ABSTRACT
Klebsiella pneumoniae is a common causative pathogen of intra-abdominal infection with concomitant bacteraemia, leading to a significant mortality risk. The time to positivity (TTP) of blood culture is postulated to be a prognostic factor in bacteraemia caused by other species. Therefore, this study aimed to investigate the prognostic value of TTP in these patients. The single-centred, retrospective, observational cohort study was conducted between 1 July 2016 and 30 June 2021. All adult emergency department patients with diagnosis of intra-abdominal infection and underwent blood culture collection which yield K. pneumoniae during this period were enrolled. A total of 196 patients were included in the study. The overall 30-day mortality rate was 12.2% (24/196), and the median TTP of the studied cohort was 12.3 h (10.5–15.8 h). TTP revealed a moderate 30-day mortality discriminative ability (area under the curve 0.73, p < 0.001). Compared with the late TTP group (>12 h, N = 109), patients in the early TTP (≤12 h, N = 87) group had a significantly higher risk of 30-day morality (21.8% vs. 4.6%, p < 0.01) and other adverse outcomes. Furthermore, TTP (odds ratio [OR] = 0.79, p = 0.02), Pitt bacteraemia score (OR = 1.30, p = 0.03), and implementation of source control (OR = 0.06, p < 0.01) were identified as independent factors related to 30-day mortality risk in patients with intra-abdominal infection and K. pneumoniae bacteraemia. Therefore, physicians can use TTP for prognosis stratification in these patients.
Introduction
The abdomen is the leading source of infection in critically ill patients, comprising a wide variety of disease spectrums and pathogens, and results in significant morbidity and mortality risks [Citation1,Citation2]. Approximately 80% of patients with intra-abdominal sepsis have identifiable pathogens, predominantly Gram-negative organisms, followed by Gram-positive and anaerobic bacteria [Citation2]. Among these pathogens, Klebsiella pneumoniae is the second most common causative Gram-negative organism and is an important cause of intra-abdominal infections [Citation2,Citation3]. It is more troublesome that approximately one-fourth of patients with intra-abdominal infection had concomitant bacteraemia (i.e. bloodstream infection), leading to a high mortality rate ranging from 36% to 63% [Citation4–6]. Nevertheless, few studies have focused on the characteristics and outcomes of patients with K. pneumoniae bacteraemia and intra-abdominal infections [Citation4,Citation7].
Blood culture is a crucial method for detecting the presence of bacteria in the blood and diagnosing bacteraemia [Citation8]. The time to positivity (TTP) is the time frame from the start of incubation to the positive bacterial growth signal [Citation3,Citation8]. TTP is associated with bacterial load and has been previously investigated as a diagnostic tool for catheter-associated or paediatric bacteraemia [Citation9–11]. Recent studies on the prognostic significance of TTP in adult patients with bacteraemia have revealed conflicting results [Citation3,Citation12]. A meta-analysis found that a short TTP was associated with a worse prognosis in Gram-positive and -negative bacteraemia [Citation3], while a recent multi-centre study failed to demonstrate a correlation between TTP and mortality risk in patients with bacteraemia [Citation12].
Notably, only two studies examined the relationship between TTP and prognosis in patients with K. pneumoniae bacteraemia, and both revealed TTP had positive correlation with prognosis [Citation13,Citation14]. Both studies enrolled bacteraemic patients with various sources of infection, and this heterogeneity can be a confounder in analysing their outcomes [Citation2,Citation12]. Since K. pneumoniae is a major causative pathogen in patients with intra-abdominal infection, this study aimed to describe the clinical characteristics of patients with intra-abdominal infection and K. pneumoniae bacteraemia, and to explore the prognostic value of TTP in these patients.
