Abstract
Postpartum depression has deleterious effects on childbearing persons globally. Existing treatments have been largely extrapolated from those for other forms of depression and have included pharmacotherapy, psychotherapy, and neuromodulation. Hormonal treatments with oestrogen and progestogens, thought to be a rational approach to treatment in response to an emerging literature on the pathophysiology of postpartum depression, have only limited evidence for efficacy to date. Novel antidepressant development with allopregnanolone analogues, in contrast, has proven a promising avenue for the development of rationally designed and efficacious treatments. This state-of-the-art review presents the evidence for the current standard-of-care pharmacotherapy, hormonal treatment, and emerging allopregnanolone analogues for the treatment of postpartum depression along with a discussion of the current understanding of its neuroactive steroid-driven pathophysiology.
Introduction
Postpartum depression (PPD) is a major complication of pregnancy and childbirth, with a pooled prevalence of 14% in one meta-analysis of global data (Liu et al. Citation2022). Although major depressive disorder with peripartum onset is defined as development of a major depressive episode during pregnancy or within four weeks postpartum by the Diagnostic and Statistical Manual of Mental Disorders 5 (American Psychiatric Association Citation2022), epidemiological studies suggest the period of elevated risk for development of postpartum depressive symptoms may be longer (Wisner et al. Citation2010), and genetic evidence indicates that episodes that arise during pregnancy may be aetiologically distinct (Figueiredo et al. Citation2015). PPD has been associated with maternal suicidal ideation, poor quality of life, more relationship difficulties, decreased household functioning, and lactation difficulties as well as adverse effects on infant motor, cognitive, language, social, and emotional development (Slomian et al. Citation2019). Mental health conditions are a leading cause of pregnancy-related deaths in the United States (US) (mTrost et al. Citation2022), and completed suicide is a leading cause of direct maternal mortality in the first postpartum year per recent data from the United Kingdom and Ireland (Knight et al. Citation2021). Depression has also been identified as a risk factor in infanticide and filicide (Flynn et al. Citation2013).
Treatment for PPD includes psychotherapy, antidepressants, hormonal treatments, and neuromodulation. Psychotherapy, particularly cognitive behavioural therapy, and interpersonal psychotherapy has a well-established evidence base for its efficacy in the treatment of PPD (Cuijpers et al. Citation2008), however, the use of medications instead of or in addition to psychotherapy may be warranted by severe symptoms or by failure to respond to psychotherapy only. Many standard-of-care (SOC) antidepressants for PPD are extrapolated from the treatment of other depressive disorders; recent advances in the understanding of the pathophysiology of PPD, however, have led to the development of novel antidepressants specific to the condition. The present article will focus on the current SOC antidepressants as well as existing evidence for hormonal treatment of PPD while highlighting novel rational antidepressant design emerging as the purported pathophysiology of PPD is increasingly described.
Antidepressants in the treatment of PPD
Selective serotonin reuptake inhibitors (SSRIs)
SSRIs are widely considered the first line of pharmacotherapy for PPD. As the present work concerns the treatment of depression in the postpartum, a review of the risks of using any of the following medications in pregnancy is considered beyond its scope. The authors recommend referring to Byatt et al. (Citation2013) for a review and discussion of that issue (Byatt et al. Citation2013). A recent meta-analysis of randomised controlled trials (RCTs) including trials of sertraline, paroxetine, and fluoxetine, as well as one study with a pragmatic approach with multiple different antidepressants, concluded that treatment with SSRIs is superior in efficacy to placebo for response and remission of depressive symptoms, with no differences between groups for acceptability of treatment (Brown et al. Citation2021). However, the authors characterised this conclusion as ‘low certainty’ due to the few studies conducted and the observed risk of bias (Brown et al. Citation2021). SSRIs are also favoured for their safety profile in breastfeeding due to their minimal expression into breastmilk and lack of significant associated adverse effects to the infant, with fluoxetine considered the least favourable of the class due to its relatively greater expression into breastmilk and long half-life (Anderson Citation2021). Vortioxetine, a newer SSRI, may be prudent to avoid given the lack of data compared to other agents in this class.
Tricyclic antidepressants (TCAs)
Only two RCTs have been conducted to date assessing the efficacy of TCAs in PPD. One RCT examining amitriptyline for PPD found it inferior to group problem solving therapy (Chabanda Citation2014). One RCT found no difference in response, time to response, remission, or proportion of adverse events reported between sertraline and nortriptyline (Wisner et al. Citation2006). Use of doxepin in the postpartum is generally avoided given reports of serious adverse events in breastfed infants, while other TCAs are considered compatible with breastfeeding (Eberhard-Gran et al. Citation2006). TCAs can be especially useful in clinical practice during pregnancy due to the ability to guide dosing with blood levels.
Other antidepressants
While open-label trials have suggested efficacy for nefazodone (Suri et al. Citation2005), bupropion (Nonacs et al. Citation2005), venlafaxine (Cohen et al. Citation2001), and desvenlafaxine (Misri et al. Citation2016) in treatment of PPD, there no RCTs evaluating the efficacy of these agents over placebo. Evidence above case-level data on efficacy of other antidepressants including mirtazapine, milnacipran, levomilnacipran, duloxetine, vilazodone, and agomelatine are lacking, although these antidepressants and others are used in clinical practice extrapolating from their efficacy in treating non-peripartum depression. Many antidepressants appear compatible with breastfeeding based on limited data, and it is prudent to consult with a comprehensive database such as LactMed (Drugs and Lactation Database (LactMed®)) Citation2006) to ensure the delivery of up-to-date information in counselling a breastfeeding patient.
