ABSTRACT
Introduction: Smoking has a significant impact on the development and progression of asthma and chronic obstructive pulmonary disease (COPD). Self-reported questionnaires and structured interviews are usually the only way to study patients’ smoking history. In this study, we aim to examine the consistency of the responses of asthma and COPD patients to repeated standardised questions on their smoking habits over the period of 10 years.
Methods: The study population consisted of 1329 asthma and 959 COPD patients, who enrolled in the study during years 2005–2007. A follow-up questionnaire was mailed to the participants 1, 2, 4, 6, 8, and 10 years after the recruitment.
Results: Among the participants who returned three or more questionnaires (N = 1454), 78.5 % of the patients reported unchanged smoking status (never smoker, ex-smoker or current smoker) across the time. In 4.5% of the answers, the reported smoking statuses were considered unreliable/conflicting (first never smoker and, later, smoker or ex-smoker). The remainder of the patients changed their status from current smoker to ex-smoker and vice versa at least once, most likely due to struggling with quitting. COPD patients were more frequently heavy ex- or current smokers compared to the asthma group. The intraclass coefficient correlations between self-reported starting (0.85) and stopping (0.94) years as well as the consumption of cigarettes (0.74) over time showed good reliability among both asthma and COPD patients.
Conclusion: Self-reported smoking data among elderly asthma and COPD patients over a 10-year follow-up is reliable. Pack years can be considered a rough estimate for their comprehensive consumption of tobacco products over time. We also observed that the questionnaire we used was not designed for dynamic changes in smoking which are rather common among heavy smokers especially when the follow-up time is several years, as in our study.
Introduction
Tobacco is considered one of the major public health threats in the world and every year it causes approximately 6 million premature deaths [Citation1–Citation3]. Smoking data provides important insight both for assessing the success of smoking cessation as well as in estimating smoking-related health risks [Citation4]. Smoking is the main risk factor for chronic obstructive pulmonary disease (COPD). Smoking has also been associated with the onset of asthma [Citation5]. It aggravates the airway inflammation and consequently worsens the symptoms of the disease. Asthmatic smokers have a greater decline of lung function and reduced efficacy of inhaled corticosteroids [Citation5,Citation6].
Currently, the only way to collect historical data on smoking is by a structured interview or using self-reported questionnaires [Citation7]. Self-reported questionnaires can be implemented rather inexpensively in large cohorts. More importantly, they are usually tested for validity. Biological indicators, such as urine, serum, and plasma levels of nicotine, cotinine [Citation8] carbon monoxide [Citation9], thiocynate [Citation10], and other smoke toxicants have been used to confirm the self-reported data [Citation11,Citation12], but these tests only detect current smoking and use of nicotine products [Citation13,Citation14]. Memory errors, misunderstandings and the honesty of the participants might, however, lead to data ambiguity [Citation15,Citation16]. In longitudinal studies, response bias is also a concern.
Cross-sectional studies in general population have shown that the accuracy of current self-reported smoking status is rather consistent with the biomarker ratings [Citation15,Citation17–Citation19]. In addition, among Finns the validity of self-reported smoking has been shown to be high [Citation20]. More variation has been observed in patient populations in which tobacco is considered a significant risk factor, such as among lung cancer [Citation21] and ischaemic heart disease patients [Citation22]. In addition, underrated results have also been reported in situations where patients perceive their smoking as socially unacceptable, such as during pregnancy [Citation23,Citation24] or after receiving medical disapproval from health care providers [Citation15,Citation16,Citation21,Citation25].
To assess the consistency and stability of responses over repeated, standardised questionnaires, ‘test-retest reliability’ assessment has been used. Several studies using self-reported smoking data have examined the reliability by repeating identical questions on two or more occasions [Citation26–Citation31]. Usually, the test-retest interval is between a few weeks and 1 to 3 months. Studies on test-retest reliability spanning years are scarce [Citation31,Citation32]. To our knowledge, in asthma and COPD, only cross-sectional studies have been done. In these studies, the validity of patients’ answer has been verified by a biological indicator such as cotinine level [Citation33–Citation38].
In the present study, we aimed to estimate the consistency of the responses made by elderly asthma and COPD patients concerning their smoking history. The same questions regarding the present smoking status, starting, and stopping year, as well as the amount of tobacco consumed were asked repeatedly six times over a period of 10 years.
