ABSTRACT
Clinical development of Ebola virus vaccines (EVV) was accelerated by the West African Ebola virus epidemic which remains the deadliest in history. To compare and rank the EVV according to their immunogenicity and safety. A total of 21 randomized controlled trial, evaluating seven different vaccines with different doses, and 5,275 participants were analyzed. The rVSVΔG-ZEBOV-GP (2 × 10 7) vaccine was more immunogenic (P-score 0.80). For pain, rVSVΔG-ZEBOV-GP (≤10 5) had few events (P-score 0.90). For fatigue and headache, the DNA-EBOV (≤ 4 mg) was the best one with P-scores of 0.94 and 0.87, respectively. For myalgia, the ChAd3 (10 10) had a lower risk (P-score 0.94). For fever, the Ad5.ZEBOV (≤ 8 × 10 10) was the best one (P-score 0.80). The best vaccine to be used to stop future outbreak of Ebola is the rVSVDG-ZEBOV-GP vaccine at dose of 2 × 107 PFU.
Introduction
According to the World Health Organization (WHO), the 2013–16 West African Ebola Virus epidemic (EBOV) was the largest and deadliest in history with more than 28,600 confirmed infections and over 11,300 deaths.Citation1,Citation2
Since this epidemic, many efforts have been made to accelerate the development of candidate vaccines against Ebola virus disease (EVD). To date, only the recombinant replication competent vesicular stomatitis virus-based vaccine expressing the glycoprotein of a Zaire Ebolavirus licensed as ERVEBO® (rVSVΔG-ZEBOV-GP) at a dose of 2 × 107 plaque-forming unit (PFU) has been proven to have a high efficacy and effectiveness to prevent EVD in contacts and contacts of contacts of recently confirmed cases in Guinea (Conakry) and Sierra-Leonne.Citation3 However, some safety concerns related to this vaccine, in particular one anaphylaxis,Citation3 four arthralgia,Citation4 and 19 arthritisCitation4 have been reported. The need for an alternative vaccine is all the more crucial since vaccination not only prevents infection but also limits its severity: in a recent therapeutic study including affected patients during the 2018 EVD outbreak in the Democratic Republic of Congo (RDC), those who had been vaccinated had less severe clinical forms at baseline.Citation5
Many other vaccines have been tested in healthy volunteers, with promising results on both safety and immunogenicity, but with very different protocols (design, dose administered or timing of evaluation).Citation6,Citation7 With the multiplicity of clinical trials against EVD, data on an effective vaccine with the optimal dose are needed to control any future outbreak like the one that happened in West Africa or more recently in the Democratic Republic of Congo.Citation5
To our knowledge, no systematic review or meta-analysis has been conducted to summarize published data on candidate vaccines from clinical trials studies in healthy adults to identify the most effective in terms of safety and immunogenicity. We therefore conducted a network meta-analysis (NMA) to compare and rank candidate vaccines tested in healthy adults in terms of safety and immunogenicity to prevent EVD.
Methods
Search strategy and selection criteria
We searched through MEDLINE, EMBASE, and Cochrane library (CENTRAL) for randomized controlled trials (RCTs) that investigated Ebola virus vaccine safety and immunogenicity in healthy adults. Despite the extensively safety and immunogenicity databases mainly for rVSV and Ad26 or MVA vaccines, we choose to restrict our search to the RCTs which gives the highest evidence level. The search was restricted to any phase trials (1, 2, and 3) conducted in human, and published in English before November 30, 2020. For studies that involve prime/boost, only data from the prime vaccination evaluated 28 days was considered. Studies in which participant were nonrandomly allocated to receive Ebola virus vaccine, or in which a combination of vaccines was used, or in which participants were aged less than 18 years, or in which information on outcomes was lacking were excluded. Using the search terms listed in the Methods in the Supplement, two authors (ADi and VW) identified all relevant studies, then independently reviewed their full texts, and in case of disagreement, differences were resolved through the arbitration by another author (MCB). Extracted data included: first author name and year of publication, country, RCTs design, study follow-up, age (range), proportion of men participants, vaccine type and dosing information, sample size, proportion or number of participants with seroconversion or seroresponse and adverse events, and study sponsorship (Government and/or Industry). The study protocol was registered in the International Register of Systematic Reviews (PROSPERO), number CRD42018109473.
