ABSTRACT
The “Adacel (Tdap5) Pregnancy Registry” was used to identify 1182 women who received the tetanus, diphtheria, acellular pertussis [5 components] (Tdap5) vaccine during pregnancy from 2005 to 2016. To evaluate the safety and use of prenatal Tdap5, we calculated the rate of maternal, obstetrical, pregnancy and neonatal outcomes following Tdap5 pregnancy exposure and assessed vaccine uptake by year and trimester of exposure. The most commonly reported maternal adverse events included injection site reactions (2.6%; 95% Confidence Interval 1.8%, 3.7%), nervous system events (1.3%; 0.8%, 2.1%) and musculoskeletal events (1.1%; 0.6%, 1.9%). The most commonly reported complications of pregnancy were hypertension/preeclampsia (5.5%; 3.3%, 8.9%) and gestational diabetes (2.5%; 1.1%, 5.3%), while those for labor and delivery were premature labor (2.9%; 1.4%, 5.7%) and premature membrane rupture (1.5%; 0.4%, 3.8%). These rates were similar to, or lower than those reported for the general population of pregnant women. Among pregnancies with known birth outcomes (N = 275), 90.4% (86.2%, 93.4%) resulted in a live birth, 5.9% (3.6%, 9.5%) in spontaneous abortion, 3.0% (1.4%, 5.8%) in stillbirth, and 0.7% (0.0%, 2.8%) in ectopic pregnancies. Most newborns had normal APGAR scores and birth weights (98.1% and 93.0%, respectively), and only two reported a congenital anomaly (0.7%; 0.0%, 2.8%). An influx of reports in 2012 with third trimester Tdap5 exposure coincided with the 2012 updated Advisory Committee on Immunization Practices recommendations. This analysis did not identify any safety concerns across the continuum of maternal, obstetrical, pregnancy, and neonatal outcomes in women who received Tdap5 vaccination during pregnancy.
Introduction
Two tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccines were licensed in the US in 2005: Adacel™ (Sanofi Pasteur), hereafter referred to as Tdap5 because of its 5 pertussis components; Boostrix (GlaxoSmithKline) with 3 pertussis components. Shortly thereafter, the Advisory Committee on Immunization Practices (ACIP) recommended Tdap vaccination in the immediate postpartum period for women who had not previously received Tdap, to help protect against transmission of pertussis to their infants.Citation1 However, the high burden of morbidity and mortality of pertussis in very young infants led to changing recommendations for Tdap vaccination during pregnancy, as evidence of conferred maternal antibody protection emerged.Citation2,Citation3 In 2011, ACIP recommended that Tdap be administered during pregnancy on or after 20 weeks of gestation,Citation4 and in 2012 the committee updated these recommendations to advocate for Tdap vaccination in all pregnancies from 27 to 36 weeks gestation, regardless of previous Tdap vaccination.Citation5 All aforementioned ACIP recommendations were endorsed by the American College of Obstetricians and Gynecologists.Citation6–8
The Adacel Pregnancy Registry, Sanofi Pasteur’s passive pregnancy exposure surveillance system, was established in June 2005 as a US Food and Drug Administration (FDA) postlicensure commitment and remains open. The aim of the registry is to capture information on Tdap5 pregnancy exposures, as well as on maternal, obstetrical, pregnancy and neonatal outcomes to monitor for any potential safety signals. While previous studies of prenatal Tdap vaccination have focused either on maternal safety or birth outcomes, the Adacel Pregnancy Registry provides an opportunity to characterize the safety of Tdap5 pregnancy exposure across a continuum of outcomes including maternal, obstetrical, pregnancy and neonatal. Furthermore, this registry also provides an opportunity to document the uptake of prenatal Tdap5 immunization following the evolving ACIP recommendations regarding the timing of immunization.
We undertook an analysis of 11 years of pregnancy registry data to evaluate the safety of prenatal Tdap5 in terms of maternal, obstetrical, pregnancy and neonatal outcomes. We also assessed trends in prenatal Tdap5 vaccination over time and by trimester of exposure.
Materials and methods
Population
The study population consisted of all women enrolled in the pregnancy registry between June 10, 2005 (date of Tdap5 licensure in the US) and 31 October, 2016.
