ABSTRACT
Actinobacillus pleuropneumoniae (APP) is an important pathogen of the porcine respiratory disease complex, which leads to huge economic losses worldwide. We previously demonstrated that Pichia pastoris-producing bovine neutrophil β-defensin-5 (B5) could resist the infection by the bovine intracellular pathogen Mycobacterium bovis. In this study, the roles of synthetic B5 in regulating mucosal innate immune response and protecting against extracellular APP infection were further investigated using a mouse model. Results showed that B5 promoted the production of tumour necrosis factor (TNF)-α, interleukin (IL)-1β, and interferon (IFN)-β in macrophages as well as dendritic cells (DC) and enhanced DC maturation in vitro. Importantly, intranasal B5 was safe and conferred effective protection against APP via reducing the bacterial load in lungs and alleviating pulmonary inflammatory damage. Furthermore, in the early stage of APP infection, we found that intranasal B5 up-regulated the secretion of TNF-α, IL-1β, IL-17, and IL-22; enhanced the rapid recruitment of macrophages, neutrophils, and DC; and facilitated the generation of group 3 innate lymphoid cells in lungs. In addition, B5 activated signalling pathways associated with cellular response to IFN-β and activation of innate immune response in APP-challenged lungs. Collectively, B5 via the intranasal route can effectively ameliorate the immune suppression caused by early APP infection and provide protection against APP. The immunization strategy may be applied to animals or human respiratory bacterial infectious diseases. Our findings highlight the potential importance of B5, enhancing mucosal defence against intracellular bacteria like APP which causes early-phase immune suppression.
Introduction
Porcine contagious pleuropneumonia (PCP) caused by Actinobacillus pleuropneumoniae (APP) is a highly contagious and lethal respiratory infectious disease threatening the worldwide pig industry. APP is an important member of pathogens causing porcine respiratory disease complex which causes great economic losses to the global pig industry [Citation1]. Acute PCP is characterized by haemorrhagic fibrinous and necrotizing pleuropneumonia and leads to a mortality rate of 80−100% [Citation2]. In addition, porcine circovirus type 2 (PCV2) promotes APP survival and their coinfections caused high mortality and huge economic losses [Citation3]. Nineteen serovars of APP have been identified based on distinct capsular polysaccharides [Citation4]. Serovars 1, 2, 3, 5, and 7 are the most frequent serovars in many countries [Citation5–7]. APP showed a high frequency of resistance to florfenicol and cephalothin in Spanish [Citation8] and a highly susceptible to ceftiofur, florfenicol, and macrolides in Spain [Citation9]. A recent study showed that 85.71% of APP isolates were sensitive to the antibiotic cefquinome in Guangxi Province of China [Citation10]. These researches present problems in controlling outbreaks of PCP [Citation8–10], resulting in an urgent need for alternatives to antibiotics. Most APP vaccines alleviate clinical symptoms but fail to protect against infection or transmission. Currently, neither antibiotics nor vaccines are able to completely eliminate the spread of APP [Citation2]. Therefore, research and development of new effective strategies for PCP prevention and control are urgently needed.
APP preferentially binds mucus and cells of the lower respiratory tract and damages lung cells via virulence factors [Citation11]. In particular, APP damages the respiratory epithelial barrier integrity and triggers serious inflammatory response [Citation12]. Airway epithelial cells play a critical defensive role in the epithelial barrier [Citation13]. Mucosal immunization may be the effective route against APP. Recently, accumulating evidence confirms that intranasal immunization confers protection against respiratory intracellular bacteria and viruses, such as Mycobacterium tuberculosis [Citation14], influenza virus [Citation15], and novel coronavirus [Citation16], and also provides protection against extracellular bacteria such as Streptococcus pneumoniae (S. pneumoniae) [Citation17], Pseudomonas aeruginosa (P. aeruginosa) [Citation18], Staphylococcus aureus (S. aureus) and Klebsiella pneumoniae (K. pneumoniae) [Citation19]. Intranasal a live auxotrophic vaccine induced mucosal immunity and conferred protection against P. aeruginosa-caused lethal pneumonia [Citation18]. Intranasal an attenuated S. pneumoniae vaccine induced IL-17 secretion and inhibited S. pneumoniae, S. aureus, and K. pneumoniae colonization [Citation19]. However, it was unclear whether intranasal administration of host defence peptide offered protection against pneumonia caused by APP.
It has been widely accepted that innate immune response plays a vital role in the antibacterial defence in the early stage of infection [Citation20]. APP leads to acute haemorrhagic pneumonia, so the host mainly depends on the innate immune system to clear APP. A recent study proved that IFN-γ and IL-18 contributed to resist APP infection [Citation21]. Mucosal innate immune cells produce cytokines, chemokines, and antibacterial peptides (AMPs) which are important for the clearance of bacteria [Citation20]. AMPs, also known as host defence peptide, are widely present in the lung and other mucosal tissues, where they play a key role as the first line of defence against pathogens. Airway epithelial cells and neutrophils, in particular, contribute to the synthesis of AMPs in the lung [Citation22]. AMPs exert their antimicrobial, antiviral, antifungal, and immunomodulatory activities [Citation23]. Defensin, one of the most common types of AMPs, possess antimicrobial and immunomodulatory functions [Citation24]. Porcine beta-defensin 2 (PBD2) displayed a direct bactericidal activity against Salmonella typhimurium and alleviated inflammatory response [Citation25]. The overexpression of PBD2 in pigs promotes resistance to APP infection [Citation26,Citation27]. Recently, increasing researches highlight the key role of defensins in antibacterial immunity [Citation28–30]. Defensins can link the innate and adaptive immune systems through the modulation of toll-like receptor and chemokine receptor [Citation23,Citation28]. A neutrophil-derived defensin (bovine neutrophil β-defensin-5, B5) was proved to induce effective mucosal immune response in the respiratory tract and facilitate protection against Mycobacterium bovis (M. bovis) [Citation29]. Epithelial-cell-derived defensins played a critical role in the neutrophil recruitment and combating S. aureus infections [Citation30]. In addition, a study found that multiple β-defensins coordinate together to promote airway antibacterial immune response [Citation31]. Our previous studies demonstrated that B5 could trigger J774A.1 macrophages to release TNF-α and IL-1β and enhance the antimicrobial effect of rifampicin [Citation32]. Intranasal B5 was vital for the efficacy generated by AH-PB vaccine against M. bovis [Citation29]. However, the immunomodulatory activity of B5 against APP was unknown.
In the current study, the roles of B5 in regulating the mucosal innate immune response and protecting against APP in mice were investigated. This study showed that B5 enhanced the secretion of pro-inflammatory cytokines in macrophages and dendritic cells (DC) and up-regulated surface molecules which are markers for DC maturation. Intranasal B5 facilitated the production of pro-inflammatory cytokines and the recruitment of innate immune cells including DC, macrophages, neutrophils, and group 3 innate lymphoid cells (ILC3s) in lungs in the early stage of the APP infection. Furthermore, intranasal B5 activated signalling pathways associated with cellular response to IFN-β, activation of innate immune response, defence response to bacterium, etc. Importantly, intranasal B5 provided effective protection against APP in mice. These findings provide insights into B5 which can be used to enhance the innate immune response in respiratory tract to restrict APP infection.
Materials and methods
Mice
All animal experiments involving mice protocols and procedures were performed according to the protocols for the care of laboratory animals, Ministry of Science and Technology People’s Republic of China, and approved according to animal care and use committee protocols at the Guangxi University (GXU20230031). The mice were purchased from SiPeiFu Biotechnology Co., Ltd (Beijing, China) and were kept under specific pathogen-free conditions. During the current study, mice received access to food and water ad libitum.
