ABSTRACT
Immunocompromised (IC) persons are at increased risk for herpes zoster (HZ) and its complications, mainly due to impairment of cell-mediated immunity (CMI). The adjuvanted recombinant zoster vaccine (RZV) demonstrated efficacy against HZ in autologous hematopoietic stem cell transplant (auto-HSCT) recipients and hematologic malignancy (HM) patients. We review immune responses to RZV in 5 adult IC populations, 4 of which were receiving multiple, concomitant immunosuppressive medications: auto-HSCT and renal transplant recipients, HM and solid tumor patients, and human immunodeficiency virus-infected adults. Although administered in most cases when immunosuppression was near its maximum, including concomitantly with chemotherapy cycles, RZV induced robust and persistent humoral and, more importantly, CMI responses in all 5 IC populations. Based on the overall clinical data generated in older adults and IC individuals, RZV is expected to provide benefit in a broad adult population at risk for HZ.
Introduction
Herpes zoster (HZ) results from reactivation of latent varicella-zoster virus (VZV) and may occur at any age. Although the incidence of HZ and its complications increases with age, it is higher in immunocompromised (IC) persons regardless of age.Citation1–4 In addition, both HZ and its complications tend to be more severe and last longer in IC individuals.
IC populations are heterogeneous and can have different levels of immune suppression depending on age, underlying disease, and the type, duration, and combination of immunosuppressive therapies.Citation5 The increased risk of HZ in IC populations may be critically influenced by the impairment of cell-mediated immunity (CMI) resulting from these specific medical conditions, their treatments, or both.Citation1,Citation2
The risk of HZ in IC populations can be mitigated by vaccination. Whereas live-attenuated vaccines are contraindicated in IC populations because of the risk of disseminated disease,Citation6,Citation7 non-live HZ vaccines have been evaluated in IC adults with the highest incidences of HZ, including chronically immunosuppressed renal transplant (RT) recipients,Citation8 hematopoietic stem cell transplant (HSCT) recipients,Citation9–12 hematologic malignancy (HM) patients,Citation12,Citation13 and solid tumor (ST) patients.Citation12,Citation14 Non-live HZ vaccines have also been evaluated in human immunodeficiency virus (HIV)-infected adults, another IC population at increased risk of HZ.Citation12,Citation15
HIV infection induces immunosuppression that directly affects CD4 T-cells. Accordingly, while the incidence of HZ ranges between 3–5/1000 person-years in the general population,Citation16 the incidence in HIV-infected individuals reached 32/1000 person-years in the pre-antiretroviral therapy (ART) era, and remains at 10–11/1000 person-years, even in the ART era.Citation4,Citation17,Citation18
Solid organ transplant recipients represent a distinct group of IC patients. To prevent allograft rejection, their immune systems are suppressed by chronic therapies, resulting in a mixed immunodeficiency, consisting mainly of T-cell-mediated immunity impairment.Citation19 HZ incidences up to 22–32/1000 person-years have been reported in recipients of various solid organ transplants.Citation4,Citation20–23
HSCT recipients are IC as a result of pre-transplant conditioning regimens, which eradicate the disease and create space for engraftment, but also increase the risk of HZ. The risk is generally highest during the first year following transplantation, with incidences of 8%-25% in autologous HSCT (auto-HSCT) recipientsCitation24–27 and 13%-28% in allogenic HSCT recipients.Citation28,Citation29 HZ risk decreases within 2–3 years post-HSCT as the transplant engrafts, matures, and reconstitutes the immune system.Citation4,Citation24,Citation30
The risk for HZ is also increased in cancer patients under treatment, of whom those with HMs appear to be most susceptible.Citation4 In patients receiving immunosuppressive cancer therapies for HMs or STs, incidences up to 31/1000 and 14/1000 person-years, respectively, have been reported.Citation31,Citation32
The increased HZ risk shows that there is a clear medical need to prevent HZ in IC populations.
Although an investigational inactivated VZV vaccine demonstrated efficacy against HZ in auto-HSCT recipients and ST patients, it was not efficacious in HM patientsCitation11,Citation33 and has not been licensed to date.
The adjuvanted recombinant zoster vaccine (RZV, Shingrix, GSK) is a recombinant VZV glycoprotein E (gE) non-live subunit vaccine that cannot cause disseminated HZ. RZV is highly immunogenic and ≥90% efficacious in preventing HZ in all age groups among adults ≥50 years.Citation34,Citation35 Efficacy is also maintained in individuals with underlying medical conditions,Citation36 including potential immune-mediated diseases.Citation37 RZV has received licensure for use in adults ≥50 years of age (YOA) in many countries since 2017, and in adults ≥18 YOA at increased HZ risk in Europe since 2020.
RZV was well tolerated in auto-HSCT and RT recipients, HM and ST patients, and HIV-infected adults.Citation38 Efficacy of RZV was demonstrated in auto-HSCT recipientsCitation10 and HM patients.Citation13 Here we present a comprehensive overview of immune responses to RZV in these 5 severely IC populations.Citation8,Citation10,Citation13–15,Citation39
Methods
Design of the reviewed studies with RZV in IC populations
All 5 studies were randomized, observer-blind, placebo-controlled, and parallel-group studies (). The study protocols were reviewed and approved by relevant institutional review boards or independent ethics committees.
