ABSTRACT
To investigate the spectrum of rat-borne pathogens circulating in Europe a systematic review spanning across 55 European countries during the years 1995–2016 was performed. The study surveyed viruses, bacteria, macroparasites and unicellular eukaryotes (protozoa). Fifty-three different infectious agents, all with zoonotic potential, were reported to be carried by commensal rats; 48 by the brown rat (Rattus norvegicus) and 20 by the black rat (R. rattus). There was a tendency for rural areas to harbour more rat-borne microbes than urban areas regarding the brown rat, but the opposite could be observed for the black rat. The study clearly indicated that an improved surveillance on wild rats is needed in Europe, and further indicated the pathogens and geographical areas where the major focus is required. For example, six zoonotic microbes seemed to be clearly more geographically widespread in Europe than others; virulent or resistant E. coli, pathogenic Leptospira spp., Hymenolepis diminuta, H. nana, Capillaria hepatica and Toxoplasma gondii.
Introduction
Several rat-borne zoonotic pathogens and their associated diseases such as hantavirus infections and leptospirosis have recently emerged or re-emerged in Europe (e.g. [Citation1,Citation2]). One pest control company handling approx. 85% of all pest control in Sweden, noted a 67% increase in the number of rat control activities over the last three years (press release 13 February 2017, https://www.anticimex.com/sv-SE/nyhetsrum/2017/saneringar-av-rattor-okar-kraftigt/). The resistance against rodenticides is increasing in rats; the geographic spread of resistance mutations is now well documented in e.g. Great Britain [Citation3]. More rats increase the probability of contact between humans and rats and accordingly the risk for transmission of severe infectious diseases [Citation4].
Brown and black rats (Rattus norvegicus, Berkenhout, 1769, and R. rattus, Linneaus, 1758) originated in Asia and are to date present all over the globe alongside humans [Citation5–Citation7]. These rodents have introduced pathogenic microorganisms such as e.g. Yersinia pestis, Bartonella and hantavirus into many new geographically areas [Citation8–Citation11]. In addition, both black and brown rats have acquired new macroparasites outside their native ranges [Citation12]. Thus, they act as efficient transmitters of pathogens between wildlife, domestic animals, vectors and humans.
The most infamous pathogen known to be transmitted from rodents to humans is Yersinia pestis, causing plague. The vectors making the transmission possible are fleas, probably the most effective being the oriental rat flea (Xenopsylla cheopsis) [Citation11]. Several mammal species can act as reservoirs for Yersinia pestis, but the black rat is a common reservoir in e.g. Madagascar, were over 1500 human plague cases were reported between year 1997 and 2001 [Citation13,Citation14]. Moreover, inhabitants of Madagascar recently suffered from a large plague outbreak with over 2000 reported cases in a period less than three months [Citation15]. Plague is wide-spread and present in large areas of North and South America, Asia and Africa [Citation11].
Lassa virus, which causes a highly fatal hemorrhagic fever common in West Africa, is mainly transmitted directly or indirectly from the multimammate rat (Mastomys natalensis) [Citation16–Citation18].
Seoul hantavirus (SEOV), causing severe disease, is mainly found in brown rats in China and Southeast Asia, but also at other locations around the world [Citation10]. The disease caused by SEOV is named haemorrhagic fever with renal syndrome (HFRS) and is characterized mainly by high fever, fatigue and severe kidney problems with a mortality of 2–3% [Citation4].
The biology of the pathogens, hosts and vectors can be altered by urbanization, which may result in an increase of disease transmission [Citation19]. Habitat fragmentation can increase the risk for disease. E.g. in New York, there was a significant increase in the density of Borrelia burgdorferi infected tick nymphs, with decreasing forest patch sizes [Citation20]. Globally, 54% of the human population live in urban areas [Citation21]. In Europe, as many as 74% of the approx. 740 million inhabitants (year 2015) live in urban areas.
The major objectives of our study were to:
Identify rat-borne pathogens circulating in Europe and reported during the years 1995–2016.
Compare pathogen diversity (number of reported pathogenic microorganisms) between different geographical areas/structures of Europe:
Compare pathogen abundance in Northern Europe with pathogen abundance in the Southern parts of Europe, and
Compare pathogen richness in urban areas with pathogen richness in rural areas and oceanic islands in Europe.