Materials and methods
Study design
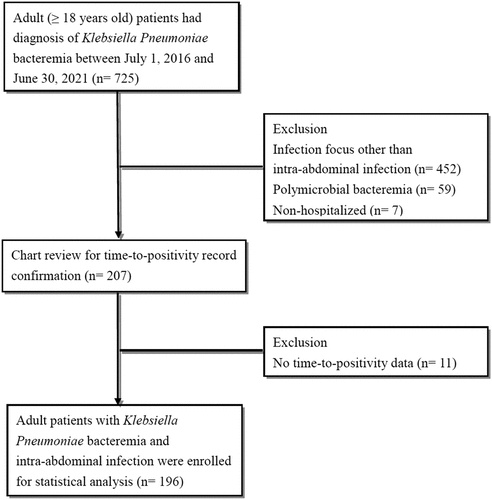
A retrospective single-centre, cohort study was conducted in a tertiary referral medical centre with approximately 1,000 beds and 54,500 emergency department (ED) visits per year. We enrolled all adult patients (aged ≥18 years) with ED visit between 1 July 2016 and 30 June 2021 and underwent blood culture collection which yielded K. pneumoniae. Patients with an infection focus other than intra-abdominal origin (e.g. urinary tract, respiratory tract, or soft tissue), polymicrobial bacteraemia, non-hospitalized, and those with incomplete TTP records were excluded. The diagnosis of intra-abdominal infection in each patient was initially screened by diagnostic code (International Classification of Diseases, 10th Revision) of each patient, then established and confirmed by their principal diagnosis and relevant manifestations (e.g. operative finding, image diagnosis). The study was approved by the local institutional review board (EMRP-110-183) and was conducted in accordance with the principles of the Declaration of Helsinki. Due to the retrospective nature of the study, the ethics committee waived the requirement for informed consent.
Data collection
All anonymized clinical information, including age, sex, comorbidities, initial ED physiological and laboratory parameters, and outcomes of each eligible patient, were retrieved from electronic medical record systems by two physicians (CPC and CCC). The implementation of source control (i.e. measures to eliminate the source of infection, reduce the bacterial inoculum, and correct anatomic derangements to restore normal physiological function [Citation1]) was recorded according to the medical records. We adopted both surgical and non-surgical procedures (e.g. endoscopic removal of the obstructed biliary tract, surgical repair of a perforated bowel, percutaneous drainage of intra-abdominal abscess, or removal of an indwelling catheter) as source control interventions. Antibiotic selection was based on the clinical decision of the treating physician. The types of intra-abdominal infection were classified by the place or origin of infection acquisition [Citation2]. The place of infection acquisition was stratified as community-acquired (infection acquired outside the hospital or within 48 h of hospital admission), hospital-acquired (infection developed after 48 h of hospital admission), or healthcare-acquired (infection developed in patients with nursing home care, chronic vascular access or dialysis status, or recent hospital admission less than 6 months) [Citation2,Citation15]. The origin of infection acquisition was classified as uncomplicated (e.g. diverticulitis, biliary tract infection) or complicated (primary peritonitis, secondary peritonitis, or tertiary peritonitis) [Citation2,Citation16].
Microbiological methods
ED physicians routinely ordered blood cultures in patients with suspected sepsis/septic shock according to practice guidelines [Citation17]. After proper disinfection, blood samples were collected from two or more separate anatomical sites from each patient. Two sets of blood cultures, each consisting of approximately 8–10 mL of blood, were immediately sent to the clinical laboratory department and processed using the BACTEC 9240 automated detection blood culture system (Becton Dickinson, NJ, USA). All blood culture bottles were incubated for 7 days before being considered negative for bacterial growth. The positive culture bacteria were further subcultured after Gram staining, and VITEK 2 Compact systems (bioMérieux, Inc., France) were used for the final species identification. Susceptibilities to antibiotics were determined using the standard disk diffusion method. TTP was recognized as the time span between the start of the blood incubation to the positive signal recorded by the automated systems. If multiple TTPs were detected in a patient, only the shortest TTP was included in the study.
Definitions
K. pneumoniae bacteraemia was defined as the growth of K. pneumoniae from at least one set of blood cultures. Systemic inflammatory response syndrome (SIRS) and quick sepsis related organ failure assessment (qSOFA) were determined in accordance with the previous consensus, and a qSOFA score ≥ 2 points was used as the prognostic cut-off value [Citation18]. The modified early warning score (MEWS) was obtained by summation of physiological parameters: respiratory rate, heart rate, systolic blood pressure, temperature, and mental status [Citation19]. The Pitt bacteraemia score (PBS) was established based on the following variables: temperature, blood pressure, need for mechanical ventilation, cardiac arrest event, and altered mental status [Citation20]. The appropriateness of antibiotic administration was determined based on its in vitro activity against the isolated pathogens.
