ABSTRACT
Rab5 and Rab7 GTPases are key regulators of endosome maturation and lysosome fusion. They activate the class III phosphoinositide 3-kinase (PI3K) Vps34 to generate pools of phosphatidylinositol-3 phosphate [PI(3)P] on endosomes. Together PI(3)P and the GTP-bound Rabs coordinate the recruitment of endosomal regulators to drive early to late endosome maturation and ultimately lysosome fusion. Counterintuitively, loss of Vps34 results in enlarged endosomes, like those seen from expressing activated Rab GTPases. Two recent papers in the Journal of Cell Science, Jaber et al., 2016 and Law, Seo et al., 2017, demonstrate that a function of Vps34 is to inactive the Rab5 and Rab7 GTPases via recruitment of the TBC1D2 family of Rab GTPase Activating Proteins (GAPs).
The Rab5 and Rab7 GTPases are important regulators of early and late endosome trafficking of cargo to the lysosome for degradation ().Citation1 Like other small GTPases, Rab GTPases cycle between a GTP-bound active state and a GDP-bound inactive state, both of which are regulated by Rab Guanine nucleotide Exchange Factors (GEFs) and Rab GTPase Activating Proteins (GAPs), respectively. In the GTP-bound state, Rab5 and Rab7 localize to early and late endosomes, respectively, where they can recruit effector proteins. Both Rab5 and Rab7 activate the Vps34 PI3K to generate PI(3)P on endosomes.Citation2–6 Many effectors bind to PI(3)P via PX (Phox) and FYVE (Fab1, YOTB, Vac1, EEA1) domains to coordinate aspects of endosome maturation and trafficking such as vesicle fusion.Citation7–14 While Vps34 and the Rab GTPases cooperate to regulate endosome trafficking, loss of Vps34 results in enlarged endosomes,Citation15,16 a phenotype that is also observed from increased Rab5 activity.Citation17 These observations suggest a potential negative feedback loop for Rab5 and Rab7 GTPases.
Figure 1. Model of mammalian Armus and C. elegans TBC-2 function and regulation during early to late endosome maturation.
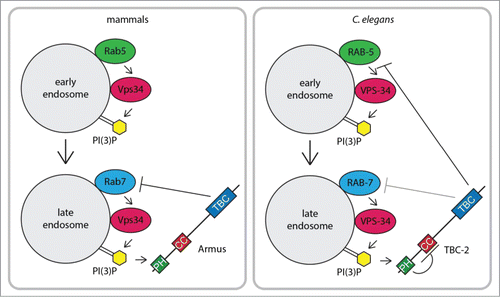
Jaber et al. (2016) and Law, Seo et al. (2017) provide evidence that specific loss of Vps34 results in increased Rab GTPase activity.Citation18,19 The Zong lab generated Vps34 knockout mouse embryonic fibroblasts (MEFs) that accumulate enlarged Rab7 positive endosomes.Citation15 They found that Vps34-null MEFs have increased Rab5 and Rab7 activities using effector pull-down assays.Citation18,19 In the nematode Caenorhabditis elegans, loss of vps-34, or its complex components vps-15 and bec-1, led to enlarged RAB-5 and RAB-7 positive endosomes full of undegraded material.Citation18,20 This phenotype had only previously been reported in animals either expressing constitutively activated RAB-5 Q78L or carrying a loss-of-function mutation in tbc-2, a gene which encodes for a RAB-5 GAP.Citation21 Together, these findings in mouse cells and C. elegans indicate that the Vps34 PI3K has a role in Rab GTPase inactivation.