Oestrogen and progestogens/progestins in the treatment of PPD
Advances in the understanding of the pathophysiology of PPD as uniquely tied to hormonal, including neuroactive steroid (NAS), flux in the postpartum has led to increased interest and study of exogenous administration of forms of oestrogen and progestogens in the treatment of PPD. To date, only one early RCT has demonstrated efficacy of transdermal oestradiol over placebo for treatment of PPD, but although use of antidepressants was not found to be a significant influence on response to oestradiol in the authors’ analysis, the inclusion of subjects using antidepressants complicates interpretation of these results (Gregoire et al. Citation1996). A subsequent small pilot RCT was unable to replicate any significant effect over placebo for transdermal oestradiol (Li et al. Citation2020), and a more recent RCT was halted when serum levels of oestradiol were found to be lower than projected (Wisner et al. Citation2015). The latter trial was able to conclude that infant growth and serum levels of oestradiol were not affected by doses administered in the study (Figueiredo et al. Citation2015). One small RCT of sublingual oestradiol for treatment of PPD similarly did not demonstrate significant efficacy over placebo (Kettunen et al. Citation2022).
Data for the use of progestins is very limited, and as one RCT of norethisterone enanthate injection versus placebo (Lawrie et al. Citation1998) and one RCT of medroxyprogesterone acetate injection versus copper intrauterine device (Singata-Madliki et al. Citation2016) both demonstrated worsening depressive symptoms in the treatment groups, progestins are not recommended for treatment of PPD (Dennis et al. Citation2008). Progestins are not contraindicated for breastfeeding (Pieh Holder Citation2015). Although ‘natural’ or ‘bio-identical’ progesterone derived from plants has been suggested to be superior to progestins due to differences in their metabolism (Stefaniak et al. Citation2023), to date, there are no data examining their efficacy for PPD.
Regarding ‘natural’ or ‘bioidentical’ formulations of oestrogen or progesterone, it is important to note that the ‘bioidentical hormone’ industry is able to escape the black box warning on Food and Drug Administration (FDA)-approved oestrogens, including increased risk of dementia, myocardial infarction, stroke, blood clots, and breast and endometrial cancers, on their products due to their status as ‘supplements’ (Santoro and Liss Citation2021). The result is a largely unregulated industry, promoting compounds for which there is little evidence of efficacy and safety. For these reasons, these products should be treated with extreme caution by clinicians pending further investigation.
Neuroactive steroids and the pathophysiology of PPD
One promising avenue for novel therapeutics in PPD is that of NAS. This umbrella term includes both neurosteroids (molecules derived from cholesterol and synthesised in the brain) as well as steroids synthesised in the periphery that act on the brain (). Synthetic NAS refer to those synthesised in a lab. Both oestrogen and progesterone are steroids derived from cholesterol, and the rapid and substantial shifts in these hormones across pregnancy and parturition, rising as much as 100-fold across pregnancy with an abrupt drop at childbirth (Hendrick et al. Citation1998; Schock et al. Citation2016), has made them a natural early target for research into the aetiology of PPD. Oestradiol withdrawal has been posited as a mechanism of PPD (Galea et al. Citation2001), and considerable attention has been paid to derivatives of progesterone, in particular to those, such as allopregnanolone, that are potent allosteric modulators (PAMs) of the gamma-aminobutyric acid (GABA) A receptor (GABAAR). GABAARs, which bind NAS, are ligand-gated ion channels that reduce neuronal excitability; they are the target not only of GABA, the chief inhibitory neurotransmitter, but also of alcohol, benzodiazepines, and barbiturates. NAS can mediate immediate effects on the central nervous system by binding neuronal membrane receptors as well as slower effects by binding nuclear steroid hormone receptors (McEvoy et al. Citation2018). Outside of the perinatal period, there is a substantial literature pointing to the role of inhibitory NAS in numerous psychiatric illnesses, including major depressive disorder, schizophrenia, anxiety disorders, and premenstrual dysphoric disorder (PMDD). This evidence demonstrates both reduced levels of inhibitory NAS in depressed individuals and increases in NAS following treatment with SSRIs, with a more complicated relationship evident in PMDD (McEvoy et al. Citation2018).
Figure 1. Neuroactive steroid synthesis.
Figure 1 shows the pathways involved in the synthesis of neuroactive steroids, including allopregnanolone, from cholesterol.
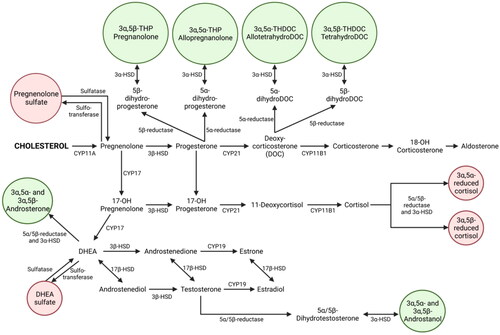
There is clear evidence for the role of GABAergic dysfunction in depression across the perinatal period, but exactly what that role is, how the GABA system connects to other systems, and whether the relationship between NAS and mood changes across the peripartum is not yet clear. There is evidence for both differential vulnerability to normal hormonal fluctuations (Bloch et al. Citation2000) and for differing levels of NAS (Hellgren et al. Citation2014; Deligiannidis et al. Citation2016; Hellgren et al. Citation2017; Osborne et al. Citation2017, Citation2019; Deligiannidis et al. Citation2020), but the evidence is not consistent. While the direct effect on GABAergic transmission is the most obvious role for NAS acting on the receptor, it may also be that NAS exert a large part of their effect through interactions with other systems, including stress reactivity, the hypothalamic-pituitary-adrenal (HPA) axis, the immune system, and regulation of other neurotransmitters including serotonin, dopamine, norepinephrine, and glutamate (McEvoy et al. Citation2018; Payne and Maguire Citation2019; Balan et al. Citation2023). Additionally, plasticity in the receptor (Maguire and Mody Citation2008, Citation2009; Maguire et al. Citation2020) may be a more important element than levels of NAS themselves. In short, while the exact mechanisms remain hazy, it is clear that dysfunction in GABAergic signalling is a key component of the pathophysiology of PPD.