Table 1. Overview of the reviewed clinical studies with RZV in immunocompromised adults
Participants
All 5 studies included participants ≥18 YOA without an HZ/varicella episode or vaccination within the year before the first dose. Administration of licensed live and other non-replicating vaccines were excluded for specific time periods. Women of childbearing potential could participate if practicing contraception from 30 days prior to study vaccination through 2 months (M) (HM and ST patients, RT recipients, HIV-infected adults) or 12 M (auto-HSCT recipients) post-dose 2.
The use of investigational or non-registered drugs/vaccines from 30 days prior to study vaccination to study end was not allowed in auto-HSCT and RT recipients, HM patients, and HIV-infected adults. However, investigational use of a registered (auto-HSCT recipients and HM patients) or non-registered (auto-HSCT recipients) product to treat the underlying disease was allowed. Other, study-specific inclusion/exclusion criteria have been described previously.Citation8,Citation10,Citation13–15
Procedures
RZV was administered intramuscularly as 3 doses at M0, M2, and M6 in HIV-infected adults,Citation15 and as 2 doses 1–2 M apart in the other IC populations.Citation8,Citation10,Citation13,Citation14 Each dose contained recombinant VZV gE antigen (50 μg) and the AS01B adjuvant system (containing 50 μg of 3-O-desacyl-4′-monophosphoryl lipid A, 50 μg of Quillaja saponaria Molina fraction 21 [licensed by GSK from Antigenics LLC, a wholly owned subsidiary of Agenus Inc., a Delaware, USA corporation] and liposome).
Immunogenicity objectives and outcomes
Anti-gE antibody concentrations and frequencies of CD4[2+] T-cells (gE-specific CD4 T-cells expressing ≥2 activation markers from among interferon-γ, interleukin-2, tumor necrosis factor-α, and cluster of differentiation 40 ligand), and the vaccine response (VR) in terms of anti-gE antibody and CD4[2+] T-cells were evaluated in all populations. Results are presented for 1 M and 12 M post-last dose overall and, where available, per age group (18–49 and ≥50 YOA). Analyses per diagnoses (auto-HSCT recipients, HM patients), timing of vaccination in relation to the immunosuppressive therapy (HM and ST patients), or immunosuppressive regimen (RT recipients) were also performed. The second dose was the last in all IC populations except for HIV-infected adults, in whom the third dose was the last. Of note, no substantial benefit of the third over the second dose was seen in HIV-infected adults in terms of humoral or CMI responses.Citation15 Finally, except for the study in auto-HSCT recipients, in which efficacy was the primary outcome, all studies had immunogenicity objectives with predefined statistical success criteria ().
Table 2. Immunogenicity objectives with predefined statistical success criteria
Main statistical considerations for immunogenicity assessment
Immunogenicity was evaluated in the according-to-protocol (ATP) cohorts for immunogenicity/persistence, which included study participants who complied with the protocol and had available immunogenicity data. The ATP cohorts for humoral immunogenicity originated from a subset (auto-HSCT recipients) or the entire study populations. The ATP cohorts for CMI originated from the entire study population (HIV-infected adults) or from subsets of study populations. The cohorts and statistical success criteria used for inferential analyses are presented in . For descriptive analyses, anti-gE antibody geometric mean concentrations (GMCs) were determined with their exact 2-sided 95% confidence intervals (CIs), and frequencies of CD4[2+] T-cells were tabulated using descriptive statistics (minimum, first quartile, median, third quartile, maximum).
Additional details about the assessment of anti-gE antibodies and CD4[2+] T-cells are presented elsewhere.Citation8,Citation10,Citation13–15,Citation40
Results
Study populations
Within each IC population, demographic characteristics were balanced between the RZV and Placebo groups in the ATP cohort for immunogenicity. Most RZV recipients were ≥50 YOA (70%, mean ages at first dose 46.5–57.0 YOA), male (58.5%-94.4%) except for ST patients (34.5% males), of White-Caucasian/European ancestry (66.1%-96.8%), and of non-Hispanic/Latino ethnicity (90.1%-100%).
In RZV recipients from the ATP cohorts for immunogenicity, 31.8% of HM patients were vaccinated during the cancer therapy course and 68.2% after the cancer therapy course, and 74.7% of ST patients were vaccinated PreChemo and 25.3% OnChemo (definitions in ). The most frequent diagnoses were multiple myeloma (53.7%) in auto-HSCT recipients, breast (51.4%) and colorectal (21.1%) cancer in ST patients, and multiple myeloma (24.0%), Hodgkin lymphoma (18.0%), chronic lymphocytic leukemia (CLL, 16.6%), and non-Hodgkin B-cell lymphoma (NHBCL, 15.2%) in HM patients. Among RT recipients, 77.3% received a maintenance immunosuppressive regimen consisting of calcineurin inhibitors/sirolimus (CIS) + mycophenolate compound (MC) + corticosteroids (CS), 17.6% received CIS+MC, and 5.0% received CIS+CS.
When assessed in all vaccinated auto-HSCT recipients, the median time between transplant and first dose was 61.0 days.
