ABSTRACT
Sonic vibration (SV), or vibroacoustic therapy, is applied to enhance local and systemic blood circulation and alleviate pain using low-frequency sine wave vibrations. However, there is limited scientific data on the mechanisms through which the benefits are achieved. In this study, we investigated the impact of SV on inflammatory responses by assessing cytokine secretion in both in vivo and in vitro models. After inducing inflammatory responses in mice and macrophages, we studied cytokine expression and the symptoms of inflammatory diseases in response to three frequencies (14, 45, or 90 Hz) of SV stimulation at 0.5 m/s2 of amplitude. The results showed that SV at 90 Hz significantly increased interelukin-10 (IL-10) secretion in mice who were administered lipopolysaccharides (LPS) and increased the expression of IL-10 transcripts in peritoneal exudate cells and macrophages. Furthermore, SV at 90 Hz improved LPS-induced lethality and alleviated symptoms in a colitis model. In conclusion, this study scientifically proves the anti-inflammatory effects of vibration therapy through its ability to increase IL-10 expression.
Introduction
Sound and vibration are forms of energy that propagate through waves, which are natural oscillations that have amplitude and frequency (Reynolds et al. Citation2018). Amplitude refers to acceleration (m/s²) passing through the equilibrium and reflects the intensity of sound and vibration (Reynolds et al. Citation2018). Frequency, measured in Hertz (Hz), denotes the time for a wave to complete a cycle. Sound and vibration are utilized as non-invasive therapies in the fields of aesthetic and sports medicine (Kennedy et al. Citation2015). Sonic vibration (SV) or vibration acoustic therapy is a therapeutic method that delivers low-frequency sinusoidal vibrations (30 to 120 Hz) to the body (Kantor et al. Citation2019). Humans can perceive frequencies ranging from 20 Hz to 20 kHz, while mice can perceive frequencies ranging from 1 to 100 kHz (Reynolds et al. Citation2018).
Excessive and chronic vibration can act as a stressor in animals, including humans, and can have various negative effects on health, potentially causing changes in normal physiology and cellular structure (Reynolds et al. Citation2018). However, localized and temporary vibration, such as SV, has benefits, including improvement in the musculoskeletal system, increased wound healing capacity, and enhanced oxygen-carrying ability (Reynolds et al. Citation2018). SV therapy has also been found to be useful in various conditions, including muscle relaxation and mild pain relief (Boyd-Brewer and McCaffrey Citation2004). Regular physical activity is essential for living a healthy life and can help regulate immune responses (Rodriguez-Miguelez et al. Citation2015). In cases where physical activity is not feasible, such as in older individuals, whole-body vibration training has shown effects similar to physical exercise (Rodriguez-Miguelez et al. Citation2015). Specifically, research has indicated that whole-body vibration can lead to improvements in inflammatory markers (Rodriguez-Miguelez et al. Citation2014; Rodriguez-Miguelez et al. Citation2015). SV has effects similar to whole-body vibration (Park et al. Citation2019).
In this study, the effects of SV on inflammatory responses were investigated in two inflammation models in mice. Exogenous and endogenous inflammatory stimuli, lipopolysaccharides (LPS), and monosodium uric crystals (MSU) were administered to the mice. Subsequently, three different SV wavelengths were applied to the inflammation models, and the secretion of pro-inflammatory and anti-inflammatory cytokines was measured. Additionally, the gene expression of cytokines was examined in cells extracted from the abdominal cavity of mice exposed to SV. Furthermore, the impact of SV on cytokine expression and secretion was investigated in human and mouse macrophages. Lastly, the ameliorative effects of SV on mice induced with lethal endotoxemia and colitis were investigated.
Materials and methods
Animal study
To induce an inflammatory response, mice (C57BL/6 mice, female, 8 weeks old, Nara Biotech, Seoul, Republic of Korea) were injected intraperitoneally (ip) with LPS (100 μg/mouse, L4130, Sigma–Aldrich Co., MO, USA) or MSU (10 mg/mouse, Sigma–Aldrich Co.). Immediately after injection, SV was applied to the mice at three different frequencies (14, 45, or 90 Hz) with an acceleration of 0.5 m/s2. SV was delivered to an acrylic cage containing mice using a function generator and audio equipment, and the vibration was monitored through the oscilloscope (Supplemental Figure S1). After 6 h of SV, the mice were sacrificed by CO2 inhalation, and their blood was collected. The peritoneal cavities of mice were flushed with 5 mL of saline to harvest peritoneal lavages and peritoneal exudate cells (PECs). The frequencies and amplitude settings were referenced from previous studies (Weinheimer-Haus et al. Citation2014; Atanasov et al. Citation2015).
For LPS-induced lethality, mice were subjected to SV stimulation (90 Hz and 0.5 m/s2) for 10d (6 h/d). After the final vibration, all mice were administered LPS (ip, 400 μg/mouse), and survival was observed for 96 h. For the colitis model, mice were provided with tap water containing 3% dextran sodium sulfate (DSS, 36–50 kDa, MP Biomedicals, Solon, OH, USA) for 7d, followed by a switch to regular tap water. SV stimulation (90 Hz, 0.5 m/s2, 6 h/d) was continued until the end of the experiment. Body weight was measured throughout the entire period, and feces were collected up to 6d after DSS consumption. The mice were supplied with normal chow and water ad libitum and maintained at room temperature (18–24°C) with a 12 h light/dark cycle. All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the Animal research: Reporting of In Vivo Experiments (ARRIVE) guidelines. The experimental protocol for the animal study was approved by the Institutional Animal Care and Use Committee.