Methods
Patient characteristics
The Finnish Chronic Obstructive Airway Disease (CAD) cohort originally comprised of 2390 asthma and COPD patients who enrolled in the study through the Pulmonary Clinics of the Helsinki (N = 2054) and Turku University Hospitals (N = 336) during the years 2005–2007. The patients were identified through the Hospital Discharge Registers. The registry was screened using ICD10 code J44.8 or J45. All patients, who were between 18 and 75 years of age, were invited to the study through a two-phase mailing campaign. By evaluating patients’ medical records, a pulmonologist reassessed the diagnosis: asthma, COPD, or asthma-COPD overlap showing the features of both diseases [Citation39,Citation40]. Out of all participants, we excluded 102 patients due defective or poorly documented diagnosis and thus, 2288 patients were included in this study. All participants visited the research nurse once. During the visit, participants donated their blood samples for DNA extraction and gave their informed consent to collect, merge, and analyse their comprehensive medical records for the past 5–10 years to confirm the diagnosis. They also gave consent to analyse the progression/outcomes of the disease in the forthcoming 10 years. The collected health care information included medication, co-morbidities, laboratory findings, lung function, and imaging results from all healthcare providers who had treated the patient. Deaths were followed from the population registry. In this study, COPD and asthma-COPD overlap groups were combined, since the results did not differ significantly from each other.
A follow-up questionnaire was mailed to the participant 1, 2, 4, 6, 8, and 10 years after recruitment. The number of withdrawals, deaths, and responders during the study years are shown in details in Supplement Table 1. The mailing address of the study subjects were updated every year from the population register. One reminder was mailed if patients did not answer within 4 weeks. The mailings were always performed at the same time of the year (± 1 month). The study subjects were given a contact number and they could withdraw from the study at any time. Each year, the questions in each section were identical, but the included sections varied slightly. Two of sections, 15D [Citation41] and the Airways Questionnaire 20 [Citation42], measured general and disease specific health-related quality of life (HRQoL), respectively [Citation43]. The other sections included smoking, current medication, and work ability. These sections were identical each year, with the exception of new medicines that were added. A standard pattern of chronic bronchitis-related questions was added in year six. Fagestrom’s Test for Nicotine Dependence was required once [Citation44] as well as a separate questionnaire on exercise habits and physical activity among patients in the COPD/Asthma-COPD overlap cohort [Citation45]. In the smoking section, we first investigated the smoking status, whether they had never been regular smokers, were current regular smokers or former regular smokers. Subsequently, the smokers were asked their starting and potential stopping year as well as the number of cigarettes and cigars they smoke or had smoked daily. The amount of tobacco used in hand-rolled cigarettes or pipes per week was also required and transformed into pack years as follows: one cigar is equal to four cigarettes, one cigarillo is equal to two cigarettes and one gram of loose tobacco is equal to two cigarettes [Citation46]. A heavy smoker was defined as a person who smoked a pack or more per day. Similar questions have been used in many Finnish epidemiological studies [Citation47]. The smoking-related questions were asked first time 1 year after the enrolment. At that point, for 1128 patients out of the 1360 former or current smokers, a complete smoking-related data set, including starting (and quitting) year as well as the number of cigarettes, was available so that the pack years could be computed (Supplementary Table 2).
Consistency of the reported smoking statuses over the years was analysed among the patients who had answered the smoking-related questions at least three times during the follow-up, regardless which survey year (Supplement Table 1). In total, 1852 patients fulfilled the criteria, out of which 1154 (62.3%) belonged to the asthma group and 698 (37.7%) to the COPD group. By choosing this subgroup for the analysis, we were able to distinguish the different trends better and thus improve the reliability of the results. After selection, participants were divided into four groups: (1) stable group that consistently reported the same status throughout the follow-up, (2) unstable type 1 – group that changed the status once, (3) unstable type 2 – group that changed the status more than once, and (4) unreliable group that reported first being a current or a former smoker but later claiming to be a never smoker. In addition, the changes in smoking statuses during the follow-up were analysed using this same subgroup, i.e. we excluded all patients who had reported their smoking statuses only once (the analyses of changes not possible) or twice (the responses gave mainly in the first and second follow-up year). This way we could get more reliable results covering the overall follow-up period.