Outcome measurement and statistical analyses
The primary outcome of this study was to examine the correlation between TTP and adverse outcomes in patients with intra-abdominal infection and K. pneumoniae bacteraemia. We included the risk of septic shock (i.e. the need of vasopressors for haemodynamic support), intensive care unit (ICU) admission, mechanical ventilation, and 30-day mortality as adverse outcomes. The secondary outcome was to identify the independent factors associated with 30-day mortality risk in these patients. Statistical significance was set at a two-tailed P-value of less than 0.05. We examined the mortality discriminative ability of TTP using the area under the receiver operating characteristic (ROC) curve, and the optimal cut-off point was recognized using the Youden index. Data for continuous variables were presented as mean ± standard deviation or median (interquartile range), and between-group differences were compared using two-sample t-test or Mann-Whitney test. For categorical variables, data were presented as percentages and analysed using the Chi-square or Fisher’s exact test. We included those potential variables with a P-value of less than 0.1 in the univariate analysis and two mandatory variables (age and sex) in a multivariate regression model to identify the independent factors associated with 30-day mortality risk in the enrolled patients. We also utilized the Kaplan-Meier survival curve to analyse the 30-day survival probability differences between the groups, and the ratio was compared using the log-rank test. Statistical analyses were performed using MedCalc Statistical Software version 18.2.1 (MedCalc Software bv, Ostend, Belgium) and Statistical Package for the Social Sciences (SPSS, IL, USA), version 22.0. Figures were drafted using GraphPad Prism version 8 (GraphPad Software, CA, USA).
Results
During the study period, 725 patients diagnosed with K. pneumoniae bacteraemia were enrolled (). We further excluded patients with polymicrobial bacteraemia (N = 59), infection focus other than intra-abdominal origin (N = 452), non-hospitalization (N = 7), and those without complete TTP records (N = 11). Finally, 196 patients were included in the analysis.
Study population
The mean age of the patients was 63.2 ± 14.0 years, and the male sex accounted for 67.9% (133/196) of the total population. Diabetes mellitus and hypertension were the most common comorbidities (). The most common place of infection acquisition was community-acquired (41.8%), followed by hospital-acquired (32.7%), and healthcare-acquired infections (25.5%). Regarding the origin of infection, most patients had complicating peritonitis (58.2%), and primary peritonitis (27.0%) and secondary peritonitis (18.4%) accounted for the majority in this category. The median TTP of the studied cohort was 12.3 h (10.5–15.8 h). We examined the mortality discriminative ability of TTP using the ROC curve, and the area under the curve (AUC) revealed moderate predictive efficacy (0.73, 95% confidence interval = 0.66–0.79, p < 0.001) (). Using the Youden index, the optimal cut-off value of TTP in our patients was 11.97 h with a sensitivity of 79.2% and specificity of 61.1%. Based on this cut-off value, we divided the patients into TTP ≤12 h (N = 87) and TTP >12 h (N = 109) groups for further comparison.
Figure 2. Receiver operating characteristic curve for 30-day mortality risk predicting ability of TTP in patients with Klebsiella pneumoniae bacteraemia and intra-abdominal infection.
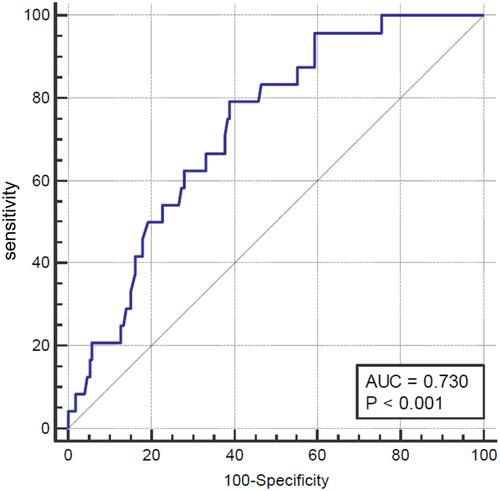
Table 1. Baseline characteristics, sepsis severity and management of patients with klebsiella pneumonia bacteraemia and intra-abdominal infection stratified by time-to-positivity (n = 196).
