ABSTRACT
Candida albicans is an immunogen for anti-Saccharomyces cerevisiae antibodies (ASCA), a serological marker of Crohn’s disease. ASCA has also been reported in other autoimmune diseases, including coeliac disease (CeD). A strong antibody response against Hwp1, a protein associated with invasive hyphal form of C. albicans which presents peptide sequence homologies with gliadin, has also been described in CeD. This observation supports the hypothesis that C. albicans hyphal transition in C. albicans may trigger CeD onset through a mechanism of molecular/antigenic mimicry. In this study, we assessed whether the anti-C. albicans oligomannose and anti-Hwp1 protein responses may be linked despite their different pathophysiological significance. The measurement of ASCA levels in a cohort of patients involved in our previous Hwp1 study showed a significant correlation between the two biomarkers. This new observation further reinforces the link between C. albicans and CeD.
Introduction
Candida albicans, a natural commensal of the human digestive tract and vagina, is the most frequent opportunistic fungal pathogen at mucosal and systemic levels [Citation1–3]. High rates of morbidity and mortality have aroused interest in identifying the molecular mechanisms associated with saprophytic-pathogenic transition [Citation2–4]. These investigations have led to the identification of human antibodies that target C. albicans antigens that are expressed under pathogenic conditions. Those of these antibodies that have been the more investigated are paradoxically named anti-S. cerevisiae antibodies or ASCA [Citation5]. This denomination originates from translational investigations on inflammatory bowel diseases where a test, based on yeast antigens, was shown to be a strong serological marker discriminating Crohn’s disease (CD) from ulcerative colitis (UC) [Citation6]. It is only later that C. albicans was demonstrated to be an immunogen for anti-yeast oligomannose antibodies supporting the ASCA response [Citation7]. Indeed, following saprophytic-pathogenic transition C. albicans expresses the oligomannose present in S. cerevisiae mannan and makes it accessible to host immune system. As a consequence, high levels of ASCA are detected in hospital patients with invasive candidiasis -IC- [Citation8]. More recently ASCA were reported in a wide range of autoimmune diseases [Citation9–14]. including CeD [Citation15]. Ongoing research into the mutual regulation between C. albicans and the immune system in the digestive tract [Citation16–18] prompted us to investigate ASCA levels in CeD.
We previously published a serological study suggesting that a molecular mimicry between Hpw1, a protein of C. albicans invasive mycelial form and gliadin, C. albicans could be a trigger of CeD [Citation19]. Hwp1 binds covalently to human epithelial cells using human transglutaminase due to sequence similarities with human proline-rich proteins, the natural substrates of transglutaminase [Citation20]. The sequence homologies between Hwp1 and the T epitopes of the dietary gliadins, involved in CeD, led to the hypothesis that C. albicans could serve as an “adjuvant” stimulating the production of anti-gluten antibodies as well as anti-transglutaminase autoantibodies [Citation21]. This hypothesis could provide a possible explanation for the commonly observed sudden occurrence of CeD in adult patients who had previously tolerated gluten. In order to assess its validity, we conducted a study in patients with CeD, patients with invasive candidiasis (IC), and healthy subjects, comparing the serological responses against Hwp1, gliadins, tissue transglutaminase, as well as to gliadin and Hwp1 peptides deamidated or not. Our results [Citation19] were consistent with the initial hypothesis that even a subclinical infection caused by this ubiquitous opportunistic commensal organism, could trigger CeD following its yeast-to-hyphal transition [Citation22,Citation23].
In this second study we intended to analyse the relations between ASCA and anti-Hwp1 responses during CeD. The rationale was that, despite being radically different in terms of molecular targets (ASCA epitopes are glycans and anti-Hwp1 epitopes are peptide sequences) these antibody responses, reported independently in CeD, are both induced by C. albicans pathogenic phase. To reach this objective, we analysed serological responses against ASCA antigens and Hwp1 in CeD, but also in sera of patients with IC as relevant controls of an immune response associated to a C. albicans pathogenic switch.