Humoral immune responses to RZV
Across the 5 IC populations, ≥95.1% of RZV recipients were seropositive for anti-gE antibody before vaccination,Citation41 and pre-vaccination anti-gE antibody GMCs ranged between 763–1354 milli-international units per milliliter (mIU/mL).
Success criteria were met for all inferential humoral immunogenicity objectives ().
Figure 1. Inferential humoral immunogenicity analyses in the reviewed studies with RZV in immunocompromised populations.
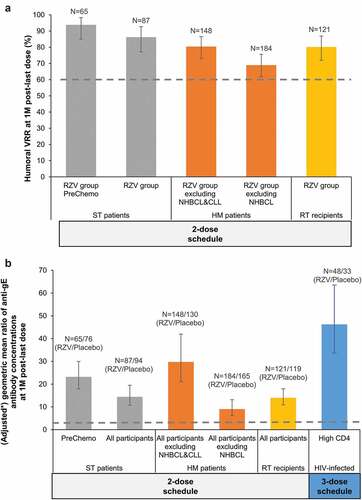
At 1 M post-last dose, humoral VR rates (VRRs) ranged between 65.4%-96.2% across the 5 IC populations (). Anti-gE antibody GMCs at 1 M post-last dose ranged between 12,753–19,164 mIU/mL across IC populations, except for HIV-infected adults, in whom it was 63,813 mIU/mL; at 12 M post-last dose these were 3184–8545 and 25,242 mIU/mL (4.2–6.3 and 20.7 times higher than pre-vaccination), respectively ().
Figure 2. Humoral immune responses to RZV in immunocompromised populations.
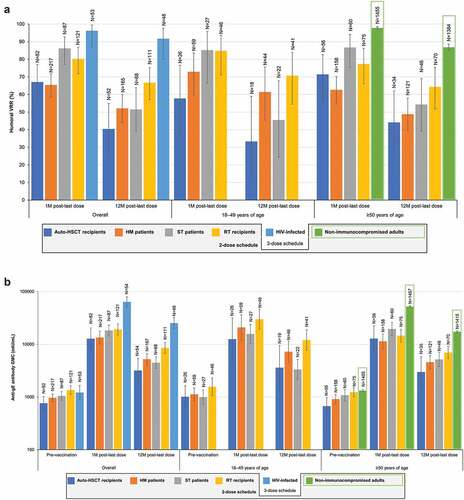
Age (18–49 or ≥50 YOA) did not appear to affect humoral immune responses in auto-HSCT recipients and ST patients (). A slight trend for stronger humoral responses in the younger age group was seen in HM patients and RT recipients.
Auto-HSCT recipients and HM patients diagnosed with NHBCL had a diminished humoral immune response compared with other diagnoses, noting that 97% of HM patients with NHBCL were treated with rituximab. HM patients with CLL (of whom 81% were treated with rituximab) also had a diminished response. At 1 M post-last dose, humoral VRR was 14.2% in auto-HSCT recipients with NHBCL compared with 67.1% in the entire study population. Similarly, humoral VRR was 45.5% and 22.2% in HM patients with NHBCL and CLL, respectively, compared with 80.4% in the rest of the study population.
HM patients vaccinated after the cancer therapy course had a stronger humoral response than those vaccinated during the cancer therapy course. In ST patients, the humoral response to RZV was higher in the PreChemo than in the OnChemo subgroup (definitions in ) at 1 M post-last dose but were similar at 12 M post-last dose. In RT recipients, humoral responses at 1 M and 12 M post-last dose 2 were similar regardless the type of chronic, daily immunosuppressive regimen (CIS+MC+CS, CIS+MC, or CIS+CS).
CMI responses to RZV
In HIV-infected adults, the geometric mean ratio of CD4[2+] T-cell frequencies at 1 M post-dose 3 over 1 M post-dose 2 was 1.0 (95%CI: 0.8–1.3), showing that the third dose had no incremental benefit over the second. In the other 4 reviewed studies, performed after evaluating the 3-dose schedule in HIV-infected adults, 2 doses were administered.
All CMI objectives met predefined statistical success criteria in RT recipients and HIV-infected adults. In the PreChemo subgroup of ST patients, although higher CD4[2+] T-cell frequencies were demonstrated in RZV versus placebo recipients, the CMI VRR was 50.0% (95%CI: 28.2–71.8) in the RZV group, and the success criterion for this objective was not met ().
Figure 3. Inferential CMI analyses in the reviewed studies with RZV in immunocompromised populations.
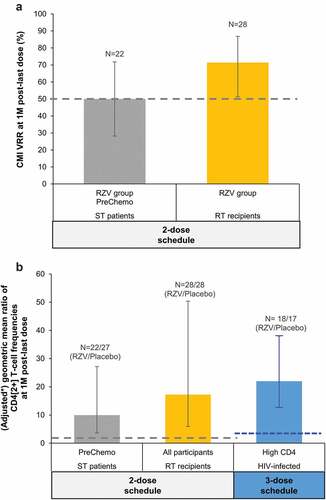
At 1 M post-last dose, CMI VRRs ranged between 71.4%-92.9% across IC populations, except for ST patients, as noted above (). Median pre-vaccination CD4[2+] T-cell frequencies ranged between 21 and 127 across the 5 IC populations. At 1 M post-last dose, median CD4[2+] T-cell frequencies ranged between 2149–6645 across IC populations, except for ST patients, in whom it was 779; at 12 M post-last dose, these were 1007–1706 and 333 (13.0–50.3 and 2.6 times higher than pre-vaccination), respectively ().
