ABSTRACT
Following viral infection, the innate immune system senses viral products, such as viral nucleic acids, to activate innate defence pathways, leading to inflammation and apoptosis, control of cell proliferation, and consequently, threat to the whole body. The ocular surface is exposed to the external environment and extremely vulnerable to viral infection. Several studies have revealed that viral infection can induce inflammation of the ocular surface and reduce tear secretion of the lacrimal gland (LG), consequently triggering ocular morphological and functional changes and resulting in dry eye disease (DED). Understanding the mechanisms of DED caused by viral infection and its potential therapeutic strategies are crucial for clinical interventional advances in DED. This review summarizes the roles of viral infection in the pathogenesis of DED, applicable diagnostic and therapeutic strategies, and potential regions of future studies.
Introduction
The clinical manifestations of a DED patient are tear volume reduction, ocular surface inflammation and damage, and high osmotic pressure of the tear film [Citation1]. In 2017, the Tear Film and Ocular Surface Society’s Dry Eye Workshop II (TFOS DEWSII) provided a new definition of DED based on the new findings, declaring that DED is a multifactorial disease of the ocular surface characterized by a loss of homoeostasis of the tear film, accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, as well as neurosensory abnormalities play aetiological roles [Citation2]. DED is widely prevalent and multifactorial in nature. Epidemiological studies explore the prevalence of DED depending on the definition and diagnosis of the disease, and the population surveyed. In Netherlands, a cross-sectional study including 79,866 participants between 2014 and 2017 reveals a 9.1% prevalence of DED. Female and older individuals are more likely to be affected by DED [Citation3]. Inflammation, electronic products, diabetes mellitus, laser-assisted in situ keratomileusis (LASIK), too little and poor sleep quality, and sex hormones contribute to DED [Citation4–7]. Ocular surface disease index (OSDI) or dry eye questionnaire (DEQ-5) are used to evaluate patients’ subjective symptoms. Tear film stability is usually evaluated by tear break-up time (TBUT). Tear film composition is evaluated by tear film osmolarity. Ocular surface staining is used to detect the damage to ocular surface. If the patients DEQ ≥ 6 or OSDI ≥ 13 with one of other diagnostic results including TBUT < 10 or osmolarity ≥ 308mosm/L/interocular difference > 8 mosm/L or ocular surface staining > 5 corneal spots, > 9 conjunctival spots/lid margin (≥2 mm length or ≥ 25% width) are diagnosed with DED [Citation8]. Meanwhile, different factors cause different types of DED. The subtype classification of DED is evaluated by the severity of meibomian gland dysfunction (MGD) feature (Meibum quality, symptoms, and corneal staining) [Citation9], lipid thickness/dynamics, and tear volume assessment [Citation8]. In 2017, TFOS DEWS II classified DED into three types: aqueous-deficient, evaporative, and mixed [Citation2].
Aqueous-deficient dry eye (ADDE) always manifests as tear volume reduction combined with lacrimal gland (LG) dysfunction and inflammation. Sjogren’s syndrome is a chronic systemic autoimmune disease, which is a form of ADDE characterized by lymphocytic infiltration of exocrine glands (lacrimal glands, abbreviated to LGs). Patients with evaporative dry eye (EDE) often show increased evaporation of the aqueous tear phase from the exposed ocular surface. Meibomian gland dysfunction (MGD) contributes to EDE. Patients with MGD display abnormal lipid biosynthesis and meibomian gland (MG) duct obstruction. Additionally, the combination of ADDE and EDE leads to tear hyperosmolarity, finally resulting in inflammation, cell death, and loss of conjunctival goblet cells. Therefore, besides ADDE and EDE, a proportion of patients are diagnosed with mixed dry eye (MDE) [Citation10–12].
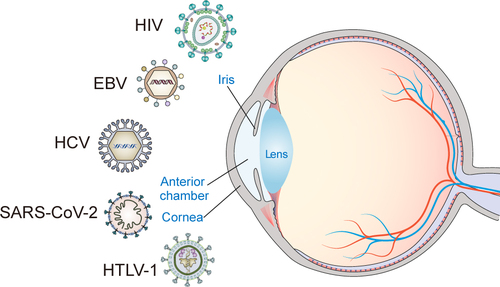
During viral infection, the host innate immune system senses viral products, such as viral nucleic acids, to activate innate defence pathways, promote inflammation and apoptosis, and reduce cell proliferation [Citation13]. Viruses, such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV), Epstein-Barr virus (EBV), and human T-cell lymphotropic virus-1 (HTLV-1) [Citation14], induce inflammation and sicca syndrome, consequently triggering morphological and functional changes on the LG and ocular surface.
This review summarizes the roles and mechanisms of viral infection in the pathogenesis of DED, applicable mechanisms, and implications for diagnostic and therapeutic strategies. Finally, we shed new light on the potential paths for future studies of viral infection-associated DED.
SARS-CoV-2
Coronavirus disease 2019 (COVID-19) is a pandemic caused by SARS-CoV-2 [Citation15]. SARS-CoV-2 infection activates both the innate and adaptive immune responses. In the later phase, infection of mononuclear cells induces a massive inflammatory response, which leads to local and systemic tissue damage. For patients with severe COVID-19, the numbers of CD4+ T cells, CD8+ T cells, B cells, and natural killer (NK) cells are decreased in the whole body [Citation16–18]. Thus, COVID-19 complications include acute liver, cardiac, and kidney injuries, as well as inflammatory responses and secondary infections [Citation16].
As of SARS-CoV-2 is transmitted by aerosols and/or droplets [Citation19], the widespread use of face mask-caused DED has become more common [Citation20,Citation21]. Meanwhile, the increase in discomfort symptoms in the ocular region is mostly part of DED, resulting in a new term called mask-associated dry eye (MADE) [Citation22]. Recently, a questionnaire was administered to 6925 Chinese participants from 29 January 2021, to 8 February 2021, among which 5973 participants wore face masks for ≥6 months. The results revealed that 7.90% of the participants had MADE. Subjects who had continuous (approximately 8 h a day for over 6 months) wearing face masks showed an increased OSDI and decreased TBUT, accompanied by increased T cells, leukocytes, and natural killer T (NKT) cells on the ocular surface [Citation23]. These data suggest that wearing masks may amplify the immune response on the ocular surface. During the ongoing COVID-19 pandemics, outdoor activities and social engagement have been reduced, while indoor activities, which are mainly attributed to screen time (time viewing screens of computers), have increased [Citation24,Citation25]. A longer blinking interval exacerbates the evaporation of tears and, consequently, increases the risk of developing DED. It has been reported that a long screen time aggravates dry eye symptoms, especially in patients with moderate DED [Citation26].