Novel allopregnanolone analogue antidepressant development for PPD
Current Food and drug administration (FDA)-approved antidepressants for the treatment of PPD
Brexanolone
In 2019, brexanolone (ZULRESSO™) was the first medication approved in the US for the indication of unipolar PPD in patients ages 15 years old and older. A soluble, intravenous preparation of synthetic allopregnanolone (Supplemental Figure 1), brexanolone is a potent, selective, PAM of extrasynaptic and synaptic GABAARs (ZULRESSO Prescribing Information Citation2022). It is administered as a 60-hour peripheral intravenous (IV) infusion through a programmable peristaltic infusion pump with a goal dose of 90 ug/kg/hour. A reduction in dosage to 60 µg/kg/hour may be considered for poor tolerability.
Brexanolone treatment has been associated with a rapid reduction of depressive symptoms in open-label (Kanes et al. Citation2017) and placebo-controlled RCTs (Kanes et al. Citation2017; Meltzer-Brody et al. Citation2018). Participants were randomised to receive placebo or brexanolone IV 90 μg/kg/hour or 60 μg/kg/hour for 60 h. Across a range of depression severity, at hour 60, there were significantly larger mean reductions from baseline in Hamilton Rating Scale for Depression (HAMD17) total scores with brexanolone IV 90 μg/kg/hour (−17.0; p < 0.001) and brexanolone IV 60 μg/kg/hour (−19.1; p < 0.001) versus placebo (−12.8). Significant differences from placebo were observed at hour 24 (both dose groups p = 0.001) and maintained through day 30 (p ≤ 0.021) (Meltzer-Brody et al. Citation2018). Posthoc analyses revealed depression remission (defined as HAMD17 total score ≤7) and response (defined as ≥50% reduction in HAMD17 total score from baseline) rates of 50% vs. 25% (brexanolone vs. placebo) and 75% vs. 55% (brexanolone vs. placebo) at hour 60, respectively. Post-hoc analyses that pooled results from the phase 2 (Kanes et al. Citation2017) and the phase 3 RCTs (Kanes et al. Citation2017; Meltzer-Brody et al. Citation2018) reported that adults receiving brexanolone IV 90 μg/kg/hour versus placebo achieved a more rapid HAMD17 response with a median time of 24 vs. 36 h (p = 0.0265) with a cumulative response rate of 81.4% versus 67.3% at hour 60 (Epperson et al. Citation2023).
The most common adverse events reported in the brexanolone group included sedation/somnolence, dry mouth, loss of consciousness, and flushing/hot flush. Adverse events involving sedation/somnolence required dose interruption or reduction in 5% of brexanolone-treated patients. 4% of the brexanolone-treated patients experienced loss or altered state of consciousness, with time to full recovery ranging from 15 to 60 min (Meltzer-Brody et al. Citation2018). Recent post marketing surveillance data demonstrated that clinical rates of excessive sedation and loss of consciousness are lower than what was observed in research trials (Garafola et al. Citation2023). Brexanolone is a Schedule IV medication with a black box warning for excessive sedation and sudden loss of consciousness. The infusion should be stopped for any signs of excessive sedation; it may be restarted after symptoms resolve with or without a dose reduction as clinically appropriate. In the event of hypoxia or loss of consciousness, the infusion should not be restarted.
In the US, brexanolone is currently available only through a restricted risk evaluation and mitigation strategy (REMS) program. The REMS program ensures that brexanolone is administered in a medically supervised setting capable of providing medical monitoring, healthcare setting certification, and a patient registry to monitor for adverse events. Implementation of brexanolone treatment programs has been hampered by the complexity of setting up the treatment in medical settings, cost, and insurance coverage (Patterson et al. Citation2022). A Phase 4 trial assessing the safety of administration of IV brexanolone in a home setting was recently completed; no results have been published to date (Sage Therapeutics Citation2023).
The use of brexanolone has been studied in healthy lactating women, where the maximum relative infant dose during infusion was between 1–2% of the maternal weight adjusted dosage (Wald et al. Citation2022).