Materials and methods
Throughout the text, bacteria (including rickettsia), helminths (including Acanthocephala), unicellular eukaryotes (hereafter called protozoa and including also Amoebas and Apicomplexan parasites) and viruses were considered. We did not include prions, fungi or ectoparasites (i.e. Arthrodopa) in this study.
Search strategy
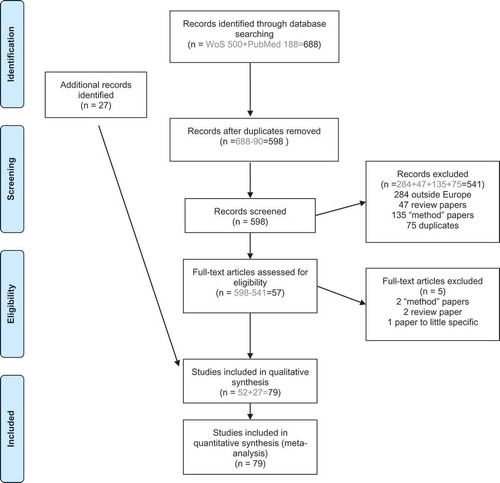
We conducted a systematic literature search to identify articles in the Web of Science ‘All databases’ (including Web of Science Core Collection, BIOSIS Citation Index, BIOSIS Previews, Zoological Records, SciELO Citation Index) and PubMed bibliographic databases as of 26th of September 2016. The time period for articles to be included was set to 1st of January 1995 to the date of search 2016 (26th of September 2016). We followed the search process described in .
Text word searches included ‘Rattus rattus’, ‘Rattus norvegicus’, ‘Norway rat’, ‘brown rat’, ‘black rat’, ‘sewage rat’, ‘urban rat’ or ‘roof rat’) AND the TOPIC: ‘Rodent-borne’, ‘rat-borne’, Zoono*, ‘human disease’, ‘one health’ or ‘Rat-associated’ or AND the TOPIC: (Babesia, Capillaria, Cestodes, Cryptosporidium, ‘Echinococcus multiloculari’, ‘Entamoeba histolytica’, Fasciolia, Giardia, Hymenolepis, Leishmania, Nematodes, Sarcocystis, Taenia, ‘Toxocara cati’, ‘Toxoplasma gondii’, Trematodes, Trichinella, Trypanosoma, Babesiosis, Capillariasis, Crypdosporidiosis, ‘alveolar echinococcosis’, ‘Amoebic dysentery’, ‘Human fasciolosis’, Giardiasis, Rodentolepiasis, Leishmaniasis, Sarcosporidiosis, Taeniasis, Toxocariasis, Toxoplasmosis, Trichinosis, ‘Chagas disease’, Anaplasma, Bartonella, ‘Borrelia burgdorferi’, Borrelia, Campylobacter, ‘Coxiella burnetii’, ‘E. coli’, ‘Francisella tularensis’, Leptospira, Listeria, ‘Orientia tsutsugamushi’, Pasteurella, Pseudomonas, Rickettsia, Salmonella, ‘Streptobacillus moniliformis’, Yersinia, ‘Bartonella Illness’, ‘Lyme disease’, ‘Relapsing fever’, Campylobacteriosis, ‘Q fever’, ‘VTEC’, Tularemia, ‘Leptospirosis’, ‘Weil’s disease’, Listeriosis, ‘Scrub typhus’, Pasterurellosis, Meliodosis, ‘typhus’, ‘murine typhus’, Salmonellosis, ‘rat bite fever’, ‘Haverhill fever’, ‘Yersiniosis’, Plague, Cowpox, ‘Hepatitis E’, HEV, ‘Lymphocytic choriomeningitis’, LCMV, Rabies, ‘Seoul hantavirus’, ‘Seoul virus’, ‘Tick-Borne Encephalitis’, TBE, ‘Haemorrhagic fever’ or ‘Hemorrhagic fever’
The investigation covered the European countries in and those were classified in the United Nations division as: Eastern Europe, Northern Europe, Southern Europe and Western Europe [Citation21].
Table 1. Countries included in the literature search.
The search language was English but articles also in German, French and Italian were included.
Data abstraction
Screening
We screened articles that by the title and abstract included the following: a) Brown or black rats, b) Studies performed within the years 1995–2016, and c) Pathogens (harmful or potentially harmful) to humans.