The key to this novel negative feedback loop is the TBC1D2 family of Rab GAPs, whose members include the C. elegans TBC-2 and its mammalian homolog Armus (aka TBC1D2A and PARIS-1).Citation21–24 This protein family is characterized by an N-terminal Pleckstrin Homology (PH) domain, a central coiled-coil (CC) domain and a C-terminal Tre-2/Bub2/Cdc16 (TBC)/Rab GAP domain (). In vitro, TBC-2 catalyzed GTP hydrolysis of RAB-5 and to a lesser extent RAB-7.Citation21 Membrane fractionation studies from C. elegans extracts confirmed that tbc-2 mutant animals have increased Rab5 activity as compared to wild-type.Citation25 Genetic and phenotypic analyses are consistent with TBC-2 functioning as a RAB-5 GAP in both the recycling and degradative pathways, but are not inconsistent with it also regulating RAB-7.Citation21,24–28 Armus had Rab7 GAP activity in vitro,Citation22 and overexpression of Armus in MEFs resulted in decreased Rab7 activity.Citation29 Armus may have additional functions earlier in the endosomal pathway, including a role in endosome recycling.Citation22,Citation30 TBC-2 and Armus localized to late endosomes where they could regulate Rab7 activity or, in the case of TBC-2, inactivate Rab5 to promote Rab5 to Rab7 conversion during endosome maturation ().Citation19,21
How are TBC-2 and Armus recruited onto endosomes? PH domains can bind phosphoinositidesCitation31; therefore, TBC-2 and Armus were obvious candidates for regulation by Vps34. Consistent with this hypothesis, knockdown of Vps34 resulted in a loss of TBC-2 and Armus localization to endosomes.Citation18,19 While PH domains do not typically bind PI(3)PCitation31, both Jaber et al (2016) and Law, Seo et al. (2017) independently found that the PH domains of TBC-2 and Armus preferentially bound to PI(3)P and PI(4)P (phosphatidylinositol-4 phosphate) using protein-lipid overlay assays.Citation18,19 In collaboration with the laboratory of Guangwei Du (University of Texas Health Science Center at Houston), they demonstrated that the PH domains of both TBC-2 and Armus preferentially bound liposomes containing PI(3)P or PI(4)P over other acidic phospholipids such as phosphatidic acid.Citation18,19 Together, these data suggest a direct role for Vps34-generated PI(3)P in recruiting C. elegans TBC-2 and mammalian Armus to endosomes as part of a negative feedback loop to inactivate Rab5 and Rab7, respectively ().
The plot thickens as the PH domain of TBC-2 is not required for localization to endosomes or phagosomes.Citation18,24 In fact, we found that deletion of the PH domain, TBC-2(ΔPH), increased TBC-2 localization to endosomes and rescued the tbc-2 mutant intestinal phenotype.Citation18 However, further deletion of the CC domain region abrogated TBC-2 localization to endosomes and did not rescue the tbc-2 mutant intestinal phenotype. These data suggest that (1) the PH domain antagonizes TBC-2 recruitment to endosomes and (2) binding of PI(3)P to the PH domain permits the CC domain region to interact with proteins and/or lipids on the endosome membrane. Interestingly, expression of Armus(ΔPH) in MEFs was more potent than full-length Armus for decreasing Rab7 activityCitation29, although we do not know if it is due to increased endosome localization as seen with TBC-2. However, the PH domain of Armus was shown to bind Armus(ΔPH), suggesting that Armus localization and/or activity is regulated by an intramolecular interaction.Citation29 We hypothesize that PI(3)P binding by the PH domain could relieve an autoinhibitory intramolecular interaction and permit TBC-2/Armus localization to endosomes.
In a second plot twist, we discovered that TBC-2(ΔPH) still required Vps34 for endosome localization.Citation18 Therefore, Vps34 appears to have dual roles in regulating the recruitment of TBC-2 to endosomes. An outstanding question remains on what mechanism regulates TBC-2 recruitment through the region containing the CC domain. This could be through a second cryptic PI(3)P binding site, or through mediating an interaction with an endosomal protein that is itself regulated by PI(3)P or its derivative, PI(3,5)P2. Alternatively, TBC-2 could directly interact with the Vps34 class III PI3K complex in a PI(3)P-independent manner.
In summary, Jaber et al. (2016) and Law, Seo et al. (2017) uncovered a negative feedback loop whereby activation of the class III PI3K Vps34 by the Rab5 and Rab7 GTPases leads to endosomal recruitment of the Armus and TBC-2 Rab GAPs in mammalian cells and C. elegans.Citation18,19 These findings echo that of a previous study, which found that non-selective PI3Ks inhibitors (Wortmannin or LY294002) increased Rab5 activity on phagosomes of hamster and murine cell lines.Citation32 Vieira et al. (2003) proposed that PI3Ks could mediate the activation and/or recruitment of a Rab5 GAP to phagosomes. We do not know if Armus and/or its paralog, TBC1D2B, have Rab5 GAP activity. If so, they could be responsible for those findings. There are still many outstanding questions to be answered. Does Vps34 dually regulate Armus and TBC1D2B as it does for TBC-2? What are the factors that recruit TBC-2 to late endosomes? Identifying the additional regulator(s) of TBC-2/Armus recruitment to endosomes should help answer some of these questions.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Wei-Xing Zong and Guangwei Du for comments on the manuscript.
Funding
Our work is supported by a Canadian Institutes of Health Research operating grant (MOP-114935) and a Natural Sciences and Engineering Research Council of Canada discovery grant (RGPIN-341579-13) to C.E.R.
References
- Wandinger-Ness A, Zerial M. Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb Perspect Biol. 2014;6(11):a022616. doi:10.1101/cshperspect.a022616. PMID:25341920
- Murray JT, Panaretou C, Stenmark H, Miaczynska M, Backer JM. Role of Rab5 in the recruitment of hVps34/p150 to the early endosome. Traffic. 2002;3(6):416–27. doi:10.1034/j.1600-0854.2002.30605.x.