Zuranolone
Zuranolone (ZURZUVAE™), a synthetic allopregnanolone analogue (Supplemental Figure 2) containing a cyanopyrazole ring at carbon 21 (Martinez Botella et al. Citation2017), has become the second medication approved in the US for unipolar PPD in adults. Zuranolone is a potent, selective extrasynaptic and synaptic GABAAR PAM (Martinez Botella et al. Citation2017) that is orally bioavailable. A double-blind, placebo-controlled phase 3 trial randomised 153 participants to a 14-day course of zuranolone 30 mg or placebo (Deligiannidis et al. Citation2021), then evaluated participants for four additional weeks. Zuranolone administration was associated with significant reduction in least mean squares (LSM) HAM-D17 total score versus placebo (−17.8 vs. −13.6, p = 0.003 at Day 15). In addition to efficacy for the primary endpoint, significant differences in efficacy of zuranolone were observed at Day 3 (p = 0.026) and were sustained through Day 45 (p = 0.003). HAM-D17 response (72% vs. 48%, p = 0.005) and remission rates (45% vs. 23%, p = 0.012) were significantly greater in the zuranolone group at Day 15, and these significant differences were maintained through Day 45, 4 weeks after zuranolone cessation. The most common adverse events reported in the zuranolone group included sedation, headache, somnolence, dizziness, upper respiratory tract infection, and diarrhoea. No loss of consciousness or increased suicidal ideation/behavior were observed.
In a similarly designed double-blind, placebo-controlled phase 3 trial in women with severe PPD, 196 participants were randomised to a 14-day course of zuranolone 50 mg or placebo (Deligiannidis et al. Citation2023). Treatment with zuranolone versus placebo resulted in statistically significant improvement in depressive symptoms at Day 15 (LSM HAMD-17 total score, −15.6 versus −11.6, p < 0.001); significant improvement in depressive symptoms was also reported as early as Day 3 (p < 0.001), and also at Day 28 (p = 0.02) and Day 45 (p = 0.007). The median time-to-first HAMD-17 response was 9 days for participants receiving zuranolone versus 43 days for placebo. The proportion of patients achieving HAMD-17 response at Day 15 was significantly higher with zuranolone (57.0%) versus placebo (38.9%). The most common adverse events associated with zuranolone were somnolence, dizziness, sedation, headache, diarrhoea, nausea, urinary tract infection, and COVID-19. No loss of consciousness, withdrawal symptoms, or increased suicidal ideation/behavior were observed (Deligiannidis et al. Citation2023). Zuranolone has been approved with a black box warning on potential impairment when driving or engaging in other hazardous activities (ZURZUVAE Prescribing Information Citation2023).
An open-label study examined the transfer of zuranolone into breast milk and its pharmacokinetics, safety, and tolerability in healthy lactating women. Zuranolone 30 mg was discovered to be highly plasma-protein bound with an estimated mean relative infant dose of 0.357% (Bullock et al. Citation2022).
Other neuroactive steroids in development for the treatment of PPD
Ganaxolone
Ganaxolone, the 3β-methylated synthetic analogue of allopregnanolone (Supplemental Figure 3), was recently in development for the treatment of PPD (Marinus Pharmaceuticals Citation2019; Hecking et al. Citation2021), however its development for this indication was halted by the sponsor. In 2022, ganaxolone was US FDA-approved for seizure-disorder associated with Cyclin-dependent Kinase-like 5 Deficiency Disorder (De Citation2023). Ganaxolone, like allopregnanolone, is an extrasynaptic and synaptic GABA-A receptor PAM, but it differs significantly in its lack of affinity for oestrogen or progesterone receptors (Bialer et al. Citation2017). Trials of ganaxolone have included both IV and oral formulations and have not been published outside of clinicaltrials.gov, a resource provided by the US National Library of Medicine.
The Magnolia study was a phase 2a, double-blind placebo controlled multiple-dose escalation study in women with severe PPD. Part 1 of the study evaluated a 48-hour IV infusion of ganaxolone (60, 90 and 140 µg/kg/h) and Part 2 evaluated a 6-hour-IV infusion followed by a 28-day oral regimen (900 mg) compared to placebo (Marinus Pharmaceuticals Citation2023a). The Amaryllis Study was a phase 2, dose-optimization study in which patients with PPD received 675 mg of oral ganaxolone at dinner for 28 days or patients received 675 mg of oral ganaxolone at dinner and bedtime for two days, followed by a dinner time dose of 1125 mg once daily for 26 days. The primary outcome measure was the change from baseline in HAMD-17 at day 29 (Marinus Pharmaceuticals Citation2023b). There are no reports that ganaxolone has been studied in lactating women. Adverse effects of ganaxolone reported in the course of published clinical trials include sedation, dizziness, fatigue, diarrhoea, anxiety, and vomiting (De Citation2023).
The pipeline: Novel allopregnanolone analogues in development
Research is underway covering other routes for administering existing drugs as well as the development of additional allopregnanolone analogues, for both PPD and other uses (Cerne et al. Citation2022). NORA-520 is an oral prodrug that is hydrolysed to brexanolone. It consists of an active moiety with rapid onset of action as a PAM of the GABAAR as well as two moieties that prolong the half-life and promote oral absorption. It is currently in Phase 2 trials for PPD and is also being investigated in preclinical trials for other disorders including major depressive disorder (MDD), generalised anxiety disorder (GAD), essential tremor, and epilepsy; no results have been posted (Gerbera Therapeutics Inc Citation2023). LYT-300 is an oral pro-drug of allopregnanolone. Phase 1 results indicated that the drug demonstrated oral bioavailability at a level associated with therapeutic benefit as well as target engagement; Phase 2 trials are expected to follow soon. No results have been published to date. BRII-296 is an extended-release injectable aqueous suspension formulation of brexanolone. Phase 1 trials in 116 subjects testing 6 dose regimens are complete with the sponsor reporting that a single intramuscular injection of 600 mg achieved dose linearity, early drug absorption, and gradual and extended-release profiles without the need for dose titration or tapering, however results have not been published (Brii Biosciences Limited Citation2023b). A significant majority (98 participants) reported treatment-emergent adverse effects of unclear severity. A Phase 1 trial to test safety, efficacy, and pharmacokinetics of the related BRII-297 is planned in 2023 (Brii Biosciences Limited Citation2023a).