Exclusion
After the reference program Endnote removed the duplications automatically, 598 papers were present. Articles were excluded if they were performed outside the European countries included in our study (284 papers). Papers that included method descriptions, modelling papers or did not include wild brown or black rats were removed and put in a category named ‘methods’ (135 articles). 47 papers were discarded because they were regarded as reviews. 75 additional duplicated papers were found manually.
Additional articles (n = 27) were added through citation searching.
Extraction
We extracted the following data from the articles: Type of pathogen (species or at genus level where a genus is known to include species that cause disease in humans, but where no specific species was reported), tissue/tissues sampled, laboratory methods used, rat species, number of rat individuals, number of infested rats, country and habitat. For each rat species and country designation into Eastern, Southern, Northern and Eastern Europe, we recorded and calculated the number of reported pathogens.
Rat studies performed on oceanic islands were designated island habitat type no matter if the study was performed in urban or rural environments. Other rat studies (according to the authors) performed in urban, periurban, suburban environment or sewer sites within these habitats were designated urban habitat. Studies performed in farms, rural habitats, forests or fields, waste disposal sites and mountain regions were designated rural habitats. If it was not possible to find out the habitat type, or if both rural and urban habitat types were mixed, they were not included in the habitat part of the analysis. As for the countries, for each rat species and habitat type, we recorded and calculated the number of pathogens.
Results
Literature search
Fifty-seven articles were extracted by the systematic screening for studies on zoonotic rat-borne microbes in Europe and read thoroughly. Four of these 57 papers were excluded since they were found to be solely methodological or reviews. One additional article was removed at this step, a virome study without the requested details presented. A total of 79 articles were finally used, including the additional 27 studies found by the authors.
Brown and black rats
Five of the 79 articles presented negative results for all rats and pathogens included in the analyses. Sixteen studies on brown rats and five studies on black rats had failed to show any positive results for one or several pathogens.
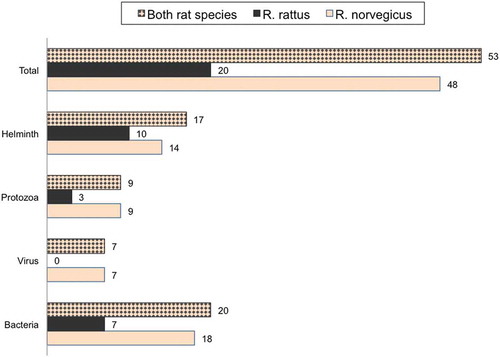
Among the 74 articles included with at least one positive finding, 55 described pathogens carried by brown rats and only seven studies reported pathogens found in black rats. Twelve studies sampled both brown and black rats for organisms pathogenic for humans. According to our literature search, 53 different infectious agents (or traces of, i.e. agent-specific antibodies) with well-known or potential zoonotic outcome were found in commensal rats in Europe, see . The more intensively studied rodent species, the brown rat, was reported to carry 48 unique infectious organisms in total, and the black rat 20 (for details, see ).
Geographical distribution of rat-borne pathogens within Europe
Rats from 22 of the 49 European countries and territories included in our initial screening (, Table S1) have been investigated for at least one rat-borne pathogen between 1995 and 2016.
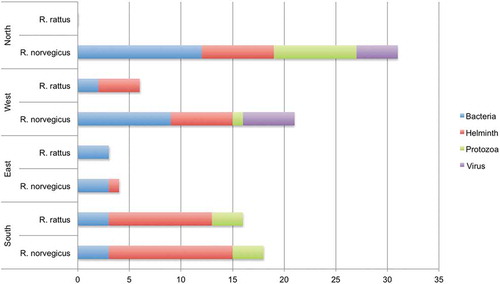
Regarding the brown rat, most pathogens were described from the Northern zone (Table S1). For the brown rat, rat-borne bacteria and helminths have been detected in all four European zones (). No pathogens have been detected in the black rat in the Northern zone (Table S1 and ). For the black rat, no virus was detected (or tested for) in any of the four zones (, Table S1). The studies on the black rat were mainly performed on Oceanic and Mediterranean islands and in countries near the Mediterranean Sea, but also in the Netherlands.