Reagents
Poly-inosinic-polycytidylic acid (Poly IC) was purchased from Sigma-Aldrich (St. Louis, MO). CCK-8 cell proliferation and cytotoxicity assay kit, nicotinamide adenine dinucleotide (NAD), RBC Lysing Buffer, and other reagents were obtained from Solarbio. Tumor necrosis factor (TNF)-α, interferon (IFN)-β, interleukin (IL)-1β, IL-23, IL-17, and IL-22 ELISA kits were purchased from Neobioscience. Bradford Protein Assay Kit was obtained from Beyotime Biotechnology. For flow cytometry, Intracellular Fixation/Permeabilization Buffer Kit, purified anti-mouse CD16/32, FITC-CD11c, PE-MHC II, APC-CD80, PerCP-CD86, PE-F4/80, APC-CD11c, and PerCP-Ly6G were from Elabscience; FITC-lineage cocktail and APC-RORγt were from eBioscience. B5 (amino acid sequence: NPQSCRWNMGVCIPISCPGNMRQIGTCFGPRVPCCRRW) was synthesized at Shanghai Apeptide Co., Ltd. M.W.: 4325.12; Purity: >95%; B5 is soluble in water, grand average of hydropathy: −0.268; Isoelectric point: 8.91; B5 stock solution (2 mg/ml in PBS) for experiments. PBD2 (amino acid sequence: DHYICAKKGGTCNFSPCPLFNRIEGTCYSGKAKCCIR) was synthesized at Shanghai Apeptide Co., Ltd. Purity: >95%.
Cell culture
RAW264.7 macrophages were obtained from Cell Culture Center, Xiehe Medical University (Beijing China). RAW264.7 were cultured in DMEM supplemented with 10% FBS, 100 µg/ml of streptomycin, and 100 U/ml of penicillin at 37°C in 5% CO2 humidified incubator. Bone marrow-derived macrophages (BMDMs) were prepared and cultured as previously described [Citation33]. Briefly, BMDM were isolated from femurs and tibias of 6–8 week-old BALB/c mice and were plated in cell culture dish in RPMI 1640 supplemented with 10% FBS, 100 µg/ml of streptomycin, 100 U/ml of penicillin, and 10 ng/ml of M-CSF. After 5–7 d of culture, the adherent cells were collected for stimulation. Bone marrow-derived dendritic cells (BMDCs) were prepared and cultured as previously described [Citation34]. In brief, bone marrow of the tibias and femurs from 6–8-week-old male BALB/c mice was flushed and depleted of red blood cells using RBC Lysing Buffer. The cells were plated in cell culture dish in RPMI 1640 supplemented with 10% FBS, 100 µg/ml streptomycin, and 100 U/ml penicillin, recombinant mouse (rm) GM-CSF (20 ng/ml) and rm IL-4 (20 ng/ml) at 37°C in 5% CO2. On days 3 and 5, floating cells were gently removed, and fresh cell culture medium was added. On day 7, proliferating DC charactered by nonadherent, and loosely adherent cells were harvested for analysis or stimulation, and ≥92% proliferating DC expressed CD11c.
Cell stimulation
RAW264.7 cells were moved to 96-well cell culture plates (5 × 103 cells in each well) and stimulated with B5 (10–80 µg/ml) for 24 h, 48 h, or 72 h and then incubated with 10 µl of cell counting kit-8 (CCK8) solution for 2 h at 37°C, and data were collected using a microplate reader (Tecan (Shanghai) Trading Co., Ltd., Infinite M200 Pro, Zürich, Switzerland) at 450 nm. Untreated cells were taken as negative control. Next, RAW264.7 cells were moved to 24-well cell culture plates (106 cells in each well) and stimulated with 5–40 µg/ml of B5 for 24 h. The cell culture supernatants were collected to test the concentrations of TNF-α and IL-1β using ELISA. BMDM and BMDC were moved to 24-well cell culture plates (106 cells in each well) and incubated with 20 μg/ml of B5 or poly IC, respectively. The cell culture supernatants were collected to quantify the concentrations of TNF-α, IL-1β, and IFN-β using ELISA, and the cells were collected to measure CD80, CD86, and MHC-II using flow cytometry.
Bacterial culture
The APP serovar 2 strain was generously donated by the Institute of Animal Husbandry and Veterinary Science, Zhejiang Academy of Agricultural Sciences. The APP serovar 7 strain was kept by the Pharmacology and Pathology Laboratory of School of Animal Science and Technology, Guangxi University. The 100 μl of bacterial suspension was added to 10 ml of tryptic soy broth (TSB) liquid medium supplemented with 10 μg/ml of NAD and 4% newborn calf serum, cultured in bacterial culture shaker at 160 rpm for 16–24 h at 37 °C. Bacterial suspension was diluted with PBS, and serial 10-fold dilutions were plated on trypticase soy agar (TSA) medium supplemented with 10 μg/ml of NAD and 4% newborn calf serum.
B5 treatment and APP challenge
Female BALB/c mice, 6 to 8 weeks old, were pretreated intranasally (i.n.) with 20 μl of PBS containing 20 μg of B5 or PBD2 two times at 2-d intervals. The dose of B5 was chosen according to our previous study [Citation29]. Negative control mice received 20 μl of PBS. Some mice were euthanized for safety evaluation 3 d after the last treatment, and the residual mice were challenged with APP via the intranasal route at 107 CFU in 20 μl of cold PBS per mouse. Next, mice were euthanized 6 h and 24 h after challenge. To observe the effects of B5 on the survival of APP-infected mice, mice were intraperitoneally challenged with an ultra-high lethal dose of APP (7.5 × 1010 CFU). To evaluate the therapeutic effects of B5 on APP-challenged mice, mice were intranasally challenged with APP, intranasally treated with single B5 (20 μg) after 3 h after challenge, and mice were euthanized 8 h after challenge.
CFU assay
CFU assay was performed by plating serial dilutions of homogenized lung tissues on a solid medium to quantify viable APP. Tissues were homogenized in 1 ml of PBS using a tissue homogenizer apparatus (Servicebio). Tissue homogenates were diluted with PBS, and serial 10-fold dilutions were plated on TSA solid plates containing 10 μg/ml of NAD and 4% newborn calf serum. Then, 100 μl of samples from each dilution was inoculated on plates in triplicate and cultured for 18–24 h at 37°C. Colonies of APP in each culture plate were counted.
Elisa
Concentrations of TNF-α, IL-1β, and IFN-β in cells culture supernatants and protein levels of mouse cytokines (TNF-α, IL-1β, IL-17, IL-22, and IL-23) in supernatants of homogenized equivalent lung tissues (1:100 dilution) using cold PBS, and cytokines in bronchoalveolar lavage fluid (BALF) were measured using mouse ELISA kits according to the manufacturer’s instructions. The optical density (OD) values were quantified via testing the absorbance at 450 nm wavelength using ELISA plate reader (Tecan (Shanghai) Trading Co., Ltd., Infinite M200 Pro, Zürich, Switzerland). In addition, protein levels in BALF were measured using a Bradford Protein Assay Kit according to the manufacturer’s instructions.
Flow cytometry
BMDC were collected and incubated with CD16/32, FITC-CD11c, APC-CD80, PerCP-CD86, and PE-MHC-II for 30 min at 4℃. Stained cells were washed with staining buffer and then fixed with 4% paraformaldehyde for the next step of detection.