Figure 4. CMI responses to RZV in immunocompromised populations.
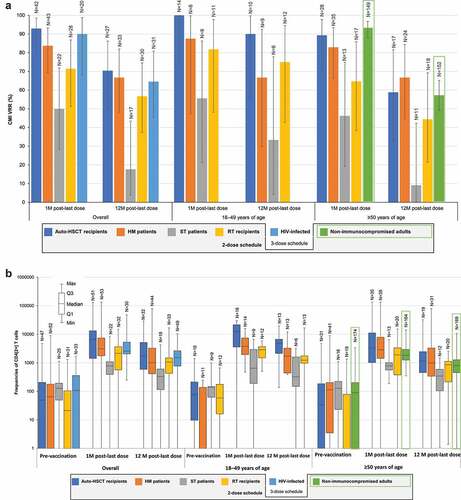
Age (18–49 or ≥50 YOA) did generally not affect the magnitude of CMI responses in auto-HSCT, RT, ST, and HM populations ().
Underlying diagnoses in auto-HSCT recipients and HM patients did not affect CMI responses. At 1 M post-last dose, the VRR was 100% in both auto-HSCT recipients and HM patients with NHBCL and 71.4% in HM patients with CLL, compared with 92.9% in all auto-HSCT recipients and 73.7% in HM patients excluding those with NHBCL or CLL. CMI responses tended to be higher in HM patients vaccinated after the cancer therapy course than in those vaccinated during cancer therapy.
Discussion
Prevention of viral infections, including the reactivation of latent VZV, in adults with immunodeficiency or immunosuppression caused by disease and/or its therapy is a large unmet medical need. Immunogenicity data show that RZV is able to induce robust and persistent gE-specific humoral and CMI responses in different populations, including adults with severely immunocompromising conditions and/or under immunosuppressive treatments. This demonstrates that, in addition to being able to overcome immunosenescence in adults ≥50 YOA,Citation40,Citation42 RZV can also overcome severe immunocompromising conditions. A plain language summary contextualizing the results and potential clinical research relevance and impact is presented in .
Age did not affect efficacy of RZV in auto-HSCT recipients,Citation10 and only had a limited effect on immune responses in IC populations with available age-based data. This has also been observed in non-IC adults, in whom efficacy and immune responses were similar across all age groups ≥50 years.Citation34,Citation35,Citation40
High proportions (65%-96%) of IC study participants mounted a humoral VR to the RZV vaccination course, and anti-gE antibody concentrations increased substantially from pre-vaccination levels in all 5 IC populations ≥18 YOA, and were maintained above pre-vaccination levels through the end of a 1-year follow-up period.Citation8,Citation10,Citation13–15
Some underlying diseases in HM patients and auto-HSCT recipients, specifically CLL and/or NHBCL, were associated with a less robust humoral response to RZV. This is likely due to B-cell depletion induced by anti-CD20 monoclonal antibody therapy (e.g., rituximab), which was frequently administered to HM patients with NHBCL and CLL.Citation13 However, this severe B-cell impairment does not appear to affect the protection offered by RZV, because the efficacy of RZV against HZ was maintained at 60.5% (95%CI: 31.0–78.2) in auto-HSCT recipients with NHBCL.Citation39
Unlike humoral immune responses, CMI responses to RZV were not affected by underlying diseases in IC populations, and were generally maintained through the end of a 1-year follow-up period at levels similar to those observed in non-IC adults ≥50 YOA (). CMI responses are regarded as the main mechanistic driver for protection against HZ,Citation43 and RZV demonstrated protection against HZ and postherpetic neuralgia (PHN) across different types of adult populations, including auto-HSCT recipients, which is the studied IC population at the greatest risk for HZ.Citation4,Citation10,Citation13,Citation34–37 Although the first RZV dose was administered early in immune reconstitution, 50–70 days post-auto-HSCT, RZV was 68.2% efficacious against HZ and 89.3% efficacious against PHN in the overall auto-HSCT population.Citation10 As mentioned above, efficacy against HZ was observed in participants with any of the underlying diseases, including NHBCL (60.5%), despite the less robust humoral response.Citation39 In a post hoc analysis, RZV also demonstrated 87.2% efficacy against HZ in HM patients, but the sample size did not allow assessment per individual underlying disease. Nevertheless, the results in auto-HSCT recipients suggest that RZV may remain efficacious in HM patients with NHBCL or CLL, in whom humoral responses were less robust.Citation13 Although efficacy was not evaluated in ST patients and RT recipients, their CMI responses were robust and similar to those in auto-HSCT recipients and HM patients, suggestive of a similar clinical benefit.