Figure 3. Number of different infectious agents per European zone, rat species and taxonomic level. Where a genus is known to include species that cause disease in humans but no species is given, the genus is counted once for each habitat and rat species.
Six infectious organisms were clearly more often reported than others, and they were all present in three geographic zones. These were two bacteria (virulent or resistant E. coli and pathogenic Leptospira spp.), three helminths (Hymenolepis diminuta, H. nana and Capillaria hepatica) and one protozoon (Toxoplasma gondii).
Habitat and pathogens
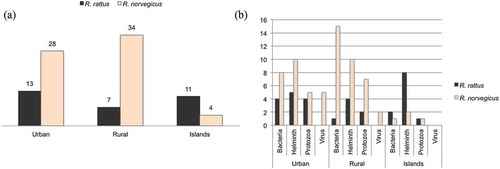
We compared the number of unique pathogens reported in rats between the specified urban, rural (including studies specified as performed on farms) and oceanic islands habitats (), most data points were independent studies for each habitat. For the brown rat, rural areas seem to harbour a larger palette of rat-borne organisms than urban areas (). The opposite seems to be the case for the black rat (). The black rat carried a higher number of pathogens, i. e. helminths, than the brown rat on oceanic islands. Brown rats were free of specific pathogens in three cases of islands habitats, 20 cases of rural environments and 24 cases of urban habitats. Black rats were free of specific pathogens in 10 cases of rural habitats and 11 cases of urban habitats.
Figure 4. (a) Number of different zoonotic infectious agents found in the literature search by rat species and habitat (urban/suburban, rural/farm, oceanic islands). Where a genus is known to include species that cause disease in humans but no species is given, the genus is counted once for each habitat and rat species. (b) Number of different zoonotic infectious agents found in the literature search by taxonomic division, rat species and habitat.
Discussion
Brown and black rats
In Europe, both the brown and the black rat are present in many geographical areas, although the black rat has disappeared from several of the countries where it used to reside, like e.g. Sweden [Citation22]. In fact, the black rat has been claimed to have been significantly reduced across most of the globe over the last century [Citation23], although exceptions seem to exist [Citation24]. Although this review is not intended as an overview for the various rat species, the brown rat seems to cover the major parts of Europe to date, in contrast to the black rat, which is mainly focused to the warmer coasts and oceanic islands.
Altogether, we found 53 different rat-borne pathogens reported, with potential consequences for the public health in Europe. In an earlier review by Cleaveland and co-workers 180 zoonotic agents that could be hosted by rodents were reported [Citation25]. Globally, there are 164 helminth species reported to be carried by R. norvegicus, 156 for R. rattus, and only 65 helminths that are common for both R. rattus and R. norvegicus [Citation12]. In the original distribution areas of the brown and the black rats (Oriental region), they harbour 21 (12.8% of the total global number) and 64 (41% of the total global number) helminths, respectively [Citation12]. According to our review, as many as 17 potentially zoonotic helminths are carried by both rat species. Generally, it is considered that commensal rats can acquire new pathogens alongside their natural pathogens in their new surroundings by so called ‘parasite spillback’ [Citation9,Citation12]. For example, Coxiella burnetii in the Netherlands is a zoonotic pathogen that is harboured mainly by goats, sheep and cattle, but was recently found also in rats [Citation26].
Geographical distribution of rat-borne pathogens within Europe
We suggest a general focus of future surveillance in Europe of the six most widespread pathogens according to this review, i.e. the virulent/resistant E. coli, pathogenic Leptospira spp., the helminths Hymenolepis diminuta, H. nana and Capillaria hepatica, and the protozoon Toxoplasma gondii.
Humans and rats are like other mammals, natural carriers of the intestinal bacteria E. coli [Citation27]. Some variants of E. coli can cause direct disease such as for enterohemorrhagic E. coli (EHEC/VTEC) that produce verotoxins, or could be harmful once you get another bacterial infection (for antibiotic resistant E. coli strains) [Citation28]. Verotoxigenic E. coli had year 2015 the highest reported incidence (12.3/100,000) on Ireland and secondly and third in Sweden (5.7/100,000) and in the Netherlands (5.1/100,000), respectively, among the 27 countries in EU reported [Citation29]. Antimicrobial resistance is widespread and is increasing in Europe for E. coli as observed by surveillance of human blood or cerebrospinal fluid [Citation30]. A majority of the isolates were resistant to at least one of the antimicrobial groups and many produced Extended-spectrum beta-lactamase (ESBL). The reports of E. coli in rats may parallel the status of E. coli in humans. Therefore, studies on E. coli in rats could be most valuable as an indicator of what strains and what level of antibiotic resistance we actually have in the surroundings. It is not clear whether rats can transmit E. coli to the human population but they might serve as excellent mirrors. For example, rats can be monitored in different neighbourhoods and possible be used as an early warning system for resistant types of E. coli.
