ABSTRACT
Objectives
Catheter-associated urinary tract infections (CAUTI) are a significant cause of morbidity and financial burden to healthcare systems. The aim of this study was to develop and implement a program designed to reduce Foley catheter use days and associated CAUTI rates.
Methods
A retrospective evaluation of a multi-modal program aimed toward reducing Foley catheter use days and associated CAUTI rates across the Indiana University Health (IUH) system was conducted. IUH is comprised of 16 hospitals and 2,703 available beds. The program included the adoption of new technologies with evidence-based clinical training materials based on change management and feedback from clinicians. National Healthcare Safety Network Standardized Infection Ratio (SIR), Standardized Utilization Ratio (SUR), and Incidence Density Ratio (IDR) rates were assessed.
Results
Implementation of the multi-modal program resulted in a significant reduction in SIR, SUR, and IDR in the post-implementation compared to the baseline periods.
Conclusions
Through CAUTI bundle workflow assessments, education, and interventions designed to standardize practice variation, the program was successfully implemented and resulted in a significant decrease in Foley Catheter use days, and associated CAUTI rates.
Introduction
Catheter-associated urinary tract infection (CAUTI) is a mandated quality metric in the United States (US) required for CMS’ (Center for Medicare and Medicaid Services) conditions of participation and thus subject to reimbursement fines in addition to the morbidity and patient safety issues inherent in hospital-acquired infections (HAI) [Citation1]. CAUTIs are thought to be preventable or partially preventable predicated on patient immune status and other comorbid conditions [Citation2,Citation3]. Given the combined patient care and regulatory requirements surrounding CAUTI, quality improvement strategies that help augment CAUTI prevention bundles recommended by the CDC often include multi-model strategies to mitigate CAUTI rates in hospitalized patients [Citation3,Citation4].
Indiana University Health (IUH) and Becton Dickinson (BD) collaborated to co-create CAUTI prevention practices based on change management theory that included assessments of clinical practice associated with indwelling Foley catheter use, training on best practices, and monitoring progress through data provided by an automated surveillance tool. In this report, we detail a multi-modal program that was initiated to reduce Foley use days and associated CAUTI rates in a healthcare setting.
Materials and methods
Setting
A retrospective evaluation of a program aimed toward reducing Foley catheter use days and associated CAUTI rates across the IUH system was conducted. IUH is the largest nonprofit academic medical center in Indiana, comprised of 16 hospitals and 2,703 available beds.
Data was retrieved retrospectively through the BD Insights database previously published on [Citation5,Citation6]. The study used a limited retrospective data set for an epidemiology study and thus was approved and exempted from consent by the New England Institutional Review Board/WCG and Human Subjects Research Committee (Wellesley, MA). The study was conducted in compliance with Health Insurance Portability and Accountability Act requirements.
Multi-modal program
The design included the creation of a multi-modal program that integrated practice assessments, education, and interventions designed to standardize practice variation with the unified goal to reduce Foley catheter use days and associated CAUTI rates. The program was designed based on a review of national guidelines to outline the best evidence-based practices for CAUTI prevention. Clinicians observed and evaluated current clinical practices related to CAUTI prevention then identified gaps in the continuum of care and opportunities for improvement. More specifically, clinical improvement opportunities identified were aimed at training and education for hospital staff on the following: (1) proper specimen management including preparation of the sample port and the order of specimen sampling and (2) adoption of urine specimen collection technology enabling urine specimen collection using a luer lock activated device (LLAD) directly from a Foley catheter to a collection tube with preservative (SurestepTM, BD, Franklin Lakes, NJ). The intervention implementation was based on clinical staff readiness evaluated using surveys. In addition, feedback was obtained from nursing staff on the new training process using QR codes on educational posters. More details about the intervention are in .
Figure 1. Comprehensive CAUTI prevention design. best practices were followed as described previously [Citation7–10]. CAUTI, catheter-associated urinary tract infection; IUH, Indiana University Hospital; IP, infection preventionist; BD, Becton Dickinson and company; HAI, hospital-associated infection; HSAI, BD healthsight infection advisor and MedMined Insights; LLAD, luer lock activated device.
