ABSTRACT
The immune checkpoint inhibitor (ICIs) as Nivolumab and Ipilimumab is a novel class of medication used in the management of several metastatic malignancies. ICIs can cause immune-related adverse events due to autoreactive T cell activation. Cardiovascular complications comprised myocarditis, conduction abnormalities, ventricular storm, and cardiomyopathy. Cardiomyopathy is one of the significant side effects highlighted in some of the case reports. The physicians should include autoimmune toxicities as the potential differential diagnosis in patients presenting with an unusual presentation and receiving ICIs. We report a case of a 66-year-old female with advanced renal cell carcinoma who developed cardiomyopathy and ventricular tachycardia from nivolumab and ipilimumab therapy.
1. Introduction
The immune checkpoint inhibitors (ICIs) Nivolumab and Ipilimumab are monoclonal antibodies directed against programmed cell death-1 (PD-1) and cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) respectively [Citation1,Citation2]. Nivolumab (anti-PD-1) with Ipilimumab (anti-CTLA-4) when combined resulted in augmented T-cell function. They are increasingly used to manage several metastatic malignancies, including non-small cell, renal and melanoma [Citation1,Citation3]. The T cell-mediated autoimmune response can affect several body organs and cause significant adverse effects [Citation4].
Cardiotoxicity is one of the adverse effects and manifests as cardiomyopathy, myocarditis, cardiac fibrosis, heart failure, and cardiac arrest [Citation1]. We report a case of cardiomyopathy and ventricular tachycardia developed due to Ipilimumab and Nivolumab in a patient with metastatic renal cell carcinoma. Only a few cases were reported cardiotoxicity with ICIs in the literature [Citation1,Citation2,Citation5]. Here, we report one to raise the awareness of these unusual and fatal side effects.
2. Case presentation
A 66-year-old female with a past medical history of essential hypertension, chronic obstructive pulmonary disease with 40 smoking years, hypothyroidism was diagnosed with metastatic renal cell carcinoma six months before the presentation. At that time, the patient had CT brain, CT chest abdomen, and pelvis which showed multiple lesions in the brain and lung. She received five cycles of Nivolumab and Ipilimumab. The last cycle was one month before the presentation.
The patient presented to the hospital with worsening shortness of breath and progressive malaise. On physical examination, the blood pressure 135/80 mmHg, heart rate 150 beats per minute, afebrile, and saturating 93% on 5 Liters of oxygen using a nasal cannula. The laboratory workup is summarized in .
Table 1. Laboratory workup
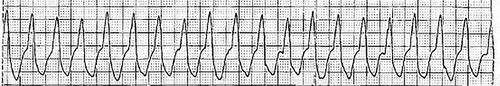
In the emergency department, she developed tachycardia and hypotension. The heart rate was 149 bpm with telemetry showing ventricular tachycardia (). She was cardioverted and was started on amiodarone. The patient underwent heart catheterization to rule out ischemic causes. The coronary angiography revealed non-obstructive coronary artery disease (Supplemental material 1, 2), left ventricular ejection fraction of 15–20%, right ventricle appears to have 20% of normal function, and cardiogenic shock indicated by hypotensive pressures below 100 and thermodilution cardiac output of 2.23, thermodilution cardiac index 1.18. The patient underwent placement of an Intra-aortic balloon pump for cardiogenic shock and a temporary pacemaker to maximize the efficiency of the balloon pump. The echocardiogram showed an ejection fraction of 25–30% with decreased left ventricular (LV) cavity size (LV compressed by enlarged RV, with LV systolic diameter 2.7 cm, LV diastolic diameter 3.5 cm and LV posterior wall diastolic thickness 1.3 cm) and severely reduced RV systolic function with RV internal diameter 4.4 cm (Supplemental material 3).