Different Leptospira serovars are transmitted by rats and other animals [Citation31]. In most cases leptospirosis in man is rather mild or asymptomatic, but occasionally it may develop to lung, liver and/or kidney disorders, and cardiovascular collapse [Citation32]. Overall, the number of cases of human leptospirosis is generally stable in Europe [Citation33]. Between 2007 and 2015, there were between 439 and 966 reported cases per year, including travel-associated cases [Citation29].
Several European countries or cities have reported increased numbers of domestically acquired leptospirosis during last years. In 2014, the Netherlands experienced an over four-fold increase in the number of autochthonous leptospirosis cases in humans, and also a significant increase of leptospirosis in dogs [Citation1]. Eight of the autochthonous 60 human cases were linked to direct contact with rats. However, several additional cases may have been connected to rats, since the transmission route can also occur indirectly through urine-contaminated water [Citation34]. In addition, the Netherlands reported the highest number of leptospirosis cases in 2015 (including travel-associated disease) (n = 86), alongside Germany (also 86 cases), among the 29 countries reported to ECDC [Citation29]. France does not report a general rise in number of reported leptospirosis cases [Citation33], but in Marseille, the reported incidence of leptospirosis steadily increased from 2001 through 2011, after an absence of cases between 1985–1995 [Citation35]. Marseille is also a town inhabited of many rats and also Leptospira-infected rats [Citation33]. In Sweden in 2015, with a recent rise in rat exterminations we detected antibodies against the highly pathogenic Leptospira serovar Icterohaemorrhagiae in wild brown rats trapped in Stockholm and Gothenburg [Citation36]. In conclusion, there are strong links established between rats and leptospirosis in humans, also in Europe [Citation34,Citation35]. Since autochthonous human leptospirosis is wide-spread at least in continental Europe [Citation29] we suggest an increased surveillance of pathogenic Leptospira in rats, also in areas where Leptospira has not (yet) been detected (like Faroe Islands) [Citation37], for an improved awareness of the future threats to the public health.
Toxoplasma gondii is a unicellular eukaryote with a life cycle that involves cats [Citation38]. Since rats are living close to humans they have been suggested to be exposed to both cats and Toxoplasma oocysts [Citation39]. As shown by this review rats are infected by T. gondii all over Europe and they might thereby serve as a good indicator of the spread of this pathogen.
The intestinal tapeworms Hymenolepis diminuta, ‘rat tapeworm’, and H. (synonym Rodentolepis) nana, ‘dwarf tapeworm’ can cause the disease hymenolepiosis but are only occasionally reported from patients in Europe [Citation40,Citation41]. In contrast, our review shows that these parasites are geographically widespread in rats all over Europe. Infection by H. nana is one of the most common tapeworm infections in man [Citation42,Citation43] and can also be transmitted from human to human [Citation42]. Both tapeworm species can cause enteritis, although infections often are asymptomatic [Citation44]. H. nana has recently been recognised to have zoonotic potential in the UK [Citation45].
Rodents are the main host of the roundworm Capillaria hepatica (synonym Calodium hepaticum) and among those, the brown rat has the highest prevalence across the globe [Citation46]. The wide distribution in rats is in line with the geographically widespread human hepatic capillariosis in Europe, although a rarely reported disease [Citation47]. It has been suggested that poor and unsanitary conditions together with the presence of rodents and domestic animals increase the risk of infection with C. hepatica. Since hepatic capillariosis can be fatal if not diagnosed correctly it is important to know the geographic range of C. hepatica.
These three zoonotic helminth parasitic species are all found in rats across a wide geographic distribution in Europe. Still there are not many human cases from Europe reported in the literature. We believe it is important to further survey these helminths to understand their current range and investigate if they are emerging.