Lungs were harvested from mice at the indicated time points after APP challenge and were placed into 20-mm dishes and then digested in 3 ml of RPMI 1640 supplemented with 10% FBS, 100 µg/ml of streptomycin, 100 U/ml of penicillin, 1 mg/ml of collagenase I, and 30 U/ml of Dnase I for 30 min at 37 ℃ using culture shaker (70 rpm). The lung cells were discharged onto a 70-μm filter and depleted of red blood cells using RBC Lysing Buffer. Staining for cell surface: cells isolated from lung tissues were incubated with CD16/32, PE-F4/80, APC-CD11c, and PerCP-Ly6G for 30 min at 4℃. Stained cells were washed with staining buffer and then fixed with 4% paraformaldehyde. Staining for intracellular cytokine: lung cells were incubated with anti-CD16/32 for 10 min at 4℃ to block Fc receptors and washed and stained with FITC-lineage cocktail for 30 min 4℃. Cells were fixed and permeabilized with Intracellular Fixation/Permeabilization Buffer Kit. Next, cells were stained with APC-RORγt for 30 min at 4°C, washed with staining buffer, and fixed with 4% paraformaldehyde. Finally, cells were analysed using a FACSVerse flow cytometer (Invitrogen) and FlowJo software.
RNA-seq
For the RNA-seq (RNA sequencing) assay, lungs were collected 6 h after APP challenge. Total RNA was extracted using Trizol reagent (Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer’s procedure. Then, mRNA was purified from total RNA (5 ug) using Dynabeads Oligo (dT) (Thermo Fisher Scientific, Waltham, MA, USA) with two rounds of purification. Following purification, the mRNA was fragmented into short fragments which were reverse-transcribed to create the cDNA. An A-base was then added to the blunt ends of each strand, preparing them for ligation to the indexed adapters. Dual-index adapters were ligated to the fragments, and size selection was performed with AMPureXP beads. After the heat-labile UDG enzyme (NEB, cat.m0280, USA) treatment of the U-labelled second-stranded DNAs, the ligated products were amplified with PCR. The average insert size for the final cDNA libraries were 300 ± 50 bp. Finally, we performed the 2 × 150 bp paired-end sequencing (PE150) on an Illumina Novaseq™ 6000 (LC-Bio Technology CO., Ltd., Hangzhou, China) following the vendor’s recommended protocol. The differentially expressed genes of RNA-seq were performed by DESeq2 software. The genes with the parameter of false discovery rate below 0.05 and absolute fold change ≥ 2 were considered differentially expressed genes, which were then subjected to enrichment analysis of Gene Ontology (GO) functions and Kyoto Encylopaedia of Genes and Genomes (KEGG) pathways. The screening threshold was set to p <0.05, and the Pearson correlation coefficient gets to 1. The R package “clusterProfiler” was used to perform gene set enrichment analysis (GSEA). Protein–protein interactions (PPIs) were predicted using STRING analysis. p <0.05 was considered statistically significant.
Statistical analysis
All data are expressed as mean ± standard deviation (SD). For comparison between two groups, Student’s t-test was applied, and for comparisons among more than two groups, one-way ANOVA followed by the Tukey’s multiple comparison test using GraphPad Prism version 8.0 software was performed. Flow cytometry results were analysed using the FlowJo software version 10. A p-value less than 0.05 was considered statistically significant.
Abbreviations
APP: Actinobacillus pleuropneumoniae; S. pneumoniae: Streptococcus pneumoniae; P. aeruginosa: Pseudomonas aeruginosa; S. aureus: Staphylococcusaureus; K. pneumoniae: Klebsiella pneumoniae. M. bovis: Mycobacterium bovis; B5: bovine neutrophil β-defensin-5; DC: dendritic cells; ILC3s: group 3 innate lymphoid cells; PCP: porcine contagious pleuropneumonia; PCV2: porcine circovirus type 2; AMPs: antibacterial peptides; PBD2: porcine beta-defensin 2; poly IC: Poly-inosinic-polycytidylic acid; NAD: nicotinamide adenine dinucleotide; BMDM: mouse bone marrow-derived macrophage; BMDC: bone marrow-derived dendritic cells; BALF: broncho alveolar lavage fluid; GO: Gene Ontology; KEGG: Kyoto Encylopaedia of Genes and Genomes; GSEA: gene set enrichment analysis; PPI: protein–protein interactions; TNF-α: tumour necrosis factor-alpha; IL: interleukin; IFN: interferon.
Results
B5 activates macrophages
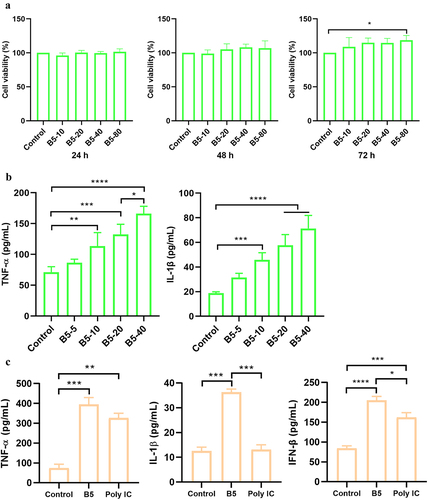
To evaluate the effect of B5 on cell viability, RAW264.7 cells were treated with B5. Results showed that B5 could significantly increase cell activity at the dose of 80 µg/ml within 72 h, B5 in the range of 10–80 µg/ml did not affect the cell viability within 24–48 h (), suggesting that B5 in the range of 10–40 µg/ml did not cause toxic effects on macrophages, and thus it was used for subsequent experiments. Moreover, 10–40 µg/ml of B5 significantly promoted the production of TNF-α and IL-1β in RAW264.7 cells (). In line with this, 20 µg/ml of B5 significantly enhanced the secretion of TNF-α and IL-1β in BMDM. Interestingly, 20 µg/ml of B5 significantly elevated the level of IFN-β in BMDM, and levels of IL-1β and IFN-β in B5 group were significantly higher than those in poly IC control group (). Collectively, these results suggest that B5 activates macrophages producing TNF-α, IL-1β and IFN-β.
Figure 1. B5 activates macrophages. a, the effects of B5 on the viability in RAW264.7 cells. Cells were incubated with 10–80 µg/ml of B5 for 24 h, 48 h, or 72 h, and cell viability was detected using CCK8 solution. b, levels of TNF-α and IL-1β in B5-treated RAW264.7 cells. Cells were incubated with 5–40 µg/ml of B5 for 24 h. c, levels of TNF-α, IL-1β and IFN-β in bone marrow-derived macrophages (BMDMs) treated with B5 or poly IC. Data shown are means ± SD. Data are representative of three independent experiments (n = 3). The significance of differences between the groups was determined by ANOVA with post-hoc Tukey’s multiple comparison test (*p < 0.033, **p < 0.0021, ***p < 0.0002, ****p < 0.0001).
B5 promotes the maturation of functional DC
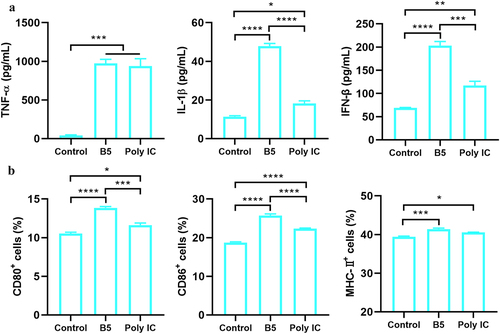
To assess whether B5 could also activate DC, BMDC were treated with 20 µg/ml of B5 and the results showed that B5 significantly enhanced the secretion of TNF-α, IL-1β, and IFN-β in BMDC. Moreover, levels of IL-1β and IFN-β in B5 group were significantly higher than those in poly IC control group (). Furthermore, the expression levels of surface molecules that are markers for DC maturation were measured. Results showed that the upregulation of CD80, CD86, and MHC-II on BMDC following B5 stimulation occurred, and B5 significantly promoted the expression of CD80 and CD86 compared with poly IC (). Overall, these findings indicate that B5 promotes the maturation of functional DC.