Cell culture and treatment
Unless indicated otherwise, all cell culturing products and plastics were supplied by Welgene (Gyeongsan, Gyeongsanbuk-do, Republic of Korea) and SPL Life Sciences (Pocheon, Gyeonggi-do, Republic of Korea). For bone marrow-derived macrophages (BMDMs), bone marrow progenitor cells were isolated from the tibiae and femurs of mice (C57BL/6 mice, six to 12 weeks old), and incubated in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS), antibiotics and 30% conditioned media containing macrophage colony-stimulating factors from L929 cells for 7d (Chen et al. Citation2022). Human monocyte-like cells (THP-1; Korea Cell Line Bank, Seoul, Republic of Korea) were differentiated into macrophages in Roswell Park Memorial Institute (RPMI) 1640 medium containing 10% FBS, antibiotics, and phorbol 12-myristate 13-acetate (200 nM, PMA; Invivogen, San Diego, CA, USA) for 24 h. All cells were incubated at 37°C in an atmosphere containing 5% CO2. The BMDMs and PMA-treated THP-1 cells (1 × 106 cells/well in a 12-well plate) were treated with LPS (10 ng/mL) alone or in combination with SB203580 (559398, Sigma–Aldrich Co.) or Bay 11-7082 (B5556, Sigma–Aldrich Co.). These cells were subjected to SV stimulation for 3 h under treatment. Total RNA was extracted using a reagent (NucleoZOL, MACHEREY-NAGEL GmbH & Co. KG, Postfach, Düren, Germany) and the cellular lysate was prepared with a lysis buffer containing Triton X-100 (0.01%), NaCl (150 mM), Tris-base (50 mM, pH 8.0), and proteinase inhibitors (HaltTM cocktail, ThermoFisher Scientific, Waltham, MA, USA).
RNA extraction and quantitative real-time polymerase chain reaction (qPCR)
The total RNA was synthesized into first-strand complementary DNA (cDNA) using a random primer (9-mer, Invitrogen, Carlsbad, CA, USA) and Moloney Murine Leukemia Virus Reverse Transcriptase (M-MLV RT, Enzynomics Co., Daejeon, Republic of Korea) (Abdellaoui et al. Citation2023). The gene expression was quantified using TOPreal™ qPCR 2X PreMIX (Enzynomics Co.), the Eco Real-Time PCR system (Illumina, San Diego, CA, USA), and the following specific primers: mouse Interleukin (IL)-1β (Gene Bank ID, NM_008361, 133 bp), 5′-CCC AAG CAA TAC CCA AAG AA-3′ and 5′-GCT TGT GCT CTG CTT GTG AG-3′; mouse IL-6 (NM_031168, 102 bp), 5′-CCG GAG AGG AGA CTT CAC AG-3′ and 5′-TCC ACG ATT TCC CAG AGA AC-3′; mouse IL-10 (NM_010548, 142 bp), 5′-GCC TGG CTC AGC ACT GCT AT-3′ and 5′-GAA GGC AGT CCG CAG CTC TA-3′; mouse glyceraldehyde-3-phosphate dehydrogenase (Gapdh, NM_001289726, 223 bp), 5′-AAC TTT GGC ATT GTG GAA GG-3′ and 5′-ACA CAT TGG GGG TAG GAA CA-3′; human IL-1β (NM_000576, 180 bp), 5′-CTG TCC TGC GTG TTG AAA GA-3′ and 5′-TTC TGC TTG AGA GGT GCT GA-3′; human IL-6 (NM_000600, 175 bp), 5′-TAC CCC CAG GAG AAG ATT CC-3′ and 5′-TTT TCT GCC AGT GCC TCT TT-3′; human IL-10 (NM_000572, 253 bp), 5′-TGA GAA CAG CTG CAC CCA CT-3′ and 5′-GTT CAC ATG CGC CTT GAT GT-3′; human Gapdh (NM_001256799, 278 bp), 5′-ACT GGC GTC TTC ACC ACC AT-3′ and 5′-GGG CCA TCC ACA GTC TTC TG-3′.
Biochemical assay
The IL-1β, IL-6, and IL-10 levels in the peritoneal lavages and the cellular lysates were measured using an enzyme-linked immunosorbent assay (ELISA) kit (DY401, DY406, and DY417, R&D Systems, Minneapolis, MN, USA) (Feng et al. Citation2022). Fecal lipocalin-2 (LCN2) secretion was analyzed using an assay kit (DY1857, R&D Systems). The plates were analyzed using a multi-microplate spectrophotometer (Synergy™ H1 Hybrid Multi-Mode Reader, BioTek, Winooski, VT, USA).
Statistical analyses
Statistical analyses were conducted using software (GraphPad Prism 6, San Diego, CA) as follows: Mann–Whitney test for the two groups, Dunn’s multiple comparisons test for multiple groups, the log-rank, and Gehan-Wilcoxon tests for survival. The p-value is shown in the figures.