Materials and methods
Study design and population
Sera were obtained from 87 patients with CeD, 46 patients with IC and 98 healthy controls (HC). For the second part of the study CeD patients were classified according to their adherence to GFD 1 = strict adherence to a GFD in the previous 2 months (53 patients); 2 = no strict adherence to a GFD in the previous 2 months (34 patients). All sera had previously been subjected to serological testing for the diagnosis of CeD and had been stored at −80°C. Anti-transglutaminase IgA and IgG (IgA-tTG and IgG-tTG, respectively) were detected using an ELISA kit purchased from Pharmacia Diagnostics (Freiburg, Germany). Anti-gliadin IgG and IgA (IgAG-AG) were detected using an ELISA kit (Biomedical Diagnostics, Noorderlaan, 139, 2030 Antwerpen, Belgium ASCA IgGAM antibodies were detected by ELISA using plates coated with phosphopeptidomannan from S. cerevisiae SU1, as previously described [Citation5,Citation6]. Detection of anti-Hwp1 antibodies were detected on ELISA plates sensitized with the recombinant N-terminal part of Hwp1 cloned, expressed, and purified as described previously [Citation19]. Binding of IgG was revealed using an anti-human IgG Bio-Rad peroxidase conjugate.
Statistical analysis
Continuous variables are expressed as medians [ranges]. Antibody levels were compared using analysis of variance (ANOVA) after rank transformation. Post-hoc analysis was performed using Bonferroni correction. The interrelation between anti-Hwp1 antibodies and ASCA and the association between changes in antibody titres and adherence to a gluten-free diet (GFD) were assessed using Spearman’s correlation coefficient. The Mann-Whitney U test was used for comparisons according to GFD adherence.
All statistical analyses were performed using the SAS software version 9.1 (SAS Institute Inc., Cary, NC 25,513). Statistical significance was set at p < 0.05.
Results
The distribution of anti-Hwp1 antibodies and ASCA in the three study groups is shown in . As reported previously, the antibody response against Hwp1 differed significantly between patients with IC and HC, as well as between HC and CeD patients suggesting that Hwp1 could be the same target for patients’ humoral response in IC and CeD. With regard to ASCA, identical results were observed, namely that this response as the one anti-Hwp1 did not differentiate an infectious process from an autoimmune pathology but was significantly different from that in HC.
Figure 1. Distribution of anti-Hwp1 antibodies (panel A) and ASCA (panel B) in sera from 78 patients with coeliac disease (CeD), 46 sera from 41 patients with C. albicans infection (IC) and 98 sera from healthy controls (HC). The significance of discrimination between the different groups is represented as the p value. A spearman correlation coefficient between ASCA titres and IgG anti-Hwp1 antibody titres (panel C) were calculated for raw data (black curve) and for data without outliers (red curve). Outliers points were automatically detected and eliminated by prism 10 software by applying a Q = 1%.
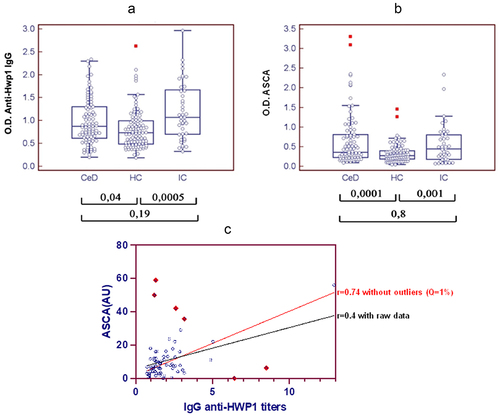
Finally, a significant correlation was shown between the two different targets of anti-C. albicans immune response, i.e Hwp1 and ASCA antigen, both in patients with invasive candidiasis and patients with coeliac disease (0.0003 and 0.01, respectively; COOR procedure, Spearman’s test). A scatter plot of Hwp1 and ASCA ELISA antibody signals illustrates this correlation ( panel C) . Although the r was not optimal for the entire population of CeD patients (0.4), the removal of outlier points, automatically detected and eliminated by Prism 10 software, led to a better correlation (r = 0.7). Noticeably, all outlier points corresponded with the rare patients with no compliance to GFD.
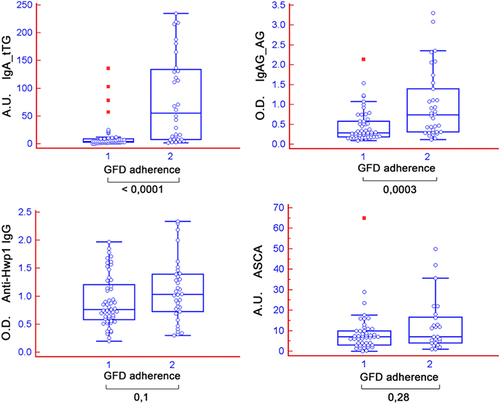
A significant decrease in anti-transglutaminase IgG (not IgA) and anti-gliadin IgA-G levels was observed in patients with good adherence to a GFD (). The decrease in anti-Hwp1 IgG was not significant because of the persistence of a subset of patients with high anti-Hwp1 antibody levels after strict GFD adherence, suggesting that different epitope-memory cells may be involved. As for Hwp1, there was a trend towards lower ASCA levels after a GFD, although this was not statistically significant.