The study approach was approved by the Coordinating Ethics Committee of the Helsinki and Uusimaa Hospital District. The permission to conduct this research was granted by the Helsinki and Turku University Hospitals.
Statistical analysis
Statistical analyses were done with IBM SPSS Statics for Mac, version 24.0 (SPSS Inc., Chicago, IL) and Excel for Windows (2013). Statistical comparisons between the groups were made by using the chi square test and T-test. All p-values below 0.05 were considered significant. The figures were made using SPSS.
Consistency between reported starting and stopping years and number of reported cigarettes smoked by a person were estimated using the intraclass correlation coefficient (ICC) [Citation48]. ICC values and their 95% confident intervals were calculated using SPSS based on a single-rating, absolute-agreement, 2-way mixed-effects model. According to the guidelines, ICC values of ≥0.90 were defined as excellent; 0.75 to 0.89 were defined as good; 0.50 to 0.74 were defined as moderate; and values less than 0.50 were defined as poor.
Results
Patient characteristics
The study cohort consisted of 1329 asthma and 959 COPD patients living in South-Western Finland and recruited through two University Hospitals (). One third of COPD patients (N = 347) showed features both asthma and COPD [Citation39]. The majority of the asthma patients were women (73.9%), whereas COPD patients were more often men (60.9%). At the time of the enrolment, asthma patients were on average 10 years younger (mean 53.9 years) than the patients in COPD group (mean 63.4 years, p < 0.001).
Table 1. Comparison of asthma and COPD patients’ characteristics and smoking status according to the first postal questionnaire.
Response rates, deaths, and withdrawals during the study
In the first year, the response rates were extremely good (95–98%) decreasing gradually over time to 67–70% in both patient groups (Supplementary Table1). The median follow-up times among asthma and COPD patients were 9.3 ± 2.4 years and 8.2 ± 2.9 years, respectively (from first to last questionnaire or from first questionnaire to death/withdrawal). Throughout the follow-up period, the response rates of asthma patients were slightly higher than among the COPD group. Of all respondents, very few left the smoking section of the questionnaire unanswered: 8.8% (192/2185) no more than once, 1.6% (32/2059) twice, and 0.5% (10/1890) three or more times. Almost 75% of the respondents, with incomplete smoking information, had stable smoking status based on the given answers.
During the follow-up, the mortality rate was significantly lower among asthma patients (82/1329 = 6.2%) when compared to that in the COPD group (381/959 = 39.7%, p < 0.001). However, withdrawing was rather even in both, asthma and COPD groups (48/1329 = 3.6% vs. 24/959 = 2.5%, p = 0.1). The smoking statuses of the withdrawals did not significantly differ in either of the disease groups.
Smoking in the beginning and changes during the follow-up
Asthma and COPD patients showed significantly different smoking statuses when evaluated for the first time 1 year after the enrolment (). Half of the asthma patients had never smoked and only 10.1% were current smokers, whereas only 3.3% of COPD patients were never smoker and 36.1% current smokers. In addition, the consumption of cigarettes was much higher in the COPD group. Of the COPD patients, 51.4% (178/346) were heavy smokers, which was significantly higher than the proportion of those among the asthma patients (33/134 = 24.6%, p < 0.001). A similar trend was seen among ex-smokers.
A heavy smoking history of COPD patients was also observed when pack years were compared between the patient groups (Supplementary Table 2).
Next, we studied the potential changes in the participants’ smoking habits and the reliability of reported outcomes. To improve the reliability of the statistical analysis, we included only the patients who had answered the smoking-related questions at least three times (N = 1852). The majority of the participants (N = 1454, 78.5%) reported an identical smoking status throughout the follow-up (). The status was changed most frequently (former smoker to current smoker and vice versa) once or more in the COPD group. To evaluate the degree of success concerning smoking cessation, we analysed participants first and last given smoking status using this same subgroup (N = 1852). Based on the first given smoking status, 1030 asthma and 420 COPD patients were smoke free. During the follow-up, out of all the current smokers 33.9% (42/124) of the asthma and 44.6% (124/278) of the COPD patients quit smoking (smoke free in their last report). In the end of the follow-up, 91.5% asthma patients and 73.1% COPD patients were smoke free. Based on the latest questionnaire, 49 of 105 smokers struggling with relapses (unstable 2 -group, changing status between current and former smoker) were smoke free. Compared to the COPD group, the asthma patients more frequently gave an unreliable pattern of responses (5.8% vs. 2.3%, p < 0.0004).