Conclusion
PPD is a serious potential consequence of childbirth for which safe, effective, easily accessible treatments are urgently needed. Psychotherapies and SOC antidepressants are effective for many patients, however time to response can be lengthy, and treatment duration is often chronic. Side effects are common with antidepressants. Evidence for the exogenous administration of oestrogen and progestogens is too limited to recommend these treatments, and in the case of progestogens, may be associated with worsening depressive symptoms. Acute treatment courses of NAS and their analogues are a promising novel approach to the treatment of PPD. Not only is their mechanism of action distinctly different from that of current SOC antidepressants, data from brexanolone and zuranolone indicate that these novel therapeutics are rapid-acting with a durable response (as studied thus far) once the acute treatment course is complete. If future NAS and NAS analogues gain regulatory approval, the field will need to study response durability over the long-term, their potential to prevent PPD in high-risk individuals, and whether there are subtypes of PPD more likely to respond to NAS-based antidepressants as compared to SOC antidepressants. NAS are not only important to our understanding of the pathophysiology and treatment of PPD, but NAS are also under active study in a broad range of neuropsychiatric illnesses (e.g. Alzheimer’s disease, stroke, intractable seizure disorders, tremor, traumatic brain injury, anxiety disorders, other depressive disorders, etc.), which makes these agents of particular importance to the broader field of neurology and psychiatry.
Authors’ contribution
Dr Deligiannidis serves as a consultant to Sage Therapeutics, Brii Biosciences, Gerbera Therapeutics, GH Research, Neuroscience Software and Reunion Neuroscience. Dr. Deligiannidis served as a study principal investigator for contracted research awarded to the Feinstein Institutes for Medical Research from Sage Therapeutics, Nesos Corporation, Woebot Health and Premier Healthcare. She also receives grants from the NIH and royalties from an NIH employee invention.
Disclosurestatement
Dr. Carlini has no financial disclosures. No potential conflict of interest was reported by the author(s).
Supplemental Material
Download MS Word (125.9 KB)Acknowledgements
Dr. Deligiannidis’s effort for manuscript development and writing was supported by NIH R01-MH120313. The authors gratefully acknowledge Ibtihal Kamal for her work in creating of the manuscript.
Additional information
Funding
References
- American Psychiatric Association 2022. Diagnostic and statistical manual of mental disorders, Fifth Edition, Text Revision. Arlington, VA: American Psychiatric Association.
- Anderson P.O 2021. Antidepressants and Breastfeeding. Breastfeed Med. 16(1):5–7. doi:10.1089/bfm.2020.0350.
- Balan I, Patterson R, Boero G, Krohn H, O'Buckley TK, Meltzer-Brody S, Morrow AL. 2023. Brexanolone therapeutics in post-partum depression involves inhibition of systemic inflammatory pathways. EBioMedicine. 89:104473. doi: 10.1016/j.ebiom.2023.104473.
- Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS. 2017. Progress report on new antiepileptic drugs: a summary of the thirteenth eilat conference on new antiepileptic drugs and devices (EILAT XIII). Epilepsia. 58(2):181–221. doi: 10.1111/epi.13634.
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. 2000. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry. 157(6):924–930. doi: 10.1176/appi.ajp.157.6.924.
- Brii Biosciences Limited 2023a. A Study of BRII-297 in healthy adult subjects. National Library of Medicine (US); [accessed 2023 May 23]. https://clinicaltrials.gov/ct2/show/NCT05845840.
- Brii Biosciences Limited 2023b. Brii Biosciences announces top-line results from phase 1 study of BRII-296, A long-acting therapy in development for postpartum depression. PRNewswire; [updated 26 Sep 2022; accessed 2023 23 May]. https://www.prnewswire.com/news-releases/brii-biosciences-announces-top-line-results-from-phase-1-study-of-brii-296-a-long-acting-therapy-in-development-for-postpartum-depression-301632804.html.
- Brown JVE, Wilson CA, Ayre K, Robertson L, South E, Molyneaux E, Trevillion K, Howard LM, Khalifeh H. 2021. Antidepressant treatment for postnatal depression. Cochrane Database Syst Rev. 2(2):CD013560.
- Bullock A, Nandy I, Garcia M, Deligiannidis K, Wald J. 2022. An open-label study to evaluate concentrations of Zuranolone in the Breast Milk of Healthy Lactating Women. 9th World Congress on Women’s Mental Health; November 6–9, 2022; Maastricht, Netherlands [Scientific poster presentation at the world congress on women' mental health].
- Byatt N, Deligiannidis KM, Freeman MP. 2013. Antidepressant use in pregnancy: a critical review focused on risks and controversies. Acta Psychiatr Scand. 127(2):94–114. doi: 10.1111/acps.12042.
- Cerne R, Lippa A, Poe MM, Smith JL, Jin X, Ping X, Golani LK, Cook JM, Witkin JM. 2022. GABAkines - Advances in the discovery, development, and commercialization of positive allosteric modulators of GABA(A) receptors. Pharmacol Ther. 234:108035. doi: 10.1016/j.pharmthera.2021.108035.
- Chibanda D, Shetty AK, Tshimanga M, Woelk G, Stranix-Chibanda L, Rusakaniko S. 2014. Group problem-solving therapy for postnatal depression among HIV-positive and HIV-negative mothers in Zimbabwe. J Int Assoc Providers AIDS Care. 13(4):335–341. doi: 10.1177/2325957413495564.