Overall, the high CD4 T-cell responses observed in IC populations may result from the ability of the AS01 adjuvant system to promote a CMI response, regardless of age, underlying medical condition, or treatment.Citation44 CD4 T-cell responses were strong in HIV-infected adults ≥18 YOA, and comparable to those observed in non-IC adults ≥50 YOA (). This might be partly because most were receiving ART with immune reconstitution and had CD4 T-cell counts ≥200 cells/mm3.Citation15 The CD4 T-cell response was particularly high in auto-HSCT recipients, which could be explained by the homeostatic proliferation following vaccination or by the low number of total CD4 T-cells, which is used as the denominator in the calculation of CD4[2+] T-cell frequencies. In auto-HSCT recipients, CD4 T-cell responses displayed polyfunctional profiles similar to those in adults ≥50 YOA,Citation39 and polyfunctional responses after vaccines against tuberculosis, malaria, melanoma, or HIV have been shown to correlate with protection.Citation45–48 While data for RZV-elicited CD8 T-cell responses are not available for IC populations,Citation8,Citation10,Citation13–15 using the same CMI assessment methods, only scarce CD8 T-cell responses were detected in older adults, which did not increase upon vaccination with RZV.Citation40,Citation42
The use of cytotoxic immunosuppressive chemotherapy is expected to interfere with the generation of antigen-specific lymphocytes, particularly when vaccination is undertaken concurrently with a chemotherapy cycle. In ST patients, the first dose was administered either 8–30 days before the first (occasionally second) cycle of a chemotherapy course (PreChemo) or at the start of and concurrently with a chemotherapy cycle (OnChemo). The second dose was administered concurrently with a chemotherapy cycle in all the ST patients. Although in ST patients, CMI responses were less robust than in HM patients, to whom RZV was administered ≥10 days before or after a chemotherapy cycle or 10 days to 6 M after completion of the immunosuppressive chemotherapy course,Citation13 humoral responses were in similar ranges. In ST patients, humoral immune responses tended to be higher in the PreChemo than in the OnChemo subgroup shortly after vaccination. The 8–30-day time window allowed PreChemo RZV recipients to develop an immune response to the first dose before immunosuppressive therapy was initiated, which likely accounts for this difference.Citation14
In addition to the high efficacy observed in older adults,Citation34,Citation35 the efficacy of RZV was also remarkable in 2 populations that are highly IC and at the highest risk of HZ.Citation10,Citation13 Although vaccine efficacy cannot be inferred for all IC populations in the absence of a correlate of protection, the overall clinical data generated in older adults and IC individuals ≥18 YOA indicate that the anticipated benefit-risk profile of RZV for the prevention of HZ in adults is favorable.Citation39,Citation49
Limitations of studies evaluating immunogenicity of RZV in IC populations result from the heterogeneity of populations and of immunosuppressive treatments within studies. In some subgroups per age, underlying disease, immunosuppressive therapy, or timing of vaccination in relation to the immunosuppressive therapy, the number of participants was very small, especially for the evaluation of CMI. As for other vaccines, due to the ever-growing field of immunosuppressive therapies and standard of care, the most beneficial timing and dosing of RZV vaccination remains to be determined.
Conclusion
RZV induced robust and persistent humoral and, more importantly, CMI responses in patients with a wide variety of IC conditions, most of whom were receiving multiple immunosuppressive medications. These include auto-HSCT recipients, who are at highest risk of HZ. In most cases, vaccination was undertaken when immunosuppression was near its maximum. Nonetheless, efficacy against HZ has been evaluated and demonstrated in auto-HSCT recipients and HM patients. Because the mechanisms leading to increased risk of HZ are believed to be shared between older adults (immunosenescent) and other adults at increased risk of HZ (individuals with IC conditions, treatments, autoimmune diseases, stress, depression, family HZ history), and because the treatments used in the IC populations studied are also used to treat other medical conditions, RZV is expected to benefit a broad adult population at risk for HZ by overcoming the CMI impairment, which is thought to be the basis for VZV reactivation.
Contributions
Conceptualization: A.F.D., D.O.W., and P.V.; Validation: A.E.S., A.F.D., B.S., D.O.W., M.D., and P.V.; Formal Analysis: M.D.; Investigation: B.S.; Resources: A.F.D, B.S., M.D., and P.V.; Writing (original draft): A.F.D.; Visualization: A.F.D., D.O.W., and P.V.; Supervision: A.E.S., A.F.D., and P.V. All authors contributed to the writing (review and editing) of the manuscript and approved the final version for submission.
Disclosure of potential conflicts of interest
The authors declare the following financial relationships: Alemnew F. Dagnew, Mamadou Drame, Bruno Salaun, Anne E. Schuind, Peter Vink, and David O. Willer were employees of the GSK group of companies at the time this manuscript was initiated and the reviewed studies were designed, commenced, and/or conducted. All authors declare they hold shares in the GSK group of companies as part of their remuneration. The authors declare no other non-financial relationships and activities or conflicts of interest.
Trademark
Shingrix is a trademark of the GSK group of companies.
Acknowledgments
The authors thank the participants, investigators, and teams of the reviewed studies,Citation8,Citation10,Citation13–15 as well as the GSK teams, including laboratory teams that undertook the immunological assessments, clinical research and development, clinical operations, statisticians and statistical analysts, medical data reviewers, and publications.
The authors also thank the Modis Platform for medical writing services, editorial assistance, and publication coordination, on behalf of GSK. Medical writing services were provided by Maria Maior and Alpár Pöllnitz (Modis on behalf of GSK). Editorial assistance and publication coordination were provided by Sander Hulsmans (Modis on behalf of GSK).