Outcomes
The primary outcome was immunogenicity assessed by either the seroconversion rate-proportion of participants who showed at least a four-fold increase in antibody titer for enzyme-linked immunosorbent assay (ELISA) 28 days after vaccination-, or seroresponse rate – proportion of participants who had a protective titer (seropositivity) measured by ELISA titer above a prespecified threshold 28 days after vaccination. Because of the variability of the method used to assess immunogenicity of Ebola virus vaccine, we considered only seroconversion or seroresponse rates based on ELISA titers at 28 days. In case both seroconversion and seroresponse were available in a study, only the seroconversion rate was used, as it is less sensitive to baseline status. Secondary outcomes were the most common Adverse Events (AEs) occurring within the first 14 days post vaccination, and recorded according to the Common Terminology Criteria for Adverse Events.Citation8 The two categories of Adverse Events outcomes considered in this network meta-analysis were the solicited local reaction, mainly injection-site pain, and the systematic reactions including fatigue, headache, fever, and myalgia. Because of the limited number of recorded Grade 3 adverse events, we subtracted them from the analysis, leaving only the reported mild (Grade 1) and moderate (Grade 2), which were analyzed together as a single outcome.
Data analysis
The original clinical trials were described using table of study characteristics and forest plot. The Cochrane risk of bias toolsCitation9 and Revman version 5.3 were used to assess the risk of bias and to generate the corresponding figures. We opted for a frequentist approach to compare safety and immunogenicity between candidates vaccine using a random-effects network meta-analysis (NMA) for binary endpoint. Summary estimates were reported as odds ratio (OR) with their reported 95% confidence intervals. For immunogenicity, beneficial vaccine effects are described by ORs > 1, while for the safety outcomes, beneficial vaccine effects are described by ORs < 1. Because of the large variety of tested dose by vaccine, we grouped them in 12 categories (≤ 4 mg, 8 mg, ≤ 105, 106, 107, 2 × 107, 5 × 107, 108, 1010, ≤8 × 1010, 1011, and 1.6 × 1011), then we considered these categories as separate nodes in the network. For each dose group, we distinguished between placebo, Ad26.ZEBOV, Ad5-EBOV, Chad3-ZEBOV, DNA-EBO, MVA-BN-Filo, rVSVN4CT1-EBOVGP1, and rVSVΔG-ZEBOV-GP, thereby giving 18 different groups of vaccine to compare. Full names of these acronyms are defined in appendix (page 6). To display the relative efficacy on immunogenicity and adverse events outcomes of all available pairwise comparisons between vaccine, league tables were used. A P-score ranging from 0 (worst vaccine) to 1 (best vaccine) was computed for each vaccine, then the vaccine with the highest P-score was selected as the preferred vaccine regimen for each considered outcome. Heterogeneity and inconsistency were quantified using the global Q test proposed by Rucker.Citation10 The Q statistic is the sum of statistic for heterogeneity, which represent the proportion of the total variation in study estimates (within-designs), and a statistic for inconsistency (between-designs), which represents the variability of the vaccines effect between direct and indirect comparisons at the meta-analytic level.Citation10 To visualize and identify nodes of single-design inconsistency, we used a network heat plot. Consistency between direct and indirect comparisons was checked using the so-called node-splitting. We conducted three sensitivity analyses, first to assess sponsorship bias by excluding studies sponsored by industrial companies, second to test for differences between health care system by excluding studies conducted in Africa, and finally to assess the impact of the risk of bias by excluding studies with a moderate risk of bias. No subgroup analysis was performed. All analyses were performed using R package ‘netmeta’;Citation10 P-values <.05 were considered significant for the difference between vaccines.
Results
Included studies
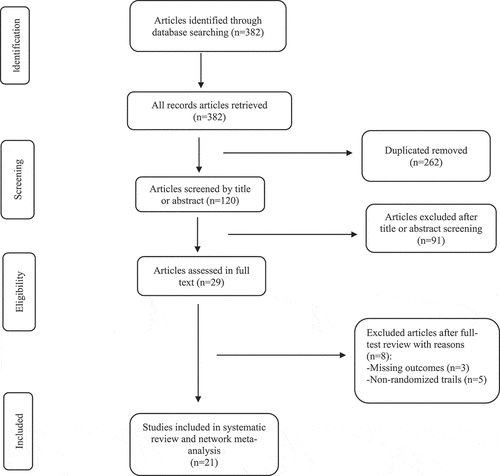
The initial search through all database identified 382 citations, of which 120 were screened by title and abstract after removing duplicates. Of the 29 full-text citations reviewed, 21 RCTsCitation4,Citation11–31 that met the inclusion criteria were finally included in the quantitative network meta-analysis (). These RCTs were published from 2006 to 2020, 13 in high impact factor journals (9 in The Lancet, 3 in The NEJM, 1 in JAMA). Fifteen were phase 1 trial, 2 were phase 3 trials, 17 were blinded, 15 were conducted in high-income countries, and 18 were sponsored by Government institutions. For the phase 3 study conducted in Sierra-Leonne, we included only the participants involved in the safety sub-study (n = 449). Together, these 21 RCTs included 5,275 healthy volunteers aged 18 and 65 years, of whom 2,983 (56.5%) were male. A total of 62 comparisons for immunogenicity () were investigated in a follow-up time ranging from 12 weeks to 12 months ().