- Stein MP, Feng Y, Cooper KL, Welford AM, Wandinger-Ness A. Human VPS34 and p150 are Rab7 interacting partners. Traffic. 2003;4(11):754–71. doi:10.1034/j.1600-0854.2003.00133.x.
- Christoforidis S, Miaczynska M, Ashman K, Wilm M, Zhao L, Yip SC, Waterfield MD, Backer JM, Zerial M. Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nat Cell Biol. 1999;1(4):249–52. doi:10.1038/12075.
- Schu PV, Takegawa K, Fry MJ, Stack JH, Waterfield MD, Emr SD. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science. 1993;260(5104):88–91. doi:10.1126/science.8385367.
- Volinia S, Dhand R, Vanhaesebroeck B, MacDougall LK, Stein R, Zvelebil MJ, Domin J, Panaretou C, Waterfield MD. A human phosphatidylinositol 3-kinase complex related to the yeast Vps34p-Vps15p protein sorting system. EMBO J. 1995;14(14):3339–48.
- Burd CG,Emr SD, Phosphatidylinositol(3)-phosphate signaling mediated by specific binding to RING FYVE domains. Mol Cell. 1998;2(1):157–62. doi:10.1016/S1097-2765(00)80125-2.
- Lawe DC, Chawla A, Merithew E, Dumas J, Carrington W, Fogarty K, Lifshitz L, Tuft R, Lambright D, Corvera S. Sequential roles for phosphatidylinositol 3-phosphate and Rab5 in tethering and fusion of early endosomes via their interaction with EEA1. J Biol Chem. 2002;277(10):8611–7. doi:10.1074/jbc.M109239200.
- Patki V, Lawe DC, Corvera S, Virbasius JV, Chawla A. A functional PtdIns(3)P-binding motif. Nature. 1998;394(6692):433–4. doi:10.1038/28771.
- Cheever ML, Sato TK, de Beer T, Kutateladze TG, Emr SD, Overduin M. Phox domain interaction with PtdIns(3)P targets the Vam7 t-SNARE to vacuole membranes. Nat Cell Biol. 2001;3(7):613–8. doi:10.1038/35083000.
- Gaullier JM, Simonsen A, D'Arrigo A, Bremnes B, Stenmark H, Aasland R. FYVE fingers bind PtdIns(3)P. Nature. 1998;394(6692):432–3. doi:10.1038/28767.
- Song X, Xu W, Zhang A, Huang G, Liang X, Virbasius JV, Czech MP, Zhou GW. Phox homology domains specifically bind phosphatidylinositol phosphates. Biochemistry. 2001;40(30):8940–4. doi:10.1021/bi0155100.
- Ellson CD, Gobert-Gosse S, Anderson KE, Davidson K, Erdjument-Bromage H, Tempst P, Thuring JW, Cooper MA, Lim ZY, Holmes AB, et al. PtdIns(3)P regulates the neutrophil oxidase complex by binding to the PX domain of p40(phox). Nat Cell Biol. 2001;3(7):679–82. doi:10.1038/35083076.
- Kanai F, Liu H, Field SJ, Akbary H, Matsuo T, Brown GE, Cantley LC, Yaffe MB. The PX domains of p47phox and p40phox bind to lipid products of PI(3)K. Nat Cell Biol. 2001;3(7):675–8. doi:10.1038/35083070.
- Jaber N, Dou Z, Chen JS, Catanzaro J, Jiang YP, Ballou LM, Selinger E, Ouyang X, Lin RZ, Zhang J, et al. Class III PI3K Vps34 plays an essential role in autophagy and in heart and liver function. Proc Natl Acad Sci U S A. 2012;109(6):2003–8. doi:10.1073/pnas.1112848109.
- Johnson EE, Overmeyer JH, Gunning WT, Maltese WA. Gene silencing reveals a specific function of hVps34 phosphatidylinositol 3-kinase in late versus early endosomes. J Cell Sci. 2006;119(Pt 7):1219–32.
- Stenmark H, Parton RG, Steele-Mortimer O, Lütcke A, Gruenberg J, Zerial M. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. Embo J. 1994;13(6):1287–96.
- Law F, Seo JH, Wang Z, DeLeon JL, Bolis Y, Brown A, Zong WX, Du G, Rocheleau CE. The VPS34 PI3K negatively regulates RAB-5 during endosome maturation. J Cell Sci. 2017;130(12):2007–2017. doi:10.1242/jcs.194746.
