage, scanner type, and estimated intracranial volume in all models. The Benjamini–Hochberg procedure was used to control for a False Discovery Rate (FDR) at the 0.05 level. Adjusted p-values were reported unless specifically noted. All analyses were performed in R (V4.1.2).
Results
Group differences in demographic characteristics and NLEs
Both AUI and CUI groups did not differ from NU participants () on basic demographics (e.g., age, gender, parental education) except that both AUI and CUI groups had more White participants, and the AUI group had more participants from high-income families (i.e., ≥$100,000). The family income and AUI association could possibly be explained by financial resources available for drug purchasing in the high-income families. This finding supports the view from a recent large-scale study that increases in real alcohol prices could lead to delayed AUI (Paraje et al. Citation2021).
eFigure 2 shows the distribution of controllable and uncontrollable NLEs. Both AUI ( p-value = 0.002) and CUI (
p-value < 0.001) groups reported a higher mean number of controllable NLEs than NU participants. However, the mean numbers of uncontrollable NLEs in NU participants did not differ from those in the AUI (
p-value = 0.121) and CUI (
p-value = 0.121) groups. Item-level information on controllable NLEs is included in eFigure 3.
Table 1. Demographic characteristics of the study sample.
Controllable NLEs link to initiating alcohol and cannabis use
Controllable NLEs increased odds of AUI ( SE = 0.12, p-value = 0.004) and CUI (
SE = 0.12, p-value < 0.001). eFigure 4 shows having one controllable NLE increased the odds of AUI by 48% (95%CI [1.17, 1.89]) and CUI by 69% (95%CI [1.34, 2.17]), respectively. Having two and three controllable NLEs in the past 12 months increased the odds by 118 and 224% for AUI and 185 and 381% for CUI, respectively. In contrast, uncontrollable NLEs were not associated (unadjusted p-value = 0.051) with AUI (
SE = 0.13, p-value = 0.103) or CUI (
SE = 0.14, p-value = 0.103).
Association among controllable NLEs, brain mediators, and onset of substance use
Cortical thickness in the left banks of the STS mediated the effects of controllable NLEs on SU initiation (). Having one controllable NLE was associated with an increase in cortical thickness by 0.026 mm ( SE = 0.011, p-value = 0.028; ) in the AUI sample and by 0.032 mm (
SE = 0.011, p-value = 0.016; ) in the CUI sample. Moreover, controlling for the effect of the exposure, increasing cortical thickness in left banks of the STS by 0.026 mm (i.e., corresponding to having one controllable NLE) increased the odds of AUI by 5.8% (
SE = 0.628, p-value = 0.002; ) and CUI by 5.5% (
SE = 0.615, p-value = 0.002; ), respectively. In addition, in the CUI sample, an increase in controllable NLEs by one event was associated with a 0.06 cm3 increase in left caudate GMV (
SE = 0.021, p-value = 0.016; ). While controlling for exposure, a 0.06 cm3 increase in left caudate gray-matter volume was associated with increasing the odds of CUI by 5.3% (
SE = 0.340, p-value = 0.011; ).
Figure 1. Mediation analysis shows how brain features explain relationships between controllable NLEs and initiation of alcohol and cannabis, respectively. Potential confounding effects due to age, gender, race/ethnicity, parental highest education level, pubertal stage, scanner type, and estimated intracrine volume were controlled in the mediation analyses. Cortical thickness of the left banks of the superior temporal sulcus mediates the effects of controllable NLEs on initiations of alcohol (A) and cannabis (B) use, respectively, (C) left caudate gray-matter volume mediates the effect of controllable NLEs on initiation of cannabis use. The figure includes the following regression coefficients: (1) the effects of controllable NLEs on each brain mediator; (2) the effects of brain mediators on initiation of alcohol and cannabis first use when adjusting for controllable NLEs; (3) the direct and indirect associations along with the 95%CIs between controllable NLEs and the onset of alcohol or cannabis use, respectively. *p < 0.05. **p < 0.001. The right column shows the location of each corresponding brain region. Note, NLEs and substance initiation data were collected at the same visit. While brain feature measures were collected at the visit before substance initiation, therefore, brain immaturity should be regarded as a pre-existing condition in the mediation analyses.