Results
Changes in cytokine secretion in mice after sonic vibration (SV)
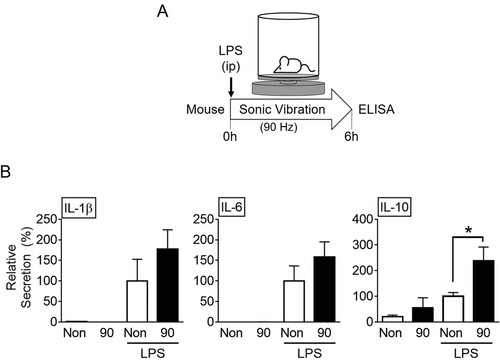
To evaluate the effect of SV on cytokine secretion, the mice were injected with inflammatory triggers (i.e. LPS and MSU) and then kept in the cages where the vibration stimuli (i.e. 14, 45, or 90 Hz) were applied for 6 h (A). Subsequently, the mice were sacrificed and the cytokine secretion in the peritoneal lavage was evaluated (B, and Supplemental Figure S2). The inflammatory triggers (LPS, a bacterial wall component, and a toll-like receptor [TLR] 4 ligand and MSU, an endogenous danger signal) increased the secretion of peritoneal cytokines (IL-1β, IL-6, and IL-10), as expected. In the LPS-injected mice, the SV stimuli did not alter cytokine secretions except that of IL-10. Vibration stimulus at 90 Hz significantly induced IL-10 production in response to LPS. However, the stimulus had no effect on the IL-10 production in the MSU-injected mice. Vibration without the triggers did not change the cytokine secretion. Based on these data, it can be concluded that SV stimuli may alter cytokine production in LPS-injected mice.
Figure 1. Effects of sound vibration (SV) on cytokine secretion in mice. A. Schematic diagram of the experimental setup: Mice were divided into four groups (n = 6 per group, total n = 24). These included the groups injected with lipopolysaccharides (LPS) and then subjected to SV at 90 Hz (90) for 6 h or no SV stimulation (Non) or the groups without LPS, subjected to SV at 90 Hz (90) for 6 h or no SV stimulation (Non). B, Peritoneal lavages were collected and analyzed for the levels of interleukin (IL)-1β, IL-6, and IL-10. The bar graph presents the mean ± SD. *, P < 0.05.
Vibration at 90 Hz enhances IL-10 production
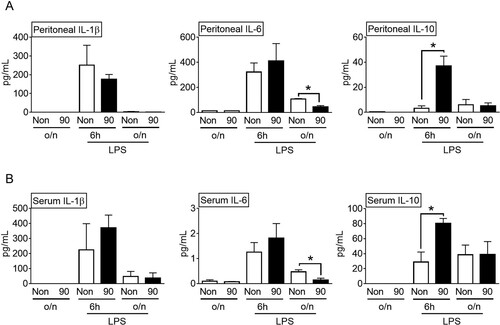
We studied the effect of vibration at 90 Hz on the cytokine secretion in the LPS-injected mice in a time-dependent manner. In the LPS-treated mice, the peritoneal IL-1β and IL-6 secretions increased at 6 h, and then decreased overnight (A). The peritoneal IL-1β and IL-6 secretions remained unchanged, irrespective of the stimulation by vibration at 90 Hz for 6 h. However, the IL-6 secretion was significantly decreased by overnight vibration at 90 Hz. Similar to the previous results (B), the peritoneal IL-10 release was significantly enhanced by the vibration at 90 Hz for 6 h, but it was not altered by the overnight vibration. Consistent with the peritoneal data (A), the serum IL-10 levels were also significantly induced by the 90 Hz vibration for 6 h, and serum IL-6 release was significantly attenuated by the overnight 90 Hz vibration (B). IL-10 inhibits the expression of proinflammatory cytokines, including IL-6 (Fiorentino et al. Citation1991). Hence, the decrease in IL-6 in the LPS-injected mice administered overnight 90 Hz vibration might be due to the increased IL-10 levels. Overall, stimulation by vibration at 90 Hz selectively induced IL-10 production in the LPS-injected mice.
Figure 2. Effects of sound vibration (SV) on cytokine secretion in a time-dependent manner. Mice (n = 6 per group, total n = 36) were injected with lipopolysaccharides (LPS) and then subjected to SV at 90 Hz (90) for 6 h or overnight (o/n, 16 h) or no-SV stimulation (Non) or were subjected to SV at 90 Hz (90) for 6 h or overnight (o/n, 16 h) or no-SV stimulation (Non) without LPS. Peritoneal lavages (A) or blood (B) were collected and analyzed for the levels of interleukin (IL)-1β, IL-6, and IL-10. The bar graph presents the mean ± SD. *, P < 0.05.
Vibration at 90 Hz regulates the cytokine expression in PECs
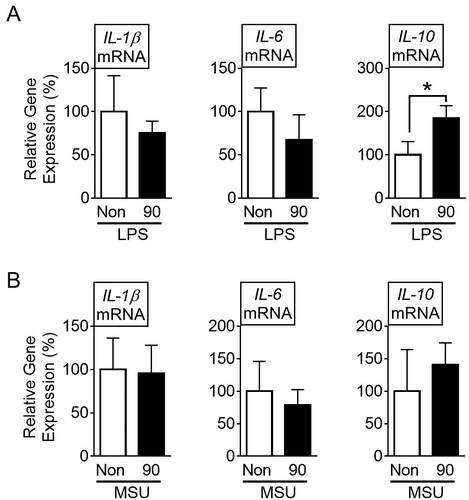
To investigate the cause of the differential expression of IL-10 due to the vibration at 90 Hz, we analyzed the cytokine transcripts in PECs derived from the LPS- or MSU-injected mice that were subjected to the vibration for 6 h. The results showed that the vibration significantly increased LPS-mediated IL-10 mRNA in PECs, while it did not change the IL-1β and IL-6 transcripts (A). In addition, the vibration at 90 Hz did not alter the cytokine transcription in the PECs which were obtained from the MSU-injected mice (B). Thus, vibration at 90 Hz stimulated the PECs of the LPS-injected mice to enhance the transcription of the IL-10 gene.