Figure 2. B5 promotes the maturation of functional DC. a, levels of TNF-α, IL-1β, and IFN-β in bone marrow-derived dendritic cells (BMDCs) stimulated with B5 or poly IC. b, expression levels of CD80, CD86, and MHC-II in BMDC stimulated with B5 or poly IC. Data shown are means ± SD. Data are representative of three independent experiments (n = 3). The significance of differences between the groups was determined by ANOVA with post-hoc Tukey’s multiple comparison test (*p < 0.033, **p < 0.0021, ***p < 0.0002, ****p < 0.0001).
Intranasal B5 safety evaluation
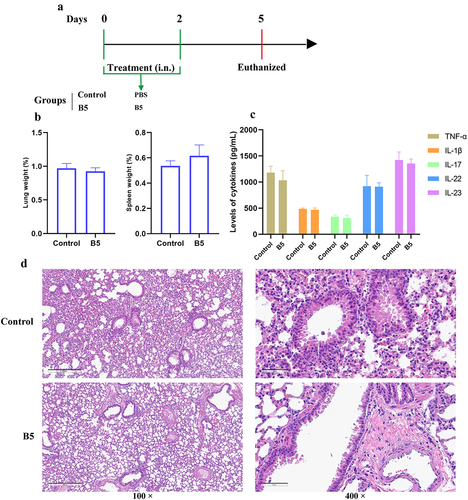
To evaluate the safety of B5 administered by the intranasal route, mice were euthanized to measure organ index, inflammatory cytokines, and pathological changes in lung tissues after 3 d of B5 administration (). Results showed that no significant difference in the weight of lung and spleen tissues (), the levels of TNF-α, IL-1β, IL-17, IL-22, and IL-23 in lungs (), and the histological morphology of lungs () between control group and B5 group was observed. Overall, these findings indicate that intranasal B5 is safe and does not cause excessive inflammatory response in lungs.
Figure 3. Intranasal B5 safety evaluation. a, schematic representation of treatment model. Mice were treated through intranasal route with 20 μg of B5 (twice, 2-d interval). Three days after the last treatment, mice were euthanized for safety evaluation. b, weight of lung and spleen tissues after treatment with B5. c, levels of TNF-α, IL-1β, IL-17, IL-22 and IL-23 in lungs. d, histopathological images of lungs performed with HE staining (left images scale bar: 300 μm; right images scale bar: 60 μm). Data shown are means ± SD. Data are representative of two independent experiments (n = 3 mice per group). The significance of differences between two groups was determined by Student’s t-test.
Intranasal B5 provides early protection against APP
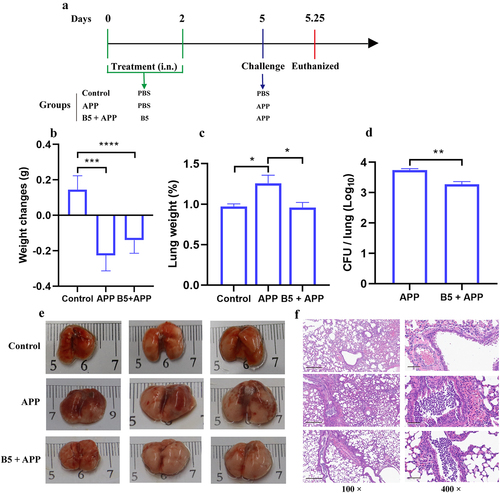
The early protection provided by B5 against APP was subsequently tested. B5-pretreated mice were challenged by APP and euthanized 6 h after challenge (). It was found that APP challenge led to body weight loss, pulmonaryedema and haemorrhagic focus, and massive inflammatory cells infiltration and haemorrhages in lungs (). However, no significant difference in body weight between APP group and B5 + APP group was observed (). Importantly, B5 significantly down-regulated the lung weight and significantly reduced bacterial load in lungs compared to APP control (). Moreover, much fewer haemorrhagic lesions and inflammatory foci were observed in B5 + APP group compared to APP group (). Moreover, B5 also provided protection against APP serovar 7 via alleviating pulmonary oedema and reducing bacterial load in lungs (Supplementary Figure S1A and B). Collectively, these results suggest that B5 provides protection against APP via reducing bacterial load in lungs and alleviating pathological damage of lung tissues in the early stage of infection.
Figure 4. Intranasal B5 provides early protection against APP. a, schematic representation of pretreatment/challenge model. Mice were pretreated intranasally with 20 μg of B5 (twice, 2-d interval) and challenged with APP 3 d after the last pretreatment and euthanized for protective evaluation 6 h after challenge. b, body weight changes. c, weight of lung tissues. d, bacterial load in lungs. e, gross pathology of lungs. f, histopathological images of lungs performed with HE staining (left images scale bar: 300 μm; right images scale bar: 60 μm). Data shown are means ± SD. Data are representative of two independent experiments (n = 6 mice per group). The significance of differences between the groups was determined by ANOVA with post-hoc Tukey’s multiple comparison test (*p < 0.033, **p < 0.0021, ***p < 0.0002, ****p < 0.0001).
Intranasal B5 maintains protection in the later stage of APP infection
To investigate protective efficacy provided by B5 in the later stage of APP infection. B5-pretreated mice were challenged by APP and euthanized 24 h after challenge (). Twenty-four hours after APP infection, compared with B5-treated mice, significant weight loss in untreated mice was observed (). Importantly, the inflammatory response in the lungs worsened, and B5 maintained the ability of reducing APP load in lungs and alleviating APP-caused pathological damage of lung tissues (). Although there was no significant difference in lung weight and gross pathology of lungs between APP group and B5 + APP group (), B5 significantly reduced pulmonary bacterial load, obviously decreased the secretion of protein-like substances, and markedly relieved haemorrhage and inflammatory cell infiltration and the damage of alveolar and bronchus structure in lungs compared to APP control (). Importantly, compared with PBD2, B5 significantly reduced bacterial load in lungs and provided better protection against ultra-high lethal APP, and the protection rate was 16.7% and 50.0%, respectively (Supplementary Figure S1C and D). In addition, a therapeutic experiment results showed that B5 significantly decreased bacterial load in lungs and ameliorated pulmonary oedema, suggesting that B5 also exhibited therapeutic effects against APP (Supplementary Figure S1E and F).
Figure 5. Intranasal B5 maintains protection in the later stage of APP infection. a, schematic representation of pretreatment/challenge model. Mice were pretreated intranasally with 20 μg of B5, challenged with APP 3 d after the last pretreatment, and euthanized for protective evaluation 24 h after challenge. b, body weight changes. c, weight of lung tissues. d, bacterial load in lungs. e, gross pathology of lungs. f, histopathological images of lungs performed with HE staining (left images scale bar: 2 mm; middle images scale bar: 300 μm; right images scale bar: 60 μm). Data shown are means ± SD. Data are representative of two independent experiments (n = 6 mice per group). The significance of differences between the groups was determined by ANOVA with post-hoc Tukey’s multiple comparison test (**p < 0.0021, ***p < 0.0002).
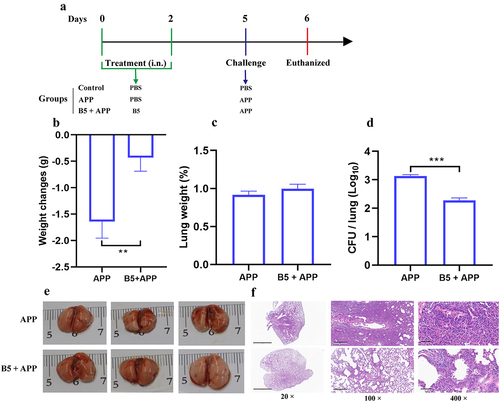
Overall, these results suggest that intranasal B5 is able to provide prolonged protection against APP.