![Figure 1. Comprehensive CAUTI prevention design. best practices were followed as described previously [Citation7–10]. CAUTI, catheter-associated urinary tract infection; IUH, Indiana University Hospital; IP, infection preventionist; BD, Becton Dickinson and company; HAI, hospital-associated infection; HSAI, BD healthsight infection advisor and MedMined Insights; LLAD, luer lock activated device.](/cms/asset/81e1f9cc-4709-4715-9a39-7a942b7962a0/ihop_a_2335099_f0001_b.gif)
Data from an automated surveillance system, BD HealthSight™ Infection Advisor with MedMined™ Insights (HSIA, BD, Franklin Lakes, NJ), was used throughout the study. The tool identified patients with a likely CAUTI, patient location, and date. Additionally, the tool supported ongoing identification of patients at high-risk for CAUTI allowing clinicians to target the highest risk units for CAUTI prevention efforts.
Measurements
To evaluate potential impact of the program on CAUTI rates, the NHSN Standardized Infection Ratio (SIR) [Citation11], Standardized Utilization Ratio (SUR) [Citation12], and the Incidence Density Rates (IDR) were calculated comparing data from the baseline and post-implementation periods.
Inputs for the SIR, SUR, and IDR were quantified during the 12-month baseline period (April 2020-March 2021) prior to the program implementation (April 2021), and the 12-month period (May 2021-April 2022) following the intervention implementation period using a combination of the automated surveillance system and trained infection preventionists utilizing the CAUTI NHSN protocol [Citation13].
Statistical analysis
The SIR, SUR, and IDR were quantified using the NHSN Statistical Calculator [Citation14].
Results
A total of 176 CAUTI were observed during the study period (105 at baseline and 71 post-implementation). The SIR at baseline showed a non-significant numerical reduction in CAUTI events relative to expected (SIR: 0.84, 95% CI: 0.69, 1.01; p = 0.06). Statistical significance was observed in the post-implementation period (SIR: 0.52, 95% CI: 0.41, 0.65; p < 0.0001). A statistical reduction in CAUTI post-implementation compared to baseline was observed (relative ratio of SIR: 0.62, 95% CI: 0.46, 0.84; p = 0.002) ().
Figure 2. Multi-modal intervention implementation effects on CAUTI. A. CAUTI count, group SIR, and relative risk ratio of SIR by baseline and post-implementation periods. B. CAUTI count, group SUR, and relative risk ratio of SUR by baseline and post-implementation periods. Data generated from the NHSN for the statistical calculator.
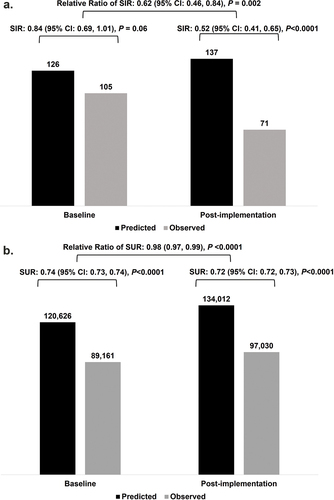
During both the baseline and post-implementation periods, the observed device days (i.e. total days a Foley catheter was in place) were significantly lower relative to the expected device days (respectively, SUR: 0.74, 95% CI: 0.73, 0.74, p < 0.0001; SUR: 0.72, 95% CI: 0.72, 0.73, p < 0.0001). The SUR was significantly lower during the post-implementation period compared to the baseline period (relative risk of SUR: 0.98, 95% CI: 0.97, 0.99, p < 0.001).
The CAUTI IDR was 1.15 per 1,000 device days during the baseline and 0.71 per 1,000 device days during the post-implementation. The IDR was significantly lower in the post-implementation period compared to baseline (p = 0.002).
For inpatients with a COVID test between 14 days prior to the admission start date and through to the discharge date, the positive COVID rate increased significantly (p < 0.0001) from 8.1 per 100 admissions (6,704/82,583) in the baseline period to 10.1 per 100 admissions (8,504/84,353) in the post-intervention period.
Discussion
In this collaboration, a multi-modal program based on education and change management workflow on specimen collection practices (including preparation of the sample port and the order of specimen sampling and adoption of urine specimen collection technology enabling urine specimen collection using a luer lock activated device (LLAD) directly from a Foley catheter to a collection tube with preservative SurestepTM, BD, Franklin Lakes, NJ) – in addition to an analytic software that provided visibility to units with higher CAUTI rates and high risk of CAUTI cases in near real time – in totality was associated with reduction in Foley catheter use days and reduction of CAUTI SIR pre and post-program implementation. The results were consistent across three different NHSN supported metrics: the SIR, SUR, and IDR. This collaboration demonstrates that the adoption of education which highlights CAUTI prevention best practices, specific urine specimen workflow and technology changes, and highlighting hospital units with CAUTI issues over time was associated with a reduction in mandated reportable CAUTI metrics.