Figure 3. Effects of sound vibration (SV) on cytokine secretion in peritoneal exudate cells (PECs). Mice (n = 12 per group, total n = 48) were injected with lipopolysaccharides (LPS) (A) or monosodium uric crystals (MSU) (B) and then subjected to SV at 90 Hz (90) for 6 h or no-SV stimulation (Non). PECs were collected and analyzed for the levels of interleukin (IL)-1β, IL-6, and IL-10. The bar graph presents the mean ± SD. *, P < 0.05.
Sonic vibration at 90 Hz increases IL-10 expression through the p38 MAPK and NF-κB signaling pathways upon TLR4 activation
We further studied the efficacy of vibration at 90 Hz in up-regulating IL-10 in mouse macrophages (BMDMs) and human macrophages (THP-1). These cells were treated with LPS and subjected to SV at 90 Hz for 3 h. Subsequently, the cytokine transcription was analyzed. As shown in A, the vibration significantly increased the level of the IL-10 mRNA of the BMDMs, while it did not alter the expression of the IL-1β and IL-6 transcripts. Also, SV (90 Hz) increased the secretion of IL-10 in the BMDMs in a time-dependent manner (B). In addition, the vibration administered to THP-1 significantly enhanced the transcription of IL-10 in response to the LPS treatment, but it did not change the levels of the IL-1β and IL-6 mRNAs (C). Overall, SV at 90 Hz up-regulated the IL-10 expression in response to LPS in human and mice macrophages.
Figure 4. Effects of sound vibration (SV) on cytokine expression in BMDMs and THP-1 cells. Bone marrow-derived macrophages (BMDMs) (A and B) were treated with lipopolysaccharides (LPS) and then subjected to SV stimulation for 3 h or as indicated. Human macrophage-like cells (THP-1) (C) were treated with LPS and then stimulated by SV for 3 h. LPS-treated THP-1 (D) and BMDM (E) were treated with SB203580 (SB) or Bay 11-7082 (Bay) and then subjected to SV stimulation for 3 h. The gene expression of interleukin (IL)-1β, IL-6, and IL-10 was analyzed by quantitative reverse transcription-polymerase chain reaction (qPCR), and the secretion of IL-10 was measured by ELISA kit. The bar graph presents the mean ± SD with at least three independent experiments. *, P < 0.05.
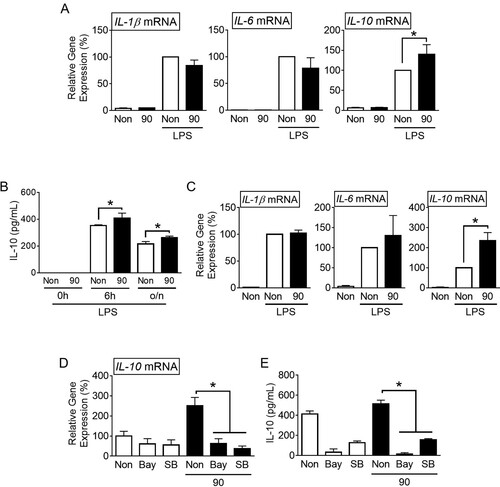
To investigate whether SV affects LPS-mediated IL-10 expression through the TLR4 signaling pathway, we treated macrophages with SB203580 (SB, a p38 MAPK inhibitor) or Bay 11-7082 (Bay, an NF-κB inhibitor) in combination with LPS and subjected them to 90 Hz SV stimulation. The results showed that both SB and Bay treatments significantly reduced the increased LPS-mediated IL-10 expression (D) in THP-1 cells and secretion (E) in BMDMs induced by SV stimulation. Additionally, examination of TLR4 mRNA expression in response to SV stimulation revealed no significant differences (Supplemental Figure S2C). In conclusion, SV stimulation may enhance IL-10 expression through the p38 MAPK and NF-κB signaling pathways dependent on TLR4 activation.
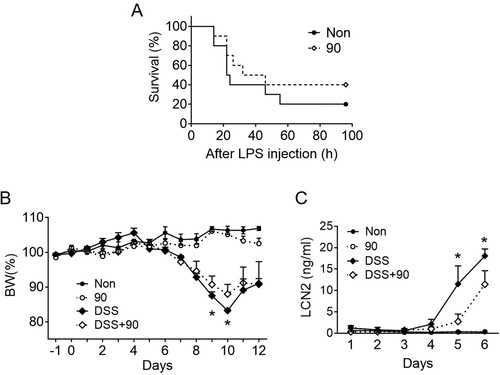
SV at 90 Hz alleviates LPS-induced lethality and DSS colitis in mice
To elucidate the role of SV in specific disease conditions, mice induced with endotoxemia and colitis were exposed to SV at 90 Hz. An LPS injection leads to lethal endotoxemia, and an IL-10 administration lowers the risk of mortality (Howard et al. Citation1993). Based on this, we hypothesized that stimulation of mice injected with LPS to SV at 90 Hz may lower the risk of mortality. The results (A) showed that the SV-stimulated mice had a survival rate twice as high as that of the non-stimulated group, although this was not statistically significant. Furthermore, IL-10-deficient mice develop spontaneous colitis and administering IL-10 in DSS-colitis models alleviates the symptoms (Kuhn et al. Citation1993; Li et al. Citation2014). Based on this, DSS-fed mice were subjected to SV at 90 Hz and were monitored for changes in body weight and fecal lipocalin-2 (LCN2) secretion, a marker for intestinal inflammation (Chassaing et al. Citation2012). As expected, DSS drinking decreased body weight (B) and increased fecal LCN2 levels (C), and SV stimulation alone did not change these indicators. Stimulation of DSS-fed mice to SV significantly reduced body weight loss and LCN2 secretion when compared with non-stimulated mice fed with DSS (B and C). Taken together, stimulation to vibration could result in the amelioration of inflammatory diseases.