The prevalence of COVID-19 has increased the number of DED patients. A recent study conducted a survey to USA COVID-19 patients who self-reported DED. In this survey, a large proportion (61%) of patients were diagnosed with Sjögren’s syndrome [Citation26]. A prospective study used different social media platforms to collect data on participants’ age, sex, profession and DED symptoms. These results confirm that a large proportion of COVID-19 patients suffer from DED. Reverse transcription polymerase chain reaction (RT-PCR) results show that SARS-CoV-2 exists in conjunctival secretions and tears of patients with conjunctivitis [Citation27]. In COVID-19 patients who have discomfort symptoms in the ocular region, tear film break-up times are decreased. Conjunctival impression cytology (CIC) results indicate that SARS-CoV-2 infection causes an alteration to ocular the surface and conjunctiva featured by decreased number and size of conjunctival goblet cells and morphological changes in corneal epithelial cells (CECs). In addition, neutrophils, which induce conjunctival inflammation and squamous metaplasia in the conjunctiva, increase the ocular surface [Citation28].
Viral infection may be transmitted through the eye route [Citation29], suggesting that the virus may enter the ocular surface to infect the eye. Studies have shown that SARS-CoV-2-associated proteins, including angiotensin-converting enzyme 2 (ACE2), transmembrane protease serine type 2 (TMPRSS2), and basigin (CD147), are expressed on human ocular surface. ACE2 acts as a SARS-CoV-2 receptor, mediating adherence of SARS-CoV-2 to host cells [Citation30]. TMPRSS2 allows the fusion of SARS-CoV-2 and cellular membranes, enabling the entry of SARS-CoV-2 into the host cells [Citation31]. Single-cell RNA sequencing (scRNA-seq) datasets show that ACE2 and TMPRSS2 are co-expressed by the limbal and conjunctival epithelium at the highest level (6.6% of the cells) [Citation32]. CD147 is a transmembrane glycoprotein of the immunoglobulin superfamily that participates in bacterial and viral infection [Citation33]. Wang et al. applied surface plasmon resonance (SPR), enzyme-linked immunosorbent assay (ELISA), and co-immunoprecipitation (Co-IP), verifying that CD147 interacts with the SARS-CoV-2 spike protein. SARS-CoV-2 enters host cells via CD147-mediated endocytosis [Citation34]. CD147 is detected in human conjunctiva, cornea, and ocular fluids [Citation35,Citation36].
Moreover, SARS-CoV-2 single guide RNA (sgRNA) has been found in COVID-19 the cornea, limbus, sclera, and retinal pigment epithelium cells [Citation37,Citation38]. SARS-CoV-2 infects the limbal and corneal cells via nuclear factor kappa B (NF-κB) [Citation39]. The signalling components RELB proto-oncogene, NF-κB subunit (RELB), interleukin-6 (IL-6), and chemokine C-X-C motif ligand 1 and 6 (CXCL1 and CXCL6), are expressed with the highest augment in SARS-CoV-2-infected corneas, limbus, and sclera following NF-κB activation [Citation38].
Increased electronic screen time and poor quality of masks lead to impaired ocular surface function. Reduction in conjunctival goblet cells decreases tear production. Experts recommend that DED patients should to ensure that the mask is worn appropriately, apply lubricating drops, decrease the time in air-conditioned environments, and take regular breaks from digital devices to reduce screen time. Researchers are currently exploring potential defensive medicines against SARS-CoV-2. Ozone (O3) is used to disinfect and treat infectious diseases and inactivate viruses, fungi, and yeast in clinical practice [Citation40]. Ozonated oil, a novel O3 derived compound, has been used to treat ocular pain, external ocular infections and inflammation. It can be stabilized for topical use by creating ozonide through a reaction between ozone and the double bonds of a monounsaturated fatty acid [Citation40]. Recently, a study reports that topical application of ozonated oil in liposome eye drop gels [Citation31] is able to reduce SARS-CoV-2 infection on the ocular surface [Citation41]. Topical use of OED can ameliorate DED pathologic changes, including reduction of corneal epithelium thickness, expression of matrix metalloproteinase 9 (MMP-9), and pro-inflammatory factor interleukin 8 (IL-8) on the ocular surface [Citation41]. The proanthocyanidin (PAC) fraction in blueberry leaves has strong antiviral activity against HCV and HTLV-1 [Citation42,Citation43]. Moreover, PAC suppressed SARS-CoV-2 infection. BB-PAC fraction 7 (Fr7) can effectively inhibit the activity of ACE2 [Citation44], reducing SARS-CoV-2 infection in the limbal and conjunctival epithelium.
HIV
HIV reduces cell-mediated and humoral immunity, leading to a wide range of infections. It directly damages the brain and lungs via mononuclear cell infection and activation. Immune activation causes subtle systemic organ damage, such as hepatic and central nervous system diseases [Citation45]. HIV primarily infects CD4+ T cells [Citation46]. Neutrophils also play an important role in HIV infection and exhibit both host defence and pathological functions. HIV-1 single-stranded RNA40 (SSRNA40) activates neutrophils, causing them to release pro-inflammatory cytokines (TNF-α and IL-6) and produce reactive oxygen species (ROS) [Citation47].
The structures and functions of exocrine glands, including salivary gland (SG) and LG, are damaged by HIV infection. The aetiology of DED in HIV patients is usually due to HIV-mediated lymphocytic infiltration in the LGs [Citation48]. A clinical case study reported that the left LG mass of an HIV-infected patient shows dense inflammatory infiltration, composed of mature lymphocytes, plasma cells, and endothelial cells [Citation49]. Chronic inflammation promotes cytokine secretion, resulting in LG dysfunction, which contributes to a reduction in tear production [Citation50]. A cross-sectional study performed a battery of comprehensive eye assessments, including tear film osmolarity, extent of MG dropout, tear film stability, and ocular surface staining, in healthy controls and HIV-positive subjects. These results demonstrate that HIV infection induces extensive MG dropout and subsequent MGD [Citation51]. DED causes changes in corneal nerve morphology and reduces the length of corneal nerves [Citation52]. One study detected retinal nerve fibre layer thickness (RNFLT) and visual acuity in HIV-positive subjects with no clinically apparent ocular infection or other pathology, confirming that HIV infection leads to decreased RNFLT and low visual acuity [Citation53]. Tear cytokines, such as epidermal growth factor (EGF) and interferon-inducible protein-10 (IP-10), are significantly elevated in HIV patients compared to those of normal subjects [Citation54].
Combined antiretroviral therapy [Citation48], defined as a combination of antiretroviral therapy with at least three drugs, dramatically decreases the prevalence of acquired immune deficiency syndrome (AIDS). With the widespread use of cART, the survival of HIV patients has been improved [Citation55]. Accordingly, the prevalence of ocular findings in the HIV-infected population has been significantly reduced, as proven by the decreased number of opportunistic ophthalmic infections and blinding disorders [Citation56]. This cross-sectional study treats HIV patients with cART for a minimum duration of 6 months. With cART, the mean CD4 count is reduced in DED patients with HIV infection [Citation56].