The reduction in CAUTI during this time period is especially notable as CAUTI and other HAI rates generally increased across other hospitals in the US during the COVID-19 pandemic [Citation15]. It has been hypothesized that the burden of HAIs increased during the pandemic due to disruptions in routine infection prevention processes, workforce challenges, gaps in continuums of care, and the increased burden of caring for COVID patients. Despite these challenges on healthcare delivery systems, the findings reported here highlight the utility and effectiveness of multi-modal CAUTI prevention practices to reduce the risk of CAUTI as measured by the NHSN. During the analysis period, positive COVID test rates increased among inpatients, indicating that the reduction in CAUTI metrics occurred despite increased COVID rates.
The current study found that CAUTI risk in the post-implementation period was lower than the baseline period using the NHSN’s SIR, SUR, and IDR. In particular, the SUR was lower than expected during the baseline and post-implementation periods. While this could be partially explained by supply chain shortages limiting the availability of Foley catheters during the COVID-19 pandemic, our findings highlight the importance of the aggregate totality of peer education, CAUTI bundle reinforcement, and that new technology roll out for urine specimen collection can be successfully accomplished during surge capacity situations, and in particular during one that on a national level increased CAUTI rates.
An additional unexpected benefit of the collaboration between IUH and BD was an update in the Lippincott Procedures, a widely used comprehensive procedure manual for practicing nurses [Citation7]. IUH submitted a recommendation advocating for obtaining specimen from Foley catheters using a preservative in urine culture tubes and a luer lock activated device (LLAD) to minimize the risk of urine culture contamination. The recommendation was accepted and incorporated into the workflow.
Limitations for this study include the COVID-19 pandemic surge during the analysis period; however, as we point out, gains in CAUTI metrics were achieved despite the COVID cases increasing in prevalence throughout the analyzed period. Although evidence by the U.S. CDC shows the COVID pandemic was associated with higher CLABSI and CAUTI rates on an aggregate national level, there have been recent reports primarily outside the U.S. that in certain populations reduction of CAUTI risks were reported, especially in non-critical acute care patients [Citation16]. Recent literature in the US reported that the majority of CAUTI cases occurred in ICU patients, and may explain the discrepancy [Citation17]. The issue of supply chain shortages on Foley catheter availability was a ubiquitous problem throughout the nation, and while this was also a potential factor that may have influenced the SUR in particular, the fact remains that nationally CAUTI rates increased during the pandemic while they decreased in this dataset. The generalizability of our results during different circumstances is not fully known.
Conclusions
Through Foley catheter care workflow assessments, education, and interventions designed to standardize practice variation, this multi-modal program was associated with a reduction in Foley catheter days and the associated CAUTI NHSN metrics, including a reduction of post implementation CAUTI SIR compared to preprogram baseline rates.
List of Abbreviations
| Word | = | Acronym |
| Catheter Associated Urinary Tract Infection | = | CAUTI |
| Indiana University Health | = | IUH |
| Standardized Infection Ratio | = | SIR |
| Standardized Utilization Ratio | = | SUR |
| Incidence Density Ratio | = | IDR |
| Hospital-Associated Infections | = | HAI |
| National Healthcare Safety Network | = | NHSN |
| Becton Dickinson | = | BD |
| Luer Lock Activated Device | = | LLAD |
| Healthsight™ Infection Advisor | = | HSIA |
Declaration of financial/other relationships
MJ and KY are employees and owners of stock in Becton Dickinson and Company. All authors disclose research support provided by Becton Dickinson and Company. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Authors’ contributions
All authors have contributed to the study design, statistical analysis, interpretation and writing of the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The study used a limited retrospective data set for an epidemiology study and thus was approved and exempted from consent by the New England Institutional Review Board/Human Subjects Research Committee (Wellesley, MA). The study was conducted in compliance with Health Insurance Portability and Accountability Act requirements.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We thank CBCC and Stephanie E. Tedford, of Pharmacologics, Inc, who, on the behalf of BD provided medical writing support.