The patient was intubated and transferred to the intensive care unit. She was persistently in cardiogenic shock, requiring norepinephrine, dopamine, dobutamine, and a balloon pump. A multidisciplinary team including intensivists, cardiologists, and oncologists was involved in the patient care. This new onset of non-ischemic cardiomyopathy was attributed to the use of nivolumab and ipilimumab. After discussing the prognosis with the patient’s husband who is the next of kin, the goal of care was tailored towards comfort measures and the patient expired on the fourth day of admission.
3. Discussion
Immune-mediated cardiotoxicity is an uncommon adverse effect of ICIs as checkpoints also regulate autoreactivity, and these reactions are found to be as high as 40% [Citation3]. This side effect on the heart has a broad spectrum of events, including myocarditis, cardiomyopathy, conduction abnormalities, and ventricular storm.
The mechanisms leading to immune-related adverse events (irAEs) have not been fully understood. Robert et al. reported that irAEs might result from the mobilization of T cells, some of which are autoreactive and involve expanding the T cell reserve [Citation4]. Besides, immune checkpoint inhibitors can influence B cell reactions and incite autoantibody production [Citation6].
Although this diagnosis could not be proven, we believe that ICIs are the reason for the patient’s presentation of a new-onset heart non-ischemic cardiomyopathy with subsequent ventricular tachycardia. Our patient did not have a prior history of heart failure, and there was a new echocardiogram finding of reduced ejection fraction after the initiation of ICIs. Finally, there was no occlusive coronary disease on the angiography to explain the cardiogenic shock.
Cardiac complications of Nivolumab therapy were reported in several case reports and case series [Citation1,Citation5,Citation7], of which ventricular arrhythmias were reported only in 3 cases. The first one was a patient of small cell lung cancer who died after developing VT and bradycardia arrest [Citation2]. The second is a case of new-onset acute decompensated heart failure and recurrent VT [Citation8]. The third one was a case of myocarditis and ventricular arrhythmia [Citation9]. Of these three, the first two had a fatal outcome [Citation2,Citation8]. To the best of our knowledge, our case is the only reported case of developed non-ischemic dilated cardiomyopathy with ventricular tachycardia on combination therapy of nivolumab and ipilimumab for metastatic renal cell carcinoma.
4. Conclusion
In this case report, we highlight the significance of cardiotoxicity associated with ICIs, especially arrhythmias, a rare but fatal complication of ICIs like Nivolumab or Ipilimumab. There are no specific guidelines on how to monitor for these side effects in patients treated with ICIs. Having a baseline echocardiogram before starting the therapy and frequent monitoring of cardiac function can help in the early identification of these side effects and have better patient outcomes. Clinicians must always entertain the possibility of these uncommon, but potentially severe complications. Patients should be made to understand the risks. The managing team, together with the patients, should have proactive plans in place for early detection and possible treatment.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Heinzerling L, Ott PA, Hodi FS, et al. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J Immunother Cancer. 2016;4:50.
- Gibson R, Delaune J, Szady A, et al. Suspected autoimmune myocarditis and cardiac conduction abnormalities with nivolumab therapy for non-small cell lung cancer. BMJ Case Rep. 2016;2016. DOI:https://doi.org/10.1136/bcr-2016-216228.
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017.
- Robert L, Tsoi J, Wang X, et al. CTLA4 blockade broadens the peripheral T-cell receptor repertoire. Clin Cancer Res. 2014;20:2424–2432.
- Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375:1749–1755.
- Weinmann SC, Pisetsky DS. Mechanisms of immune-related adverse events during the treatment of cancer with immune checkpoint inhibitors. Rheumatology (Oxford). 2019;58:vii59–vii67.
- Salem JE, Manouchehri A, Moey M, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–1589.
- Hassam T, Ramasubbu K. Fatal myocarditis: a rare but life threatening adverse effect of nivolumab chemotherapy. Chest. 2017;152:A692.
- Tay RY, Blackley E, McLean C, et al. Successful use of equine anti-thymocyte globulin (ATGAM) for fulminant myocarditis secondary to nivolumab therapy. Br J Cancer. 2017;117:921–924.