HTLV-1
HTLV-1 spreads worldwide, especially in Central Africa, South America, and Southwest of Japan [Citation57]. The prevalence of HTLV-1 infection gradually increases with age, especially among the female. HTLV-1 is transmitted through three main pathways: mother-to-child, sexual, and contaminated blood products [Citation57]. HTLV-1 infects T cells, B cells, and myeloid cell lineages, ultimately causing weakened immunity [Citation58]. HTLV-1 infection is associated with ocular inflammatory diseases including conjunctivitis, Sjögren’s syndrome, interstitial keratitis, and polyneuropathies [Citation59]. Additionally, HTLV-1 infection can induce eye inflammation and neoplastic infiltration [Citation60].
In year 2009–2011, a cross-sectional study recruited 272 HTLV-1-infected individuals. Results shows that 21.7% of HTLV-1 patients presented with sicca syndrome. Pro-inflammatory cytokine TNF-α expression is higher in the peripheral blood mononuclear cells (PBMCs) of patients with sicca syndrome than in those without sicca syndrome [Citation61]. The OSDI, TBUT test, Schirmer I test, and Rose Bengal staining were used to evaluate 96 HTLV-1-infected subjects’ DED symptoms, revealing that half of HTLV-infected subjects were diagnosed with DED [Citation62]. A clinical measure targeting 129 HTLV-1-infected subjects showed that 44 (34.1%) subjects complained of dry mouth, whereas 18 (13.9%) had dry eye. The Schirmer’s test of only two subjects showed abnormal results. Eight subjects showed hyposalivation [Citation63]. These studies suggest that HTLV-1 changes correspond to the patient’s clinical manifestations, without damaging the structures and functions of the exocrine glands.
Meanwhile, a case report showed that fluorescein and lissamine green staining of an HTLV-1-infected patient showed a defect in the cornea and conjunctiva, whereas the Schirmer test indicated tear deficiency. Consequently, this HTLV-1-infected patient is diagnosed with aqueous tear-deficient keratoconjunctivitis sicca [Citation64]. The average RNFLT for HTLV-1-infected patients is less than that for healthy individuals [Citation65].
HTLV-1 infection activates CD4+ T cells, which produce inflammatory cytokines that cause ocular inflammation. Topical and/or oral corticosteroid treatment improves intraocular inflammation by inhibiting cytokine production in HTLV-1-infected CD4+ T cells [Citation60]. A recent study indicates that tacrolimus was effective in resolving autoimmune manifestations in HTLV-1-related overlap syndrome (dermatomyositis/Sjögren’s syndrome) [Citation66]. Similarly, allogeneic haematopoietic stem cell transplantation (HSCT) is considered the optimal curative therapy. Following allogeneic HSCT, eye complications display significant improvement with decreased HTLV-1 proviral load (PVL) [Citation60]. Adalimumab, infliximab (IFX), and fully human monoclonal TNF-α antibodies are common strategies for the treatment of inflammatory diseases. Furthermore, ADA and IFX can’t exacerbate inflammatory cytokines and chemokines or increase PVL in HTLV-1-infected T cells, suggesting that ADA and IFX are safe for use in HTLV-1 infectious conditions safely [Citation67,Citation68].
EBV
EBV belongs to the human herpes virus (HHVs) family, also known as human herpesvirus 4 (HHV4), which is a gamma-type HHV [Citation69,Citation70]. The course of EBV infection is determined by the virus load and immune system state of an individual, along with gene composition, other infection history, and environmental factors [Citation71]. EBV is transmitted to recipients through saliva but rarely through semen or blood. Primary EBV infection causes infectious mononucleosis (IM), that typically manifests as fever, pharyngitis, lymphadenopathy, and fatigue [Citation72]. EBV infection is associated with lymphoid and epithelial malignancies, including diffuse large B-cell lymphoma (DLBCL), Hodgkin lymphoma (HL), and various types of T/NK-cell lymphomas [Citation73].
Likewise, EBV infection is related to Sjögren’s syndrome [Citation74,Citation75], which usually manifests as dysfunctional exocrine glands with keratoconjunctivitis sicca and xerostomia [Citation76]. A recent seroepidemiological study determines that EBV is closely correlated with Sjögren’s syndrome [Citation77]. Initially, EBV infects epithelial cells of the oropharynx, gains access to the underlying tissue, releases it from the oropharyngeal epithelium, and finally activates B cells [Citation71]. In India, an EBV-infected person is diagnosed with acute DED, as evidenced by a significant reduction in tear meniscus height derived from slit lamp examination and easily peeled thick membranes from the tarsal surface in both eyes. Nevertheless, lubricants and topical steroids can’t improve tear production [Citation78].
Patients with DED are characterized by increased tear film osmotic pressure and ocular surface inflammation, causing severe ocular surface damage. c-Jun N-terminal kinase (JNK), mitogen-activated protein kinase (MAPK), and NF-κB pathways mediate inflammation in CECs, leading to ocular surface damage [Citation79]. EBV infects human corneal epithelial cells (HCECs) via NF-κB activation. One study ascertained that EBV infection increased the expression of NF-κB subunits, including p65 and p50/p52, in HCECs [Citation80]. EBV induces increased Toll-like receptor 3 (TLR3) expression in HCECs. Receptor-interacting protein-1 (RIP-1) and tumour necrosis factor receptor-associated factor 6 (TRAF6), which are recruited by the protein toll/interleukin-1 receptor (TIR) domain-containing adaptor, are expressed at higher levels in HCECs/EBV in EBV-induced HCECs than in normal HCECs. These data suggest that both TLR3/Toll/IL-1 R domain-containing adaptor-inducing IFN-β (TRIF) and retinoic acid-inducible gene I (RIG-I)/RIP-1 pathways regulate the activation of IRFs and NF-κB in EBV-infected HCECs [Citation80]. High levels of MMP-9 impair corneal epithelial function when EBV infection increases the levels of pro-inflammatory cytokines IL-6 and TNF-α and matrix metalloproteinases (MMP2 and MMP9). In addition, EBV-infected HCECs exhibit increased migratory and invasive capabilities compared with uninfected HCECs [Citation81].
Punctual cautery increases tear meniscus height and alleviates epitheliopathy in acute DED [Citation78]. Previous studies corroborate that acyclovir is effective in some cases of EBV-infection-associated ocular disease [Citation82]. The topical application of steroids mitigates inflammation and oedema in the cornea [Citation83]. Cyclosporin A (CsA) is thought to inhibit the activation of T lymphocytes and effectively control ocular inflammation in consequence [Citation84].
HCV
HCV is an RNA virus that belongs to the Flaviviridae family. HCV infection causes acute hepatitis C, and part of acute hepatitis C transforms into a chronic inflammatory disease process, which might lead to liver fibrosis, hepatocellular carcinoma, and terminal death [Citation85]. HCV is primarily transmitted through percutaneous exposure to the blood caused by iatrogenic infections. Blood transfusion or the administration of clotting factors can cause iatrogenic infections.
HCV and DED have a direct causal relationship. The manifestations of chronic extrahepatic HCV infection include DED, which leads to tear film instability and ocular surface damage [Citation86]. A study collects 36 tear samples from patients with dry eye, and 21 tear samples are positive for HCV RNA [Citation86].