Additional information
Funding
References
- Klevens RM, Edwards JR, Richards CL, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122(2):160–166. doi: 10.1177/003335490712200205
- Umscheid CA, Mitchell MD, Doshi JA, et al. Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect Control Hosp Epidemiol. 2011;32(2):101–114. doi: 10.1086/657912
- Gould CV, Umscheid CA, Agarwal RK, et al. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319–326. doi: 10.1086/651091
- Meddings J, Rogers M, Macy M, et al. Systematic review and meta-analysis: reminder systems to reduce catheter-associated urinary tract infections and urinary catheter use in hospitalized patients. Clinical Infectious Diseases. 2010;51(5):550–560. doi: 10.1086/655133
- Kaye KS, Gupta V, Mulgirigama A, et al. Antimicrobial resistance trends in urine Escherichia coli isolates from adult and adolescent females in the United States from 2011 to 2019: rising ESBL strains and impact on patient management. Clin Infect Dis. 2021;73(11):1992–1999. doi: 10.1093/cid/ciab560
- Yu KC, Ye G, Edwards JR, et al. Treated, hospital-onset clostridiodes difficile infection: an evaluation of predictors and feasibility of benchmarking comparing 2 risk-adjusted models among 265 hospitals. Infect Control Hosp Epidemiol: 2023;45(1): 1–9. doi: 10.1017/ice.2023.124
- Lippincott Procedures for Nurses. Urine specimen collection from an indwelling urinary catheter (Foley). 2021. [Accessed 2024 Mar 24]. https://www.wolterskluwer.com/en/solutions/lippincott-solutions/lippincott-procedures
- Garcia R, Spitzer ED. Promoting appropriate urine culture management to improve health care outcomes and the accuracy of catheter-associated urinary tract infections. Am J Infect Control. 2017;45(10):1143–1153. doi: 10.1016/j.ajic.2017.03.006
- Frontera JA, Wang E, Phillips M, et al. Protocolized urine sampling is associated with reduced catheter-associated urinary tract infections: a pre- and postintervention study. Clin Infect Dis. 2021;73(9):e2690–e2696. doi: 10.1093/cid/ciaa1152
- Dolan VJ, Cornish NE. Urine specimen collection: how a multidisciplinary team improved patient outcomes using best practices. Urol Nurs. 2013;33(5):249–256. doi: 10.7257/1053-816X.2013.33.5.249
- Centers for Disease Control and Prevention. The NHSN standardized infection ratio (SIR); aguide to the SIR. 2024. [Accessed 2024 Mar 24]. https://www.cdc.gov/nhsn/pdfs/ps-analysis-resources/nhsn-sir-guide.pdf
- Centers for Disease Control and Prevention. The NHSN standardized utilization ratio (SUR): aguide to the SUR. 2024. [Accessed 2024 Mar 24]. https://www.cdc.gov/nhsn/pdfs/ps-analysis-resources/nhsn-sur-guide-508.pdf
- National Healthcare Safety Network. Urinary tract infection (Catheter-Associated urinary tract infection [CAUTI] and non-catheter-associated urinary tract infection [UTI]) events. 2024. [Accessed 2024 Mar 24]. https://www.cdc.gov/nhsn/pdfs/pscmanual/7psccauticurrent.pdf
- National Healthcare Safety Network. Patient Safety Component: Calculators And Worksheets. [cited 2023 Apr]. Available from: https://www.cdc.gov/nhsn/psc/calculators.html
- Baker MA, Sands KE, Huang SS, et al. The impact of coronavirus disease 2019 (COVID-19) on healthcare-associated infections. Clinical Infectious Diseases. 2022;74(10):1748–1754. doi: 10.1093/cid/ciab688
- Garcell HG, Al-Ajmi J, Arias AV, et al. Catheter-associated urinary tract infection and urinary catheter utilization ratio over 9 years, and the impact of the COVID-19 pandemic on the incidence of infection in medical and surgical wards in a single facility in Western Qatar. Qatar Med J. 2023;2023(1):14. Published May 2. doi: 10.5339/qmj.2023.14
- Kelly T, Ai, C, Jung, M, et al. Catheter-associated urinary tract infections (CAUTIs) and non-CAUTI hospital-onset urinary tract infections: relative burden, cost, outcomes and related hospital-onset bacteremia and fungemia infections. Infect Control Hosp Epidemiol. 2024:1–8. doi: 10.1017/ice.2024.26