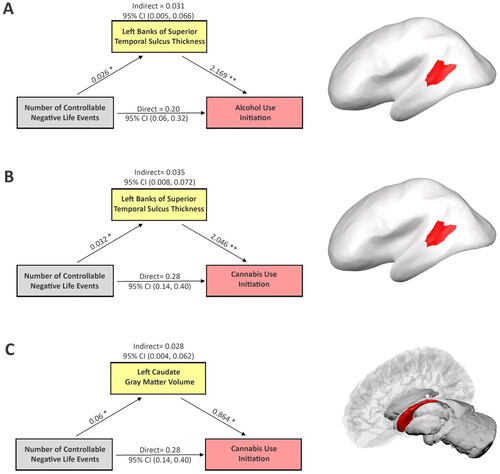
Left banks of the STS thickness partially mediated the effects of controllable NLEs on both AUI (indirect effect = 0.031, 95% CI: (0.005, 0.066), p-value = 0.04, proportion mediated:13.1%, ) and CUI (indirect effect = 0.035, 95% CI: (0.008, 0.072), p-value = 0.02, proportion mediated:11.0%, ). Additionally, left caudate GMV mediated the effect of controllable NLEs on CUI (indirect effect = 0.028, 95% CI: (0.004, 0.062), p-value = 0.04, proportion mediated:8.7%, ). Taken together, findings suggest children and adolescents with less mature brains in the left banks of STS and/or caudate are at increased risk of initiating SU in response to controllable NLEs.
Discussion
This investigation examined relationships between types of NLEs and SU initiation and explored brain structural mediators. Our findings partially supported hypotheses and showed that controllable, but not uncontrollable, NLEs were associated with AUI and CUI. Moreover, mediation analyses suggest that individuals with less mature brain structures at the time of the NLEs in regions involved in social brain networks (left banks of the STS) and impulsivity and other cognitive functions (left caudate) might be at greater risk for SU initiation.
Controllable NLEs as a modifiable risk factor for AUI and CUI
Prior research has assessed the impacts of total or uncontrollable NLEs on SU problems including heavy drinking and SU disorders (Kilpatrick et al. Citation2000; Hyman et al. Citation2006; McBride et al. Citation2009; Pilowsky et al. Citation2009; Enoch Citation2011; Fite et al. Citation2016; Zilberman et al. Citation2019; Schmaling et al. Citation2020). Only limited retrospective studies have explored the negative impacts of uncontrollable NLEs (e.g., childhood emotional abuse) on onset (Rothman et al. Citation2008) and onset age of alcohol use (Hyman et al. Citation2006). This investigation separated controllable from uncontrollable NLEs and established a dose-response relationship between controllable, but not uncontrollable, NLEs and early initiation of alcohol and cannabis use. This indicates that controllable NLEs may be more critical than uncontrollable NLEs in impacting SU initiation, thus highlighting an important but arguably overlooked role of controllable NLEs. Unlike uncontrollable NLEs (e.g., sexual assault, death of close ones), controllable NLEs represents a modifiable risk factor, as the occurrences of controllable NLEs (e.g., arguments with parents or siblings, poor academic performance) may be controlled and preventable. Moreover, controllable NLEs are closely related to individual’s behaviours and may occur more frequently during adolescence (Jhang Citation2020). The observation that controllable events may have a bigger impact on a person’s substance use trajectory may be because they can create a sense of personal responsibility or accountability. When a negative outcome is perceived as being within a person’s control, they may feel a strong sense of guilt or regret for not making different choices. This can lead to feelings of failure, low self-esteem, and negative self-perceptions. Furthermore, the sense of control over one’s life is an important aspect of a person’s sense of well-being, so any perceived loss of control can have a significant impact. On the other hand, uncontrollable events are often seen as random or outside of a person’s control, which can reduce feelings of guilt or responsibility and mitigate their impact on a person’s life. Taken together, focusing on these psychological processes might be an important target for preventing youth alcohol and substance use initiation.