Patients with HCV infection have corneal inflammation, which contributes to DED. The HCV core is recognized by Toll-like receptors (TLR1, TLR2, and TLR6), and then the signal is transferred to myeloid differentiation factor 88 (MyD88), resulting in activation of the NF-κB pathway. HCV infection increases the protein levels of proinflammatory factors, including IL-6, IL-8, and TNF-α, in CECs and conjunctival fibroblasts. HCV core protein mediates nitric oxide (NO) production via the activation of inducible nitric oxide synthase (iNOS), facilitating the apoptosis of CECs and conjunctival fibroblasts [Citation87,Citation88]. The OSDI questionnaire, Schirmer I, TBUT, and ocular surface fluorescein were used to evaluate chronic hepatitis C (CHC) patients’ changes in ocular surface and tear function parameters in CHC patients. The results show that DED symptoms in CHC patients include reduced tear production, with increased OSDI and corneal straining scores [Citation89].
Long-term artificial tears and topical use of Cyclosporin A in both eyes can cure the severe form of DED. Non-structural (NS) proteins, including NS3, NS5A, and NS5B, are required for HCV replication. Daclatasvir is an NS5A replication inhibitor that suppresses viral RNA synthesis and release [Citation90]. Sofosbuvir is a nucleotide NS5B polymerase inhibitor. Combination medication with two or more direct-acting agents (DAAs) can effectively treat HCV infections. No intraocular complications were detected during the follow-up period of sofosbuvir ± daclatasvir ± ribavirin treatment for HCV-infected patients, indicating the safety of these treatments [Citation91].
IFN is the drug of choice for treating HCV infection. After treatment with interferon α 2b and ribavirin for 16 weeks, serum HCV RNA decreased from the initial value to an undetectable level in HCV-infected patients [Citation92]. IFN-free DAAs are widely used for the treatment of HCV. Schirmer’s test, TBUT, and PCR showed that IFN-free DAAs can improve ocular manifestations and reduce HCV RNA levels with sufficient safety [Citation93].
Potential therapeutic strategies for viral infection-associated dry eye
Evidence has shown that viral infections are associated with autoimmune disorders. SARS-CoV-2, HTLV-1, and EBV infections are associated with the sicca syndrome. HTLV-1 infection-related HSCT may contribute to severe complications such as chronic ocular graft-versus-host disease (GVHD) [Citation94]. However, few direct drugs are available for curing viral infection-associated dry eye. Hydrocortisone combined with topical CsA can effectively treat Sjögren’s Syndrome [Citation95]. As a well-known antiviral agent, type I IFN triggers an antiviral response within infected and target cells, as well as activates innate immune cells, which ultimately control viral replication, activate adaptive immune response to clear viral infection, and generate memory to create a rapid response against future infections [Citation96]. The pathogenesis of DED includes inflammation, and keratitis can cause DED. Meanwhile, viral infection induces conjunctivitis and keratitis, as evidenced by a case report of a patient diagnosed with EBV retinitis [Citation97]. Topical application of anti-inflammatory drugs can inhibit inflammatory mediators and relieve the symptoms and signs of DED. Traditional ocular topical anti-inflammatory drugs include tetracycline, glucocorticoids and nonsteroidal anti-inflammatory drugs. Rebamipide is an oral drug that increases the number of conjunctival goblet cells on the ocular surface. Furthermore, it suppresses T cell activation and cytokine production to exert anti-inflammatory effects [Citation98]. Here, we summarize the treatment and effect of dry eye associated with viral infection ().
Table 1. The treatments and effects of DED associated with viral infection.
Conclusions and prospect
Multiple studies have revealed that systemic and ocular infections, as well as other environmental factors, may contribute to the development of DED. Similarly, many studies have confirmed the intimate relationship between viral infections and the ocular surface, lacrimal gland, and conjunctiva. A new multiplex solid-phase strip PCR can be used to identify common pathogens that cause ocular infectious diseases. Each well of the strip PCR targets one to three types of pathogens. The wells are coated with primers and probes containing the fluorescent reporter dye [6-carboxyfluorescein (6-FAM), hexachlorofluorescein (HEX), or cyanine-5 (Cy5)] on the 5’-end and the non-fluorescent quencher on the 3’-end [Citation101]. Both strip PCR and capillary PCR have detected human herpesvirus 7 (HHV-7) in conjunctivitis and the tears of dry eye patients. Acanthamoeba, herpes simplex virus type 1 (HSV-1), bacterial 16S, human herpesvirus 6 (HHV-6), aspergillus, and fungal 28S were detected in the corneas of keratitis patients. Adenovirus, Propionibacterium acnes (P. acnes), and EBV were detected in the conjunctivas of infectious conjunctivitis patients [Citation102]. A study indicated that interferon regulatory factor (irf3) −/− mice infected with HSV-1 display symptoms of DED, including corneal keratinization, corneal cell apoptosis, and tear production reduction. Irf3−/− mice have pathological changes and functional impairment in the lacrimal glands (LGs) caused by increased levels of HSV-1 [Citation103]. Here, we summarize the symptoms, causes, diagnosis, and treatment of viral infection-associated DED. A variety of viruses, including HIV, EBV, HCV, SARS-CoV-2, and HTLV-1, infect the ocular surface, and cause DED (). We also generalized the receptors of EBV, HCV, and SARS-CoV-2 infecting CECs as well as the downstream pathways in DED associated with their infections (). All three viruses activate the NF-κB pathway by binding to diverse receptors on the CECs. These findings raise the question of whether there are other mechanisms besides the NF-κB pathway that mediate EBV, HCV, and SARS-CoV-2 infections-associated DED. More importantly, the cellular and molecular mechanisms of HIV and HTLV-1 infection-associated DED remain unclear and require further investigation.
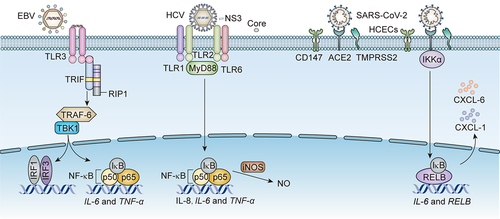
Figure 2. The receptors on CECs and downstream pathways for EBV, HCV, and SARS-CoV-2 infection-associated DED.
EBV activates NF-κB to produce IL-6 and TNF-α via binding to TLR3 on CECs. Meanwhile, EBV promotes the transcription of IRF3 via potentiating the same upstream TRIF/RIP1/tumour necrosis factor receptor-associated factor 6 (TRAF-6)/TANK-binding kinase 1 (TBK1) signalling. The NS3 of HCV binds to TLR1 and TLR6 on CECs to activate NF-κB, producing IL-8, IL-6 and TNF-α via boosting upstream MyD88. The core protein of HCV enhances the generation of NO via activating iNOS. SARS-CoV-2 binds to CD147, ACE2, and TMPRSS2 on CECs, activating NF-κB pathway to produce IL-6, CXCL-1 and CXCL-6 through upstream inhibitor of kappa B kinase α (IKKα).