Intranasal B5 promotes pro-inflammatory cytokines secretion in lungs
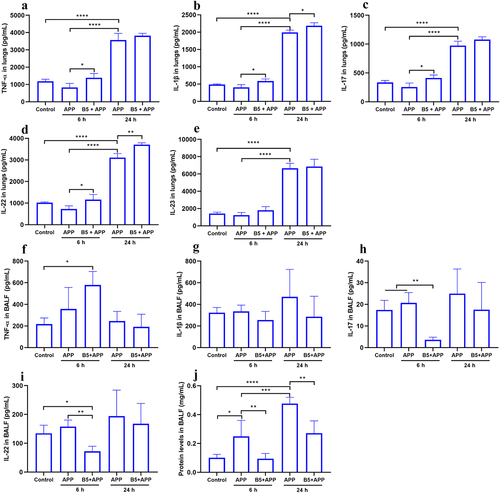
In the early stages of infection, high levels of pro-inflammatory cytokines in the lungs are beneficial for protection against APP [Citation21]. In the current study, we found that there was no significant difference in levels of TNF-α, IL-1β, IL-17, IL-22, and IL-23 in lungs between control group and APP group 6 h after APP challenge, suggesting pulmonary immunosuppression in the early stage of infection, which was in line with Bao C et al. [Citation35]. However, the secytokines production significantly increased 24 h after APP challenge (), suggesting severe inflammatory response in lungs in the later stage of infection, this was in line with pathological changes in lungs (). Moreover, B5 pretreatment significantly up-regulated expression levels of TNF-α, IL-1β, IL-17, and IL-22 in lungs 6 h after challenge and markedly increased expression levels of IL-1β and IL-22 in lungs 24 h after challenge compared with APP control. However, no significant difference in TNF-α and IL-17 secretion between APP group and B5 + APP group was observed after 24 h of APP challenge, and B5 failed to affect IL-23 secretion (). It was worth mentioning that B5 mildly up-regulated TNF-α, IL-1β, IL-17, and IL-22 expression in lungs and levels of these cytokines increased by less than twice, suggesting that B5 failed to cause excessive inflammatory response in lungs. Surprisingly, B5 significantly up-regulated TNF-α production, but markedly down-regulated IL-17 and IL-22 production in BALF 6 h after APP challenge compared with APP control, while no significant difference in cytokines expression in BALF between two groups was observed 24 h after APP challenge (). We speculated that IL-17/IL-22-producing cells migrated from trachea to lungs. In addition, APP challenge led to protein hyper secretion in BALF, and B5 significantly reduced protein levels in BALF compared with APP control (), suggesting that B5 inhibited airway mucus hyper secretion caused by APP. Collectively, these results indicate that intranasal B5 promotes TNF-α, IL-1β, IL-17, and IL-22 secretion in lungs in the early stage of APP infection and reduces protein levels in BALF during the APP infection.
Figure 6. Intranasal B5 promotes cytokine secretion in lungs during APP infection. Mice were pretreated intranasally with 20 μg of B5, challenged with APP 3 d after the last pretreatment, and euthanized for cytokines detection 6 h and 24 h after challenge. a-e, levels of TNF-α (a), IL-1β (b), IL-17 (c), IL-22 (d), and IL-23 (e) in lungs. f-i, levels of TNF-α (f), IL-1β (g), IL-17 (h), and IL-22 (i) in BALF. j, protein levels in BALF. Data shown are means ± SD. Data are representative of two independent experiments (n = 3–4 mice per group). The significance of differences between the groups was determined by ANOVA with post-hoc Tukey’s multiple comparison test (*p < 0.033, **p < 0.0021, ***p < 0.0002, ****p < 0.0001).
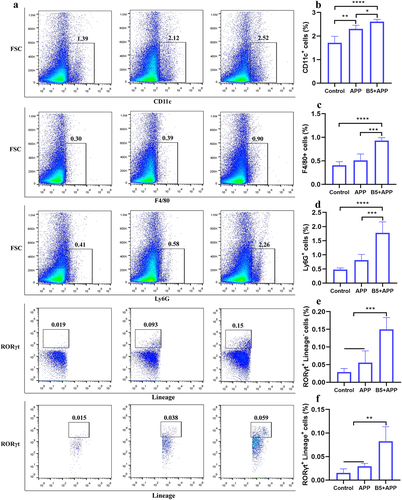
Intranasal B5 induces the generation of innate immune cells in lungs
Innate immune cells including DC, macrophages, neutrophils, and ILC3s play an important protective role in the early stages of bacterial infection [Citation36–38]. To evaluate the ability of B5 to prime lung innate immune cells, lung cells were isolated 6 h after APP challenge. Results showed that APP infection caused an increase in DC marked with CD11c, while there was no change in the number of macrophages marked with F4/80, neutrophils marked with Ly6G, and ILC3s marked with Lineage−Rorγt+. However, B5 pretreatment significantly increased the number of DC, macrophages, neutrophils and ILC3s in lungs compared with uninfected control and APP control (). Moreover, B5 pretreatment also significantly increased the number of Lineage+Rorγt+cells in lungs (). Collectively, these results suggest that intranasal B5 induces the generation of innate immune cells in the lung.
Figure 7. Intranasal B5 induces the generation of innate immune cells in lungs. Mice were pretreated intranasally with 20 μg of B5, challenged with APP 3 d after the last pretreatment, and euthanized for innate immune cells detection 6 h after challenge. a, Representative FACS blots of DC (CD11c+), macrophages (F4/80+), neutrophils (Ly6G+), ILC3s (Lineage− Rorγt+), and Lineage+ Rorγt+ cells in lungs. b-f, percentage of DC (b), macrophages (c), neutrophils (d), ILC3s (e), and Lineage+ Rorγt+ cells (f). Data shown are means ± SD. Data are representative of two independent experiments (n = 4–6 mice per group). The significance of differences between the groups was determined by ANOVA with post-hoc Tukey’s multiple comparison test (*p < 0.033, **p < 0.0021, ***p < 0.0002, ****p < 0.0001).