Brain maturity in social-brain-network regions partially mediates effects of controllable NLEs
The underlying processes involved in NLE/SU-initiation relationships remain unclear; however, our results provide some evidence that social cognition could contribute significantly. Specifically, adolescents are more sensitive than children to acceptance and rejection by their peers and others (Blakemore Citation2008), and this period is important for social competence development. Four of the five most endorsed NLEs are social in nature (eFigure 1), including breaking up with boyfriend/girlfriend, having too many arguments with siblings, having too many arguments with parents, and not getting into a group/activity in which he/she wanted to participate. The fifth item, performing worse than expected on an important exam/in a course, might also be related to social skills, given that academic underachievement has been frequently associated with more child-parent conflicts, peer shaming, and negative social and behavioural skills (Dyck and Piek Citation2010). Longitudinally, more NLEs that are social in nature (e.g., personal conflicts) have been associated with poorer social skills (Segrin Citation2001). These findings along with the self-change model (Kiecolt Citation1994) suggest that individuals experiencing controllable NLEs may have diminished self-perceived competence and poor social skills (Segrin Citation2001). Consequently, as a ‘self-coping’ or ‘self-medication’ strategy (Khantzian Citation1985), they may choose to use substances over the natural rewards of substance-free social interactions to experience positive feelings. Mediation analyses also provide supporting evidence for the involvement of social skills in the impacts of controllable NLEs on SU initiation. We found that greater thickness of the left banks of the STS and greater left caudate GMV mediated the effects of controllable NLEs on SU initiation. Both brain structures play important roles in social cognition. Taken together, the nature of the NLE and the brain structures mediating associations between NLE and SU initiation are consistent with the notion that altered social cognition (e.g., exaggerated response to acceptance or rejection) may contribute to early-onset SU in these participants.
First, the STS bank is a region exhibiting robust changes in cortical thickness during adolescence (Modabbernia et al. Citation2021) and is located mainly in posterior STS (eFigure 3). The posterior STS is a key component in the social brain network (Blakemore Citation2008) implicated in social cognition including language comprehension and social processing (Beauchamp Citation2015). This region processes information about the nature of social interactions (Isik et al. Citation2017), is involved in simulating the mental processes of others (Beauchamp Citation2015), and responds to learning from romantic interest and rejection (Cooper et al. Citation2014). Although the posterior STS has been mainly studied in social cognition research, there is an increasing understanding of functional specialisation within the STS for different social tasks (Beauchamp Citation2015). The left posterior STS has been associated with addictive behaviours, such as internet gaming disorder (Lee et al. Citation2020) and cue-induced craving in recently abstinent patients with alcohol use disorder (Schneider et al. Citation2001). Thus, it is conceivable that brain immaturity in the banks of the STS may be linked to poor social processing skills in children and adolescents who are experiencing controllable NLEs, rendering them more vulnerable to risk behaviours.
Second, the role of caudate nucleus in addictions has been well documented (Koob and Volkow Citation2010), and its link to social intelligence has emerged. The caudate is an information hub that receives axons from nearly all parts of the cortex apart from primary visual, auditory, and olfactory cortices (Grahn et al. Citation2008). Increasing evidence supports the contribution of the caudate to goal-directed learning and incentive motivation (see Grahn et al. Citation2008 and references therein). The caudate may also play a causal role in decision-making that balances internal reward and external uncertain visual information (Doi et al. Citation2020). Interestingly, the functional integration between the caudate and brain regions involved in the Theory of Mind network was recently shown to be positively correlated with social intelligence, where social intelligence involves abilities to have effective interpersonal interactions (Votinov et al. Citation2021). Taken together, the mediation findings suggest that imbalanced maturation of different brain regions involved in social-information processing may mediate the effects of controllable NLEs on SU initiation in adolescents.