- Jaber N, Mohd-Naim N, Wang Z, DeLeon JL, Kim S, Zhong H, Sheshadri N, Dou Z, Edinger AL, Du G, Braga VM, Vps34 regulates Rab7 and late endocytic trafficking through recruitment of the GTPase-activating protein Armus. J Cell Sci. 2016;129(23):4424–4435. doi:10.1242/jcs.192260.
- Ruck A, Attonito J, Garces KT, Núnez L, Palmisano NJ, Rubel Z, Bai Z, Nguyen KC, Sun L, Grant BD, Hall DH, et al. The Atg6/Vps30/Beclin 1 ortholog BEC-1 mediates endocytic retrograde transport in addition to autophagy in C. elegans. Autophagy. 2011;7(4):386–400. doi:10.4161/auto.7.4.14391.
- Chotard L, Mishra AK, Sylvain MA, Tuck S, Lambright DG, Rocheleau CE. TBC-2 regulates RAB-5/RAB-7-mediated endosomal trafficking in Caenorhabditis elegans. Mol Biol Cell. 2010;21(13):2285–96. doi:10.1091/mbc.E09-11-0947.
- Frasa MA, Maximiano FC, Smolarczyk K, Francis RE, Betson ME, Lozano E, Goldenring J, Seabra MC, Rak A, Ahmadian MR, et al. Armus is a Rac1 effector that inactivates Rab7 and regulates E-cadherin degradation. Curr Biol. 2010;20(3):198–208. doi:10.1016/j.cub.2009.12.053.
- Zhou Y, Toth M, Hamman MS, Monahan SJ, Lodge PA, Boynton AL, Salgaller ML. Serological cloning of PARIS-1: a new TBC domain-containing, immunogenic tumor antigen from a prostate cancer cell line. Biochem Biophys Res Commun. 2002;290(2):830–8. doi:10.1006/bbrc.2001.6257.
- Li W, Zou W, Zhao D, Yan J, Zhu Z, Lu J, Wang X. C. elegans Rab GTPase activating protein TBC-2 promotes cell corpse degradation by regulating the small GTPase RAB-5. Development. 2009;136(14):2445–55. doi:10.1242/dev.035949.
- Liu O, Grant BD, Basolateral Endocytic Recycling Requires RAB-10 and AMPH-1 Mediated Recruitment of RAB-5 GAP TBC-2 to Endosomes. PLoS Genet. 2015;11(9):e1005514. doi:10.1371/journal.pgen.1005514. PMID:26393361
- Chotard L, Skorobogata O, Sylvain MA, Shrivastava S, Rocheleau CE. TBC-2 is required for embryonic yolk protein storage and larval survival during L1 diapause in Caenorhabditis elegans. PLoS One. 2010;5(12):e15662. doi:10.1371/journal.pone.0015662. PMID:21203392
- Sasidharan N, Sumakovic M, Hannemann M, Hegermann J, Liewald JF, Olendrowitz C, Koenig S, Grant BD, Rizzoli SO, Gottschalk A, et al. RAB-5 and RAB-10 cooperate to regulate neuropeptide release in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2012;109(46):18944–9. doi:10.1073/pnas.1203306109.
- Sun L, Liu O, Desai J, Karbassi F, Sylvain MA, Shi A, Zhou Z, Rocheleau CE, Grant BD. CED-10/Rac1 regulates endocytic recycling through the RAB-5 GAP TBC-2. PLoS Genet. 2012;8(7):e1002785. doi:10.1371/journal.pgen.1002785. PMID:22807685
- Toyofuku T, Morimoto K, Sasawatari S, Kumanogoh A. Leucine-Rich Repeat Kinase 1 Regulates Autophagy through Turning On TBC1D2-Dependent Rab7 Inactivation. Mol Cell Biol. 2015;35(17):3044–58. doi:10.1128/MCB.00085-15.
- Serva A, Knapp B, Tsai YT, Claas C, Lisauskas T, Matula P, Harder N, Kaderali L, Rohr K, Erfle H, et al. miR-17-5p regulates endocytic trafficking through targeting TBC1D2/Armus. PLoS One. 2012;7(12):e52555. doi:10.1371/journal.pone.0052555. PMID:23285084
- Lemmon MA. Pleckstrin homology (PH) domains and phosphoinositides. Biochem Soc Symp 2007;74:81–93.
- Vieira OV, Bucci C, Harrison RE, Trimble WS, Lanzetti L, Gruenberg J, Schreiber AD, Stahl PD, Grinstein S. Modulation of Rab5 and Rab7 recruitment to phagosomes by phosphatidylinositol 3-kinase. Mol Cell Biol. 2003;23(7):2501–14. doi:10.1128/MCB.23.7.2501-2514.2003.