Table 2. Comparison of smoking status between the disease groups throughout the 10-year follow-up.
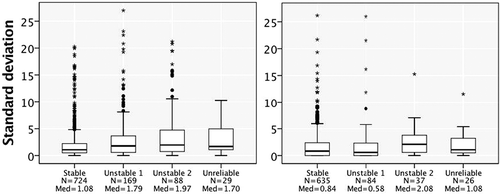
To understand how reliably the patients reported the year they started and stopped smoking, we studied the variances and ICCs among their answers during the follow-up (, ). When interpreting ICCs, we found good reliability of starting years in all groups. The reliability of stopping years was excellent in the stable and the unreliable -groups. In addition, to find out the possible differences between the disease group and the smoking -group, we also calculated ICC values for these groups. The reliability of starting years was higher among asthmatics (ICC 0.90, CI 0.88–0.91) than among COPD -patients (ICC 0.83, CI 0.81–0.85). When comparing ICC -values among smokers, we found good reliability of starting years among former smokers (ICC 0.83, CI 0.80–0.85) and excellent reliability among current smokers (ICC 0.91, CI 0.88–0.93).
Table 3. Variation of self-reported starting year, stopping year and consumption of cigarettes.
Discussion
In the present study, we analysed the consistency and reliability of self-reported smoking history among elderly asthma and COPD patients over 10 years. Similar questions have been used previously in many Finnish epidemiological studies before ours. The participants’ self-reported smoking statuses were analysed between the follow-up years to find out the unreliable path of responses. Only 5.8% of asthma and 2.3% of COPD patients reported unreliable results. Most of these unreliable answers (67/83) belonged to asthma patients. The results might suggest that asthma patients have a stronger response to social pressure that is caused by the rising disapproval towards smoking.
We used a test-retest method for assessing the consistency of the other smoking-related variables such as starting and quitting year, and number of smoked cigarettes. Intra individual variation between questionnaires (ICC values) for starting year was good, for stopping year excellent, and for cigarette consumption moderate. In COPD, only the less advantaged cases who survived longer during the follow-up could be included. Therefore, the results observed here may not be applicable in end-of-life COPD. Obviously, the quitting year was remembered better since it was closer to the time of study. Most variation was seen in tobacco consumption, due to misremembering or real fluctuation. Nowadays, smoking may not be as stable a habit as it was previously, especially in patient-cohorts who are actively reminded of the dangers of smoking and encouraged to quit. The pack years among COPD patients were higher even though the younger average age of the asthma cohort was taken into account. The smoking initiation ages were rather equal in both groups (mean 17–19 years).
In the majority of these elderly patients, the smoking status remained consistent throughout the study, but 8.5% of asthma and 26.9% of COPD patients struggled with smoking cessation. In the latter group, almost all patients were either current or former smokers, which explains the observed fluctuation. Smoking is an addictive habit, caused by nicotine [Citation49] and behaviour dependency. It typically requires three to four attempts before a smoker is able to quit smoking [Citation50]. In the present study, 92% asthma patients and 73% COPD patients were smoke free in the end of the follow-up. Out of all current smokers, 41.3% succeeded in quitting during the follow-up. We have shown previously, in this same cohort, that smoking cessation is associated with the severity of airway obstruction [Citation44]. COPD is a progressive disease and its symptoms often force the patient to quit smoking.
The set of questions about smoking was rather simple in our questionnaire, but unfortunately, it was not optimally designed to take into account the dynamic changes in patients’ smoking habits. For example, the questions did not take into account potential gaps in smoking. Therefore, pack years has to be considered only as a rough estimate. Some of the participants realised the problem and additionally described their complete smoking history as free text on the questionnaire. Another downside in the questionnaire was that the participants were unable to report possible changes in the type and amount of tobacco products they have used over time. The patients remembered the starting year rather well also in cases where the patient had remained an ex-smoker for many years (mean variation 2.1–4.1 years).