It is worth noting that the impacts of NLEs on SU initiation may also be mediated by other processes (e.g., emotional regulation). For example, activation of key brain regions including the PFC (along with the insula, cingulate, and hypothalamus), hippocampus, amygdala, and temporal cortex in response to emotional treatments has been found to mediate the effect of NLEs in the development of anxiety or depression disorders (Gollier-Briant et al. Citation2016; Brieant et al. Citation2021). Given that emotional regulation undergoes considerable maturation in childhood and early-to-mid adolescence and is implicated in addictions (Arain et al. Citation2013), it will be important to assess whether such processes may also mediate the effects of controllable NLEs on SU initiation.
Limitations
Finding interpretations are limited by several factors. First, the estimated effect sizes for uncontrollable NLEs/SU-initiations were small (i.e., odds ratio = 1.28 for AUI and 1.3 for CUI). Given these effect sizes and the current sample sizes (n = 335 for AUI and 296 for CUI), post-hoc power analyses revealed that our study had ∼40% power to detect a significant uncontrollable NLEs/SU-initiation association. In fact, one prior study (n = 3592) has linked uncontrollable NLEs with an increased likelihood of initiating alcohol use before age 14 (Rothman et al. Citation2008). Therefore, future research on uncontrollable NLEs and SU initiation is needed. Second, the data-driven approach was used to select brain mediators of controllable NLEs. Brain features not identified as top predictors in the RF analyses may also mediate the effects of controllable NLEs. Third, the thickness of STS banks as a common brain mediator of alcohol and cannabis initiation should be interpreted with caution. Since a large portion of the participants (n = 100, corresponding to 48.3% of AUI and 59.5% of CUI) initiated both alcohol and cannabis use, further research is warranted. Moreover, it is a correlational study by nature, which inherently restricts us from making causal inferences. Fourth, the psychiatric history of the study sample is unclear, which could introduce bias in the analysis of the results. Fifth, the study did not measure impulsivity or social processing so the interpretation of the results regarding these functions is limited. Finally, additional factors could contribute to the onset of substance use. For example, in certain cultures, the consumption of alcohol by children is more common as normal meal-time activities. Within these contexts, adolescent-onset of alcohol use may be less likely to cause negative health consequences (DeWit et al. Citation2000). Similarly, cannabis use may have different underlying motivations. Taken together, some adolescents may view their first drink or use of cannabis as a positive rewarding event personally, not necessarily as an escape from emotional and/or social problems. Nonetheless, considerable evidence suggests negative social and mental health consequences of adolescent-onset of cannabis (Meier et al. Citation2012; Melchior et al. Citation2017) and alcohol (DeWit et al. Citation2000) use. Therefore, it is important to understand which circumstances related to SU initiation are detrimental and which are not. Given that negative urgency prospectively statistically predicts greater occurrences of controllable NLEs (Liu and Kleiman Citation2012) and has been linked to problematic SU (Kaiser et al. Citation2012; Um et al. Citation2019), we suspect SU initiation induced by NLEs could lead to potentially detrimental effects, suggesting the need for future research on characterising neural mechanisms underlying developmental pathways from negative urgency to NLEs and subsequent problematic SU.
Conclusion
In conclusion, this investigation showed that controllable, but not uncontrollable, NLEs were associated with substance initiation in adolescents. Moreover, mediation analyses suggested an important role for the banks of the STS and caudate in exerting the effects of controllable NLEs on substance initiation, pointing towards immature social processing as a potential target. Future investigation is to examine these relationships longitudinally in larger cohorts.
Author’s contributions
Y.Z. and M.P.P. designed the analytic plan for this paper. Y.Z. conducted the statistical analysis and wrote the first draft of the manuscript. All authors revised, contributed to, and approved the final manuscript.
Acknowledgements
The authors would like to extend special thanks to Duncan B. Clark, MD, Ph.D. (Department of Psychiatry, University of Pittsburgh) for sharing critical information on negative life events.