We did not find any support for our initial hypothesis that there is a smaller panel of rat-borne pathogens in Northern Europe as compared to more southern areas. In fact, despite the absence of black rats in Fennoscandia [Citation48], we found the majority of the reported rat-borne bacteria in the Northern Europe zone (, Table S1). Within Europe, brown rats are more well studied as compared to black rats, they are spread all over Europe and harbour many types of pathogens in large parts of their total geographical range.
Habitat and pathogens
If there are less species available to serve as reservoirs or carrier-species for pathogens in settled areas, it is possible that at least certain types of pathogens will decrease [Citation19]. One study found that there was a significantly lower total number of zoonotic helminth intensity in rodents in human settlements than in the forests, upland or lowland habitats [Citation49]. On the other hand, if commensal rats are the most competent reservoirs for a certain microbe and are most abundant and other mammal decrease in species and numbers, the microbe may increase in a reverse process of ‘dilution effect’ [Citation50]. Where the rats increase in numbers, the pathogens they harbour may increase too.
Another reason for the possible higher pathogen richness reported from rural areas compared to urban areas in Europe can be that farm areas may have more ‘vacant slots’ for invading brown rats than what is present in towns. Both brown and black rats are thought to not welcome newcomers in already existing populations [Citation5,Citation51]. This may have the effect that exotic rat-borne pathogens do not enter cities as often as they enter rural settings.
One potential limitation of this study is the fact that we were not able to control how publication trends may bias our results. Several factors may influence reporting trends such as varying research fundings and surveillance efforts, which may of course bias the results.
There were not many studies on pathogens in black rats found by our literature screening but the black rat seems to harbour many species of helminths, especially on the Oceanic islands (). We therefore have to reject our hypothesis about a smaller panel of pathogens in oceanic islands than other areas (urban and rural). Both rat species colonised the world at different routes and periods [Citation5,Citation6,Citation23,Citation52]. The black rat is generally thought to have been established in Europe earlier than the brown rat [Citation23,Citation52]. Molecular results indicate that the black rat has a long colonisation history on the Canary Islands and at least the helminths pathogenic to man may have had a long time to co-evolve with the black rat.
We recommend future studies to include and present data both from rural and urban surveys of rat-borne pathogens in the same article to make comparisons possible. For surveillance, we recommend a sample size calculated from an expected prevalence.
Conclusion
These results will ultimately serve as guidelines for future monitoring of rat-borne zoonoses in areas where data is lacking.
Our results also identified a number of important human pathogens that are carried by increasing populations of rats, and thereby may constitute significant and/or increasing threats to the Public Health. We suggest that wild rats in Europe are monitored regularly at least for the six most widespread pathogens identified, i.e. virulent/resistant E. coli, pathogenic Leptospira spp., the helminths Hymenolepis diminuta, H. nana and Capillaria hepatica, and the protozoon Toxoplasma gondii.
Supplemental Material
Download MS Word (289 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplementary data for this article can be accessed here.
Additional information
Funding
Notes on contributors
Tanja Maria Strand
Tanja Strand defended her PhD in population biology at Uppsala University. Since then, she has performed two postdocs in infection biology. Currently, Tanja Strand is Coordinator of One Health Sweden and Scientific administrator at the National Veterinary Institute.
Åke Lundkvist
Åke Lundkvist is Professor in virology and one of the founders of the Zoonosis Science Center at Uppsala University. His work focus on basic virological, pathogenetic, ecological and epidemiological studies on zoonotic viruses and their vectors and hosts.
References
- Pijnacker R, Goris MG, Te Wierik MJ, et al. Marked increase in leptospirosis infections in humans and dogs in the Netherlands, 2014. Eurosurveillance. 2016;21:1–9.
- Verner-Carlsson J, Lõhmus M, Sundström K, et al. First evidence of Seoul hantavirus in the wild rat population in the Netherlands. Infect Ecol Epidemiol. 2015;5:27215.
- Buckle A. Anticoagulant resistance in the UK and a new guideline for the management of resistant infestations of Norway rats (Rattus norvegicus berk.). Pest Manag Sci. 2013;69:334–341.
- Meerburg BG, Singleton GR, Kijlstra A. Rodent-borne diseases and their risks for public health. Crit Rev Microbiol. 2009;35:221–270.