Baseline characteristics
As shown in , the mean ages of the two groups are comparable, and no significant differences in the proportion of sex and comorbidities between the two groups. Regarding laboratory results, patients in the early TTP group had significantly lower serum platelet count (148 ± 97 × 103/μL vs. 178 ± 90 × 103/μL, p = 0.03) and higher lactate level (3.4 [1.5–6.3] mmol/L vs. 2.0 [1.4–3.1] mmol/L, p < 0.01) than those in the late group. The early TTP group had a significantly higher proportion of high qSOFA scores (25.3% vs. 11.0%, p = 0.01) than that in the late group. The median PBS (2 [0–3] vs. 1 [0–2], p < 0.01) and mean MEWS (4 ± 2 vs. 3 ± 2, p = 0.02) were significantly higher in the early TTP group (). There was a comparable proportion of appropriate antibiotic and source control implementation between the two groups.
Clinical outcomes
The overall 30-day mortality rate of the enrolled patients was 12.2% (24/196) (). Compared with the late group, patients in the early TTP group had a significantly higher risk of adverse outcomes, including septic shock (46.0% vs. 22.0%, p < 0.01), ICU admission (26.4% vs. 11.9%, p = 0.02), mechanical ventilation (13.8% vs. 1.8%, p = 0.01), and 30-day morality (21.8% vs. 4.6%, p < 0.01). We further investigated the independent factors associated with 30-day mortality risk in these patients. As shown in , only TTP (odds ratio [OR] = 0.79, p = 0.02), PBS (OR = 1.30, p = 0.03), and implementation of source control (OR = 0.06, p < 0.01) remained independent factors related to 30-day mortality risk in patients with intra-abdominal infection and K. pneumoniae bacteraemia. Finally, the Kaplan-Meier curve demonstrated a significantly lower 30-day cumulative survival probability in the early TTP group (log-rank p < 0.01) ().
Figure 3. The kaplan-meier survival analysis for a 30-day cumulative survival probability stratified by TTP in patients with Klebsiella pneumoniae bacteraemia and intra-abdominal infection.
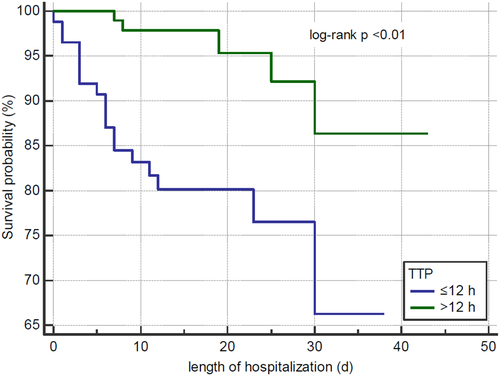
Table 2. Outcome analysis of patients with klebsiella pneumonia bacteraemia and intra-abdominal infection stratified by time-to-positivity (n = 196).
Table 3. Univariate and multivariate regression analysis of factors associated with 30-day mortality in patients with klebsiella pneumonia bacteraemia and intra-abdominal infection (n = 196).
Discussion
In this single-centre, ED-based retrospective cohort study, we described the clinical characteristics of patients with intra-abdominal infection and K. pneumoniae bacteraemia and explored the prognostic value of TTP in these patients. We found that TTP had a moderate mortality discrimination ability, and was associated with other adverse outcomes in these patients, including the risk of septic shock, ICU admission, and mechanical ventilation. Furthermore, TTP, PBS, and source control implementation were recognized as independent factors related to 30-day mortality in patients with intra-abdominal infection and K. pneumoniae bacteraemia. To the best of our knowledge, this is the first study to investigate the prognostic significance of TTP in patients with concomitant intra-abdominal and bloodstream infection caused by a single pathogen.