Figure 5. Effects of SV on LPS lethality and DSS-colitis. A, Mice (n = 10 per group, total n = 20) were subjected to daily sound vibration (SV, 90 Hz, 0.5 m/s2, 6 h/d) stimulation for 10d and then injected with a lethal dosage of lipopolysaccharides (LPS). Mortality was monitored after the LPS injection for up to 96 h and presented as a Kaplan-Meier survival plot. B and C, Mice (n = 4 per group, total n = 16) were provided with tap water containing dextran sodium sulfate (DSS) for 7d on day 0, and then the water was replaced with normal tap water. Body weight (B) was monitored daily under SV for 12d, and fecal lipocalin (LCN2) levels (C) were measured for 6d. The line graph presents the mean ± SD. *, P < 0.05 between DSS only vs DSS + 90.
Discussion
In this study, we elucidated the impact of SV on cytokine secretion in response to inflammatory stimuli both in vitro and in vivo. The results confirmed that stimulation through vibration at 90 Hz increased serum and peritoneal IL-10 secretion, as well as IL-10 transcription in peritoneal exudate cells. Furthermore, SV increased IL-10 expression in macrophages treated with LPS. In LPS-induced lethality, vibration at 90 Hz reduced mortality rates and mitigated weight loss and fecal LCN2 secretion in DSS colitis. Through this study, SV has been demonstrated to have anti-inflammatory effects by increasing IL-10 expression.
We selected three different frequencies (14, 45, and 90 Hz) for our experiment, based on previous research (Weinheimer-Haus et al. Citation2014; Atanasov et al. Citation2015). We believe that the effects were observed at 90 Hz for the following reasons: Objects have specific resonance frequencies influenced by their physical composition, with the resonance frequency range (RFR) amplifying vibrations as they approach the resonance frequency. The approximate resonance frequency range identified in mice was calculated to be 30 to 100 Hz (Reynolds et al. Citation2018). Additionally, measurements of physiological changes in response to vibrations revealed that mice exhibited behavioral changes in the range of 70–100 Hz (Garner et al. Citation2018). This suggests that within the 70–100 Hz RFR, mice are sensitive to low-level vibrations, which may have the most significant impact on the physiology and behavior of animals. Therefore, the effectiveness observed at 90 Hz in this study is believed to be due to its inclusion in the resonance frequency range for mice.
Previously, some studies have been conducted on the impact of vibration therapy on inflammatory responses. Whole-body vibration is employed as one of the passive exercise methods for elderly individuals (Rodriguez-Miguelez et al. Citation2014; Rodriguez-Miguelez et al. Citation2015). Results from measuring the expression of inflammatory cytokines in the blood of elderly individuals subjected to whole-body vibration showed no change or a decrease in pro-inflammatory cytokines, while the expression of IL-10 increased (Rodriguez-Miguelez et al. Citation2014; Rodriguez-Miguelez et al. Citation2015). Vibration stimulation suppressed TLR 2/4 signaling, such as extracellular signal-regulated kinases 1 and 2 and heat shock proteins, in peripheral blood mononuclear cells, leading to an increased expression of IL-10 (Rodriguez-Miguelez et al. Citation2014; Rodriguez-Miguelez et al. Citation2015). Additionally, whole-body vibration increased IL-10 expression in patients with chronic obstructive pulmonary disease, and similar effects were observed in healthy individuals (Lage et al. Citation2018; Jawed et al. Citation2020). In animal models using rats, vibration stimulation enhanced IL-10 expression in the tissues (Chow et al. Citation2019). To summarize, vibration stimulation is considered to have an anti-inflammatory effect through an increase in the IL-10 expression. The results of this study, which show increased IL-10 expression due to SV at 90 Hz alleviate symptoms in the disease animal model and align with previous research.
IL-10 plays a crucial role in infections by serving as an anti-inflammatory cytokine that limits the immune response to pathogens, thereby preventing damage to the host (Saraiva and O’Garra Citation2010). Evolution has shaped immune responses to protect hosts from a wide range of pathogenic microorganisms, but it is equally important to regulate excessive immune reactions and to limit damage to the host by preventing self-reactivity (Saraiva and O’Garra Citation2010). IL-10 is secreted by various cells, namely, both innate (macrophages, dendritic cells, neutrophils, etc.) and adaptive (Th1, Th2, Th17, B cells, etc.) immune cells (Moore et al. Citation2001; Gabrysova et al. Citation2014; Lobo-Silva et al. Citation2016). Moreover, IL-10 is produced in tissue-resident macrophages like microglia and epithelial cells (Jarry et al. Citation2008; Lobo-Silva et al. Citation2016). Additionally, tumor cells induce immunosuppression through IL-10 production (Itakura et al. Citation2011). IL-10 is a feedback regulator of various immune responses, is secreted by diverse cells, and operates in different immune reactions (Saraiva and O’Garra Citation2010). Stimulation of TLR signaling in macrophages results in substantial IL-10 production. When TLRs are activated, they induce the production of IL-10 and pro-inflammatory cytokines through the mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB) pathways (Akira and Takeda Citation2004; Saraiva et al. Citation2020). MAPK is considered the most critical pathway for IL-10 production (Saraiva et al. Citation2020). Activated NF-κB downstream of the pattern recognition receptors is also crucial for IL-10 production in myeloid cells, particularly in TLR4-stimulated macrophages (Saraiva et al. Citation2020). Vibration has been shown to activate p38 MAPK signaling in osteogenesis and induce NF-κB activation in human periodontal ligament cells and osteocytes (Sanyal and Schubert Citation1993; Lu et al. Citation2018; Sakamoto et al. Citation2019; Phusuntornsakul et al. Citation2020). Based on this, we investigated whether the effect of SV stimulation on LPS-mediated IL-10 expression could be attributed to their role in p38 MAPK and NF-κB signaling. The results indicate that these signaling pathways are crucially involved in promoting IL-10 transcription in response to SV stimulation. However, IL-10 expression is complex, involving post-transcriptional modifications and intricate regulation in epigenetics. Therefore, the increased IL-10 expression due to SV in this study is considered a product of a multifaceted response that cannot be confined to a single signaling pathway.