Methods
The Adacel Pregnancy Registry, included in Sanofi Pasteur’s Global Pharmacovigilance database, captures voluntary reports from health care providers and consumers about Tdap5 vaccine administration to women who were pregnant at the time of vaccination. In the US, health care providers and pregnant women are encouraged to register all cases of Tdap5 pregnancy exposure, with or without adverse events, through the provision of information about the pregnancy vaccination passive surveillance program included in the product prescribing information and on the company’s sponsored pregnancy registry website (http://www.sanofipasteurpregnancyregistry.com/). Information on maternal socio-demographics, relevant medical histories, and all outcomes are obtained from the reporter (exposed pregnant woman or her health care provider) using a structured questionnaire completed voluntarily and returned by mail; details of the data collected can be found in Appendix A. Information on pregnancy, birth, and neonatal outcomes are obtained shortly following the time of estimated delivery; three follow-up attempts are made before the case is considered “lost to follow up” and closed. Missing data was attributed to loss to follow up. To aid in reducing bias, reports are classified as “prospective” if received before the condition of the fetus has been assessed through prenatal testing (e.g., targeted ultrasound, amniocentesis), otherwise they are classified as “retrospective.” All adverse events temporally associated with Tdap5 pregnancy exposure are differentiated into diagnoses (i.e., clinical manifestations) and symptoms (i.e., co-manifestations), and coded according to preferred terms in the Medical Dictionary of Regulatory Activities (MedDRA).Citation9 Each report is medically assessed for seriousness (per regulatory definitionCitation10), expectedness (listed in the label) and causal relationship to vaccination.
For the purposes of this study, both diagnoses and symptoms were included in the counts of adverse events. Outcomes were classified as “maternal” if they impacted solely maternal health and were independent of the pregnancy; “obstetrical” if they were directly related to the pregnancy; “birth’ (including live births, stillbirths/fetal deaths, elective terminations, spontaneous abortions/miscarriages); or “neonatal” if they were related to the infant and were evaluated at or after birth (including birth weights, APGAR scores and congenital anomalies). Elective terminations were reviewed on a per-case basis to determine whether they were attributable to Tdap5 vaccination; those unrelated were excluded from the analysis as they did not constitute adverse events. Spontaneous abortion (miscarriage) and fetal death (stillbirth) were defined as pregnancy loss before and after 20 weeks gestation, respectively. A full-term delivery was defined as 37–42 weeks from last menstrual period, preterm births were defined as <37 weeks gestation, and very preterm births were those occurring at <32 weeks. Low birth weight was defined as <5.5 lbs, very low birth weight as <3.3 lbs, and normal if between 5.5 lbs and 8.8 lbs at term. APGAR scores were considered normal if they were >7 at 5 minutes post-birth. A complete case narrative review was conducted to determine birth and neonatal outcomes.
The analysis of maternal adverse events was based on the number of women who were vaccinated with Tdap5 during pregnancy, whereas the analysis of obstetrical outcomes was based on the number of pregnancies with known outcomes. The 95% confidence intervals for the corresponding frequencies were calculated using the Agresti-Coull method, as recommended for binomial proportions,Citation11 when the proportion was used for inference (e.g., external comparisons). Similarly, the analyses of pregnancy and neonatal outcomes were based on the number of births for which the outcomes were known. As such, the latter excluded elective terminations since the “natural” outcome of a terminated pregnancy cannot be determined except in cases when prenatal testing predicted a birth defect. It was not possible to stratify the safety analysis according to the status of the report (i.e., prospective vs. retrospective) owing to the small number of retrospective reports.
For the analysis of trends in prenatal Tdap5 vaccination, the percentage of the study population vaccinated each calendar year and each trimester (first [0–13 weeks], second [14–27 weeks] and third [≥28 weeks] trimester) was calculated using the number of vaccinated pregnant women as the denominator. When information on trimester was missing, the date of vaccination and date of last menstrual period were used to estimate the trimester of exposure.
The analyses were conducted using SAS statistical software version 9.2 (SAS Institute Inc., Cary, North Carolina) and Microsoft Excel.
Ethics
Ethics review was not needed as the decision to participate in the registry or disclose follow-up information was voluntary. Moreover, participation in the pregnancy registry did not provide direct benefit nor present any risk to either the pregnant woman or the fetus. This study complies with the Health Insurance Portability and Accountability Act (HIPAA) for the protection of all personal data.
Results
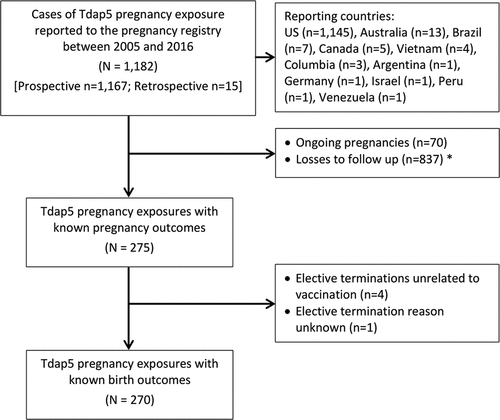
From June 10, 2005 through October 31, 2016, 1,182 cases of Tdap5 vaccination during pregnancy were submitted to the registry; the majority (98.7%) of which were reported prospectively (). Among these, 96.9% (n = 1,145) originated from the US while the remaining 3.1% were received from Australia (n = 13), Brazil (n = 7), Canada (n = 5), Vietnam (n = 4), Colombia (n = 3), and one report each from Argentina, Germany, Israel, Peru and Venezuela. At the time of the analysis, 70 (5.9%) pregnancies were ongoing and 837 (70.8%) had been lost to follow up, leaving 275 pregnancies with known outcomes. The losses to follow up were primarily due to the reporter not returning the completed structured questionnaire. Furthermore, 4 pregnancies were terminated electively for reasons unrelated to vaccination (social circumstances and unplanned pregnancy) and one aborted for unknown reasons, leaving 270 pregnancies for the analysis of birth outcomes.