Intranasal B5 regulates gene expression profiles in lungs
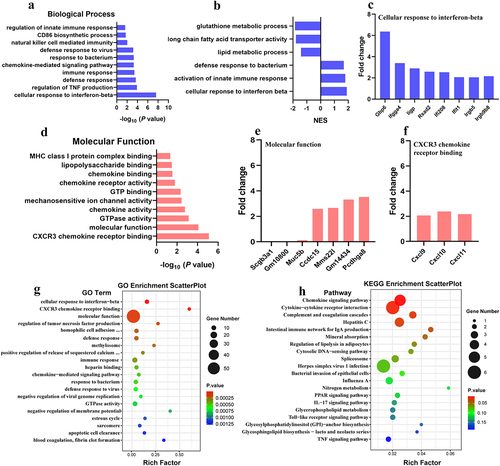
To explore the potential mechanism of B5 regulating the pulmonary immune response. B5-pretreated mice were challenged with APP and lungs were collected 6 h after challenge and carried out RNA-seq experiments to measure molecular changes at gene expression level. We found that 126 genes were significantly up-regulated (fold change, FC > 2) and 143 genes were significantly down-regulated (FC < 0.5) in B5 + APP group compared with APP group (). In addition to antimicrobial mediators, airway mucins including Muc5b are produced by secretory epithelial cells and contribute to the first layer of host defence [Citation39]. We found that APP infection led to increases in genes expression involving airway mucus secretion (Muc5b, Bpifb1, ltf and Scgb3a1); however, B5 obviously significantly down-regulated these genes expression (), suggesting that B5 regulates mucous secretion in airway, this was in line with protein levels in BALF (). Next, GO analysis showed that genes were mainly enriched in cellular response to IFN-β, regulation of TNF production, defence response, immune response, etc., in the biological process of B5 + APP group and APP group (). GSEA showed that B5 activated signalling pathway related to cellular response to IFN-β, activation of innate immune response and defense response to bacterium (). In line with this, we found B5 pretreatment significantly increased expression levels of IFN-stimulated genes (Gbp6, Ifgga4, Iigp, Rsad2, Ifi208, Ifit1, Irgb5 and Irgb9b8) () and also significantly up-regulated IFN-β-related genes (Cxcl9 and Cxcl10) () compared with untreated and infected control, indicating that B5 may play a role in regulating the IFN signalling. In addition, genes were mainly enriched in CXCR3 chemokine receptor binding, molecular function, GTPase activity, chemokine activity, etc., in the molecular function of B5 + APP group and APP group (). Compared with APP group, B5 + APP group showed significantly down-regulated levels of Scgb3a1 and Muc5b and significantly up-regulated levels of Cxcl9, Cxcl10, and Cxcl11 (). Moreover, KEGG analysis showed that genes were mainly enriched in chemokine signalling pathway, cytokine–cytokine receptor interaction, complement and coagulation cascades, hepatitis C, and intestinalimmune network for IgA production in B5 + APP group and APP group (). Collectively, these results suggest that intranasal B5 activates innate immune response in the lung.
Figure 8. Analysis of differentially expressed genes. a, differentially expressed genes were shown in volcano plots (B5 + APP group vs APP group). b, representative differentially expressed genes were shown in heat map.
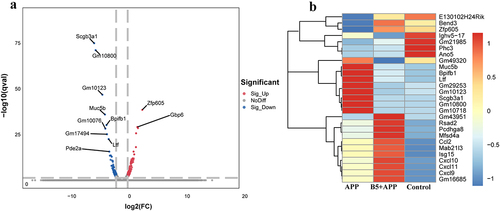
Figure 9. Gene ontology (GO) term analysis of differentially expressed genes. B5 + APP group vs APP group: a, gene enrichment in biological process. b, gene set enrichment analysis (GSEA). c, fold changes of genes involving cellular response to interferon-beta d, gene enrichment in molecular function. e-f, fold changes of genes involving molecular function (e) and CXCR3 chemokine receptor binding (f). g-h, GO enrichment scatterplot (g) and KEGG enrichment scatterplot (h).
Disscussion
Here, we provide evidence that intranasal B5 activates the innate immune response in respiratory tract and limits airway inflammation and provides protection against APP. B5-induced antibacterial immunity involves the activation of macrophages and DC, the rapid recruitment of macrophages, neutrophils, and ILC3s at the site of bacteria invasion, and the activation of cellular response to IFN-β signalling pathway. We propose that B5 promotes pro-inflammatory response in the early stage of APP infection, ameliorates early immune suppression caused by APP, and inhibits APP-caused airway mucus hypersecretion.
The clearance of APP is dependent on the innate immune response of the host. However, early APP infection inhibited the pulmonary innate immune response [Citation35]. Similarly, we found that early APP failed to change pro-inflammatory cytokine secretion and phagocyte recruitment in lungs. Previously, our study showed that B5 could amplify cell-mediated immunity and humoral immunity in respiratory tract against M. bovis [Citation29]. In this study, we found that the anti-APP ability (e.g. clearing APP in lungs and alleviating haemorrhage, inflammatory cells infiltration and the damage of alveolar and bronchus structure) of B5-pretreated mice was significantly stronger than that of untreated mice, so we suppose that B5 may promote the generation of the innate immune cells to eliminate APP. Consistent with these findings, a recent study found that IL-8 supplement promoted the expression of inflammatory cytokines and reduced the susceptibility to APP [Citation21]. Macrophages and neutrophils are the first line of host defence and play a vital role in the resistance of bacteria [Citation20]. APP-producing Apx toxins lead to the lysis of macrophages, neutrophils, alveolar epithelial cells, endothelial cells, and red blood cells [Citation11,Citation40]. In the current study, early recruitment of macrophages, neutrophils, and DC in lungs of B5-treated mice 6 h after APP challenge were observed. Importantly, B5 pretreatment also controlled APP replication and alleviated pulmonary inflammatory response. ILC3s are found at mucosal sites which respond rapidly in the event of an infection. They promote bacterial clearance and tissue repair during bacterial pneumonia [Citation37,Citation38]. Our study first found that the host defence peptide B5 promoted thegeneration of ILC3s in lungs, suggesting that B5 may contribute to pulmonary epithelial repair. The porcine antibacterial peptide PR-39 mainly produced by neutrophils plays a vital role in the innate immune defense of pigs against APP infection [Citation41]. However, exogenous PR-39 failed to effectively kill APP due to resistance [Citation42,Citation43] and it was proposed that this peptide was conducive to fibrotic tissue repair processes rather than high-level antibacterial activity [Citation2]. Our study highlight that B5 protected mice from APP challenge through the regulation of mucosal innate immune rather than strong direct antibacterial activity. Notably, PBD2 was demonstrated to protect pigs against APP [Citation26]; however, compared with PBD2, B5 offered better protection. More importantly, B5 also provided protection against APP serovar 7 and exhibited a good therapeutic effect. It is worth mentioning that AMPs has faced the challenges of drug resistance, AMPs from animals and plants are suggested to replace human AMPs [Citation44]. Therefore, it is possible to use B5 in PCP treatment, but further study on its safety and effectiveness in pigs will be needed.
TNF-α and IL-1β are vital to control pathogens in the early stages ofinfection, but their over production results in inflammatory lung injury [Citation45]. In this study, we found that B5 promoted the secretion of TNF-α and IL-1β in macrophages and DC in vitro and also mildly up-regulated levels of TNF-α and IL-1β in lungs. However, the hyper secretion of pro-inflammatory cytokines and the aggregation of inflammatory cells in lungs were not observed after B5 pretreatment, suggesting that intranasal B5 was safe. Moreover, the early production of IL-17 and IL-22 is important for neutrophil recruitment and host defence against S. pneumoniae, K. pneumoniae and P. aeruginosa [Citation38]. IL-17 and IL-22 produced by ILC3s facilitate bacteria clearance and tissue repair in pneumonia [Citation37], but they may contribute to chronic inflammation in the presence of allergens [Citation46]. Here, our study showed that B5 pretreatment enhanced the secretion of IL-17 and IL-22 in lungs after APP challenge. Surprisingly, in the early stages of infection, B5 down-regulated the levels of IL-17 and IL-22 in BALF, we speculated that B5 pretreatment enhanced IL-17-producing or IL-22-producing cells in BALF migration to the lungs where APP invaded and colonized. Overall, intranasal B5 ameliorated early pulmonary immunosuppression caused by APP but did not cause excessive inflammatory response in lungs.