Figure 2. Reactivity of sera from patients with coeliac disease (CeD) according to their adherence to a gluten-free diet (GFD) and sera from patients with C. albicans infection. 1= strict adherence to a GFD in the previous 2 months; 2 = no strict adherence to a GFD in the previous 2 months. IgA-tTG, IgAG-AG, anti-Hwp1 IgG, and ASCA results are expressed as optical density (450 nm), and all other results are expressed as AU.
Discussion
This brief report shows for the first time that two types of antibody responses generated by C. albicans infection, targeting a protein expressed during the pathogenic phase [Citation19] and oligomannoses expressed in the mannan and polysaccharide moieties of glycoproteins of the pathogenic phase [Citation7], are markers of IC [Citation8], but also markers of CeD [Citation24]. Of note, as native C. albicans Hwp1 is mannosylated the use of a non-glycosylated form produced in E. coli remove ambiguity about possible overlaps.
While our previous study on Hwp1 strengthened the hypothesis that C. albicans could trigger CeD through molecular mimicry with gliadin involving transglutaminase, the present observation addresses the question of the simultaneous presence of ASCA in this disease. In CeD, it is conceivable that ASCA positivity correlates with (auto) immune inflammation [Citation25]. Previous reports interpreted the presence of ASCA as a result of increased intestinal permeability secondary to an impaired small-bowel mucosa [Citation26] or as a loss of self-tolerance [Citation27]. We also noted a decrease in ASCA after a GFD. However this was not statistically significant due to the limited number of patients enrolled in the second part of our study. We also noticed that complete absence of compliance impacted more ASCA than anti-Hwp1 levels ( panel C).Previous studies actually described a decrease in ASCA IgA and IgG in larger cohorts of patients with CeD on GFD [Citation28] with a longer follow-up. Another study reporting the decrease of ASCA after GFD in CeD suggested that a microbial target could play a role in early stages [Citation29]. Interestingly, in both CD and CeD [Citation30,Citation31], ASCA were shown to correlate with antibodies against pancreatic secretory granule membrane glycoprotein 2 (GP2), the first human antigen against which an antibody response has been described in CD [Citation32]; in CeD anti-GP2 antibodies were shown to decrease after GFD, but their persistence associated with CeD refractory to GFD [Citation33]. A study showing that first degree relatives of CeD patients showed increased ASCA seroreactivity unrelated to HLA, suggested a role for other genetic and/or environmental factors [Citation34]. However, as far as a genetic basis is discussed for ASCA in CeD, there is an important difference to consider with CD where, once the disease is triggered, ASCA remain stable throughout patient’s life regardless of medical or surgical treatments [Citation24].
One of the limitation of the current study is the absence of comparison of IgG and IgA against each molecular target throughout that would have provided more analytical clues [Citation16]. Another limitation could be that the coincidence established between ASCA and Hwp1 is only related to hyphal antigens and not yeast antigens. However, it should be first mentioned that ASCA antigens are expressed in vivo on the surface of yeasts forms infecting human tissues [Citation7]. Furthermore although regulatory circuits are different between yeast and hyphal growth forms, no antigen specific for yeast form -excepted oligomannosides- could be identified so far for testing this hypothesis. Nevertheless this question of morphogenic shift influence would be interesting to address by testing antibody response against another germ tube protein antigen than Hwp1. Among these, Als3, against which human IgA antibody response is preferentially targeted to maintain C. albicans intestinal fitting, would be as strong candidate [Citation35].
In conclusion, besides being a possible trigger of CeD autoimmune processes via Hwp1, C. albicans is an ASCA immunogen in this condition. The correlation between antibodies against these two virulence antigens of C. albicans reinforces the hypothesis that C. albicans could act as a trigger and a contributor to inflammation in CeD.