The abdominal cavity was postulated to be a high-risk source of bacteraemia, imposing a greater mortality risk than other infection sources [Citation4,Citation21]. It is noteworthy that compared with other management strategies in patients with intra-abdominal infection (e.g. source control, antimicrobial therapy), there is no consensus regarding routine blood culture for pathogen incubation in these patients, and practice guidelines state that it should be performed in patients with septic shock to guide further choice of antimicrobial regimen [Citation22,Citation23]. In accordance with this recommendation, patients with intra-abdominal infection and bacteraemia had a poorer prognosis than non-bacteraemic patients in an ICU-based study [Citation6].
The SIRS criteria remain an important tool for inflammation and complication detection, and a high qSOFA score is recognized as a marker of in-hospital mortality in patients with intra-abdominal infection [Citation23,Citation24]. Early warning scores comprise physiological parameters that allow rapid assessment and risk stratification [Citation19]. Combined early warning scores with abdominal signs and symptoms have screening and prognostic value in patients with intra-abdominal infections [Citation1]. Although our patients in the early TTP group had significantly higher MEWS and a higher proportion of high qSOFA scores, neither was identified as an independent mortality prediction factor in the regression analysis. Future large-scale prospective studies are warranted to validate their prognostic value in these patients.
Compared with previous studies, our patients had substantially lower 30-day mortality rates [Citation2,Citation4,Citation6], which may be attributed to their high proportion of appropriate antibiotic administration (87.8%) and source control implementation (56.6%). Fluid resuscitation, empiric antibiotic therapy, and source control are the three pillars of intra-abdominal infection management strategies [Citation22–24]. Inadequate source control and inappropriate antibiotic administration were independent predictors of mortality in critically ill patients with intra-abdominal infection and bacteraemia [Citation4]. While studies regarding the adequacy and optimal timing of source control are still lacking, most guidelines recommend that either surgical or non-surgical manoeuvres to eliminate the infection source are both acceptable, and ideally be implemented within 6 to 12 h of infection source recognition [Citation1,Citation17,Citation24]. Antimicrobial agent should be administered as soon as possible in critically ill patients with intra-abdominal infection and titrated based on local resistance epidemiology, individual patient risk factors, clinical disease severity, and infection source [Citation1,Citation22,Citation23]. The trend towards increased mortality risk in our patients with no source control measures or inappropriate antibiotic administration further strengthened their roles in these patients.
PBS has been a well-established scoring system for decades and has been developed as a prognostic tool in patients with bacteraemia [Citation20,Citation25]. The simplicity and easy calculation of PBS by summation of physiological parameters allow for rapid assessment and risk stratification in critically ill patients, and PBS outperformed other commonly-used risk scores (e.g. Acute Physiology and Chronic Health Evaluation and SOFA scores) in previous bacteraemia studies [Citation25]. A PBS score of 4 was traditionally deemed as the cut-off value of the critically ill and mortality risk threshold [Citation20]. Consistent with these findings, the relatively low PBS in our patients correlated with their low mortality rate, and it is also not surprising that PBS was identified as an independent prognostic factor in our study.
The median and optimal cut-off values of TTP in our study were similar to previous paediatric K. pneumoniae bacteraemia results [Citation14]. As TTP varies among different pathogen species, the cut-off TTP values were also widely ranged from 7 h to 7 d among different microorganisms in previous studies [Citation3,Citation8]. TTP provides potential biomass information regarding bacteraemia load and microbial growth rate, correlated to the extent of systemic inflammatory response and organ injury [Citation26,Citation27]. A meta-analysis analysed previous TTP studies for comparison, and most (72%) studies reported a significant relationship between mortality and short TTP, regardless of the pathogen species [Citation3]. Nevertheless, a recent large-scale prospective study did not reveal a positive correlation between TTP and mortality risk, except in patients with Streptococcus and Candida infection [Citation12]. The discrepancy was possibly influenced by various host factors (individual immune condition, pathogen tolerance or resistance) and processing factors in different hospital laboratories, and infection source variety can be another confounding factor [Citation3,Citation12]. Our patients were relatively homogeneous (i.e. pathogen, infection source) and were investigated in a single institution; thus, these factors can be minimized in our study. Furthermore, the short TTP associated with other adverse outcomes (e.g. ICU admission, septic shock, and mechanical ventilation) in our study can intensify its prognostic value in these patients.