IFN-β in host response to viruses is well known, recently, increasing evidence showed that IFN-β mediated anti-bacterial defence [Citation47,Citation48]. IFN-β produced by resolution phase macrophages plays a pivotal rolein resolving bacterial inflammation [Citation47]. Recently, IFN-β was demonstrated to regulate proresolving lipids to enhance there solution of acute airway inflammation [Citation49]. Our study showed that B5 could promoted the secretion of IFN-β in macrophages and DC in vitro and up-regulated IFN-stimulated gene expression in vivo. Moreover, B5 pretreatment induced a gene expression signature profile, consistent with upregulated IFN-β expression. These genes were mainly IFN-stimulated genes such as Gbp6, Rsad2 and Ifit1, and IFN-β-related genes such as Cxcl9 and Cxcl10. Consistently, GSEA showed that B5 activated signalling pathway related to cellular response to IFN-β. Moreover, B5 also enhanced the activation of innate immune response and the defence response to bacterium. Importantly, B5 pretreatment protected APP-challenged mice. However, the potential mechanism of B5 regulating IFN-β will be further studied. Although the role of IFN-β in host defence against bacterial infections is enigmatic [Citation50], our current study showed that up-regulated IFN-β expression was conducive to host defence against APP infection.
In summary, B5 activated macrophages and DC in vitro. Furthermore, intranasal B5 up-regulated levels of TNF-α, IL-1β, IL-17, and IL-22 promoted the rapid recruitment of macrophages and neutrophils, enhanced the generation of ILC3s, and activated signaling pathways associated with cellular response to IFN-β and activation of innate immune response in the early stage of APP infection. Importantly, intranasal B5 significantly reduced the bacterial load in lungs and markedly alleviated pulmonary inflammatory damage. Overall, B5 effectively ameliorated the pulmonary immune suppression caused by early APP infection and provided protection against APP. Nonetheless, further studies will be needed to evaluate its safe dose, protection, and duration in pigs. Collectively, this study has offered a possibility that B5 can be used as an alternative drug enhancing the initiation of airway innate immune response against APP infection.
Author contributions
Z.L. and J.H. prepared the manuscript. Z.L., J.H. and J.K designed experiments. J.H., W.K. and D.Y performed experiments. S.Z., Y.X., Y.W., C.L., H.L., D.D. and J.S. assisted to complete experiments and contributed to acquisition of date. Z.L. provided funding. All authors contributed to date analysis.
3-Supplementary information.docx
Download MS Word (210.1 KB)Acknowledgements
This research was supported by the Guangxi Key Technologies R&D Program (Project No. GUIKE AB23026082) and the Specific Research Project of Guangxi for Research Base and Talents (Project No. GUIKE AD23026289), and Guangxi Key Laboratory of Animal Breeding, Disease Control and Prevention (No. ABDC-b202305).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data Availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request ([email protected]).
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/21505594.2024.2316459
Additional information
Funding
References
- Gottschalk M, Broes A. Actinobacillosis. In Zimmerman JJ, Karriker LA, Ramirez A, et al., editors. Diseases of swine. Chichester: Wiley; 2019. p. 749–17.
- Sassu EL, Bossé JT, Tobias TJ, et al. Update on Actinobacillus pleuropneumoniae-knowledge, gaps and challenges. Transbound Emerg Dis. 2018;1:72–90. doi: 10.1111/tbed.12739
- Qi W, Zhu R, Bao C, et al. Porcine circovirus type 2 promotes Actinobacillus pleuropneumoniae survival during coinfection of porcine alveolar macrophages by inhibiting ROS production. Vet Microbiol. 2019;233:93–101. doi: 10.1016/j.vetmic.2019.04.028
- Stringer OW, Bossé JT, Lacouture S, et al. Proposal of Actinobacillus pleuropneumoniae serovar 19, and reformulation of previous multiplex PCRs for capsule-specific typing of all known serovars. Veterinary Microbiology. 2021;255:109021. doi: 10.1016/j.vetmic.2021.109021
- Kardos G, Sárközi R, Laczkó L, et al. Genetic Diversity of Actinobacillus pleuropneumoniae Serovars in Hungary[J]. Vet Sci. 2022;9(10):511. doi: 10.3390/vetsci9100511
- Shunsuke K, Toshiya S, Hiroya I. Serovar and antimicrobial resistance profiles of actinobacillus pleuropneumoniae isolated in Japan from 2006 to 2011. JARQ. 2016;50(1):73–77. doi: 10.6090/jarq.50.73
- Nahar N, Turni C, Tram G, et al. Actinobacillus pleuropneumoniae: The molecular determinants of virulence and pathogenesis[J]. Adv Microb Physiol. 2021;78:179–216.
- Gutiérrez-Martín CB, Blanco N G D, Blanco M, et al. Changes in antimicrobial susceptibility of Actinobacillus pleuropneumoniae isolated from pigs in Spain during the last decade[J]. Vet Microbiol. 2006;115(1–3):218–222. doi: 10.1016/j.vetmic.2005.12.014
- Vilaró A, Novell E, Enrique-Tarancón V, et al. Antimicrobial Susceptibility Pattern of Porcine Respiratory Bacteria in Spain[J]. Antibiotics. 2020;9(7):402. doi: 10.3390/antibiotics9070402
- Rao J, Wei X, Li H, et al. Novel multiplex PCR assay and its application in detecting prevalence and antibiotic susceptibility of porcine respiratory bacterial pathogens in Guangxi, China. Microbiol Spectr. 2023;11(2):e0397122. doi: 10.1128/spectrum.03971-22
- Chiers K, De Waele T, Pasmans F, et al. Virulence factors of Actinobacillus pleuropneumoniae involved in colonization, persistence and induction of lesions in its porcine host. Vet Res. 2010;41(5):65. doi: 10.1051/vetres/2010037
- Bercier P, Gottschalk M, Grenier D. Effects of Actinobacillus pleuropneumoniae on barrier function and inflammatory response of pig tracheal epithelial cells. Pathog Dis. 2019;77(1):fty079. doi: 10.1093/femspd/fty079
- Gao N, Rezaee F. Airway epithelial cell junctions as targets for pathogens and antimicrobial therapy. Pharmaceutics. 2022;14(12):2619. doi: 10.3390/pharmaceutics14122619
- Counoupas C, Ferrell KC, Ashhurst A, et al. Mucosal delivery of a multistage subunit vaccine promotes development of lung-resident memory T cells and affords interleukin-17-dependent protection against pulmonary tuberculosis[J]. NPJ Vaccin. 2020;5(1):105. doi: 10.1038/s41541-020-00255-7
- Eiden J, Fierro C, Schwartz H, et al. Intranasal M2SR (M2-deficient single replication) H3N2 influenza vaccine provides enhanced mucosal and serum antibodies in Adults. J Infect Dis. 2022;227(1):103–112. doi: 10.1093/infdis/jiac433
- Deng S, Liu Y, Tam RC, et al. An intranasal influenza virus-vectored vaccine prevents SARS-CoV-2 replication in respiratory tissues of mice and hamsters. Nat Commun. 2023;14(1):2081. doi: 10.1038/s41467-023-37697-1
- Figueiredo DB, Kaneko K, Rodrigues TDC, et al. Pneumococcal Surface Protein A-Hybrid Nanoparticles Protect Mice from Lethal Challenge after Mucosal Immunization Targeting the Lungs[J]. Pharmaceutics. 2022;14(6):1238. doi: 10.3390/pharmaceutics14061238