Acknowledgements
We thank Val Hopwood for editing assistance, and Nadine François for serum bank management.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data Availability statement
The data that support the findings of this study are available on https://entrepot.recherche.data.gouv.fr/dataset.xhtml?persistentId=doi:10.57745/GUQ1QR
Additional information
Funding
References
- Lopes JP, Lionakis MS. Pathogenesis and virulence of Candida albicans. Virulence. 2022;13(1):89–6. doi: 10.1080/21505594.2021.2019950
- Pappas PG, Lionakis MS, Arendrup MC, et al. Invasive candidiasis. Nat Rev Dis Primers. 2018;4(1):18026. doi: 10.1038/nrdp.2018.26
- Poulain D. Candida albicans, plasticity and pathogenesis. Crit Rev Microbiol. 2015;41(2):208–217. doi: 10.3109/1040841X.2013.813904
- Brown AJ, Brown GD, Netea MG, et al. Metabolism impacts upon Candida immunogenicity and pathogenicity at multiple levels. Trends Microbiol. 2014;22(11):614–622. doi: 10.1016/j.tim.2014.07.001
- Sendid B, Colombel JF, Jacquinot PM, et al. Specific antibody response to oligomannosidic epitopes in Crohn’s disease, Clin Diagn Lab Immunol. Clin Diagn Lab Immunol. 1996;3(2):219–226. doi: 10.1128/cdli.3.2.219-226.1996
- Quinton JF, Sendid B, Reumaux D, et al. Anti-Saccharomyces cerevisiae mannan antibodies combined with antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease: prevalence and diagnostic role. Gut. 1998;42(6):788–791. doi: 10.1136/gut.42.6.788
- Standaert-Vitse A, Jouault T, Vandewalle P, et al. Candida albicans is an Immunogen for anti–Saccharomyces cerevisiae antibody markers of Crohn’s disease. Gastroenterology. 2006;130(6):1764–1775. doi: 10.1053/j.gastro.2006.02.009
- Sendid B, Dotan N, Nseir S, et al. Antibodies against Glucan, chitin, and Saccharomyces cerevisiae Mannan as new biomarkers of Candida albicans infection that complement tests based on C. albicans Mannan. Albicans Mannan, Clin Vaccine Immunol. 2008;15(12):1868–1877. doi: 10.1128/CVI.00200-08
- Alunno A, Bistoni O, Carubbi F, et al. Prevalence and significance of anti-Saccharomyces cerevisiae antibodies in primary Sjogren’s syndrome. Clin Exp Rheumatol. 2018;36(Suppl 112):73–79.
- Assan F, Gottlieb J, Tubach F, et al. Anti-Saccharomyces cerevisiae IgG and IgA antibodies are associated with systemic inflammation and advanced disease in hidradenitis suppurativa. J Allergy Clin Immunol. 2020;146(2):452–455 e5. doi: 10.1016/j.jaci.2020.01.045
- Kvehaugen AS, Aasbrenn M, Farup PG. Anti-Saccharomyces cerevisiae antibodies (ASCA) are associated with body fat mass and systemic inflammation, but not with dietary yeast consumption: a cross-sectional study. BMC Obes. 2017;4(1):28. doi: 10.1186/s40608-017-0164-2
- Lang S, Duan Y, Liu J, et al. Intestinal fungal dysbiosis and systemic immune response to fungi in patients with alcoholic hepatitis. Hepatology. 2020;71:522–538. doi: 10.1002/hep.30832
- Maillet J, Ottaviani S, Tubach F, et al. Anti-Saccharomyces cerevisiae antibodies (ASCA) in spondyloarthritis: prevalence and associated phenotype. Joint Bone Spine. 2016;83(6):665–668. doi: 10.1016/j.jbspin.2015.10.011
- Mankai A, Sakly W, Thabet Y, et al. Anti-Saccharomyces cerevisiae antibodies in patients with systemic lupus erythematosus. Rheumatol Int. 2013;33(3):665–669. doi: 10.1007/s00296-012-2431-3
- Kotze LM, Nisihara RM, Utiyama SR, et al. Antibodies anti-Saccharomyces cerevisiae (ASCA) do not differentiate Crohn’s disease from celiac disease. Arq Gastroenterol. 2010;47:242–245. doi: 10.1590/S0004-28032010000300006
- Doron I, Mesko M, Li XV, et al. Mycobiota-induced IgA antibodies regulate fungal commensalism in the gut and are dysregulated in Crohn’s disease. Nat Microbiol. 2021;6(12):1493–1504. doi: 10.1038/s41564-021-00983-z