In this review, the importance of timely diagnosis of viral infections is emphasized. A deeper and wider understanding of the direct relationship between viral infections and DED is helpful for ophthalmologists to make accurate judgements in time and achieve effective treatments.
Dry eye symptoms in virus-infected patients, such as SARS-CoV-2, EBV, and HSV, have been reported. However, the relationship between other viruses, such as HSV, HTLV-1, HIV, and DED, and their symptoms and pathogenesis warrant further study. Here, we summarize DED symptoms in virus-infected patients and the routes of viral transmission (). Cell and animal models are urgently need to explore the pathogenesis of viral infection-associated DED. However, only a few animal models of viral infection-associated DED have been developed. Cell and animal models of viral infection-associated ocular surface diseases such as keratitis and keratouveitis have already been successfully constructed (). These models are beneficial for the investigation of viral infection-associated DED.
Table 2. The manifestations and routes of DED caused by viruses.
Table 3. The cell and animal models associated with viral infection.
Currently, antiviral drugs (type I IFN and cART) are combined with drugs for dry eye (artificial tears and corticosteroids) and anti-inflammatory drugs, such as ADA and IFX. However, the current approaches to tackling DES remains suboptimal. Palliative medication using ophthalmic lubricants remains the backbone of DED treatment [Citation117]. FDA-approved DED treatments, such as 5% lifitegrast ophthalmic solution (Xiidra®, Novartis, Switzerland) and 0.05% cyclosporine ophthalmic emulsion (Restasis®, Allergan, CA), show only finite efficacy for treating DED signs and symptoms, and are associated with related adverse events that hinder their widespread use in general patients globally [Citation37,Citation118]. Thus, it is pivotal to develop effective and safe DED treatments that target their authentic pathophysiology, which is a wicked cycle of desiccating stress-induced hyperosmolar tissue damage and inflammation of the surface [Citation119,Citation120]. Likewise, there is a need to investigate more drugs that target viral infection-associated DED.
CRediT author contribution statement
Min Wu, Cuilian Sun wrote the original draft, review and editing. Manhui Zhu, Xiaojuan Liu revised the manuscript critically for important intellectual content. Qin Shi, Yalu Luo, Ziyu Wang, Jianxiang Wang, Yun Qin, Weihang Cui, Chufeng Yan, Huangyi Dai, Zhiyang Wang, Jia Zeng, Yamei Zhou participated in data curation, writing the original draft, review and editing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Tsubota K, Pflugfelder SC, Liu Z, et al. Defining dry eye from a clinical perspective. Int J Mol Sci. 2020;21(23):21.
- Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–12.
- Vehof J, Snieder H, Jansonius N, et al. Prevalence and risk factors of dry eye in 79,866 participants of the population-based lifelines cohort study in the Netherlands. Ocul Surf. 2021;19:83–93. doi: 10.1016/j.jtos.2020.04.005
- Toda I. Dry eye after LASIK. Invest Ophthalmol Vis Sci. 2018;59(14):DES109–DES15. doi: 10.1167/iovs.17-23538
- Chen Y, Chauhan SK, Lee HS, et al. Chronic dry eye disease is principally mediated by effector memory Th17 cells. Mucosal Immunol. 2014;7(1):38–45.
- Schaumberg DA, Dana R, Buring JE, et al. Prevalence of dry eye disease among US men: estimates from the Physicians Health Studies. Arch Ophthalmol. 2009;127(6):763–8. doi: 10.1001/archophthalmol.2009.103
- Li S, Tang L, Zhou J, et al. Sleep deprivation induces corneal epithelial progenitor cell over-expansion through disruption of redox homeostasis in the tear film. Stem Cell Rep. 2022;17(5):1105–1119.
- Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II diagnostic methodology report. Ocular Surf. 2017;15(3):539–574.
- Geerling G, Tauber J, Baudouin C, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52(4):2050–2064.
- Baudouin C, Aragona P, Messmer EM, et al. Role of hyperosmolarity in the pathogenesis and management of dry eye disease: proceedings of the OCEAN group meeting. Ocul Surf. 2013;11(4):246–258.
- McMonnies CW. Aqueous deficiency is a contributor to evaporation-related dry eye disease. Eye Vis (Lond). 2020;7(1):6. doi: 10.1186/s40662-019-0172-z
- Chhadva P, Goldhardt R, Galor A. Meibomian gland disease: the role of gland dysfunction in dry eye disease. Ophthalmol. 2017;124(11):S20–S6. doi: 10.1016/j.ophtha.2017.05.031
- Song K, Li S. The role of ubiquitination in NF-κB signaling during virus infection. Viruses. 2021;13(2):145. doi: 10.3390/v13020145
- Schiller JT, Lowy DR. An introduction to virus infections and human cancer. Recent Results Cancer Res. 2021;217:1–11.
- Sidiq Z, Hanif M, Dwivedi KK, et al. Benefits and limitations of serological assays in COVID-19 infection. Indian J Tuberc. 2020;67(4):S163–S6. doi: 10.1016/j.ijtb.2020.07.034
- Felsenstein S, Herbert JA, McNamara PS, et al. COVID-19: immunology and treatment options. Clin Immunol. 2020;215:108448. doi: 10.1016/j.clim.2020.108448
- Tan M, Liu Y, Zhou R, et al. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. Immunology. 2020;160(3):261–268.
- Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762–768.
- Jayaweera M, Perera H, Gunawardana B, et al. Transmission of COVID-19 virus by droplets and aerosols: a critical review on the unresolved dichotomy. Environ Res. 2020;188:109819. doi: 10.1016/j.envres.2020.109819
- Boccardo L. Self-reported symptoms of mask-associated dry eye: a survey study of 3,605 people. Cont Lens Anterior Eye. 2022;45(2):101408. doi: 10.1016/j.clae.2021.01.003
- Rouen PA, White ML. Dry eye disease: prevalence, assessment, and management. Home healthcare now. Home Healthc Now. 2018;36(2):74–83. doi: 10.1097/NHH.0000000000000652
- Krolo I, Blazeka M, Merdzo I, et al. Mask-associated dry eye during COVID-19 pandemic-How face masks contribute to dry eye disease symptoms. Med Arch. 2021;75(2):144–148.
- Fan Q, Liang M, Kong W, et al. Wearing face masks and possibility for dry eye during the COVID-19 pandemic. Sci Rep. 2022;12(1):6214.
- Meyer J, McDowell C, Lansing J, et al. Changes in physical activity and sedentary behavior in response to COVID-19 and their associations with Mental Health in 3052 US adults. Int J Environ Res Public Health. 2020;17(18):17.
- Xiang M, Zhang Z, Kuwahara K. Impact of COVID-19 pandemic on children and adolescents’ lifestyle behavior larger than expected. Prog Cardiovasc Dis. 2020;63(4):531–532. doi: 10.1016/j.pcad.2020.04.013
- Saldanha IJ, Petris R, Makara M, et al. Impact of the COVID-19 pandemic on eye strain and dry eye symptoms. Ocul Surf. 2021;22:38–46. doi: 10.1016/j.jtos.2021.06.004
- Xia J, Tong J, Liu M, et al. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020;92(6):589–594.