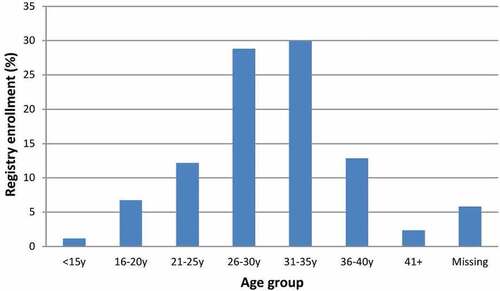
Although cases of prenatal Tdap5 exposure represented women of all childbearing ages, the majority of pregnancy exposures occurred in women 26 to 35 years of age, with nearly equal representation from the 26- 30-year and 31- to 35-year age groups ().
Most women vaccinated with Tdap5 during pregnancy did not experience an adverse event (88.5%; 95% confidence interval [CI] 86.5%, 90.2%; n = 1,046); however, 6.3% (95% CI 5.1%, 7.9%; n = 75) of pregnancy exposure cases reported a serious adverse event (AE) and 5.2% (95% CI 4.0%, 6.6%; n = 61) a nonserious AE (data not shown). The number and frequency of maternal adverse events are shown in . The most frequent maternal adverse events included injection site reactions (2.6%, 95% CI 1.8%, 3.7%; n = 31), followed by nervous system-related events (1.3%, 95% CI 0.8%, 2.1%; n = 15) and musculoskeletal events (1.1%, 95% CI 0.6%, 1.9%; n = 13). All other maternal adverse events reported were experienced by less than 0.5% of women.
Table 1. Maternal adverse events reported following prenatal tetanus-diphtheria-acellular pertussis (Tdap5) vaccination*
The most frequently reported complication of pregnancy among women with a known pregnancy outcome (N = 275) was hypertension and preeclampsia (5.5%, 95% CI 3.3%, 8.9%; n = 15), followed by gestational diabetes (2.5%, 95% CI 1.1%, 5.3%; n = 7), amniotic cavity infection (1.5%, 95% CI 0.4%, 3.8%; n = 4) and fetal growth restriction (1.5%, 95% CI 0.4%, 3.8%; n = 4), while the most common complications of labor and delivery were premature labor (2.9%, 95% CI 1.4%, 5.7%; n = 8) and premature membrane rupture (1.5%, 95% CI 0.4%, 3.8%; n = 4) (). The only adverse events reported during the puerperium period were postpartum hemorrhage (0.4%, 95% CI 0.0%, 2.2%; n = 1) and postoperative wound complication (0.4%, 95% CI 0.0%, 2.2%; n = 1).
Table 2. Complications of pregnancy, labor, delivery and puerperium reported following prenatal tetanus-diphtheria-acellular pertussis (Tdap5) vaccination
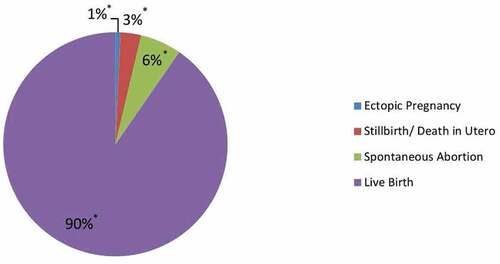
Among the 270 pregnancies with known birth outcomes, 90.4% (95% CI 86.2%, 93.4%; n = 244) resulted in a live birth, 5.9% (95% CI 3.6%, 9.5%; n = 16) in spontaneous abortions, 3.0% (95% CI 1.4%, 5.8%; n = 8) in stillbirths, and 0.7% (95% CI 0.0%, 2.8%; n = 2) were ectopic pregnancies ().
Figure 3. Pregnancy birth outcomes following prenatal tetanus-diphtheria-acellular pertussis (Tdap5) vaccination; Legend: blue = Ectopic Pregnancy, red = Stillbirth/Death in Utero, green = Spontaneous Abortion, and purple = Live Birth.