The response rates across time were very good decreasing gradually during the last year to the level of 69%. As expected, the mortality rate was significantly higher among COPD patients compared to that in asthma (39.7% vs. 6.2%, p < 0.001). In total, 72 (3.1%) of the participants withdrew from the study, but the withdrawals did not show any difference concerning their smoking habits. This suggests that no significant response bias was developed in the results. Among the respondents, the smoking-related questions were rather often left unanswered. Almost 9% of all participants who returned the questionnaire omitted the smoking section once or more. One reason for this might be frustration over answering the repeated questions, especially if their status had remained unchanged (75% had a stable smoking status).
Reliability of self-reported smoking data among adult COPD and asthma patients have been examined before in cross-sectional studies [Citation33–Citation38]. All of the studies have used biochemical validation for the patients’ current smoking status. The misreporting rates have varied greatly from 1 up to 52%. Compared to our study, the sample sizes have mainly been small and only Murray’s study for early stage COPD patients has had over 1000 participants [Citation33]. Higher misreporting rates have been reported in smoking-cessation studies [Citation33,Citation36–Citation38], whereas epidemiological studies without any expectations of succeeding in smoking cessation have announced misreporting rates of around 10% or lower [Citation33,Citation35]. The intensity of a cessation programme and cultural factors seems to affect results. However, the comparison is not completely straightforward. Some studies have compared the number of unreliable answers to the whole study population while others compared it to subgroups (for example those taking part in the cessation programme).
Our study showed that the great majority of elderly asthma and COPD patients showed good adherence to the longitudinal study design. Their self-reported smoking data was shown to be reliable and consistent with the exception of pack years that can only be used as a rough estimate for comprehensive consumption of tobacco products over time. In about 20% of the patients, the smoking status can fluctuate or the responses can have uncertainties to some extent.
Supplemental Material
Download MS Word (53.5 KB)Acknowledgments
The authors would like to thank clinical research nurses Ms Kerstin Ahlskog, Kirsi Lindgren, and Päivi Laakso for their skilful patient recruitment, Ms Tuula Lahtinen for the monitoring of the project, Ms Sari Nummijoki, Tinja Kanerva, and Elli Niemi for transforming the questionnaires into electronic format.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Eveliina Hirvonen
Eveliina Hirvonen, MD, is a PhD student at the Department of Pulmonary Diseases, Turku University Hospital and University of Turku. Main research interests are asthma and COPD.
Mikhail Stepanov
Mikhail Stepanov, MSc, is a Statistician at Clinical Research Centre, Turku University Hospital.
Maritta Kilpeläinen
Maritta Kilpeläinen, MD, PhD, is a Consultant at the Department of Pulmonary Diseases, Turku University Hospital. Main research interests are allergic and fibrotic pulmonary diseases.
Ari Lindqvist
Ari Lindqvist, MD, PhD, is a Senior Medical Officer and the Head of the Clinical Research Unit for Pulmonary Diseases, Helsinki University Hospital. Main research topics are in clinical trials in many pulmonary diseases and clinical physiology of lungs.
Tarja Laitinen
Tarja Laitinen, MD, PhD, is Professor and the Chief Physician at the Department of Pulmonary Diseases, Turku University Hospital and University of Turku. Main research topics are genetics of complex disorders and registry based real world studies in variety of diseases.
References
- Danaei G, El D, Mozaffarian D, et al. The preventable causes of death in the USA: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6(4):e1000058.
- Centers for Disease Control and Prevention (CDC). CDC grand rounds: current opportunities in tobacco control. MMWR Morb Mortal Wkly Rep. 2010;59(16):487–8.
- Warren GW, Alberg AJ, Kraft AS, et al. The 2014 surgeon general’s report: ‘The Health Consequences of Smoking-50 Years of Progress’: a paradigm shift in cancer care. Cancer. 2014;120(13):1914–1916.
- Etter JF, Perneger TV. Measurement of self reported active exposure to cigarette smoke. J Epidemiol Community Health. 2001;55(9):674–680.
- Polosa R, Thomson NC. Smoking and asthma: dangerous liaisons. Eur Respir J. 2013;41(3):716–726.