IL-10 has broad anti-inflammatory activity. In particular, IL-10 receptors (IL-10R) are highly expressed in macrophages, and experimental results with macrophage-selective IL-10R deficiency indicate that macrophages play a key role in IL-10 responsiveness (Moore et al. Citation2001; Shouval et al. Citation2014). IL-10 initiates a robust immunosuppressive response in innate immune cells, including macrophages, by regulating the transcription of cytokines and chemokines, and it inhibits the generation of reactive oxygen species during pathogenic infections (Bogdan et al. Citation1991; Saraiva et al. Citation2020). IL-10 indirectly inhibits T cell responses by suppressing antigen-presenting cells and directly regulates memory/effect T cells and T helper 17 cells (Macatonia et al. Citation1993; Huber et al. Citation2011; Kamanaka et al. Citation2011). Consequently, research on disease therapy through the modulation of IL-10 is actively underway (Saraiva et al. Citation2020). Significant effects have been observed in immune diseases such as rheumatoid arthritis (Trachsel et al. Citation2007), psoriasis, and allergic asthma. In addition, inflammatory bowel disease (IBD) is most commonly associated with IL-10 (McInnes et al. Citation2001; Trachsel et al. Citation2007; Galeazzi et al. Citation2014). Colitis develops in IL-10-deficient mice, and genetic evidence found in IBD patients suggests a deficiency in IL-10 signaling (Kuhn et al. Citation1993; Jostins et al. Citation2012; Ellinghaus et al. Citation2016). Furthermore, IL-10 administration or overexpression in colitis animal models has been consistently proven to be beneficial (Li et al. Citation2014; Cardoso et al. Citation2018). However, systemic administration of recombinant IL-10 in human clinical trials did not result in significant improvement, probably because IL-10 acts locally (Colombel et al. Citation2001). Therefore, research has focused on delivering IL-10 specifically to the intestine. Interestingly, engineered probiotics (e.g. Lactococcus lactis and Bifidobacterium bifidum) capable of expressing human IL-10 were administered to DSS-colitis mice, resulting in significant benefits (Steidler et al. Citation2000; Mauras et al. Citation2018). In this context, it can be suggested that the SV used in this study, which can transmit vibrations to the intestine when administered to the abdomen, may locally increase the IL-10 levels in the intestine and improve the symptoms of IBD.
In conclusion, vibration at 90 Hz within the resonance frequency range accelerates the increase in IL-10 expression without affecting pro-inflammatory cytokine secretion induced by inflammatory triggers such as LPS. Stimulation with SV at 90 Hz improves inflammatory symptoms in disease mice, such as LPS lethality and DSS-colitis. Based on this study, we demonstrated the anti-inflammatory effect of SV, manifested by the increased secretion of IL-10.
Ethics approval
All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the Animal research: Reporting of In Vivo Experiments (ARRIVE) guidelines. The experimental protocol for the animal study was approved by the Institutional Animal Care and Use Committee of Kangwon National University (KW-220908-4).
Supplemental Material
Download PDF (437.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data used to support the findings of this study are available from the corresponding author upon request.
Additional information
Funding
References
- Abdellaoui N, Kim SY, Kim MS. 2023. Effect of TRAF6-knockout on gene expression and lncRNA expression in Epithelioma papulosum cyprini (EPC) cells. Animal Cells Syst. 27:197–207. doi:10.1080/19768354.2023.2263070.
- Akira S, Takeda K. 2004. Toll-like receptor signalling. Nat Rev Immunol. Jul; 4:499–511. doi:10.1038/nri1391.
- Atanasov NA, Sargent JL, Parmigiani JP, Palme R, Diggs HE. 2015. Characterization of train-induced vibration and its effect on fecal corticosterone metabolites in mice. J Am Assoc Lab Anim Sci: JAALAS. Nov;54:737–744.
- Bogdan C, Vodovotz Y, Nathan C. 1991. Macrophage deactivation by interleukin 10. J Exp Med. Dec 1;174:1549–1555. doi:10.1084/jem.174.6.1549.
- Boyd-Brewer C, McCaffrey R. 2004. Vibroacoustic sound therapy improves pain management and more. Holistic Nurs Pract. May-Jun;18:111–118; quiz 118-119. doi:10.1097/00004650-200405000-00002.
- Cardoso A, Gil Castro A, Martins AC, Carriche GM, Murigneux V, Castro I, Cumano A, Vieira P, Saraiva M. 2018. The dynamics of interleukin-10-afforded protection during dextran sulfate sodium-induced colitis. Front Immunol. 9:400.
- Chassaing B, Srinivasan G, Delgado MA, Young AN, Gewirtz AT, Vijay-Kumar M. 2012. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS One. 7:e44328.
- Chen YS, Chuang WC, Kung HN, Cheng CY, Huang DY, Sekar P, Lin WW. 2022. Pan-caspase inhibitor zVAD induces necroptotic and autophagic cell death in TLR3/4-stimulated macrophages. Mol Cells. Apr 30;45:257–272. doi:10.14348/molcells.2021.0193.