APGAR scores and birth weights were available for 107 (39.6%) and 104 (38.1%) of newborns, respectively (). The vast majority of newborns had normal APGAR scores (98.1%, 95% CI 93.0%, 99.9%) and birth weights (95.0%, 95% CI 89.0%, 98.2%). In addition, there were no instances of infants with very low birth weight. There were two cases of congenital anomaly reported (0.7 per 100 live births, 95% CI 0.0–2.8 per 100 live births): one with congenital deafness and the other with peripheral pulmonic stenosis with patent foramen ovale. Both of these infants were born to women enrolled retrospectively in the registry.
Table 3. Neonatal outcomes following prenatal tetanus-diphtheria-acellular pertussis (Tdap5) vaccination
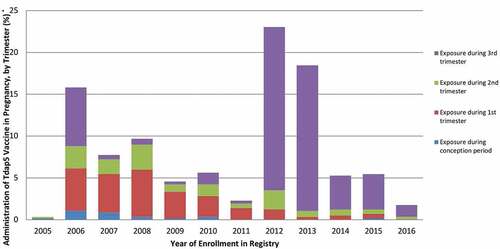
The trimester of Tdap5 vaccination was available or could be derived in 569 (48.1%) pregnancies. Eighteen cases (3.2%, 95% CI 2.0%, 5.0%) reported being vaccinated during the conception period, 142 (25.0%, 95% CI 21.6%, 28.7%) during the first trimester, 85 (14.9%, 95% CI 12.2%, 18.1%) during the second trimester, and 324 (56.9%, 95% CI 52.8%, 61.0%) during the third trimester (data not shown). Trends in trimester of vaccination changed over the course of the registry in keeping with the updated ACIP recommendations (). Most importantly, we noted a rapid shift from vaccination during the first trimester to vaccination during the third trimester starting in 2012. In addition, except for 2012–2013, we observed a decrease over time in the reporting of prenatal Tdap5 vaccination.
Discussion
This analysis of 11 years of pregnancy registry data finds no unusual or unexpected trends in the pattern or frequencies of reported adverse events following prenatal Tdap5 vaccination. The rates of maternal adverse events reported are consistent with those listed in the product label and the existing body of evidence on the safety of prenatal Tdap, while the rates of complications of pregnancy, labor, delivery, and births are similar to or lower than those observed for pregnant women in the general population. Similarly, no safety concerns were observed in newborns exposed to Tdap5 during pregnancy. With regards to the timing of vaccination, a marked shift toward late third trimester vaccination is noted following the publication of the updated ACIP recommendations in 2012.
Although a number of serious adverse events were reported following prenatal Tdap5 vaccination, the types and frequencies of these are similar or lower than those observed in the general population of unvaccinated pregnant women in the US. For example, this study finds that hypertensive disorders and gestational diabetes were the most commonly reported adverse events of pregnancy similar to that reported for the general population of pregnant women.Citation12–14 Likewise, the rates of these events were also within the range of what is expected. For example, 5.5% (95% CI 3.3%, 8.9%) of women in the pregnancy registry experienced a hypertensive disorder, whereas the background rate ranged from 7.1% to 9.0% during the study period.Citation12,Citation13 Gestational diabetes and postpartum hemorrhage were reported in 2.5% (95% CI 1.1%, 5.3%) and 0.4% (95% CI 0.0%, 2.2%) of vaccinated pregnant women while the expected rates are 4.5%-9.0% and 0.3%-0.4%, respectively.Citation12,Citation13,Citation15,Citation16 These results are consistent with the existing body of evidence on the safety of prenatal Tdap including, seven large cohort studies (N ≥ 10,000 pregnancies),Citation17–23 five smaller cohort studies (N = 470 to 7,378 pregnancies),Citation24–28 and two analyses of the US Vaccine Adverse Event Reporting System (VAERS) database.Citation29,Citation30
In contrast, the rates of some complications of labor and delivery observed in the registry population are lower than those of the US population. For example, the background rates of premature labor and premature membrane rupture are 9.6%-12% and 3%,Citation31,Citation32 respectively, compared with 2.9% (95% CI 1.4%, 5.7%) and 1.5% (95% CI, 0.4%, 2.2%), respectively, for women in the pregnancy registry.