- Cohen L, Viguera A, Bouffard S, Nonacs R, Morabito C, Collins M, Ablon J. 2001. Venlafaxine in the treatment of postpartum depression. J Clin Psychiatry. 62(8):592–596. doi: 10.4088/jcp.v62n0803.
- Cuijpers P, Brannmark JG, van Straten A. 2008. Psychological treatment of postpartum depression: a meta‐analysis. J Clin Psychol. 64(1):103–118. doi: 10.1002/jclp.20432.
- De SK. 2023. Ganaxolone: first FDA-approved medicine for the treatment of seizures associated with cyclin-dependent kinase-like 5 deficiency disorder. Curr Med Chem. 31(4):388–392. doi: 10.2174/0929867330666230320123952.
- Deligiannidis KM, Kroll-Desrosiers AR, Mo S, Nguyen HP, Svenson A, Jaitly N, Hall JE, Barton BA, Rothschild AJ, Shaffer SA. 2016. Peripartum neuroactive steroid and gamma-aminobutyric acid profiles in women at-risk for postpartum depression. Psychoneuroendocrinology. 70:98–107. doi: 10.1016/j.psyneuen.2016.05.010.
- Deligiannidis KM, Kroll-Desrosiers AR, Tan Y, Dubuke ML, Shaffer SA. 2020. Longitudinal proneuroactive and neuroactive steroid profiles in medication-free women with, without and at-risk for perinatal depression: a liquid chromatography-tandem mass spectrometry analysis. Psychoneuroendocrinology. 121:104827. doi: 10.1016/j.psyneuen.2020.104827.
- Deligiannidis KM, Meltzer-Brody S, Gunduz-Bruce H, Doherty J, Jonas J, Li S, Sankoh AJ, Silber C, Campbell AD, Werneburg B, et al. 2021. Effect of Zuranolone vs placebo in postpartum depression: a randomized clinical trial. JAMA Psychiatry. 78(9):951–959. doi: 10.1001/jamapsychiatry.2021.1559.
- Deligiannidis KM, Meltzer-Brody S, Maximos B, Peeper EQ, Freeman MP, Lasser R, Bullock A, Kotecha M, Li S, Forrestal F, et al. 2023. Zuranolone for the treatment of adults with postpartum depression. Am J Psychiatry. 180(9):668–675. doi: 10.1176/appi.ajp.20220785.
- Dennis C, Ross L, Herxheimer A. 2008. Oestrogens and progestins for preventing and treating postpartum depression. Cochrane Database Syst Rev. 2008(4):CD001690. doi:10.1002/14651858.CD001690.pub2.
- Drugs and Lactation Database (LactMed®) 2006. National Institute of Child Health and Human Development.
- Eberhard-Gran M, Eskild A, Opjordsmoen S. 2006. Use of psychotropic medications in treating mood disorders during lactation: practical recommendations. CNS Drugs. 20(3):187–198. doi: 10.2165/00023210-200620030-00002.
- Epperson CN, Rubinow DR, Meltzer-Brody S, Deligiannidis KM, Riesenberg R, Krystal AD, Bankole K, Huang MY, Li H, Brown C, et al. 2023. Effect of brexanolone on depressive symptoms, anxiety, and insomnia in women with postpartum depression: pooled analyses from 3 double-blind, randomized, placebo-controlled clinical trials in the HUMMINGBIRD clinical program. J Affect Disord. 320:353–359. doi: 10.1016/j.jad.2022.09.143.
- Figueiredo F, Parada A, de Araujo L, Silva W, Del-Ben C. 2015. The influence of genetic factors on peripartum depression: a systematic review. J Affect Disord. 172:265–273. doi: 10.1016/j.jad.2014.10.016.
- Flynn S, Shaw J, Abel K. 2013. Filicide: mental illness in those who kill their children. PLOS One. 8(4):e58981. doi: 10.1371/journal.pone.0058981.
- Galea LA, Wide JK, Barr AM. 2001. Estradiol alleviates depressive-like symptoms in a novel animal model of post-partum depression. Behav Brain Res. 122(1):1–9. doi: 10.1016/s0166-4328(01)00170-x.
- Garafola S, Shiferaw E, Dev V. 2023. Safety of brexanolone in adults with postpartum depression: postmarketing surveillance data. Drugs Real World Outcomes. 10(3):351–356. doi: 10.1007/s40801-023-00372-4.
- Gerbera Therapeutics Inc 2023. Nora 520. https://www.gerberarx.com/en/product/detail/NORA520. [accessed 2023 May 23].
- Gregoire A, Kumar R, Everitt B, Henderson A, Studd J. 1996. Transdermal oestrogen for treatment of severe postnatal depression. Lancet. 347(9006):930–933. doi: 10.1016/s0140-6736(96)91414-2.
- Hecking J, Davoudian PA, Wilkinson ST. 2021. Emerging therapeutics based on the amino acid neurotransmitter system: an update on the pharmaceutical pipeline for mood disorders. Chronic Stress . 5:24705470211020446. doi: 10.1177/24705470211020446.
- Hellgren C, Akerud H, Skalkidou A, Backstrom T, Sundstrom-Poromaa I. 2014. Low serum allopregnanolone is associated with symptoms of depression in late pregnancy. Neuropsychobiology. 69(3):147–153. doi: 10.1159/000358838.