- Chow SK, Chim YN, Wang J, Zhang N, Wong RM, Tang N, Leung KS, Cheung WH. 2019. Vibration treatment modulates macrophage polarisation and enhances early inflammatory response in oestrogen-deficient osteoporotic-fracture healing. Eur Cells Mater. Nov 7;38:228–245. doi:10.22203/eCM.v038a16.
- Colombel JF, Rutgeerts P, Malchow H, Jacyna M, Nielsen OH, Rask-Madsen J, Van Deventer S, Ferguson A, Desreumaux P, Forbes A, et al. 2001. Interleukin 10 (Tenovil) in the prevention of postoperative recurrence of Crohn’s disease. Gut. Jul;49:42–46. doi:10.1136/gut.49.1.42.
- Ellinghaus D, Jostins L, Spain SL, Cortes A, Bethune J, Han B, Park YR, Raychaudhuri S, Pouget JG, Hubenthal M, et al. 2016. Analysis of five chronic inflammatory diseases identifies 27 new associations and highlights disease-specific patterns at shared loci. Nat Genet. May;48:510–518. doi:10.1038/ng.3528.
- Feng JH, Jung JS, Hwang SH, Lee SK, Lee SY, Kwak YG, Kim DH, Song CY, Kim MJ, Suh HW, et al. 2022. The mixture of Agrimonia pilosa Ledeb. and Salvia miltiorrhiza Bunge. extract produces analgesic and anti-inflammatory effects in a collagen-induced arthritis mouse model. Anim Cells Syst. 26:166–173. doi:10.1080/19768354.2022.2106302.
- Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O’Garra A. 1991. IL-10 inhibits cytokine production by activated macrophages. J Immunol. Dec 1;147:3815–3822. doi:10.4049/jimmunol.147.11.3815.
- Gabrysova L, Howes A, Saraiva M, O’Garra A. 2014. The regulation of IL-10 expression. Curr Top Microbiol Immunol. 380:157–190.
- Galeazzi M, Bazzichi L, Sebastiani GD, Neri D, Garcia E, Ravenni N, Giovannoni L, Wilton J, Bardelli M, Baldi C, et al. 2014. A phase IB clinical trial with Dekavil (F8-IL10), an immunoregulatory ‘armed antibody’ for the treatment of rheumatoid arthritis, used in combination wiIh methotrexate. Israel Med Assoc J: IMAJ. Oct;16:666.
- Garner AM, Norton JN, Kinard WL, Kissling GE, Reynolds RP. 2018. Vibration-induced behavioral responses and response threshold in female C57BL/6 mice. J Am Assoc Lab Anim Sci: JAALAS. Sep 1;57:447–455. doi:10.30802/AALAS-JAALAS-17-00092.
- Howard M, Muchamuel T, Andrade S, Menon S. 1993. Interleukin 10 protects mice from lethal endotoxemia. J Exp Med. Apr 1;177:1205–1208. doi:10.1084/jem.177.4.1205.
- Huber S, Gagliani N, Esplugues E, O’Connor W, Huber FJ, Chaudhry A, Kamanaka M, Kobayashi Y, Booth CJ, Rudensky AY, et al. 2011. Th17 cells express interleukin-10 receptor and are controlled by Foxp3(-) and Foxp3 + regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. Apr 22;34:554–565. doi:10.1016/j.immuni.2011.01.020.
- Itakura E, Huang RR, Wen DR, Paul E, Wunsch PH, Cochran AJ. 2011. IL-10 expression by primary tumor cells correlates with melanoma progression from radial to vertical growth phase and development of metastatic competence. Modern Pathol. Jun;24:801–809. doi:10.1038/modpathol.2011.5.
- Jarry A, Bossard C, Bou-Hanna C, Masson D, Espaze E, Denis MG, Laboisse CL. 2008. Mucosal IL-10 and TGF-beta play crucial roles in preventing LPS-driven, IFN-gamma-mediated epithelial damage in human colon explants. J Clin Investig. Mar;118:1132–1142.
- Jawed Y, Beli E, March K, Kaleth A, Loghmani MT. 2020. Whole-body vibration training increases stem/progenitor cell circulation levels and may attenuate inflammation. Milit Med. Jan 7;185:404–412. doi:10.1093/milmed/usz247.
- Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, et al. 2012. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. Nov 1;491:119–124. doi:10.1038/nature11582.
- Kamanaka M, Huber S, Zenewicz LA, Gagliani N, Rathinam C, O’Connor W, Wan YY, Nakae S, Iwakura Y, Hao L, et al. 2011. Memory/effector (CD45RB(lo)) CD4 T cells are controlled directly by IL-10 and cause IL-22-dependent intestinal pathology. J Exp Med. May 9;208:1027–1040. doi:10.1084/jem.20102149.
- Kantor J, Kantorova L, Mareckova J, Peng D, Vilimek Z. 2019. Potential of vibroacoustic therapy in persons with cerebral palsy: An advanced narrative review. Int J Environ Res Public Health. Oct;16:16.
- Kennedy J, Verne S, Griffith R, Falto-Aizpurua L, Nouri K. 2015 Sep. Non-invasive subcutaneous fat reduction: a review. J Eur Acad Dermatol Venereol: JEADV. 29:1679–1688. doi:10.1111/jdv.12994.
- Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. 1993. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. Oct 22;75:263–274. doi:10.1016/0092-8674(93)80068-P.