Similar to the pattern observed for complications of labor and delivery, the rates of adverse pregnancy and neonatal outcomes for women and their offspring in the registry were lower than those observed in the general population. For example, the observed rate of fetal loss (spontaneous abortions/miscarriages and stillbirths combined) was 8.9% compared with an expected rate of 17.0%,Citation33 and the observed rate of ectopic pregnancy was 0.7% (95% CI, 0.00%, 2.8%) compared with an expected rate of 2.0% to 2.45% in the general population of unvaccinated pregnant women.Citation34–36 The rate of induced abortions (1.8%; 5/275) was also lower than expected (18.4%).Citation34 Likewise, the rate of low birth weight (4.8%; 95% CI, 1.8%, 11.0%) was lower than that in the general population of unvaccinated pregnant women (8.1%).Citation32
The lower rates of preterm births, premature membrane rupture, and fetal losses (spontaneous abortions/miscarriages and stillbirths) observed in this study can be explained by the high proportion of registry women vaccinated late in the third trimester, thereby reducing the probability of observing preterm births, premature membrane rupture and stillbirths, and excluding the diagnosis of spontaneous abortion (miscarriage). On the other hand, the lower rates of ectopic pregnancies and adverse neonatal outcomes suggest that women enrolled in the pregnancy registry represent a healthier subset of women eligible for prenatal Tdap vaccination. Indeed, research on the determinants of prenatal vaccination acceptance has shown that women of higher socioeconomic status, higher education level, and those demonstrating healthier behaviors such as being nonsmokers and receiving more prenatal care are more likely to accept prenatal vaccination.Citation37,Citation38 Moreover, the two largest cohort studies published to date also found that women who received prenatal Tdap were more likely to have received early and adequate to superior prenatal care, more likely to have received ultrasound and prenatal flu vaccination, more likely to live in a large metropolitan area, and less likely to have been hospitalized in the first 20 weeks of gestation. Therefore, women who agree to receive prenatal Tdap appear to be at lower risk of adverse pregnancy and neonatal outcomes.Citation18,Citation19
The Adacel Pregnancy Registry did not receive any reports of chorioamnionitis following prenatal Tdap5 vaccination over a period of 11 years. This finding contradicts the results of two recent large retrospective cohort studies reporting a small but statistically significant increased risk of chorioamnionitis.Citation18,Citation19 However, the authors of these studies suggested their results be interpreted with caution given the lack of direct biological mechanism for the observed association, the absence of increased risks for related infant outcomes, and the possibility of residual confounding.
During 11 years of registry data, two cases of congenital anomaly were reported in women who received prenatal Tdap5; both cases were reported retrospectively. In the case of congenital deafness, Tdap5 vaccination occurred at 37 weeks gestation, thereby making the vaccine an unlikely cause of this anomaly. Moreover, the majority of cases of congenital malformation are due to genetic factors, most often a single gene defect,Citation39 and the pregnancy was also complicated by single umbilical artery, a known risk factor for congenital anomalies.Citation40 The second anomaly reported was patent foramen ovale with peripheral pulmonic stenosis in the offspring of a 31 year old women who received Tdap5 at 2 ½ weeks of gestation and influenza vaccination at week 10. These congenital anomalies are among the most common types of heart defects observed in neonates and infants,Citation41,Citation42 with approximately 0.6% to 0.8% of infants having a cardiovascular malformation;Citation43 a rate well within that reported to the registry. In addition, more than half of cases of peripheral pulmonic stenosis are accompanied by an associated cardiac defect.Citation44 As such, it is not surprising that at least one case of patent foramen ovale accompanied with peripheral pulmonic stenosis was reported to the registry. In addition, this case’s mother had two infections during pregnancy, including one during the first trimester, a known risk factor for congenital heart defects.Citation45–47
The high number of reports of prenatal Tdap5 exposure observed in the year following the launch of this vaccine and subsequent decrease in reporting over time, with the exception of 2012 and 2013, is a pattern that has been previously described for vaccines and drugs and is known as the Webber effect.Citation48 The observed trend is consistent with increasing acceptance of prenatal Tdap vaccination over time as evidence about the safety and benefits of this practice accumulated. On the other hand, the sudden increase in reporting observed in 2012 and 2013 is likely due to increasing use and acceptance of prenatal Tdap vaccination during one of the largest pertussis epidemics recorded to date in the US.Citation49
The shift from first to third trimester Tdap5 vaccination observed during the study period corresponds to ACIP’s changing recommendations over the same time period and demonstrates the rapid adoption of the 2012 recommendations regarding the timing of prenatal Tdap vaccination. To the best of our knowledge, this is the first study to assess the impact of the ACIP recommendations on clinical practice. The ability of the pregnancy registry to capture this practice change, consistent with the expected change following the publication of the ACIP recommendations, demonstrates the internal validity of the data captured by the registry despite the voluntary nature of the reports and the high losses to follow up.