Disclosure statement
Dr. Paulus is an advisor to Spring Care, Inc., a behavioural health start-up, he has received royalties for an article about methamphetamine in UpToDate. Dr. Potenza has consulted for Opiant Therapeutics, Game Day Data, the Addiction Policy Forum, AXA, and Idorsia Pharmaceuticals; has been involved in a patent application with Yale University and Novartis; has received research support from Mohegan Sun Casino and the National Centre for Responsible Gaming; has participated in surveys, mailings or telephone consultations related to drug addiction, impulse-control disorders or other health topics; has consulted for and/or advised gambling and legal entities on issues related to impulse-control/addictive disorders; has provided clinical care in a problem gambling services program; has performed grant reviews for research-funding agencies; has edited journals and journal sections; has given academic lectures in grand rounds, CME events and other clinical or scientific venues; and has generated books or book chapters for publishers of mental health texts. The other authors do not report disclosures.
Data availability statement
The data used in this paper was retrieved on 21 October 2021, from Sage Bionetworks Synapse (https://doi.org/10.7303/syn22213272).
Additional information
Funding
References
- Arain M, Haque M, Johal L, Mathur P, Nel W, Rais A, Sandhu R, Sharma S. 2013. Maturation of the adolescent brain. Neuropsychiatr Dis Treat. 9:449–461. doi: 10.2147/NDT.S39776.
- Bagot KS, Milin R, Kaminer Y. 2015. Adolescent initiation of cannabis use and early-onset psychosis. Subst Abus. 36(4):524–533. doi: 10.1080/08897077.2014.995332.
- Basu S, Kumbier K, Brown JB, Yu B. 2018. Iterative random forests to discover predictive and stable high-order interactions. Proc Natl Acad Sci USA. 115(8):1943–1948. doi: 10.1073/pnas.1711236115.
- Beauchamp MS. 2015. The social mysteries of the superior temporal sulcus. Trends Cogn Sci. 19(9):489–490. doi: 10.1016/j.tics.2015.07.002.
- Blakemore SJ. 2008. The social brain in adolescence. Nat Rev Neurosci. 9(4):267–277. doi: 10.1038/nrn2353.
- Brieant AE, Sisk LM, Gee DG. 2021. Associations among negative life events, changes in cortico-limbic connectivity, and psychopathology in the ABCD Study. Dev Cogn Neurosci. 52:101022. doi: 10.1016/j.dcn.2021.101022.
- Brown SA, Brumback T, Tomlinson K, Cummins K, Thompson WK, Nagel BJ, De Bellis MD, Hooper SR, Clark DB, Chung T, et al. 2015. The National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA): a multisite study of adolescent development and substance use. J Stud Alcohol Drugs. 76(6):895–908. doi: 10.15288/jsad.2015.76.895.
- Casey BJ, Getz S, Galvan A. 2008. The adolescent brain. Dev Rev. 28(1):62–77. doi: 10.1016/j.dr.2007.08.003.
- Christensen AI, Davidsen M, Koushede V, Juel K. 2022. Mental health and the risk of negative social life events: a prospective cohort study among the adult Danish population. Scand J Public Health. 50(2):189–198. doi: 10.1177/1403494820944718.
- Cooper JC, Dunne S, Furey T, O'Doherty JP. 2014. The role of the posterior temporal and medial prefrontal cortices in mediating learning from romantic interest and rejection. Cereb Cortex. 24(9):2502–2511. doi: 10.1093/cercor/bht102.
- Crews F, He J, Hodge C. 2007. Adolescent cortical development: a critical period of vulnerability for addiction [Research Support, N.I.H., Extramural Review]. Pharmacol Biochem Behav. 86(2):189–199. doi: 10.1016/j.pbb.2006.12.001.
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, et al. 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021.
- DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. 2000. Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry. 157(5):745–750. doi: 10.1176/appi.ajp.157.5.745.
- Doi T, Fan Y, Gold JI, Ding L. 2020. The caudate nucleus contributes causally to decisions that balance reward and uncertain visual information. eLife. 9:e56694. doi: 10.7554/eLife.56694.