- Thomson NC, Chaudhuri R, Livingston E. Asthma and cigarette smoking. Eur Respir J. 2004;24(5):822–833.
- Axelsson M, Ekerljung L, Eriksson J, et al. Chronic bronchitis in West Sweden - a matter of smoking and social class. Eur Clin Respir J. 2016;3:30319.
- Binnie V, McHugh S, Macpherson L, et al. The validation of self-reported smoking status by analysing cotinine levels in stimulated and unstimulated saliva, serum and urine. Oral Dis. 2004;10(5):287–293.
- Pearce MS, Hayes L. Newcastle heart project, newcastle thousand families study. self-reported smoking status and exhaled carbon monoxide. Chest. 2005;128(3):1233–1238.
- Morabia A, Bernstein MS, Curtin F, et al. Validation of self-reported smoking status by simultaneous measurement of carbon monoxide and salivary thiocyanate. Prev Med. 2001;32(1):82–88.
- Blank MD, Breland AB, Enlow PT, et al. Measurement of smoking behavior: comparison of self-reports, returned cigarette butts, and toxicant levels. Exp Clin Psychopharmacol. 2016;24(5):348–355.
- Joseph AM, Hecht SS, Murphy SE, et al. Relationships between cigarette consumption and biomarkers of tobacco toxin exposure. Cancer Epidemiol Biomarkers Prev. 2005;14(12):2963–2968.
- Dolcini MM, Adler NE, Lee P, et al. An assessment of the validity of adolescent self-reported smoking using three biological indicators. Nicotine Tob Res. 2003;5(4):473–483.
- Park SW, Kim JY. Validity of self-reported smoking using urinary cotinine among vocational high school students. J Prev Med Public Health. 2009;42(4):223–230.
- Patrick DL, Cheadle A, Thompson DC, et al. The validity of self-reported smoking: a review and meta-analysis. Am J Public Health. 1994;84(7):1086–1093.
- Velicer WF, Prochaska JO, Rossi JS, et al. Assessing outcome in smoking cessation studies. Psychol Bull. 1992;111(1):23–41.
- Gorber SC, Schofield-Hurwitz S, Hardt J, et al. The accuracy of self-reported smoking: A systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res. 2009;11(1):12–24.
- West R, Zatonski W, Przewozniak K, et al. Can we trust national smoking prevalence figures? Discrepancies between biochemically assessed and self-reported smoking rates in three countries. Cancer Epidemiol Biomarkers Prev. 2007;16(4):820–822.
- Wong SL, Shields M, Leatherdale S, et al. Assessment of validity of self-reported smoking status. Heal Rep. 2012;23(1):47–53.
- Vartiainen E, Seppälä T, Lillsunde P, et al. Validation of self reported smoking by serum cotinine measurement in a community-based study. J Epidemiol Community Health. 2002;56(3):167–170.
- Studts JL, Ghate SR, Gill JL, et al. Validity of self-reported smoking status among participants in a lung cancer screening trial. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1825–1828.
- From Attebring M, Herlitz J, Berndt AK, et al. Are patients truthful about their smoking habits? A validation of self-report about smoking cessation with biochemical markers of smoking activity amongst patients with ischaemic heart disease. J Intern Med. 2001;249(2):145–151.
- Campbell E, Sanson-Fisher R, Walsh R. Smoking status in pregnant women assessment of self-report against carbon monoxide (CO). Addict Behav. 2001;26(1):1–9.
- Boyd NR, Windsor RA, Perkins LL, et al. Quality of measurement of smoking status by self-report and saliva cotinine among pregnant women. Matern Child Health J. 1998;2(2):77–83.
- Stuber J, Galea S. Who conceals their smoking status from their health care provider? Nicotine Tob Res. 2009;11(3):303–307.
- Huerta M, Chodick G, Balicer RD, et al. Reliability of self-reported smoking history and age at initial tobacco use. Prev Med. 2005;41(2):646–650.
- Johnson TP, Mott JA. The reliability of self-reported age of onset of tobacco, alcohol and illicit drug use. Addiction. 2001;96(8):1187–1198.
- Soulakova JN, Hartman AM, Liu B, et al. Reliability of adult self-reported smoking history: data from the tobacco use supplement to the current population survey 2002-2003 cohort. Nicotine Tob Res. 2012;14(8):952–960.