The limitations of this study need to be considered. First, exposure to Tdap5 during pregnancy is likely greatly underreported, even though one provider tended to report, at least initially, every inadvertent Tdap5 pregnancy exposure even in the absence of an adverse event. Nevertheless, the rates of many of the reported adverse outcomes are similar to those of unvaccinated pregnant women in the US, and adverse event rates that are lower than expected can be explained by factors other than underreporting such as vaccination late in the third trimester excluding events such as miscarriages, and acceptance of prenatal vaccination by women at lower risk of adverse outcomes, as previously discussed. Second, passive surveillance systems are susceptible to reporting bias as evidenced by the fact that nearly a third of cases of Tdap5 pregnancy exposures were received from one health care provider. As such, our results need to be interpreted with caution. Third, outcomes are available for only 23% of women enrolled in the pregnancy registry and the corresponding extent of losses to follow up could have introduced bias. However, the types and rates of outcomes observed are similar to those expected for pregnant women. Fourth, this study likely overestimates the rate of serious cases for three reasons: (i) exposure to Tdap5 during pregnancy was classified by some reporters as “serious,” whether an adverse event occurred or not, (ii) in some cases, seriousness was attributed to the case report (i.e., inadvertent pregnancy exposure), rather than to a particular event or symptom; as such, all events reported for a serious case were also classified as serious regardless of their nature, and (iii) seriousness was attributed to concurrent events in a case report, such as the occurrence of both nausea and syncope during pregnancy. Fifth, the number of vaccinated pregnant women studied was small. Finally, the results of this study may not be generalizable to pregnancies occurring in adolescents and young adults as these age groups were underrepresented in the registry. On the other hand, there is no reason to believe that the safety profile of prenatal Tdap5 vaccination would differ in a younger population.
In conclusion, no safety concerns were identified with the administration of prenatal Tdap5 with regard to maternal, obstetrical, pregnancy and neonatal outcomes in this comprehensive review of 11 years of pregnancy registry data. These findings add to the existing body of evidence on the safety of prenatal Tdap vaccination and provide additional evidence in support of ACIP’s recommendation regarding the routine use of prenatal Tdap vaccination. Moreover, these findings provide additional reassurance to women and health-care providers considering prenatal Tdap vaccination. This study also demonstrates the rapid adoption of the 2012 ACIP recommendations on the timing of prenatal vaccination.
Disclosure of potential conflicts of interest
CS and IT were employees of Sanofi Pasteur at the time the study was conducted, and AK, VP, LEL are employees of Sanofi Pasteur. IT and AK hold stocks and/or shares in the company.
Additional information
Funding
References
- Centers for Disease Control and Prevention (CDC). Preventing tetanus, diphtheria, and pertussis among pregnant women and postpartum women and their infants. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2008;57(RR–4):1–41.
- Gall SA, Myers J, Pichichero M. Maternal immunization with tetanus-diphtheria-pertussis vaccine: effect on maternal and neonatal serum antibody levels. Am J Obstet Gynecol. 2011;203(4):334.e1–5. doi:10.1016/j.ajog.2010.11.024.
- Leuridan E, Hens N, Peeters N, de Witte L, Van der Meeren O, Van Damme P. Effect of a prepregnancy pertussis booster dose on maternal antibody titers in young infants. Pediatr Infect Dis J. 2011;30(7):603–10. doi:10.1097/INF.0b013e3182093814.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) in pregnant women and persons who have or anticipate having close contact with an infant aged <12 months - Advisory Committee on Immunizations Practices (ACIP), 2011. MMWR. 2011;60(41):1424–26.
- Centers for Disease Control Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women – Advisory Committee on Immunization Practices (ACIP), 2012. MMWR. 2013;62(7):131–35.
- American College of Obstetricians and Gynecologists. Update on immunization and pregnancy: tetanus, diphtheria, and pertussis vaccination. Committee opinion no. 438. Obstet Gynecol. 2009;114(2 Pt 1):398–400. doi:10.1097/AOG.0b013e3181b48f07.
- American College of Obstetricians and Gynecologists. Update on immunization and pregnancy: tetanus, diphtheria, and pertussis vaccination ACOG committee opinion no. 521. Obstet Gynecol. 2012;119(3):690–91. doi:10.1097/AOG.0b013e31824e1327.
- American College of Obstetricians and Gynecologists. Update on immunization and pregnancy: tetanus, diphtheria, and pertussis vaccination. Committee opinion no. 566. Obstet Gynecol. 2013;121(6):1411–14. doi:10.1097/01.AOG.0000431054.33593.e3.
- Medical Dictionary for Regulatory Activities (MedDRA). [accessed 2018 Aug 28]. http://www.meddra.org.
- US Food and Drug Administration (FDA). 21 code of federal regulation part 600.80 postmarketing reporting of adverse experiences. Fed Regist. 1997 [accessed 2018 Aug 28];62:52252–53. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=600.80.
- Brown LD, Cai TT, DasGupta A. Interval estimation for a binomial proportion. Stat Sci. 2001;16(2):101–33. doi:10.1214/ss/1009213286.
- Center for Disease Control and Prevention (CDC). Data on selected pregnancy complications in the United States. [accessed 2019 Feb 18]. http://www.cdc.gov/reproductiveheatlh/maternalinfanthealth/pregnancy-complications.html.
- US Department of Health and Human Services, National Institute of Child Health and Human Development, National Institute of Health. Most common complications of pregnancy. [accessed 2019 Feb 18]. http://www.nichd.nih.gov/health/topics/pregnancy/conditioninfo/complications.