- Puckett EE, Park J, Combs M, et al. Global population divergence and admixture of the brown rat (Rattus norvegicus). Proc R Soc B. 2016;283. DOI:10.1098/rspb.2016.1762
- Zeng L, Ming C, Li Y, et al. Out of Southern East Asia of the brown rat revealed by large-scale genome sequencing. Mol Biol Evol. 2017. msx276. DOI:10.1093/molbev/msx276
- Aplin KP, Chesser T, Have JT. Evolutionary biology of the genus Rattus: profile of an archetypal rodent pest. ACIAR Monograph Ser. 2003;96:487–498.
- Ellis BA, Regnery RL, Beati L, et al. Rats of the genus Rattus are reservoir hosts for pathogenic Bartonella species: an Old World origin for a New World disease? J Infect Dis. 1999;180:220–224.
- Morand S, Bordes F, Chen H-W, et al. Global parasite and Rattus rodent invasions: the consequences for rodent-borne diseases. Integr Zool. 2015;10:409–423.
- Lin X-D, Guo W-P, Wang W, et al. Migration of Norway rats resulted in the worldwide distribution of Seoul hantavirus today. J Virol. 2012;86:972–981.
- Gage KL, Kosoy MY. Natural history of plague: perspectives from more than a century of research. Annu Rev Entomol. 2005;50:505–528.
- Wells K, O’Hara RB, Morand S, et al. The importance of parasite geography and spillover effects for global patterns of host–parasite associations in two invasive species. Diversity Distrib. 2015;21:477–486.
- Migliani R, Chanteau S, Rahalison L, et al. Epidemiological trends for human plague in Madagascar during the second half of the 20th century: a survey of 20,900 notified cases. Trop Med Int Health. 2006;11:1228–1237.
- Andrianaivoarimanana V, Kreppel K, Elissa N, et al. Understanding the persistence of plague foci in Madagascar. PLoS Negl Trop Dis. 2013;7:e2382.
- World Health Organisation Africa. Plague outbreak Madagascar. External Situation Report 12; 2017 Nov 20.
- Ogbu O, Ajuluchukwu E, Uneke C. Lassa fever in West African sub-region: an overview. J Vector Borne Dis. 2007;44:1–11.
- Azeez-Akande O. Review of Lassa fever, an emerging old world haemorrhagic viral disease in sub-Saharan Africa. Afr J Clin Exp Microbiol. 2016;17:282–289.
- Lecompte E, Fichet-Calvet E, Daffis S, et al. Mastomys natalensis and lassa fever, West Africa. Emerg Infect Dis. 2006;12:1971–1974.
- Bradley CA, Altizer S. Urbanization and the ecology of wildlife diseases. Trends Ecol Evol. 2007;22:95–102.
- Allan BF, Keesing F, Ostfeld RS. Effect of forest fragmentation on lyme disease risk. Conserv Biol. 2003;17:267–272.
- United Nations. World population prospects: the 2015 revision, volume 1, comprehensive tables. (Comprehensive tables no 1.). New York: Department of Economic and Social Affairs, Population Division; 2015.
- Bergström U. Smågnagarna. In: Notini G, Haglund B, editors. Svenska djur - däggdjuren. Stockholm: Norstedt och Söners förlag; 1948, 385-444. Swedish.
- Kosoy M, Khlyap L, Cosson J-F, et al. Aboriginal and invasive rats of genus Rattus as hosts of infectious agents. Vector-Borne Zoonotic Dis. 2015;15:3–12.
- Cavia R, Cueto GR, Suárez OV. Changes in rodent communities according to the landscape structure in an urban ecosystem. Landscape Urban Plann. 2009;90:11–19.
- Cleaveland S, Laurenson M, Taylor L. Diseases of humans and their domestic mammals: pathogen characteristics, host range and the risk of emergence. Philos Trans R Soc London B. 2001;356:991–999.
- Reusken C, van der Plaats R, Opsteegh M, et al. Coxiella burnetii (Q fever) in Rattus norvegicus and Rattus rattus at livestock farms and urban locations in the Netherlands; could Rattus spp. represent reservoirs for (re)introduction? Prev Vet Med. 2011;101:124–130.
- Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638.