- Leonardi I, Gao IH, Lin WY, et al. Mucosal fungi promote gut barrier function and social behavior via type 17 immunity. Cell. 2022;185(5):831–846 e14. doi: 10.1016/j.cell.2022.01.017
- Li XV, Leonardi I, Putzel GG, et al. Immune regulation by fungal strain diversity in inflammatory bowel disease. Nature. 2022;603(7902):672–678. doi: 10.1038/s41586-022-04502-w
- Corouge M, Loridant S, Fradin C, et al. Humoral immunity links Candida albicans infection and celiac disease. PLoS One. 2015;10(3):e0121776. doi: 10.1371/journal.pone.0121776
- Staab JF, Bradway SD, Fidel PL, et al. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science. 1999;283(5407):1535–1538. doi: 10.1126/science.283.5407.1535
- Nieuwenhuizen WF, Pieters RH, Knippels LM, et al. Is Candida albicans a trigger in the onset of coeliac disease? Lancet. 2003;361(9375):2152–2154. doi: 10.1016/S0140-6736(03)13695-1
- Aaron L, Torsten M. Candida albicans in celiac disease: a wolf in sheep’s clothing. Autoimmun Rev. 2020;19(9):102621. doi: 10.1016/j.autrev.2020.102621
- Renga G, Bellet MM, Stincardini C, et al. To Be or not to Be a pathogen. Candida albicans And Celiac Disease, Front Immunol. 2019;10:2844. doi: 10.3389/fimmu.2019.02844
- Poulain D, Sendid B, Standaert-Vitse A, et al. Yeasts: neglected pathogens. Dig Dis. 2009;27(Suppl 1):104–110. doi: 10.1159/000268129
- Barta Z. Seroreactivity against Saccharomyces cerevisiae in patients with Crohn’s disease and celiac disease. World J Gastroenterol. 2003;9(10):2308–2312. doi: 10.3748/wjg.v9.i10.2308
- Papp M, Foldi I, Altorjay I, et al. Anti-microbial antibodies in celiac disease: trick or treat? World J Gastroenterol. 2009;15(31):3891–3900. doi: 10.3748/wjg.15.3891
- Wolters VM, Alizadeh BZ, Weijerman ME, et al. Intestinal barrier gene variants may not explain the increased levels of antigliadin antibodies, suggesting other mechanisms than altered permeability. Hum Immunol. 2010;71(4):392–396. doi: 10.1016/j.humimm.2010.01.016
- Ashorn S, Valineva T, Kaukinen K, et al. Serological responses to microbial antigens in celiac disease patients during a gluten-free diet. J Clin Immunol. 2009;29(2):190–195. doi: 10.1007/s10875-008-9255-7
- Viitasalo L, Niemi L, Ashorn M, et al. Early microbial markers of celiac disease. J Clin Gastroenterol. 2014;48(7):620–624. doi: 10.1097/MCG.0000000000000089
- Roggenbuck D, Goihl A, Sowa M, et al. Human glycoprotein-2 expressed in Brunner glands - a putative autoimmune target and link between Crohn’s and coeliac disease. Clin Immunol. 2023;247:109214. doi: 10.1016/j.clim.2022.109214
- Laass MW, Rober N, Range U, et al. Loss and gain of tolerance to pancreatic glycoprotein 2 in Celiac disease. PLoS One. 2015;10(6):e0128104. doi: 10.1371/journal.pone.0128104
- Bonneau J, Dumestre-Perard C, Rinaudo-Gaujous M, et al. Systematic review: new serological markers (anti-glycan, anti-GP2, anti-GM-CSF ab) in the prediction of IBD patient outcomes. Autoimmun Rev. 2015;14(3):231–245. doi: 10.1016/j.autrev.2014.11.004
- Gross S, Bakker SF, van Bodegraven AA, et al. Increased IgA glycoprotein-2 specific antibody titres in refractory celiac disease. J Gastrointestin Liver Dis. 2014;23(2):127–133. doi: 10.15403/jgld.2014.1121.232.sg1
- Viitasalo L, Iltanen S, Huhtala H, et al. First-degree relatives of celiac disease patients have increased seroreactivity to serum microbial markers. Nutrients. 2020;12(4):1073. doi: 10.3390/nu12041073
- Ost KS, O’Meara TR, Stephens WZ, et al. Adaptive immunity induces mutualism between commensal eukaryotes. Nature. 2021;596(7870):114–118. doi: 10.1038/s41586-021-03722-w