- Bozkurt E, Ozates S, Muhafiz E, et al. Ocular surface and conjunctival cytology findings in patients with confirmed COVID-19. Eye Contact Lens. 2021;47(4):168–73. doi: 10.1097/ICL.0000000000000752
- Inomata T, Kitazawa K, Kuno T, et al. Clinical and prodromal ocular symptoms in coronavirus disease: a systematic review and meta-analysis. Invest Ophthalmol Vis Sci. 2020;61(10):29.
- Yan R, Zhang Y, Li Y, et al. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448.
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry Depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–80 e8.
- Collin J, Queen R, Zerti D, et al. Co-expression of SARS-CoV-2 entry genes in the superficial adult human conjunctival, limbal and corneal epithelium suggests an additional route of entry via the ocular surface. Ocul Surf. 2021;19:190–200. doi: 10.1016/j.jtos.2020.05.013
- Kitazawa K, Deinhardt-Emmer S, Inomata T, et al. The transmission of SARS-CoV-2 infection on the ocular surface and prevention strategies. Cells. 2021;10(4):10. doi: 10.3390/cells10040796
- Wang K, Chen W, Zhang Z, et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Target Ther. 2020;5(1):283.
- Maatta M, Tervahartiala T, Kaarniranta K, et al. Immunolocalization of EMMPRIN (CD147) in the human eye and detection of soluble form of EMMPRIN in ocular fluids. Curr Eye Res. 2006;31(11):917–924.
- Li YP, Ma Y, Wang N, et al. Eyes on coronavirus. Stem Cell Res. 2021;51:102200. doi: 10.1016/j.scr.2021.102200
- Shen Lee B, Toyos M, Karpecki P, et al. Selective pharmacologic therapies for dry eye disease treatment: efficacy, tolerability, and safety data review from preclinical studies and pivotal trials. Ophthalmol Ther. 2022;11(4):1333–1369. doi: 10.1007/s40123-022-00516-9
- Eriksen AZ, Moller R, Makovoz B, et al. SARS-CoV-2 infects human adult donor eyes and hESC-derived ocular epithelium. Cell Stem Cell. 2021;28(7):1205–20 e7. doi: 10.1016/j.stem.2021.04.028
- Gudowska-Sawczuk M, Mroczko B. The role of nuclear factor kappa B (NF-κB) in development and treatment of COVID-19: review. Int J Mol Sci. 2022;23(9):23. doi: 10.3390/ijms23095283
- Valacchi G, Fortino V, Bocci V. The dual action of ozone on the skin. Br J Dermatol. 2005;153(6):1096–1100. doi: 10.1111/j.1365-2133.2005.06939.x
- Rizzo S, Savastano MC, Bortolotti D, et al. COVID-19 ocular prophylaxis: the potential role of ozonated-oils in liposome eyedrop gel. Transl Vis Sci Technol. 2021;10(9):7.
- Takeshita M, Ishida Y, Akamatsu E, et al. Proanthocyanidin from blueberry leaves suppresses expression of subgenomic hepatitis C virus RNA. J Biol Chem. 2009;284(32):21165–21176.
- Kai H, Fuse T, Kunitake H, et al. Comparison of cultivars and seasonal variation in blueberry (Vaccinium Species) leaf extract on adult T-Cell leukemia cell line growth suppression. Medicines (Basel). 2014;1(1):3–11. doi: 10.3390/medicines1010003
- Sugamoto K, Tanaka YL, Saito A, et al. Highly polymerized proanthocyanidins (PAC) components from blueberry leaf and stem significantly inhibit SARS-CoV-2 infection via inhibition of ACE2 and viral 3CLpro enzymes. Biochem Biophys Res Commun. 2022;615:56–62. doi: 10.1016/j.bbrc.2022.04.072
- Lucas S, Nelson AM. HIV and the spectrum of human disease. J Pathol. 2015;235(2):229–241. doi: 10.1002/path.4449
- Deeks SG, Overbaugh J, Phillips A, et al. HIV infection. Nat Rev Dis Primers. 2015;1(1):15035.
- Giraldo DM, Hernandez JC, Urcuqui-Inchima S. HIV-1-derived single-stranded RNA acts as activator of human neutrophils. Immunol Res. 2016;64(5–6):1185–1194. doi: 10.1007/s12026-016-8876-9
- Nizamuddin I, Koulen P, McArthur CP. Contribution of HIV infection, AIDS, and antiretroviral therapy to exocrine pathogenesis in salivary and lacrimal glands. Int J Mol Sci. 2018;19(9):2747. doi: 10.3390/ijms19092747
- Kumari P, Kasturi N, Nagarajan G, et al. Spontaneous regression of angiolymphoid hyperplasia with eosinophilia of lacrimal gland in an HIV-positive patient. Indian J Ophthalmol. 2019;67(8):1334–1335.
- Stern ME, Gao J, Siemasko KF, et al. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res. 2004;78(3):409–416.
- Nguyen BN, Chung AW, Lopez E, et al. Meibomian gland dropout is associated with immunodeficiency at HIV diagnosis: implications for dry eye disease. Ocul Surf. 2020;18(2):206–213.
- Tran BN, Maass M, Musial G, et al. Topical application of cannabinoid-ligands ameliorates experimental dry-eye disease. Ocul Surf. 2022;23:131–139. doi: 10.1016/j.jtos.2021.12.008
- Paul R, Ghosh AK, Nag A, et al. Study of retinal nerve fibre Layer thickness and visual contrast sensitivity in HIV positive individuals. J Clin Diagn Res. 2017;11:OC01–OC4. doi: 10.7860/JCDR/2017/24751.9956
- Agrawal R, Balne PK, Veerappan A, et al. A distinct cytokines profile in tear film of dry eye disease (DED) patients with HIV infection. Cytokine. 2016;88:77–84. doi: 10.1016/j.cyto.2016.08.026
- Venkatesh KK, Biswas J, Kumarasamy N. Impact of highly active antiretroviral therapy on ophthalmic manifestations in human immunodeficiency virus/acquired immune deficiency syndrome. Indian J Ophthalmol. 2008;56(5):391–393. doi: 10.4103/0301-4738.42415
- Arora R, Sandhu N, Dokania P, et al. Ocular manifestations in patients of HIV(Human immunodeficiency virus) infection on combined anti-retroviral therapy (CART). Ocul Immunol Inflamm. 2022;30(6):1399–1407.