This study had several limitations. The retrospective and single-centre nature of the analysis inevitably limits its extrapolation potential to other institutions. We adopted both surgical and non-surgical manoeuvres as source control methods but did not consider their timing, which also influenced the outcomes [Citation1,Citation24]. The interpretation of TTP has been associated with other confounding factors in previous studies, including blood sampling volume and methods, culture conditions, prior antibiotic therapy, and time of transportation for incubation [Citation26]. As the sampling and culture methods are unified in our institution, we admitted that the transportation time and concomitant antibiotic administration were not controlled in our study, which is similar to most previous TTP studies [Citation3]. Finally, the relatively small sample size in this analysis warrants future larger multi-centre studies to validate these findings.
Conclusions
In conclusion, TTP was associated with mortality risk and revealed moderate mortality discrimination ability in patients with intra-abdominal infection and K. pneumoniae bacteraemia. Physicians can utilize this simple and readily available information for rapid assessment, risk stratification, and commencement of prompt treatment in patients with shorter TTP.
Author contributions
Yong-Ye Yang: Conceptualization, Writing – original draft
I-Ting Tsai: Methodology, Writing – original draft
Chung-Hsu Lai: Visualization, Formal analysis
Chih-Ping Chen: Data curation, Visualization
Chia‐Chi Chen: Data curation, Software
Yin-Chou Hsu: Conceptualization, Supervision, Writing – review & editing
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analysed during the present study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Sartelli M, Chichom-Mefire A, Labricciosa FM, et al. The management of intra-abdominal infections from a global perspective: 2017 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2017;12(1):1–9. doi: 10.1186/s13017-016-0112-3
- Blot S, Antonelli M, Arvaniti K, et al. Epidemiology of intra-abdominal infection and sepsis in critically ill patients: “AbSeS”, a multinational observational cohort study and ESICM trials group project. Intensive Care Med. 2019;45(12):1703–17. doi: 10.1007/s00134-019-05819-3
- Hsieh Y-C, Chen H-L, Lin S-Y, et al. Short time to positivity of blood culture predicts mortality and septic shock in bacteremic patients: a systematic review and meta-analysis. BMC Infect Dis. 2022;22(1):1–18. doi: 10.1186/s12879-022-07098-8
- Tellor B, Skrupky LP, Symons W, et al. Inadequate source control and inappropriate antibiotics are key determinants of mortality in patients with intra-abdominal sepsis and associated bacteremia. Surg Infect (Larchmt). 2015;16(6):785–93. doi: 10.1089/sur.2014.166
- Gauzit R, Péan Y, Barth X, et al. Epidemiology, management, and prognosis of secondary non-postoperative peritonitis: a French prospective observational multicenter study. Surg Infect (Larchmt). 2009;10(2):119–127. doi: 10.1089/sur.2007.092
- Alqarni A, Kantor E, Grall N, et al. Clinical characteristics and prognosis of bacteraemia during postoperative intra-abdominal infections. Crit Care. 2018;22(1):1–10. doi: 10.1186/s13054-018-2099-5
- Liu Q, Wu J, Wang Z, et al. Polymicrobial bacteremia involving Klebsiella pneumoniae in patients with complicated intra-abdominal infections: frequency, co-pathogens, risk factors, and clinical outcomes. Surg Infect (Larchmt). 2019;20(4):317–25. doi: 10.1089/sur.2018.207
- Ning Y, Hu R, Yao G, et al. Time to positivity of blood culture and its prognostic value in bloodstream infection. Eur J Clin Microbiol Infect Dis. 2016;35(4):619–24. doi: 10.1007/s10096-016-2580-5