- Dyck M, Piek J. 2010. How to distinguish normal from disordered children with poor language or motor skills. Int J Lang Commun Disord. 45(3):336–344. doi: 10.3109/13682820903009503.
- Enoch MA. 2011. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology. 214(1):17–31. doi: 10.1007/s00213-010-1916-6.
- Feinstein EC, Richter L, Foster SE. 2012. Addressing the critical health problem of adolescent substance use through health care, research, and public policy. J Adolesc Health. 50(5):431–436. doi: 10.1016/j.jadohealth.2011.12.033.
- Fite PJ, Gabrielli J, Cooley JL, Rubens SL, Pederson CA, Vernberg EM. 2016. Associations between physical and relational forms of peer aggression and victimization and risk for substance use among elementary school-age youth. J Child Adolesc Subst Abuse. 25(1):1–10. doi: 10.1080/1067828X.2013.872589.
- Gollier-Briant F, Paillère-Martinot M-L, Lemaitre H, Miranda R, Vulser H, Goodman R, Penttilä J, Struve M, Fadai T, Kappel V, et al. 2016. Neural correlates of three types of negative life events during angry face processing in adolescents. Soc Cogn Affect Neurosci. 11(12):1961–1969. doi: 10.1093/scan/nsw100.
- Grahn JA, Parkinson JA, Owen AM. 2008. The cognitive functions of the caudate nucleus. Prog Neurobiol. 86(3):141–155. doi: 10.1016/j.pneurobio.2008.09.004.
- Gray KM, Squeglia LM. 2018. Research review: what have we learned about adolescent substance use? [research support, non-U.S. Gov’t review]. J Child Psychol Psychiatry. 59(6):618–627.
- Hardee JE, Cope LM, Martz ME, Heitzeg MM. 2018. Review of neurobiological influences on externalizing and internalizing pathways to alcohol use disorder. Curr Behav Neurosci Rep. 5(4):249–262. doi: 10.1007/s40473-018-0166-5.
- Hyman SM, Garcia M, Sinha R. 2006. Gender specific associations between types of childhood maltreatment and the onset, escalation and severity of substance use in cocaine dependent adults. Am J Drug Alcohol Abuse. 32(4):655–664. doi: 10.1080/10623320600919193.
- Hyman SM, Sinha R. 2009. Stress-related factors in cannabis use and misuse: implications for prevention and treatment. J Subst Abuse Treat. 36(4):400–413. doi: 10.1016/j.jsat.2008.08.005.
- Imai K, Keele L, Tingley D. 2010. A general approach to causal mediation analysis [research support, U.S. Gov’t, non-P.H.S.]. Psychol Methods. 15(4):309–334. doi: 10.1037/a0020761.
- Isik L, Koldewyn K, Beeler D, Kanwisher N. 2017. Perceiving social interactions in the posterior superior temporal sulcus. Proc Natl Acad Sci USA. 114(43):E9145–E9152. doi: 10.1073/pnas.1714471114.
- Jhang F-H. 2020. Uncontrollable and controllable negative life events and changes in mental health problems: exploring the moderation effects of family support and self-efficacy in economically disadvantaged adolescents. Child Youth Serv Rev. 118:105417. doi: 10.1016/j.childyouth.2020.105417.
- Jordan CJ, Andersen SL. 2017. Sensitive periods of substance abuse: early risk for the transition to dependence [review]. Dev Cogn Neurosci. 25:29–44. doi: 10.1016/j.dcn.2016.10.004.
- Kaiser AJ, Milich R, Lynam DR, Charnigo RJ. 2012. Negative urgency, distress tolerance, and substance abuse among college students. Addict Behav. 37(10):1075–1083. doi: 10.1016/j.addbeh.2012.04.017.
- Khantzian EJ. 1985. The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. Am J Psychiatry. 142(11):1259–1264.
- Kiecolt KJ. 1994. Stress and the decision to change oneself: a theoretical model. Soc Psychol Q. 57(1):49–63. doi: 10.2307/2786974.