- Law A, McCoy M, Lynen R, Curkendall SM, Gatwood J, Juneau PL, Landsman-Blumberg P. The prevalence of complications and healthcare costs during pregnancy. J Med Econ. 2015;18(7):533–41. doi:10.3111/13696998.2015.1016229.
- DeSisto CL, Kim SY, Sharma AJ. Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007–2010. Prev Chronic Dis. 2014;11:E104. doi:10.58888/pcd11.130415.
- Chen Y, Quick WW, Yang W, Zhang Y, Baldwin A, Moran J, Moore V, Sahai N, Dall TM. Cost of gestational diabetes mellitus in the United States in 2007. Popul Health Manage. 2009;12(3):165–74. doi:10.1089/pop.2009.12303.
- Griffin JB, Yu L, Watson D, Turner N, Walls T, Howe AS, Jiang Y, Pertussis P-H-H. Immunization in pregnancy safety (PIPS) study: a retrospective cohort study of safety outcomes in pregnant women vaccinated with Tdap vaccine. Vaccine. 2018;36(34):5173–79. doi:10.1016/j.vaccine.2018.07.011.
- Layton JB, Butler AM, Li D, Boggess KA, Weber DJ, McGrath LJ, Prenatal B-DS. Tdap immunization and risk of maternal and newborn adverse events. Vaccine. 2017;35(33):4072–78. doi:10.1016/j.vaccine.2017.06.071.
- DeSilva M, Vazquez-Benitez G, Nordin JD, Lipkind HS, Klein NP, Cheetham TC, Naleway AL, Hambidge SJ, Lee GM, Jackson ML, et al. Maternal Tdap vaccination and risk of infant morbidity. Vaccine. 2017;35(29):3655–60. doi:10.1016/j.vaccine.2017.05.041.
- Sukumaran L, McCarthy NL, Kharbanda EO, McNeil MM, Naleway AL, Klein NP, Jackson ML, Hambidge SJ, Lugg MM, Li R, et al. Association of Tdap vaccination with acute events and adverse birth outcomes among pregnant women with prior tetanus-containing immunizations. JAMA. 2015;314(15):1581–87. doi:10.1001/jama.2015.12790.
- Sukumaran L, McCarthy NL, Kharbanda EO, Weintraub ES, Vazquez-Benitez G, McNeil MM, Li R, Klein NP, Hambidge SJ, Naleway AL, et al. Safety of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis and influenza vaccinations in pregnancy. Obstet Gynecol. 2015;126(5):1069–74. doi:10.1097/AOG.0000000000001066.
- Donegan K, King B, Bryan P. Safety of pertussis vaccination in pregnant women in UK: observational study. BMJ. 2014;349:g4219. doi:10.1136/bmj.g4219.
- Kharbanda EO, Vazquez-Benitez G, Lipkind HS, Klein NP, Cheetham TC, Naleway A, Omer SB, Hambidge SJ, Lee GM, Jackson ML, et al. Evaluation of the association of maternal pertussis vaccination with obstetric events and birth outcomes. JAMA. 2014;312(18):1897–904. doi:10.1001/jama.2014.14825.
- Walls T, Graham P, Petousis-Harris H, Hill L, Austin N. Infant outcomes after exposure to Tdap vaccine in pregnancy: an observational study. BMJ Open. 2016;6(1):e009536. doi:10.1136/bmjopen-2015-009536.
- Petousis-Harris H, Walls T, Watson D, Paynter J, Graham P, Turner N. Safety of Tdap vaccine in pregnant women: an observational study. BMJ Open. 2016;6(4):e010911. doi:10.1136/bmjopen-2015-010911.
- Berenson AB, Hirth JM, Rahman M, Laz TH, Rupp RE, Sarpong KO. Maternal and infant outcomes among women vaccinated against pertussis during pregnancy. Hum Vaccin Immunother. 2016;12(8):1965–71. doi:10.1080/21645515.2016.1157241.
- Morgan JL, Baggari SR, McIntire DD, Sheffield JS. Pregnancy outcomes after antepartum tetanus, diphtheria, and acellular pertussis vaccination. Obstet Gynecol. 2015;125(6):1433–38. doi:10.1097/AOG.0000000000000862.
- Shakib JH, Korgenski K, Sheng X, Varner MW, Pavia AT, Byington CL. Tetanus, diphtheria, acellular pertussis vaccine during pregnancy: pregnancy and infant health outcomes. J Pediatr. 2013;163(5):1422–26. doi:10.1016/j.jpeds.2013.06.021.
- Moro PL, Cragan J, Tepper N, Zheteyeva Y, Museru O, Lewis P, Broder K. Enhanced surveillance of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccines in pregnancy in the Vaccine Adverse Event Reporting System (VAERS), 2011–2015. Vaccine. 2016;34(20):2349–53. doi:10.1016/j.vaccine.2016.03.049.