- Gessain A, Cassar O. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol. 2012;3:388. doi: 10.3389/fmicb.2012.00388
- Yoshida M. Multiple viral strategies of HTLV-1 for dysregulation of cell growth control. Annu Rev Immunol. 2001;19(1):475–96. doi: 10.1146/annurev.immunol.19.1.475
- Eusebio-Ponce E, Anguita E, Paulino-Ramirez R, et al. HTLV-1 infection: an emerging risk. Pathogenesis, epidemiology, diagnosis and associated diseases. Rev Esp Quimioter. 2019;32(6):485–96. doi: 10.1073/pnas.77.12.7415
- Kamoi K, Mochizuki M. HTLV infection and the eye. Curr Opin Ophthalmol. 2012;23(6):557–561. doi: 10.1097/ICU.0b013e328358b9ec
- Lima CM, Santos S, Dourado A, et al. Association of sicca syndrome with proviral load and proinflammatory cytokines in HTLV-1 infection. J Immunol Res. 2016;2016:8402059. doi: 10.1155/2016/8402059
- Castro-Lima-Vargens C, Grassi MFR, Boa-Sorte N, et al. Algorithm for dry eye disease diagnosis in individuals infected with human T-cell lymphotropic virus type 1. Arq Bras Oftalmol. 2017;80(6):369–72. doi: 10.5935/0004-2749.20170090
- Vale DAD, Casseb J, de Oliveira ACP, et al. Prevalence of Sjögren’s syndrome in Brazilian patients infected with human T-cell lymphotropic virus. J Oral Pathol Med. 2017;46(7):543–8. doi: 10.1111/jop.12530
- Buggage RR, Levy-Clarke GA, Smith JA. New corneal findings in human T-cell lymphotrophic virus type 1 infection. Am J Ophthalmol. 2001;131(3):309–313. doi: 10.1016/S0002-9394(00)00881-3
- Merle H, Hage R, Jeannin S, et al. Retinal nerve fiber Layer thickness in human T-cell lymphotropic virus type 1 patients. Curr Eye Res. 2017;42(12):1644–1649.
- Izumi Y, Kojima H, Koga Y, et al. Successful treatment of HTLV-1-related overlap syndrome using tacrolimus. Intern Med. 2011;50(17):1849–1853.
- Kurozumi-Karube H, Kamoi K, Ando N, et al. In vitro Evaluation of the safety of adalimumab for the eye under HTLV-1 infection status: a preliminary study. Front Microbiol. 2020;11:522579. doi: 10.3389/fmicb.2020.522579
- Uchida M, Kamoi K, Ando N, et al. Safety of infliximab for the eye under human T-Cell leukemia virus type 1 infectious conditions in vitro. Front Microbiol. 2019;10:2148. doi: 10.3389/fmicb.2019.02148
- Jouanguy E, Beziat V, Mogensen TH, et al. Human inborn errors of immunity to herpes viruses. Curr Opin Immunol. 2020;62:106–122. doi: 10.1016/j.coi.2020.01.004
- Siakallis G, Spandidos DA, Sourvinos G. Herpesviridae and novel inhibitors. Antivir Ther. 2009;14(8):1051–1064. doi: 10.3851/IMP1467
- Houen G, Trier NH. Epstein-Barr Virus and Systemic Autoimmune Diseases. Front Immunol. 2020;11:587380. doi: 10.3389/fimmu.2020.587380
- Jayasooriya S, de Silva TI, Njie-Jobe J, et al. Early virological and immunological events in asymptomatic Epstein-Barr virus infection in African children. PLOS Pathog. 2015;11(3):e1004746.
- Shannon-Lowe C, Rickinson AB, Bell AI. Epstein–Barr virus-associated lymphomas. Philos Trans R Soc Lond B Biol Sci. 2017;372(1732):20160271. doi: 10.1098/rstb.2016.0271
- Maslinska M. The role of Epstein–Barr virus infection in primary Sjögren’s syndrome. Curr Opin Rheumatol. 2019;31(5):475–83. doi: 10.1097/BOR.0000000000000622
- Tsubota K, Fujishima H, Toda I, et al. Increased levels of Epstein-Barr virus DNA in lacrimal glands of Sjögren’s syndrome patients. Acta Ophthalmologica Scandinavica. 1995;73(5):425–430.
- Stefanski AL, Tomiak C, Pleyer U, et al. The diagnosis and treatment of Sjogren’s syndrome. Dtsch Arztebl Int. 2017;114:354–61. doi: 10.3238/arztebl.2017.0354
- Xuan J, Ji Z, Wang B, et al. Serological Evidence for the Association between Epstein-Barr virus infection and Sjogren’s syndrome. Front Immunol. 2020;11:590444. doi: 10.3389/fimmu.2020.590444
- Chatterjee S, Iyer G, Srinivasan B, et al. Severe acute onset dry eye following presumed Epstein.Barr viral infection. Indian J Ophthalmol. 2020;68(4):642–644.
- Li DQ, Luo L, Chen Z, et al. JNK and ERK MAP kinases mediate induction of IL-1β, TNF-α and IL-8 following hyperosmolar stress in human limbal epithelial cells. Exp Eye Res. 2006;82(4):588–96. doi: 10.1016/j.exer.2005.08.019
- Park GB, Hur DY, Kim YS, et al. TLR 3/ TRIF signalling pathway regulates IL -32 and IFN -β secretion through activation of RIP -1 and TRAF in the human cornea. J Cell Mol Med. 2015;19(5):1042–54. doi: 10.1111/jcmm.12495
- Park GB, Kim D, Kim YS, et al. The Epstein-Barr virus causes epithelial–mesenchymal transition in human corneal epithelial cells via Syk/src and Akt/Erk signaling pathways. Invest Ophthalmol Vis Sci. 2014;55(3):1770–9.
- Keorochana N. A case report of Epstein-Barr virus-associated retinal vasculitis: successful treatment using only acyclovir therapy. Int Med Case Rep J. 2016;9:213–8. doi: 10.2147/IMCRJ.S107089
- Mohanty A, Behera HS, Barik MR, et al. Microsporidia-induced stromal keratitis: a new cause of presumed immune stromal (interstitial) keratitis. Br J Ophthalmol. 2021;107(5):607–613.
- Chen Y, Xu H. Epstein-Barr virus-associated Hemophagocytic Lymphohistiocytosis following cyclosporine for uveitis. Ocul Immunol Inflamm. 2020;28(4):549–551. doi: 10.1080/09273948.2019.1606258
- Stanaway JD, Flaxman AD, Naghavi M, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the global burden of disease study 2013. Lancet. 2016;388(10049):1081–1088.
- Rajalakshmy AR, Malathi J, Madhavan HN, et al. Patients with dry eye without hepatitis C virus infection possess the viral RNA in their tears. Cornea. 2015;34(1):28–31. doi: 10.1097/ICO.0000000000000304
- Rajalakshmy AR, Malathi J, Madhavan HN. HCV core and NS3 proteins mediate toll like receptor induced innate immune response in corneal epithelium. Exp Eye Res. 2014;128:117–128. doi: 10.1016/j.exer.2014.09.011
- Rajalakshmy AR, Malathi J, Madhavan HN, et al. Hepatitis C virus core and NS3 antigens induced conjunctival inflammation via toll-like receptor-mediated signaling. Mol Vis. 2014;20:1388–97.
- Karaman Erdur S, Kulac Karadeniz D, Kocabora MS, et al. Ocular surface and tear parameters in patients with chronic hepatitis C at initial stages of hepatic fibrosis. Eye Contact Lens. 2015;41(2):117–20.