- Kilpatrick DG, Acierno R, Saunders B, Resnick HS, Best CL, Schnurr PP. 2000. Risk factors for adolescent substance abuse and dependence: data from a national sample. J Consult Clin Psychol. 68(1):19–30. doi: 10.1037//0022-006x.68.1.19.
- Klugah-Brown B, Di X, Zweerings J, Mathiak K, Becker B, Biswal B. 2020. Common and separable neural alterations in substance use disorders: a coordinate-based meta-analyses of functional neuroimaging studies in humans. Hum Brain Mapp. 41(16):4459–4477. doi: 10.1002/hbm.25085.
- Koob GF, Volkow ND. 2010. Neurocircuitry of addiction. Neuropsychopharmacology. 35(1):217–238. doi: 10.1038/npp.2009.110.
- Koob GF, Volkow ND. 2016. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 3(8):760–773. doi: 10.1016/S2215-0366(16)00104-8.
- Lee J, Lee D, Namkoong K, Jung YC. 2020. Aberrant posterior superior temporal sulcus functional connectivity and executive dysfunction in adolescents with internet gaming disorder. J Behav Addict. 9(3):589–597. doi: 10.1556/2006.2020.00060.
- Liu R, Kleiman E. 2012. Impulsivity and the generation of negative life events: the role of negative urgency. Pers Indiv Differ. 53(5):609–612. doi: 10.1016/j.paid.2012.05.003.
- Low NC, Dugas E, O'Loughlin E, Rodriguez D, Contreras G, Chaiton M, O'Loughlin J. 2012. Common stressful life events and difficulties are associated with mental health symptoms and substance use in young adolescents. BMC Psychiatry. 12(1):116. doi: 10.1186/1471-244X-12-116.
- Masten A, Neemann J, Andenas S. 1994. Life events and adjustment in adolescents: the significance of event independence desirability, and chronicity. J Res Adolesc. 4(1):71–97. doi: 10.1207/s15327795jra0401_5.
- McBride O, Adamson G, Bunting BP, McCann S. 2009. Characteristics of DSM-IV alcohol diagnostic orphans: drinking patterns, physical illness, and negative life events. Drug Alcohol Depend. 99(1–3):272–279. doi: 10.1016/j.drugalcdep.2008.08.008.
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, McDonald K, Ward A, Poulton R, Moffitt TE. 2012. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci USA. 109(40):E2657–E2664. doi: 10.1073/pnas.1206820109.
- Melchior M, Bolze C, Fombonne E, Surkan PJ, Pryor L, Jauffret-Roustide M. 2017. Early cannabis initiation and educational attainment: is the association causal? Data from the French TEMPO study. Int J Epidemiol. 46(5):1641–1650. doi: 10.1093/ije/dyx065.
- Modabbernia A, Reichenberg A, Ing A, Moser DA, Doucet GE, Artiges E, Banaschewski T, Barker GJ, Becker A, Bokde ALW, et al. 2021. Linked patterns of biological and environmental covariation with brain structure in adolescence: a population-based longitudinal study. Mol Psychiatry. 26(9):4905–4918. doi: 10.1038/s41380-020-0757-x.
- Paraje GR, Guindon GE, Chaloupka FJ. 2021. Prices, alcohol use initiation and heavy episodic drinking among Chilean youth. Addiction. 116(3):485–494. doi: 10.1111/add.15167.
- Pfefferbaum A, Kwon D, Brumback T, Thompson WK, Cummins K, Tapert SF, Brown SA, Colrain IM, Baker FC, Prouty D, et al. 2018. Altered brain developmental trajectories in adolescents after initiating drinking. Am J Psychiatry. 175(4):370–380. doi: 10.1176/appi.ajp.2017.17040469.
- Pilowsky DJ, Keyes KM, Hasin DS. 2009. Adverse childhood events and lifetime alcohol dependence. Am J Public Health. 99(2):258–263. doi: 10.2105/AJPH.2008.139006.