- Brigham J, Lessov-Schlaggar CN, Javitz HS, et al. Test-retest reliability of web-based retrospective self-report of tobacco exposure and risk. J Med Internet Res. 2009;11(3):e35.
- Bernaards CM, Twisk JW, Snel J, et al. Is calculating pack-years retrospectively a valid method to estimate life-time tobacco smoking? A comparison between prospectively calculated pack-years and retrospectively calculated pack-years. Addiction. 2001;96(11):1653–1661.
- Brigham J, Lessov-Schlaggar C, Javitz H, et al. Reliability of adult retrospective recall of lifetime tobacco use. Nicotine Tob Res. 2008;10(2):287–299.
- Hudmon KS, Pomerleau CS, Brigham J, et al. Validity of retrospective assessments of nicotine dependence: a preliminary report. Addict Behav. 2005;30(3):613–617.
- Murray RP, Connett JE, Lauger GG, et al. Error in smoking measures: effects of intervention on relations of cotinine and carbon monoxide to self-reported smoking. The Lung Health Study Research Group. Am J Public Health. 1993;83(9):1251–1257.
- Lores Obradors L, Monsó Molas E, Rosell Gratacós A, et al. Do patients lie about smoking during follow-up in the respiratory medicine clinic?. Arch Bronconeumol. 1999;35(5):219–222.
- Sato S, Nishimura K, Koyama H, et al. Optimal cutoff level of breath carbon monoxide for assessing smoking status in patients with asthma and COPD. Chest. 2003;124(5):1749–1754.
- Monninkhof E, van der VP, van der PJ, et al. The effect of a minimal contact smoking cessation programme in out-patients with chronic obstructive pulmonary disease: a pre–post-test study. Patient Educ Couns. 2004;52(3):231–236.
- Hilberink SR, Jacobs JE, van Opstal S, et al. Validation of smoking cessation self-reported by patients with chronic obstructive pulmonary disease. Int J Gen Med. 2011;4:85–90.
- Stelmach R, Fernandes FLA, Carvalho-Pinto RM, et al. Comparison between objective measures of smoking and self-reported smoking status in patients with asthma or COPD: are our patients telling us the truth? J Bras Pneumol. 2015;41(2):124–132.
- Kauppi P, Kupiainen H, Lindqvist A, et al. Overlap syndrome of asthma and COPD predicts low quality of life. J Asthma. 2011;48(3):279–285.
- Laitinen T, Hodgson U, Kupiainen H, et al. Real-world clinical data identifies gender-related profiles in chronic obstructive pulmonary disease. Copd. 2009;6(4):256–262.
- Sintonen H. 15D Instrument. http://www.15d-instrument.net/15d/
- Barley EA, Quirk FH, Jones PW. Asthma health status measurement in clinical practice: validity of a new short and simple instrument. Respir Med. 1998;92(10):1207–1214.
- Koskela J, Kilpeläinen M, Kupiainen H, et al. Co-morbidities are the key nominators of the health related quality of life in mild and moderate COPD. BMC Pulm Med. 2014;14(1):102.
- Kupiainen H, Kinnula VL, Lindqvist A, et al. Successful smoking cessation in COPD: association with comorbidities and mortality. Pulm Med. 2012;2012:725024.
- Katajisto M, Kupiainen H, Rantanen P, et al. Physical inactivity in COPD and increased patient perception of dyspnea. Int J Chron Obstruct Pulmon Dis. 2012;7:743–755.
- Wood DM, Mould MG, Ong SBY, et al. Pack year” smoking histories: what about patients who use loose tobacco? Tob Control. 2005;14:141–142.
- Laatikainen T, Tapanainen H, Alfthan G et al. FINRISKI 2002. Tutkimuksen Toteutus Ja Tulokset 1. Perusraportti [The FINRISK Study 2002: Methods and Results 1. Main Report]. Helsinki; 2003.
- Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–163.
- Schwartz RS, Benowitz NL. Nicotine Addiction. N Engl J Med. 2010;362(24):2295–2303.
- Curry SJ, McBride CM. Relapse prevention for smoking cessation: review and evaluation of concepts and interventions. Annu Rev Public Health. 1994;15(1):345–366.