- Brolund A. Overview of ESBL-producing Enterobacteriaceae from a Nordic perspective. Infect Ecol Epidemiol. 2014;1,4. DOI:10.3402/iee.v4.24555
- European Centre for Disease Prevention and Control. ECDC Surveillance Atlas. [ updated 2016; cited 2016 Dec 15th]. Available from: http://atlas.ecdc.europa.eu/public/index.aspx
- European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2014. Annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net), Stockholm; 2015.
- Mwachui MA, Crump L, Hartskeerl R, et al. Environmental and behavioural determinants of leptospirosis transmission: a systematic review. PLoS Negl Trop Dis. 2015;9:e0003843.
- Bharti AR, Nally JE, Ricaldi JN, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3:757–771.
- Dupouey J, Faucher B, Edouard S, et al. Human leptospirosis: an emerging risk in Europe? Comp Immunol Microbiol Infect Dis. 2014;37:77–83.
- Aviat F, Blanchard B, Michel V, et al. Leptospira exposure in the human environment in France: A survey in feral rodents and in fresh water. Comp Immunol Microbiol Infect Dis. 2009;32:463–476.
- Socolovschi C, Angelakis E, Renvoisé A, et al. Strikes, flooding, rats, and leptospirosis in Marseille, France. Inter J Infect Dis. 2011;15:e710–e715.
- Strand TM, Lohmus M, Persson Vinnersten T, et al. Highly pathogenic leptospira found in urban brown rats (Rattus norvegicus) in the largest cities of Sweden. Vector Borne Zoonotic Dis. 2015;15:779–781.
- Jensen PM, Magnussen E. Is it too cold for Leptospira interrrogans transmission on the Faroese Islands? Infect Dis (Auckl). 2016;48:156–160.
- Hunter CA, Sibley LD. Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nature Rev Microbiol. 2012;10:766–778.
- Gotteland C, Chaval Y, Villena I, et al. Species or local environment, what determines the infection of rodents by Toxoplasma gondii? Parasitology. 2014;141:259–268.
- Tena D, Simón MP, Gimeno C, et al. Human infection with Hymenolepis diminuta: case report from Spain. J Clin Microbiol. 1998;36:2375–2376.
- Kolodziej P, Rzymowska J, Stepien-Rukasz H, et al. Analysis of a child infected with Hymenolepis diminuta in Poland. Ann Agric Environ Med. 2014;21:510–511.
- Craig P, Ito A. Intestinal cestodes. Curr Opin Infect Dis. 2007;20:524–532.
- Crompton D. How much human helminthiasis is there in the world?. J Parasitol. 1999;85:397–403.
- Marangi M, Zechini B, Fileti A, et al. Hymenolepis diminuta infection in a child living in the urban area of Rome, Italy. J Clin Microbiol. 2003;41:3994–3995.
- McGarry JW, Higgins A, White NG, et al. Zoonotic helminths of urban brown rats (Rattus norvegicus) in the UK: neglected public health considerations? Zoonoses Public Health. 2015;62:44–52.
- Fuehrer H-P. An overview of the host spectrumand distribution of Calodium hepaticum (syn. Capillaria hepatica): part 1-Muroidea. Parasitol Res. 2014;113:619–640.
- Fuehrer H-P, Igel P, Auer H. Capillaria hepatica in man—an overview of hepatic capillariosis and spurious infections. Parasitol Res. 2011;109:969–979.
- Mitchell-Jones AJ, Mitchell JAmori G, et al. The atlas of european mammals. London: Academic Press; 1999.
- Chaisiri K, Siribat P, Ribas A, et al. Potentially zoonotic helminthiases of murid rodents from the Indo-Chinese peninsula: impact of habitat and the risk of human infection. Vector-Borne Zoonotic Dis. 2015;15:73–85.
- Swaddle JP, Calos SE. Increased avian diversity is associated with lower incidence of human West Nile infection: observation of the dilution effect. PloS one. 2008;3:e2488.
- Colangelo P, Abiadh A, Aloise G, et al. Mitochondrial phylogeography of the black rat supports a single invasion of the western Mediterranean basin. Biol Invasions. 2015;17:1859–1868.
- Vigne J-D, Valladas HÈ. Small mammal fossil assemblages as indicators of environmental change in Northern Corsica during the last 2500 Years. J Archaeol Sci. 1996;23:199–215.