Data availability statement
Anonymized individual participant data and study documents for the 5 reviewed studies can be requested for further research from www.clinicalstudydatarequest.com
Additional information
Funding
References
- Gershon AA, Gershon MD, Breuer J, Levin MJ, Oaklander AL, Griffiths PD. Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J Clin Virol. 2010;48(Suppl 1):S2–7. doi:10.1016/S1386-6532(10)70002-0.
- Cohen JI. Clinical practice: herpes zoster. N Engl J Med. 2013;369(3):255–63. doi:10.1056/NEJMcp1302674.
- Yawn BP, Gilden D. The global epidemiology of herpes zoster. Neurology. 2013;81(10):928–30. doi:10.1212/WNL.0b013e3182a3516e.
- McKay SL, Guo A, Pergam SA, Dooling K. Herpes zoster risk in immunocompromised adults in the United States: a systematic review. Clin Infect Dis. 2020;71(7):e125–e134. doi:10.1093/cid/ciz1090.
- Levin MJ, Bresnitz E, Popmihajlov Z, Weinberg A, Liaw KL, Willis E, Curtis JR. Studies with herpes zoster vaccines in immune compromised patients. Expert Rev Vaccines. 2017;16(12):1217–30. doi:10.1080/14760584.2017.1395703.
- Willis ED, Woodward M, Brown E, Popmihajlov Z, Saddier P, Annunziato PW, Halsey NA, Gershon AA. Herpes zoster vaccine live: a 10 year review of post-marketing safety experience. Vaccine. 2017;35(52):7231–39. doi:10.1016/j.vaccine.2017.11.013.
- Alexander KE, Tong PL, Macartney K, Beresford R, Sheppeard V, Gupta M. Live zoster vaccination in an immunocompromised patient leading to death secondary to disseminated varicella zoster virus infection. Vaccine. 2018;36(27):3890–93. doi:10.1016/j.vaccine.2018.05.078.
- Vink P, Ramon Torrell JM, Sanchez Fructuoso A, Kim S-J, Kim S-I, Zaltzman J, Ortiz F, Campistol Plana JM, Fernandez Rodriguez AM, Rebollo Rodrigo H, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in chronically immunosuppressed adults following renal transplant: a phase III, randomized clinical trial. Clin Infect Dis. 2020;70(2):181–90. doi:10.1093/cid/ciz177.
- Stadtmauer EA, Sullivan KM, Marty FM, Dadwal SS, Papanicolaou GA, Shea TC, Mossad SB, Andreadis C, Young JAH, Buadi FK, et al. A phase 1/2 study of an adjuvanted varicella-zoster virus subunit vaccine in autologous hematopoietic cell transplant recipients. Blood. 2014;124(19):2921–29. doi:10.1182/blood-2014-04-573048.
- Bastidas A, De La Serna J, El Idrissi M, Oostvogels L, Quittet P, López-Jiménez J, Vural F, Pohlreich D, Zuckerman T, Issa NC, et al. Effect of recombinant zoster vaccine on incidence of herpes zoster after autologous stem cell transplantation: a randomized clinical trial. JAMA. 2019;322(2):123–33. doi:10.1001/jama.2019.9053.
- Winston DJ, Mullane KM, Cornely OA, Boeckh MJ, Brown JW, Pergam SA, Trociukas I, Žák P, Craig MD, Papanicolaou GA, et al. Inactivated varicella zoster vaccine in autologous haemopoietic stem-cell transplant recipients: an international, multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391(10135):2116–27. doi:10.1016/s0140-6736(18)30631-7.
- Mullane KM, Winston DJ, Wertheim MS, Betts RF, Poretz DM, Camacho LH, Pergam SA, Mullane MR, Stek JE, Sterling TM, et al. Safety and immunogenicity of heat-treated zoster vaccine (ZVHT) in immunocompromised adults. J Infect Dis. 2013;208(9):1375–85. doi:10.1093/infdis/jit344.
- Dagnew AF, Ilhan O, Lee WS, Woszczyk D, Kwak JY, Bowcock S, Sohn SK, Rodriguez Macías G, Chiou T-J, Quiel D, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis. Lancet Infect Dis. 2019;19(9):988–1000. doi:10.1016/s1473-3099(19)30163-x.
- Vink P, Delgado Mingorance I, Maximiano Alonso C, Rubio‐Viqueira B, Jung KH, Rodriguez Moreno JF, Grande E, Marrupe Gonzalez D, Lowndes S, Puente J, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in patients with solid tumors, vaccinated before or during chemotherapy: a randomized trial. Cancer. 2019;125(8):1301–12. doi:10.1002/cncr.31909.
- Berkowitz EM, Moyle G, Stellbrink HJ, Schurmann D, Kegg S, Stoll M, El Idrissi M, Oostvogels L, Heineman TC, Brockmeyer N, et al. Safety and immunogenicity of an adjuvanted herpes zoster subunit candidate vaccine in HIV-infected adults: a phase 1/2a randomized, placebo-controlled study. J Infect Dis. 2015;211(8):1279–87. doi:10.1093/infdis/jiu606.
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4(6):e004833. doi:10.1136/bmjopen-2014-004833.