- Lage VKS, Lacerda ACR, Neves CDC, Chaves MGA, Soares AA, Lima LP, Martins JB, Matos MA, Vieira ELM, Teixeira AL, et al. 2018. Acute effects of whole-body vibration on inflammatory markers in people with chronic obstructive pulmonary disease: a pilot study. Rehab Res Pract. 2018:5480214.
- Li B, Alli R, Vogel P, Geiger TL. 2014. IL-10 modulates DSS-induced colitis through a macrophage-ROS-NO axis. Mucosal Immunol. Jul;7:869–878. doi:10.1038/mi.2013.103.
- Lobo-Silva D, Carriche GM, Castro AG, Roque S, Saraiva M. 2016. Balancing the immune response in the brain: IL-10 and its regulation. J Neuroinflamm. Nov 24;13:297. doi:10.1186/s12974-016-0763-8.
- Lu Y, Zhao Q, Liu Y, Zhang L, Li D, Zhu Z, Gan X, Yu H. 2018. Vibration loading promotes osteogenic differentiation of bone marrow-derived mesenchymal stem cells via p38 MAPK signaling pathway. J Biomech. Apr 11;71:67–75. doi:10.1016/j.jbiomech.2018.01.039.
- Macatonia SE, Doherty TM, Knight SC, O’Garra A. 1993. Differential effect of IL-10 on dendritic cell-induced T cell proliferation and IFN-gamma production. J Immunol. May 1;150:3755–3765. doi:10.4049/jimmunol.150.9.3755.
- Mauras A, Chain F, Faucheux A, Ruffie P, Gontier S, Ryffel B, Butel MJ, Langella P, Bermudez-Humaran LG, Waligora-Dupriet AJ. 2018. A new bifidobacteria expression SysTem (BEST) to produce and deliver interleukin-10 in bifidobacterium bifidum. Front Microbiol. 9:3075.
- McInnes IB, Illei GG, Danning CL, Yarboro CH, Crane M, Kuroiwa T, Schlimgen R, Lee E, Foster B, Flemming D, et al. 2001. IL-10 improves skin disease and modulates endothelial activation and leukocyte effector function in patients with psoriatic arthritis. J Immunol. Oct 1;167:4075–4082. doi:10.4049/jimmunol.167.7.4075.
- Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. 2001. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 19:683–765.
- Park JM, Park S, Jee YS. 2019. Rehabilitation program combined with local vibroacoustics improves psychophysiological conditions in patients with ACL reconstruction. Medicina (B Aires). Sep 30;55: 659. doi:10.3390/medicina55100659.
- Phusuntornsakul P, Jitpukdeebodintra S, Pavasant P, Leethanakul C. 2020. Vibration activates the actin/NF-kappaB axis and upregulates IL-6 and IL-8 expression in human periodontal ligament cells. Cell Biol Int. Feb;44:661–670. doi:10.1002/cbin.11267.
- Reynolds RP, Li Y, Garner A, Norton JN. 2018. Vibration in mice: a review of comparative effects and use in translational research. Anim Models Exp Med. Jun;1:116–124. doi:10.1002/ame2.12024.
- Rodriguez-Miguelez P, Fernandez-Gonzalo R, Almar M, Mejias Y, Rivas A, de Paz JA, Cuevas MJ, Gonzalez-Gallego J. 2014. Role of toll-like receptor 2 and 4 signaling pathways on the inflammatory response to resistance training in elderly subjects. Age (Omaha). 36:9734.
- Rodriguez-Miguelez P, Fernandez-Gonzalo R, Collado PS, Almar M, Martinez-Florez S, de Paz JA, Gonzalez-Gallego J, Cuevas MJ. 2015. Whole-body vibration improves the anti-inflammatory status in elderly subjects through toll-like receptor 2 and 4 signaling pathways. Mech Ageing Dev. Sep; 150:12–19. doi:10.1016/j.mad.2015.08.002.
- Sakamoto M, Fukunaga T, Sasaki K, Seiryu M, Yoshizawa M, Takeshita N, Takano-Yamamoto T. 2019. Vibration enhances osteoclastogenesis by inducing RANKL expression via NF-kappaB signaling in osteocytes. Bone. Jun;123:56–66. doi:10.1016/j.bone.2019.03.024.
- Sanyal AJ, Schubert ML. 1993. Endoscopic ligation versus sclerotherapy: is it time to jump on the bandwagon? Gastroenterology. Dec;105:1915–1916.
- Saraiva M, O’Garra A. 2010. The regulation of IL-10 production by immune cells. Nat Rev Immunol. Mar;10:170–181. doi:10.1038/nri2711.
- Saraiva M, Vieira P, O’Garra A. 2020. Biology and therapeutic potential of interleukin-10. J Exp Med. Jan 6;217:e20190418. doi:10.1084/jem.20190418 .
- Shouval DS, Biswas A, Goettel JA, McCann K, Conaway E, Redhu NS, Mascanfroni ID, Al Adham Z, Lavoie S, Ibourk M, et al. 2014. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity. May 15;40:706–719. doi:10.1016/j.immuni.2014.03.011.
- Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, Falk W, Fiers W, Remaut E. 2000. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. Aug 25;289:1352–1355.
- Trachsel E, Bootz F, Silacci M, Kaspar M, Kosmehl H, Neri D. 2007. Antibody-mediated delivery of IL-10 inhibits the progression of established collagen-induced arthritis. Arthritis Res Therapy. 9:R9.
- Weinheimer-Haus EM, Judex S, Ennis WJ, Koh TJ. 2014. Low-intensity vibration improves angiogenesis and wound healing in diabetic mice. PLoS One. 9:e91355.