- Hellgren C, Comasco E, Skalkidou A, Sundstrom-Poromaa I. 2017. Allopregnanolone levels and depressive symptoms during pregnancy in relation to single nucleotide polymorphisms in the allopregnanolone synthesis pathway. Horm Behav. 94:106–113. doi: 10.1016/j.yhbeh.2017.06.008.
- Hendrick V, Altshuler LL, Suri R. 1998. Hormonal changes in the postpartum and implications for postpartum depression. Psychosomatics. 39(2):93–101. doi: 10.1016/S0033-3182(98)71355-6.
- Kanes S, Colquhoun H, Gunduz-Bruce H, Raines S, Arnold R, Schacterle A, Doherty J, Epperson CN, Deligiannidis KM, Riesenberg R, et al. 2017. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. 390(10093):480–489. doi: 10.1016/S0140-6736(17)31264-3.
- Kanes SJ, Colquhoun H, Doherty J, Raines S, Hoffmann E, Rubinow DR, Meltzer-Brody S. 2017. Open-label, proof-of-concept study of brexanolone in the treatment of severe postpartum depression. Hum Psychopharmacol Clin Exp. 32:e2576. https://doi.org/10.1002/hug.2576.
- Kettunen P, Koistinen E, Hintikka J, Perheentupa A. 2022. Oestrogen therapy for postpartum depression: efficacy and adverse effects. A double-blind, randomized, placebo-controlled pilot study. Nord J Psychiatry. 76(5):348–357. doi: 10.1080/08039488.2021.1974556.
- Knight M, Bunch K, Tuffnell D, Patel R, Shakespeare J, Kotnis R, Kenyon S, Kurinczuk J. 2021. Saving Lives, Improving Mothers’ Care - Lessons learned to inform maternity care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2017-19. Oxford: National Perinatal Epidemiology Unit, University of Oxford.
- Lawrie T, Hofmeyr G, De Jager M, Berk M, Paiker J, Viljoen E. 1998. A double-blind randomised placebo controlled trial of postnatal norethisterone enanthate: the effect on postnatal depression and serum hormones. Br J Obstet Gynaecol. 105(10):1082–1090. doi: 10.1111/j.1471-0528.1998.tb09940.x.
- Li H, Martinez P, Li X, Schenkel L, Nieman L, Rubinow D, Schmidt P. 2020. Transdermal estradiol for postpartum depression: results from a pilot randomized, double-blind, placebo-controlled study. Arch Womens Ment Health. 23(3):401–412. doi: 10.1007/s00737-019-00991-3.
- Liu X, Wang S, Wang G. 2022. Prevalence and risk factors of postpartum depression in women: a systematic review and meta-analysis. J Clin Nurs. 31(19-–20):2665–2677. doi: 10.1111/jocn.16121.
- Maguire J, McCormack C, Mitchell A, Monk C. 2020. Neurobiology of maternal mental illness. Handb Clin Neurol. 171:97–116.–
- Maguire J, Mody I. 2008. GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron. 59(2):207–213. doi: 10.1016/j.neuron.2008.06.019.
- Maguire J, Mody I. 2009. Steroid hormone fluctuations and GABA(A)R plasticity. Psychoneuroendocrinology. 34 (Suppl 1):S84–S90. doi: 10.1016/j.psyneuen.2009.06.019.
- Marinus Pharmaceuticals 2019. Marinus pharmaceuticals announces data from Magnolia and Amaryllis Phase 2 studies in women with postpartum depression. [accessed 2023 May 23]. https://www.globenewswire.com/news-release/2019/07/23/1886335/0/en/Marinus-Pharmaceuticals-Announces-Data-from-Magnolia-and-Amaryllis-Phase-2-Studies-in-Women-with-Postpartum-Depression.html.
- Marinus Pharmaceuticals 2023a. A clinical trial of intravenous (IV) ganaxolone in women with postpartum depression. National Library of Medicine (S); [updated Feb 8; accessed 2023 1 June]. https://clinicaltrials.gov/study/NCT03228394.
- Marinus Pharmaceuticals 2023b. A clinical trial of oral ganaxolone in women with postpartum depression. National Library of Medicine (US); [updated 30 Jan 2023; accessed 2023 1 June]. https://clinicaltrials.gov/study/NCT03460756.
- Martinez Botella G, Salituro FG, Harrison BL, Beresis RT, Bai Z, Blanco MJ, Belfort GM, Dai J, Loya CM, Ackley MA, et al. 2017. Neuroactive steroids. 2. 3α-Hydroxy-3β-methyl-21-(4-cyano-1H-pyrazol-1'-yl)-19-nor-5β-pregnan-20-one (SAGE-217): a clinical next generation neuroactive steroid positive allosteric modulator of the (γ-Aminobutyric Acid)(A) receptor. J Med Chem. 60(18):7810–7819. doi: 10.1021/acs.jmedchem.7b00846.
- McEvoy K, Payne J, Osborne L. 2018. Neuroactive steroids and perinatal depression: a review of recent literature. Curr Psychiatry Rep. 20(9):78. doi: 10.1007/s11920-018-0937-4.
- Meltzer-Brody S, Colquhoun H, Riesenberg R, Epperson CN, Deligiannidis KM, Rubinow DR, Li H, Sankoh AJ, Clemson C, Schacterle A, et al. 2018. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 392(10152):1058–1070. doi: 10.1016/S0140-6736(18)31551-4.