- Pohl KM, Sullivan EV, Pfefferbaum A. 2023a. The ‘NCANDA_PUBLIC_3Y_STRUCTURAL_V01’ Data Release of the National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA). Sage Bionetworks Synapse.
- Pohl KM, Sullivan EV, Pfefferbaum A. 2023b. The ‘NCANDA_PUBLIC_4Y_REDCAP_V01’ Data Release of the National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA). Sage Bionetworks Synapse.
- Rothman EF, Edwards EM, Heeren T, Hingson RW. 2008. Adverse childhood experiences predict earlier age of drinking onset: results from a representative US sample of current or former drinkers. Pediatrics. 122(2):e298–e304. doi: 10.1542/peds.2007-3412.
- Schmaling KB, Blume AW, Skewes MC. 2020. Negative life events and incident alcohol use disorders among ethnic minorities. J Ethn Subst Abuse. 19(2):327–342. doi: 10.1080/15332640.2018.1548322.
- Schneider F, Habel U, Wagner M, Franke P, Salloum JB, Shah NJ, Toni I, Sulzbach C, Honig K, Maier W, et al. 2001. Subcortical correlates of craving in recently abstinent alcoholic patients. Am J Psychiatry. 158(7):1075–1083. doi: 10.1176/appi.ajp.158.7.1075.
- Segrin C. 2001. Social skills and negative life events: testing the deficit stress generation hypothesis. Curr Psychol. 20(1):19–35. doi: 10.1007/s12144-001-1001-8.
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. 2008. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Dev Psychol. 44(6):1764–1778. doi: 10.1037/a0012955.
- Stern GS, McCants TR, Pettine PW. 1982. Stress and illness: controllable and uncontrollable life events’ relative contributions. Pers Soc Psychol Bull. 8(1):140–145. doi: 10.1177/014616728281022.
- Swahn MH, Bossarte RM, Choquet M, Hassler C, Falissard B, Chau N. 2012. Early substance use initiation and suicide ideation and attempts among students in France and the United States. Int J Public Health. 57(1):95–105. doi: 10.1007/s00038-011-0255-7.
- Um M, Whitt ZT, Revilla R, Hunton T, Cyders MA. 2019. Shared neural correlates underlying addictive disorders and negative urgency. Brain Sci. 9(2):36. doi: 10.3390/brainsci9020036.
- Veenstra MY, Lemmens PH, Friesema IH, Garretsen HF, Knottnerus JA, Zwietering PJ. 2006. A literature overview of the relationship between life-events and alcohol use in the general population. Alcohol Alcohol. 41(4):455–463. doi: 10.1093/alcalc/agl023.
- Votinov M, Myznikov A, Zheltyakova M, Masharipov R, Korotkov A, Cherednichenko D, Habel U, Kireev M. 2021. The interaction between caudate nucleus and regions within the theory of mind network as a neural basis for social intelligence. Front Neural Circuits. 15:727960. doi: 10.3389/fncir.2021.727960.
- Zhao Y, Castellanos FX. 2016. Annual research review: discovery science strategies in studies of the pathophysiology of child and adolescent psychiatric disorders – promises and limitations [review]. J Child Psychol Psychiatry. 57(3):421–439. doi: 10.1111/jcpp.12503.
- Zhao Y, Constable RT, Hien D, Chung T, Potenza MN. 2021. Brain anatomical covariation patterns linked to binge drinking and age at first full drink. Neuroimage Clin. 29:102529. doi: 10.1016/j.nicl.2020.102529.
- Zhao Y, Paulus M, Bagot KS, Constable RT, Yaggi HK, Redeker NS, Potenza MN. 2022. Brain structural covariation linked to screen media activity and externalizing behaviors in children. J Behav Addict. 11(2):417–426. doi: 10.1556/2006.2022.00044.
- Zilberman N, Yadid G, Efrati Y, Rassovsky Y. 2019. Negative and positive life events and their relation to substance and behavioral addictions. Drug Alcohol Depend. 204:107562. doi: 10.1016/j.drugalcdep.2019.107562.