- Gilbert L, Wang X, Deiss R, Okulicz J, Maves R, Schofield C, Ferguson T, Whitman T, Kronmann K, Agan B, et al. Herpes zoster rates continue to decline in people living with human immunodeficiency virus but remain higher than rates reported in the general US population. Clin Infect Dis. 2019;69(1):155–58. doi:10.1093/cid/ciy1041.
- Moanna A, Rimland D. Decreasing incidence of herpes zoster in the highly active antiretroviral therapy era. Clin Infect Dis. 2013;57(1):122–25. doi:10.1093/cid/cit165.
- Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351(26):2715–29. doi:10.1056/NEJMra033540.
- Arness T, Pedersen R, Dierkhising R, Kremers W, Patel R. Varicella zoster virus-associated disease in adult kidney transplant recipients: incidence and risk-factor analysis. Transpl Infect Dis. 2008;10(4):260–68. doi:10.1111/j.1399-3062.2007.00289.x.
- Gourishankar S, McDermid JC, Jhangri GS, Preiksaitis JK. Herpes zoster infection following solid organ transplantation: incidence, risk factors and outcomes in the current immunosuppressive era. Am J Transplant. 2004;4(1):108–15. doi:10.1046/j.1600-6143.2003.00287.x.
- Pergam SA, Forsberg CW, Boeckh MJ, Maynard C, Limaye AP, Wald A, Smith NL, Young BA. Herpes zoster incidence in a multicenter cohort of solid organ transplant recipients. Transpl Infect Dis. 2011;13(1):15–23. doi:10.1111/j.1399-3062.2010.00547.x.
- Koo S, Gagne LS, Lee P, Pratibhu PP, James LM, Givertz MM, Marty FM. Incidence and risk factors for herpes zoster following heart transplantation. Transpl Infect Dis. 2014;16(1):17–25. doi:10.1111/tid.12149.
- Schuchter LM, Wingard JR, Piantadosi S, Burns WH, Santos GW, Saral R. Herpes zoster infection after autologous bone marrow transplantation. Blood. 1989;74(4):1424–27. doi:10.1182/blood.V74.4.1424.1424.
- Offidani M, Corvatta L, Olivieri A, Mele A, Brunori M, Montanari M, Rupoli S, Scalari P, Leoni P. A predictive model of varicella-zoster virus infection after autologous peripheral blood progenitor cell transplantation. Clin Infect Dis. 2001;32(10):1414–22. doi:10.1086/320157.
- Barton T, Collis T, Stadtmauer E, Schuster M. Infectious complications the year after autologous bone marrow transplantation or peripheral stem cell transplantation for treatment of breast cancer. Clin Infect Dis. 2001;32(3):391–95. doi:10.1086/318491.
- Kamber C, Zimmerli S, Suter-Riniker F, Mueller BU, Taleghani BM, Betticher D, Zander T, Pabst T. Varicella zoster virus reactivation after autologous SCT is a frequent event and associated with favorable outcome in myeloma patients. Bone Marrow Transplant. 2015;50(4):573–78. doi:10.1038/bmt.2014.290.
- Koc Y, Miller KB, Schenkein DP, Griffith J, Akhtar M, DesJardin J, Snydman DR. Varicella zoster virus infections following allogeneic bone marrow transplantation: frequency, risk factors, and clinical outcome. Biol Blood Marrow Transplant. 2000;6(1):44–49. doi:10.1016/s1083-8791(00)70051-6.
- Steer CB, Szer J, Sasadeusz J, Matthews JP, Beresford JA, Grigg A. Varicella-zoster infection after allogeneic bone marrow transplantation: incidence, risk factors and prevention with low-dose aciclovir and ganciclovir. Bone Marrow Transplant. 2000;25(6):657–64. doi:10.1038/sj.bmt.1702190.
- Asano-Mori Y, Kanda Y, Oshima K, Kako S, Shinohara A, Nakasone H, Sato H, Watanabe T, Hosoya N, Izutsu K, et al. Long-term ultra-low-dose acyclovir against varicella-zoster virus reactivation after allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2008;83(6):472–76. doi:10.1002/ajh.21152.
- Habel LA, Ray GT, Silverberg MJ, Horberg MA, Yawn BP, Castillo AL, Quesenberry CP, Li Y, Sadier P, Tran TN, et al. The epidemiology of herpes zoster in patients with newly diagnosed cancer. Cancer Epidemiol Biomarkers Prev. 2013;22(1):82–90. doi:10.1158/1055-9965.epi-12-0815.
- Mao J, McPheeters JT, Finelli L. Healthcare utilization and costs among patients with herpes zoster and solid tumor malignancy on chemotherapy: a retrospective cohort study. Medicine (Baltimore). 2017;96(48):e8746. doi:10.1097/MD.0000000000008746.
- Mullane KM, Morrison VA, Camacho LH, Arvin A, McNeil SA, Durrand J, Campbell B, Su S-C, Chan ISF, Parrino J, et al. Safety and efficacy of inactivated varicella zoster virus vaccine in immunocompromised patients with malignancies: a two-arm, randomised, double-blind, phase 3 trial. Lancet Infect Dis. 2019;19(9):1001–12. doi:10.1016/s1473-3099(19)30310-x.
- Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang S-J, Levin MJ, McElhaney JE, Poder A, Puig-Barberà J, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087–96. doi:10.1056/NEJMoa1501184.