Table 1. Characteristics of included trials investigating the immunogenicity and safety of Ebola virus vaccination
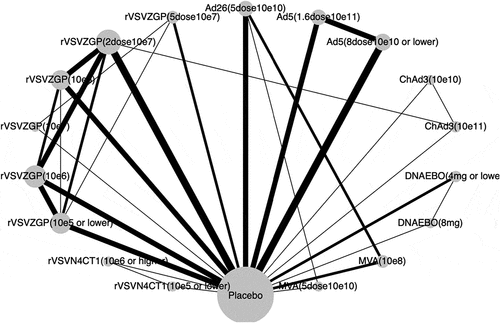
Figure 2. Network graph of eligible Ebola vaccines comparisons for immunogenicity
The methodological quality of the included RCTs is shown in . Overall, the risk of bias was low in nine RCTs, and moderate in the others (Figure S1 and Table S1 in the Supplement). A higher risk of attrition bias (incomplete outcome data), detection bias (blinding outcome assessment), and performance bias (blinding participants and personnel) occurred in 4, 2, and 2 of 21 RCTs respectively.
Immunogenicity
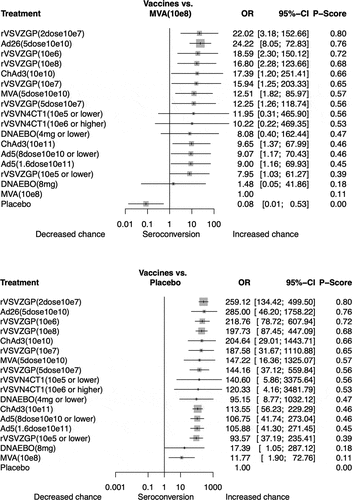
Of the 5,275 participants, 3,110 (59.0%) had seroconverted at 28-days post-vaccination. shows the network for immunogenicity captured by the immunogenicity of candidate vaccine against Ebola virus. All vaccines were significantly more immunogenic than placebo (), and the corresponding pairwise comparisons are summarized in the Supplement (Table S2). The vaccine with the highest probability of giving the highest seroconversion rate was the recombinant vesicular virus-based vaccine expressing a Zaire Ebola virus glycoprotein (rVSVΔG-ZEBOV-GP) at the dose of 2 × 107 PFU, with a P-score of 0.80, and an OR over Placebo of 259.1 (95% CI 134.4–449.5). Among vaccines, we found that the MVA-BN-Filo at dose of 108 TCID50 (50% tissue culture infectious doses) was significantly less immunogenic. Compared to the latter, patients in the adenovirus type-26 vector-based Ebola group (Ad26-ZEBOV) at doses of 5 × 1010 VP had 24 times more likely to be immunized 28-days after vaccination (Figure S2). Only 2 others pairwise comparisons were significant: the rVSVΔG-ZEBOV-GP vaccine with doses of 2 × 107 PFU (OR 2.77, 1.24–6.21) and 106 PFU (2.34, 1.08–5.06) conferred more immunogenicity than lower dose (≤ 5 × 105 PFU). Likewise, no significant differences were found between direct and indirect treatment estimates comparisons or evidence of publication bias according to the comparison-adjusted funnel plot (Figure S3 in the Supplement).
Safety
A total of 9,194 adverse events were reported between seven- and 14-days post-vaccination, in which 127 (1.4%) were severe (SAEs). The ratio of adverse events per participant was 1.7 (9,194/5,275) meaning that on average each participant reported at least more than one adverse reaction (local or systemic). The most commonly reported mild-to-moderate AEs were injection-site pain (1,544 AEs), headache (1,578), fatigue (1,007), myalgia (810), and fever (1,145). Likewise, among the 127 severe reported adverse reactions, the most prevalent were headache (33), fever (32), fatigue (20), chills (15), and myalgia (13).