- Zheteyeva YA, Moro PL, Tepper NK, Rasmussen SA, Barash FE, Revzina NV, Kissin D, Lewis PW, Yue X, Haber P, et al. Adverse event reports after tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccines in pregnant women. Am J Obstet Gynecol. 2012;207(1):59.e1–7. doi:10.1016/j.ajog.2012.05.006.
- American College of Obstetrics and Gynecology (ACOG). Practice bulletin no. 172: premature rupture of membranes. Obstet Gynecol. 2016;128(4):e165–e177. doi:10.1097/AOG.0000000000001712.
- Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Mathews TJ. Births: final data for 2015. National Vital Statistics Reports; 66( 1). Hyattsville, MD: National Centre for Health Statistics; 2017. [accessed 2019 Feb 18]. http://www.cdc.gov/nchs/data/nvsr/vnsr67/nvsr67_01.pdf.
- Ventura SJ, Curtin SC, Abma JC, Henshaw SK. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990-2008. National Vital Stat Rep. 2012;60(7):1–21.
- Stulberg DB, Cain LR, Dahlquist I, Lauderdale DS. Ectopic pregnancy rates in the Medicaid population. Am J Obstet Gynecol. 2013;208(4):274.e7. doi:10.1016/j.ajog.2012.12.038.
- Trabert B, Holt VL, Yu O, Van den Eeden SK, Scholes D. Population-based ectopic pregnancy trends, 1993–2007. Am J Prev Med. 2011;40(5):556–60. doi:10.1016/j.amepre.2010.12.026.
- Van den Eeden SK, Shan J, Bruce C, Glasser M. Ectopic pregnancy rate and treatment utilization in a large managed care organization. Obstet Gynecol. 2005;105:1052–57. doi:10.1097/01.AOG.0000158860.26939.2d.
- Yuen CYS, Tarrant M. Determinants of uptake of influenza vaccination among pregnant women – a systematic review. Vaccine. 2014;32(36):4602–13. doi:10.1016/j.vaccine.2014.06.067.
- Legge A, Dodds L, MacDonald NE, Scott J, McNeil S. Rates and determinants of seasonal influenza vaccination in pregnancy and association with neonatal outcomes. CMAJ. 2014;186(4):E157–64. doi:10.1503/cmaj.130499.
- Korver AM, Smith RJ, Van Camp G, Schleiss MR, Bitner-Glindzicz MAK, Lustig LR, Usami S-I, Boudewyns AN. Congenital hearing loss. Nat Rev Dis Primers. 2017;3(1):16094. doi:10.1038/nrdp.2016.94.
- Murphy-Kaulbeck L, Dodds L, Joseph KS, Van den Hof M. Single umbilical artery risk factors and pregnancy outcomes. Obstet Gynecol. 2010;116(4):843–50. doi:10.1097/AOG.0b013e3181f0bc08.
- American Heart Association. Patent Foramen Ovale (PFO). [accessed 2019 Feb 18]. http://www.heart.org/en/health-topics/congenital-heart-defects/about-congenital-heart-defects/patent-foramen-ovale.
- Parker SE, MMai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, Anderson P, Mason CA, Collins JS, Kirby RS, et al. Updated national birth prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res Part A. 2010;88(12):1008–16. doi:10.1002/bdra.20735.
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890–900. doi:10.1016/S0735-1097(02)01886-7.
- Franch RH, Gay BB Jr. Congenital stenosis of the pulmonary artery branches: a classification, with postmortem findings in two cases. Am J Med. 1963;35(4):512–29. doi:10.1016/0002-9343(63)90149-9.
- Stanford Children’s Health. Factors contributing to congenital birth defects [accessed 2019 Feb 23]. http://www.stanfordchildrens.org/en/topic/default?id=factors-contributing-to-congenital-heart-disease-90-P01788.
- Ul Haq F, Jalil F, Hashmi S, Jumani MI, Imdad A, Jabeen M, Hashmi JT, Irfan FB, Imran M, Atiq M. Risk factors predisposing to congenital heart defects. Ann Pediatr Cardiol. 2011;4(2):117–21. doi:10.4103/0974-2069.84641.
- Abqari S, Gupta A, Shahab T, Rabbani MU, Ali SM, Firdaus U. Profile and risk factors for congenital heart defects: a study in a tertiary care hospital. Ann Pediatr Cardiol. 2011;9(3):216–21. doi:10.4103/0974-2069.189119.
- Weber JCP. Epidemiology of adverse reactions to nonsteroidal anti-inflammatory drugs. Adv Inflam Res. 1984;6:1–7.
- Centers for Disease Control and Prevention (CDC). Summary of notifiable diseases, 2012. MMWR. 2014;61(53):1–121.