- McGivern DR, Masaki T, Williford S, et al. Kinetic analyses reveal potent and early blockade of hepatitis C virus assembly by NS5A inhibitors. Gastroenterology. 2014;147(2):453–62 e7.
- Abd Elaziz MS, Nada ASE, ElSayed SH, et al. Ocular comorbidities with direct-acting antiviral treatment for chronic hepatitis C virus (HCV) patients. Int Ophthalmol. 2020;40(5):1245–1251.
- Anisia-Iuliana A, Alina C, Elena CR, et al. Ophthalmological implications of the chronic infections with the hepatitis C virus. Rom J Ophthalmol. 2015;59(4):263–8.
- Caroleo B, Colangelo L, Donato M, et al. Direct-acting antivirals inducing HCV-RNA sustained suppression improve xerophthalmia in HCV-infected patients. Curr Rev Clin Exp Pharmacol. 2022;17(2):156–60.
- Shimizu E, Ogawa Y, Saijo Y, et al. Commensal microflora in human conjunctiva; characteristics of microflora in the patients with chronic ocular graft-versus-host disease. Ocul Surf. 2019;17(2):265–271.
- Fondi K, Mihaltz K, Vecsei-Marlovits PV. Efficacy of topical hydrocortisone in combination with topical ciclosporin a for the treatment of dry eye disease in patients with Sjogren syndrome. J Ophthalmol. 2021;2021:7584370. doi: 10.1155/2021/7584370
- Lee AJ, Ashkar AA. Herpes simplex virus-2 in the genital mucosa: insights into the mucosal host response and vaccine development. Curr Opin Infect Dis. 2012;25(1):92–99. doi: 10.1097/QCO.0b013e32834e9a56
- Mushiga Y, Komoto T, Nagai N, et al. Effects of intraocular treatments for Epstein-Barr virus (EBV) retinitis: a case report. Medicine (Baltimore). 2021;100(48):e28101.
- Mohamed HB, El-Hamid BN A, Fathalla D, et al. Current trends in pharmaceutical treatment of dry eye disease: a review. Eur J Pharm Sci. 2022;175:106206. doi: 10.1016/j.ejps.2022.106206
- Yener AÜ. COVID-19 and the eye: ocular manifestations, treatment and protection measures. Ocul Immunol Inflamm. 2021;29(6):1225–1233. doi: 10.1080/09273948.2021.1977829
- Merayo-Lloves J, Baltatzis S, Foster CS. Epstein-Barr virus dacryoadenitis resulting in keratoconjunctivitis sicca in a child. Am J Ophthalmol. 2001;132(6):922–923. doi: 10.1016/S0002-9394(01)01182-5
- Nejati F, Junne S, Kurreck J, et al. Quantification of Major Bacteria and Yeast Species in kefir consortia by multiplex TaqMan qPCR. Front Microbiol. 2020;11:1291. doi: 10.3389/fmicb.2020.01291
- Nakano S, Sugita S, Tomaru Y, et al. Establishment of multiplex solid-phase strip PCR test for detection of 24 ocular infectious disease pathogens. Invest Ophthalmol Vis Sci. 2017;58(3):1553–1559.
- Zhu JY, Zhang X, Zheng X, et al. Dry eye symptoms in interferon regulatory factor 3-deficient mice due to herpes simplex virus infection in harderian gland and lacrimal gland. Exp Eye Res. 2022;219:109053. doi: 10.1016/j.exer.2022.109053
- Sterczewska A, Wojtyniak A, Mrukwa-Kominek E. Ocular complaints from students during COVID-19 pandemic. Adv Clin Exp Med. 2022;31(2):197–202. doi: 10.17219/acem/144199
- Gambini G, Savastano MC, Savastano A, et al. Ocular surface impairment after coronavirus disease 2019: a cohort study. Cornea. 2021;40(4):477–483.
- Hu Y, Chen T, Liu M, et al. Positive detection of SARS‐CoV‐2 combined HSV1 and HHV6B virus nucleic acid in tear and conjunctival secretions of a non‐conjunctivitis COVID‐19 patient with obstruction of common lacrimal duct. Acta Ophthalmol. 2020;98(8):859–863.
- Taylor JD. AIDS and hepatitis B and C: contamination risk at transurethral resection. A study using sodium fluorescein as a marker. Med J Aust. 1990;153(5):257–260. doi: 10.5694/j.1326-5377.1990.tb136896.x
- Goncalves DU, Proietti FA, Ribas J, et al. Epidemiology, treatment, and prevention of human T-Cell leukemia virus type 1-associated diseases. Clin Microbiol Rev. 2010;23(3):577–89.
- Jacobi C, Wenkel H, Jacobi A, et al. Hepatitis C and ocular surface disease. Am J Ophthalmol. 2007;144(5):705–11.e1.
- Li S, Li A, Ruan F, et al. Evaluation of the clinical characteristics of dry eye secondary to different types of liver diseases. Ophthalmol Ther. 2023;12(5):2493–503.
- Rao P, McKown RL, Laurie GW, et al. Development of lacrimal gland inflammation in the mouse model of herpes stromal keratitis. Exp Eye Res. 2019;184:101–106. doi: 10.1016/j.exer.2019.04.022
- Wan S, Zhou Y, Huang Q, et al. Dot1l aggravates keratitis induced by herpes simplex virus type 1 in mice via p38 MAPK-Mediated oxidative stress. Oxid Med Cell Longev. 2021;2021:6612689. doi: 10.1155/2021/6612689
- Zhou X, Ramke M, Chintakuntlawar AV, et al. Role of MyD88 in adenovirus keratitis. Immunol Cell Biol. 2017;95(1):108–116.
- Zhang S, Zang Y, Lu Q, et al. Establishing an animal model of cytomegalovirus keratouveitis in rats: broad infection of anterior segment tissue by cytomegalovirus. Invest Ophthalmol Vis Sci. 2021;62(13):22.
- Larsen IV, Clausius H, Kolb AW, et al. Both CD8+ and CD4+ T cells contribute to corneal clouding and viral clearance following vaccinia virus infection in C57BL/6 mice. J Virol. 2016;90(14):6557–72. doi: 10.1128/JVI.00570-16
- Wang J, Kaplan N, Wysocki J, et al. The ACE2-deficient mouse: a model for a cytokine storm-driven inflammation. FASEB J. 2020;34(8):10505–10515.
- Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575–628.
- Chan YH, Sun CC. Efficacy and safety of topical cyclosporine 0.1% in moderate-to-severe dry eye disease refractory to topical cyclosporine 0.05% regimen. Taiwan J Ophthalmol. 2023;13(1):68–74. doi: 10.4103/tjo.TJO-D-22-00140
- Clayton JA, Longo DL. Dry Eye. N Engl J Med. 2018;378(23):2212–2223. doi: 10.1056/NEJMra1407936
- Huang R, Su C, Fang L, et al. Dry eye syndrome: comprehensive etiologies and recent clinical trials. Int Ophthalmol. 2022;42(10):3253–3272.