- Misri S, Swift E, Abizadeh J, Shankar R. 2016. Overcoming functional impairment in postpartum depressed or anxious women: a pilot trial of desvenlafaxine with flexible dosing. Ther Adv Psychopharmacol. 6(4):269–276. doi: 10.1177/2045125316656297.
- Nonacs R, Soares C, Viguera A, Pearson K, Poitras J, Cohen L. 2005. Bupropion SR for the treatment of postpartum depression: a pilot study. Int J Neuropsychopharmacol. 8(3):445–449. doi: 10.1017/S1461145705005079.
- Osborne LM, Betz JF, Yenokyan G, Standeven LR, Payne JL. 2019. The role of allopregnanolone in pregnancy in predicting postpartum anxiety symptoms. Front Psychol. 10:1033. doi: 10.3389/fpsyg.2019.01033.
- Osborne LM, Gispen F, Sanyal A, Yenokyan G, Meilman S, Payne JL. 2017. Lower allopregnanolone during pregnancy predicts postpartum depression: an exploratory study. Psychoneuroendocrinology. 79:116–121. doi: 10.1016/j.psyneuen.2017.02.012.
- Patterson R, Krohn H, Richardson E, Kimmel M, Meltzer-Brody S. 2022. A brexanolone treatment program at an academic medical center: patient selection, 90-day posttreatment outcomes, and lessons learned. J Acad Consult Liaison Psychiatry. 63(1):14–22. doi: 10.1016/j.jaclp.2021.08.001.
- Payne JL, Maguire J. 2019. Pathophysiological mechanisms implicated in postpartum depression. Front Neuroendocrinol. 52:165–180. doi: 10.1016/j.yfrne.2018.12.001.
- Pieh Holder K. 2015. Contraception and breastfeeding. Clin Obstet Gynecol. 58(4):928–935. doi: 10.1097/GRF.0000000000000157.
- Sage Therapeutics 2023. A study to assess the safe-use conditions for administration of ZULRESSO® in a home setting. National Library of medicine (US); [accessed 2023 June 1]. https://clinicaltrials.gov/ct2/show/NCT05059600.
- Santoro N, Liss J. 2021. Compounded bioidentical hormones: myths and realities. Clin Obstet Gynecol. 64(4):793–802. doi: 10.1097/GRF.0000000000000650.
- Schock H, Zeleniuch-Jacquotte A, Lundin E, Grankvist K, Lakso H, Idahl A, Lehtinen M, Surcel HM, Fortner RT. 2016. Hormone concentrations throughout uncomplicated pregnancies: a longitudinal study. BMC Pregnancy Childbirth. 16(1):146. doi: 10.1186/s12884-016-0937-5.
- Singata-Madliki M, Hofmeyr G, Lawrie T. 2016. The effect of depot medroxyprogesterone acetate on postnatal depression: a randomised controlled trial. J Fam Plann Reprod Health Care. 42(3):171–176. doi: 10.1136/jfprhc-2015-101334.
- Slomian J, Honvo G, Emonts P, Reginster J, Bruyère O. 2019. Consequences of maternal postpartum depression: a systematic review of maternal and infant outcomes. Womens Health. 15:1745506519844044. doi: 10.1177/1745506519844044.
- Stefaniak M, Dmoch-Gajzlerska E, Jankowska K, Rogowski A, Kajdy A, Maksym R. 2023. Progesterone and its metabolites play a beneficial role in affect regulation in the female brain. Pharmaceuticals. 16(4):520. doi: 10.3390/ph16040520.
- Suri R, Burt V, Altshuler L. 2005. Nefazodone for the treatment of postpartum depression. Arch Womens Ment Health. 8(1):55–56. doi: 10.1007/s00737-005-0071-2.
- mTrost S, Beauregard J, Chandra G, Njie F, Berry J, Harvey A, Goodman D. 2022. Pregnancy-related deaths: data from maternal mortality review committees in 36 US States, 2017–2019. Atlanta, GA: Centers for Disease Control and Prevention US Department of Health and Human Services.
- Wald J, Henningsson A, Hanze E, Hoffmann E, Li H, Colquhoun H, Deligiannidis KM. 2022. Allopregnanolone concentrations in breast milk and plasma from healthy volunteers receiving brexanolone injection, with population pharmacokinetic modeling of potential relative infant dose. Clin Pharmacokinet. 61(9):1307–1319. doi: 10.1007/s40262-022-01155-w.
- Wisner K, Hanusa B, Perel J, Peindl K, Piontek C, Sit D, Findling R, Moses-Kolko E. 2006. Postpartum depression: a randomized trial of sertraline versus nortriptyline. J Clin Psychopharmacol. 26(4):353–360. doi: 10.1097/01.jcp.0000227706.56870.dd.
- Wisner K, Sit D, Moses-Kolko E, Driscoll K, Prairie B, Stika C, Eng H, Dills J, Luther J, Wisniewski S. 2015. Transdermal estradiol treatment for postpartum depression: a pilot, randomized trial. J Clin Psychopharmacol. 35(4):389–395. doi: 10.1097/JCP.0000000000000351.
- Wisner KL, Moses-Kolko EL, Sit DKY. 2010. Postpartum depression: a disorder in search of a definition [Letter]. Arch Womens Ment Health. 13(1):37–40. doi: 10.1007/s00737-009-0119-9.
- ZULRESSO Prescribing Information 2022. Cambridge, MA: Sage Therapeutics, Inc.
- ZURZUVAE Prescribing Information 2023. Cambridge, MA: Biogen, Inc. and Sage Therapeutics, Inc.