- Cunningham AL, Lal H, Kovac M, Chlibek R, Hwang S-J, Diez-Domingo J, Godeaux O, Levin MJ, McElhaney JE, Puig-Barberà J, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375(11):1019–32. doi:10.1056/NEJMoa1603800.
- Oostvogels L, Heineman TC, Johnson RW, Levin MJ, McElhaney JE, Van Den Steen P, Zahaf T, Dagnew AF, Chlibek R, Diez-Domingo J, et al. Medical conditions at enrollment do not impact efficacy and safety of the adjuvanted recombinant zoster vaccine: a pooled post-hoc analysis of two parallel randomized trials. Hum Vaccin Immunother. 2019;15(12):2865–72. doi:10.1080/21645515.2019.1627818.
- Dagnew AF, Rausch D, Hervé C, Zahaf T, Levin MJ, Schuind A. Efficacy and serious adverse events profile of the adjuvanted recombinant zoster vaccine in adults with pre-existing potential immune-mediated diseases: a pooled post hoc analysis on two parallel randomized trials. Rheumatology (Oxford). 2020. doi:10.1093/rheumatology/keaa1424.
- Lopez-Fauqued M, Co M, Bastidas A, Beukelaers P, Dagnew AF, Fernandez Garcia JJ, Schuind A, Tavares da Silva F. Safety profile of the adjuvanted recombinant zoster vaccine (RZV) in immunocompromised populations: an overview of 6 trials. Open Forum Infect Dis. 2020;7(S1):S42–S43. doi:10.1093/ofid/ofaa439.082
- Stadtmauer EA, Sullivan KM, El Idrissi M, Salaun B, Alonso Alonso A, Andreadis C, Anttila VJ, Bloor AJC, Broady R, Cellini C, et al. Adjuvanted recombinant zoster vaccine in adult autologous stem cell transplant recipients: polyfunctional immune responses and lessons for clinical practice. Hum Vaccin Immunother. Under consideration.
- Cunningham AL, Heineman TC, Lal H, Godeaux O, Chlibek R, Hwang S-J, McElhaney JE, Vesikari T, Andrews C, Choi WS, et al. Immune responses to a recombinant glycoprotein E herpes zoster vaccine in adults aged 50 years or older. J Infect Dis. 2018;217(11):1750–60. doi:10.1093/infdis/jiy095.
- GSK Study Register. Clinical study reports for study IDs 115523, 116428, and 116886. [accessed 9 Sept 2020]. https://www.gsk-studyregister.com/en/
- Chlibek R, Smetana J, Pauksens K, Rombo L, Van Den Hoek JA, Richardus JH, Plassmann G, Schwarz TF, Ledent E, Heineman TC, et al. Safety and immunogenicity of three different formulations of an adjuvanted varicella-zoster virus subunit candidate vaccine in older adults: a phase II, randomized, controlled study. Vaccine. 2014;32(15):1745–53. doi:10.1016/j.vaccine.2014.01.019.
- Weinberg A, Levin MJ. VZV T cell-mediated immunity. Curr Top Microbiol Immunol. 2010;342:341–57. doi:10.1007/82_2010_31.
- Didierlaurent AM, Laupeze B, Di Pasquale A, Hergli N, Collignon C, Garcon N. Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expert Rev Vaccines. 2017;16(1):55–63. doi:10.1080/14760584.2016.1213632.
- Berry N, Manoussaka M, Ham C, Ferguson D, Tudor H, Mattiuzzo G, Klaver B, Page M, Stebbings R, Das AT, et al. Role of occult and post-acute phase replication in protective immunity induced with a novel live attenuated SIV vaccine. PLoS Pathog. 2016;12(12):e1006083. doi:10.1371/journal.ppat.1006083.
- Maggioli MF, Palmer MV, Thacker TC, Vordermeier HM, McGill JL, Whelan AO, Larsen MH, Jacobs Jr. WR, Waters WR. Increased TNF-alpha/IFN-gamma/IL-2 and Decreased TNF-alpha/IFN-gamma production by central memory T cells are associated with protective responses against bovine tuberculosis following BCG vaccination. Front Immunol. 2016;7:421. doi:10.3389/fimmu.2016.00421.
- Mordmuller B, Surat G, Lagler H, Chakravarty S, Ishizuka AS, Lalremruata A, Gmeiner M, Campo JJ, Esen M, Ruben AJ, et al. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature. 2017;542(7642):445–49. doi:10.1038/nature21060.
- Gross S, Erdmann M, Haendle I, Voland S, Berger T, Schultz E, Strasser E, Dankerl P, Janka R, Schliep S, et al. Twelve-year survival and immune correlates in dendritic cell-vaccinated melanoma patients. JCI Insight. 2017;2(8):e91438. doi:10.1172/jci.insight.91438.
- Lopez-Fauqued M, Campora L, Delannois F, El Idrissi M, Oostvogels L, De Looze FJ, Diez-Domingo J, Heineman TC, Lal H, McElhaney JE, et al. Safety profile of the adjuvanted recombinant zoster vaccine: pooled analysis of two large randomised phase 3 trials. Vaccine. 2019;37(18):2482–93. doi:10.1016/j.vaccine.2019.03.043.