- Cabral MP, Correia A, Vilanova M, et al. A live auxotrophic vaccine confers mucosal immunity and protection against lethal pneumonia caused by Pseudomonas aeruginosa[J]. PLOS Pathog. 2020;16(2):e1008311. doi: 10.1371/journal.ppat.1008311
- Kim GL, Lee S, Kim SJ, et al. Pulmonary colonization resistance to pathogens via noncanonical wnt and interleukin-17A by intranasal pep27 mutant Immunization. The Journal Of Infectious Diseases. 2018;217(12):1977–1986. doi: 10.1093/infdis/jiy158
- Galeas-Pena M, Mclaughlin N, Pociask D. The role of the innate immune system on pulmonary infections. Biol Chem. 2019;400(4):443–456. doi: 10.1515/hsz-2018-0304
- Bao C, Liu B, Zhu R, et al. IFN-γ(-/-) mice resist Actinobacillus pleuropneumoniae infection by promoting early lung IL-18 release and PMN-I Accumulation. Infect Immun. 2021;89(6):e00069–21. doi: 10.1128/IAI.00069-21
- Hiemstra PS, Amatngalim GD, Van Der Does AM, et al. Antimicrobial peptides and innate lung defenses: role in infectious and noninfectious lung diseases and therapeutic Applications. Chest. 2016;149(2):545–551. doi: 10.1378/chest.15-1353
- Mookherjee N, Anderson MA, Haagsman HP, et al. Antimicrobial host defence peptides: functions and clinical potential[J]. Nat Rev Drug Discov. 2020;19(5):311–332. doi: 10.1038/s41573-019-0058-8
- Gao X, Ding J, Liao C, et al. Defensins: The natural peptide antibiotic[J]. Adv Drug Deliv Rev. 2021;179:114008. doi: 10.1016/j.addr.2021.114008
- Huang C, Yang X, Huang J, et al. Porcine beta-defensin 2 provides protection against bacterial infection by a direct bactericidal activity and alleviates inflammation via interference with the TLR4/NF-κB pathway. Front Immunol. 2019;10:1673. doi: 10.3389/fimmu.2019.01673
- Yang X, Cheng YT, Tan MF, et al. Overexpression of porcine beta-defensin 2 enhances resistance to Actinobacillus pleuropneumoniae infection in pigs. Infect Immun. 2015;83(7):2836–2843. doi: 10.1128/IAI.03101-14
- Huang J, Yang X, Wang A, et al. Pigs Overexpressing Porcine β-Defensin 2 Display Increased Resilience to Glaesserella parasuis Infection[J]. Antibiotics. 2020;9(12):903. doi: 10.3390/antibiotics9120903
- Lee EY, Lee MW, Wong GCL. Modulation of toll-like receptor signaling by antimicrobial peptides. Semin Cell Dev Biol. 2019;88:173–184. doi: 10.1016/j.semcdb.2018.02.002
- Liang Z, Li H, Qu M, et al. Intranasal bovine β-defensin-5 enhances antituberculosis immunity in a mouse model by a novel protein-based respiratory mucosal vaccine[J]. Virulence. 2022;13(1):949–962. doi: 10.1080/21505594.2022.2080342
- Dong X, Limjunyawong N, Sypek EI, et al. Keratinocyte-derived defensins activate neutrophil-specific receptors Mrgpra2a/b to prevent skin dysbiosis and bacterial infection. Immunity. 2022;55(9):1645–1662. doi: 10.1016/j.immuni.2022.06.021
- Ryan LK, Wu J, Schwartz K, et al. β-defensins coordinate in vivo to inhibit bacterial infections of the Trachea. Vaccines (Basel). 2018;6(3):57. doi: 10.3390/vaccines6030057
- Liang Z, Liu Y, Sun X, et al. Immunoregulatory and antimicrobial activity of bovine neutrophil β-defensin-5-loaded PLGA nanoparticles against Mycobacterium bovis. Pharmaceutics. 2020;12(12):1172. doi: 10.3390/pharmaceutics12121172
- Song Y, Ge X, Chen Y, et al. Mycobacterium bovis induces mitophagy to suppress host xenophagy for its intracellular survival. Autophagy. 2022;18(6):1401–1415. doi: 10.1080/15548627.2021.1987671
- Jung ID, Shin SJ, G LM, et al. Enhancement of tumor-specific T cell-mediated immunity in dendritic cell-based vaccines by Mycobacterium tuberculosis heat shock protein X. J Immunol. 2014;193(3):1233–1245. doi: 10.4049/jimmunol.1400656
- Bao C, Jiang H, Zhu R, et al. Differences in pig respiratory tract and peripheral blood immune responses to Actinobacillus pleuropneumoniae. Vet Microbiol. 2020;247:108755. doi: 10.1016/j.vetmic.2020.108755
- Gonzalez-Ferrer S, Peñaloza HF, Budnick JA, et al. Finding Order in the Chaos: Outstanding Questions in Klebsiella pneumoniae Pathogenesis[J]. Infect Immun. 2021;89(4):e00693–20. doi: 10.1128/IAI.00693-20
- Hoffmann JP, Kolls JK, Mccombs JE. Regulation and function of ILC3s in pulmonary Infections. Front Immunol. 2021;12:672523. doi: 10.3389/fimmu.2021.672523
- Stehle C, Hernández DC, Romagnani C. Innate lymphoid cells in lung infection and immunity. Immunol Rev. 2018;286(1):102–119. doi: 10.1111/imr.12712
- Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363(23):2233–2247. doi: 10.1056/NEJMra0910061
- Cruijsen TL, Leengoed L A V, Dekker-Nooren TC, et al. Phagocytosis and killing of Actinobacillus pleuropneumoniae by alveolar macrophages and polymorphonuclear leukocytes isolated from pigs. Infect Immun. 1992;60(11):4867–4871. doi: 10.1128/iai.60.11.4867-4871.1992
- Hennig-Pauka I, Koch R, Hoeltig D, et al. PR-39, a porcine host defence peptide, is prominent in mucosa and lymphatic tissue of the respiratory tract in healthy pigs and pigs infected with Actinobacillus pleuropneumoniae. BMC Res Notes. 2012;5(1):539. doi: 10.1186/1756-0500-5-539
- Hennig-Pauka I, Jacobsen I, Blecha F, et al. Differential proteomic analysis reveals increased cathelicidin expression in porcine bronchoalveolar lavage fluid after an Actinobacillus pleuropneumoniae infection. Vet Res. 2006;37(1):75–87. doi: 10.1051/vetres:2005043
- Xie F, Wang Y, Li G, et al. The SapA Protein Is Involved in Resistance to Antimicrobial Peptide PR-39 and Virulence of Actinobacillus pleuropneumoniae[J]. Front Microbiol. 2017;8:811. doi: 10.3389/fmicb.2017.00811
- Lazzaro BP, Zasloff M, Rolff J. Antimicrobial peptides: application informed by evolution. Science. 2020;368(6490):eaau5480. doi: 10.1126/science.aau5480
- Li B, Fang J, Zuo Z, et al. Activation of porcine alveolar macrophages by Actinobacillus pleuropneumoniae lipopolysaccharide via the toll-like receptor 4/nf-κb-mediated pathway. Infect Immun. 2018;86(3):e00642–17. doi: 10.1128/IAI.00642-17
- McAleer JP, Kolls JK. Directing traffic: IL-17 and IL-22 coordinate pulmonary immune defense. Immunol Rev. 2014;260(1):129–144. doi: 10.1111/imr.12183
- Kumaran Satyanarayanan S, El Kebir D, Soboh S, et al. IFN-β is a macrophage-derived effector cytokine facilitating the resolution of bacterial inflammation. Nat Commun. 2019;10(1):3471. doi: 10.1038/s41467-019-10903-9
- Boxx GM, Cheng G. The roles of type I interferon in bacterial infection. Cell Host Microbe. 2016;19(6):760–769. doi: 10.1016/j.chom.2016.05.016
- Sekheri M, Rizo-Téllez SA, Othman A, et al. Interferon-β regulates proresolving lipids to promote the resolution of acute airway inflammation. Proc Natl Acad Sci USA. 2022;119(31):e2201146119. doi: 10.1073/pnas.2201146119
- Boxx GM, Cheng G. The roles of type I interferon in bacterial Infection [J]. Cell Host Microbe. 2016;19(6):760–769.