- Kumar Y, Qunibi M, Neal T, et al. Time to positivity of neonatal blood cultures. Arch Dis Childhood-Fetal Neonatal Ed. 2001;85(3):F182–F6. doi: 10.1136/fn.85.3.F182
- Raad I, Hanna HA, Alakech B, et al. Differential time to positivity: a useful method for diagnosing catheter-related bloodstream infections. Ann Internal Med. 2004;140(1):18–25. doi: 10.7326/0003-4819-140-1-200401060-00007
- Biondi EA, Mischler M, Jerardi KE, et al. Blood culture time to positivity in febrile infants with bacteremia. JAMA Pediatr. 2014;168(9):844–9. doi: 10.1001/jamapediatrics.2014.895
- Hamilton F, Evans R, Ghazal P, et al. Time to positivity in bloodstream infection is not a prognostic marker for mortality: analysis of a prospective multicentre randomized control trial. Clin Microbiol Infect. 2022;28(1):. e136. e7– e13. doi: 10.1016/j.cmi.2021.05.043
- Liao C-H, Lai C-C, Hsu M-S, et al. Correlation between time to positivity of blood cultures with clinical presentation and outcomes in patients with Klebsiella pneumoniae bacteraemia: prospective cohort study. Clin Microbiol Infect. 2009;15(12):1119–25. doi: 10.1111/j.1469-0691.2009.02720.x
- Cheng J, Zhang G, Li Q, et al. Time to positivity of Klebsiella pneumoniae in blood culture as prognostic indicator for pediatric bloodstream infections. Eur J Pediatr. 2020;179(11):1689–1698. doi: 10.1007/s00431-020-03675-8
- Silva-Nunes J, Cardoso T. Intra-abdominal infections: the role of different classifications on the selection of the best antibiotic treatment. BMC Infect Dis. 2019;19(1):1–9. doi: 10.1186/s12879-019-4604-0
- Sartelli M A focus on intra-abdominal infections. World J Emerg Surg. 2010;5(1):1–20. doi: 10.1186/1749-7922-5-9
- Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181–247. doi: 10.1007/s00134-021-06506-y
- Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):762–774.
- Van der Woude S, Van Doormaal F, Hutten B, et al. Classifying sepsis patients in the emergency department using SIRS, qSOFA or MEWS. Neth J Med. 2018;76(4):158–66.
- Al-Hasan MN, Baddour LM. Resilience of the pitt bacteremia score: 3 decades and counting. Clinical Infectious Diseases. 2020;70(9):1834–6. doi: 10.1093/cid/ciz535
- Tabah A, Koulenti D, Laupland K, et al. Characteristics and determinants of outcome of hospital-acquired bloodstream infections in intensive Care units: the EUROBACT International cohort study. Intensive care Med. 2012;38(12):1930–45. doi: 10.1007/s00134-012-2695-9
- Montravers P, Dupont H, Leone M, et al. Guidelines for management of intra-abdominal infections. Anaesth Crit Care Pain Med. 2015;34(2):117–30. doi: 10.1016/j.accpm.2015.03.005
- Sartelli M, Coccolini F, Kluger Y, et al. WSES/GAIS/SIS-E/WSIS/AAST global clinical pathways for patients with intra-abdominal infections. World J Emerg Surg. 2021;16(1):1–48. doi: 10.1186/s13017-021-00387-8
- Hecker A, Reichert M, Reuß C, et al. Intra-abdominal sepsis: new definitions and current clinical standards. Langenbecks Arch Surg. 2019;404(3):257–71. doi: 10.1007/s00423-019-01752-7
- Henderson H, Luterbach CL, Cober E, et al. The pitt bacteremia score predicts mortality in nonbacteremic infections. Clinical Infectious Diseases. 2020;70(9):1826–33. doi: 10.1093/cid/ciz528
- Lamy B. Reprint of: blood culture time-to-positivity: making use of the hidden information. Clin Microbiol Infect. 2019;25(4):399–402. doi: 10.1016/j.cmi.2019.03.005
- Mosevoll KA, Skrede S, Markussen DL, et al. Inflammatory mediator profiles differ in sepsis patients with and without bacteremia. Front Immunol. 2018;9:691. doi: 10.3389/fimmu.2018.00691