rVSVΔG-ZEBOV-GP (≤105) was associated with a 90% lower probability of injection-site pain (P-score 0.90). Importantly, the lower dose (≤ 105 PFU) of the rVSVΔG-ZEBOV-GP vaccine induced a lower risk of pain compared to the same vaccine at higher doses. The corresponding risk reduction of pain were 78% (0.22, 0.08–0.60) for 106 PFU, 85% (0.15, 0.06–0.41) for 2 × 107 PFU, 88% (0.12, 0.04–0.40) for 108 PFU, 93% (0.07, 0.01–0.76) for 107 PFU, and 93% (0.07, 0.02–0.33) for 5 × 107 PFU. In addition, the lower dose (105 PFU) of the rVSVΔG-ZEBOV-GP vaccine reduced by 83% (0.17, 0.04–0.67) the risk of injection site pain when compared to the Ad5 (1.6 × 1011) vaccine (Table S3 in the Supplement).
For fatigue, the network meta-analysis was conducted in 19 RCTs involving 17 groups of vaccines and 59 pairwise comparisons. We found that the DNA-EBOV vaccine at dose of ≤ 4 mg was associated with less fatigue symptoms with a higher probability (P-score 0.94) of being the best one. The fatigue risk reduction for DNA-EBOV vaccine (≤ 4 mg) was 82% (0.18, 0.03–0.95) compared to the ChAd3 vaccine at dose of 1011, 84% (0.16, 0.03–0.80) for 106 PFU, 84% (0.16, 0.03–0.73) for 2 × 107 PFU, and 88% (0.12, 0.02–0.61) for 108 PFU. Compared to placebo, the highest dose of ChAd3 vaccine (1011) multiplied by 2.76 (1.26–6.03) the risk of fatigue (). The corresponding increased risk of fatigue compared with rVSVΔG-ZEBOV-GP vaccine were 3.12 (1.62–6.00) for 106 PFU, 3.19 (1.98–5.15) for 2 × 107 PFU, and 4.18 (2.12–8.02) for 108 PFU. In addition, a higher dose (106 PFU) of rVSVN4CT1-EBOVGP1 vaccine was significantly associated with an increased risk of fatigue compared with other vaccines, except for the rVSVΔG-ZEBOV-GP vaccine at dose of 107 PFU (0.05, 0.00–1.42) and 108 PFU (0.06, 0.00–1.29) (Table S4 in the Supplement). Finally, a lower dose (≤ 105) of rVSVΔG-ZEBOV-GP vaccine was associated with 57% (0.43, 0.20–0.91) risk reduction of fatigue compared with a higher dose (108 PFU).
Figure 5. Pairwise comparisons in network meta-analysis for safety outcomes (injection site-pain, fatigue, headache, myalgia, and fever)

For headache, pairwise comparisons were performed in all RCTs. As for fatigue, the DNA-EBOV vaccine at dose of ≤ 4 mg gave the best results (P-score 0.87). A significantly lower risk of headache was seen in 39 of the 63 indirect comparisons (Table S5 in the Supplement). The specific risk reductions of headache for DNA-EBOV vaccine (≤ 4 mg) were 80% (0.20, 0.04–0.97) and 99% (0.01, 0.00–0.20) compared to the ChAd3 vaccine at dose of 1011 and to the rVSVN4CT1-EBOVGP1 at dose of 106 PFU, respectively. The corresponding risk reductions of headache compared to the rVSVΔG-ZEBOV-GP vaccine were 77% (0.23, 0.07–0.79) for 106 PFU, 77% (0.23, 0.07–0.75) for 2 × 107 PFU, 78% (0.22, 0.07–0.76) for 108 PFU, and 82% (0.18, 0.03–0.97) for 107 PFU. In addition, when compared with rVSVΔG-ZEBOV-GP at dose of 2 × 107 PFU, the risk of headache was reduced by 67% (0.33, 0.12–0.94) for MVA-BN-Filo (108 TCID50), 62% (0.38, 0.19–0.78) for Ad5-EBOV (≤ 8 × 1010), 60% (0.40, 0.25–0.64) for rVSVΔG-ZEBOV-GP (≤ 105), and 60% (0.40, 0.25–0.64) for Ad5-EBOV (1.6 × 1011). The corresponding risk reduction of headache compared with rVSVΔG-ZEBOV-GP at dose of 106 and 108 PFU were shown in supplemental Table S5. As for fatigue, a higher dose (106 PFU) of rVSVN4CT1-EBOVGP1 vaccine was significantly associated with an increased risk of headache, except for the rVSVΔG-ZEBOV-GP vaccine at dose of 107 PFU (0.03, 0.00–1.13).
The risk of myalgia was assessed in 19 RCTs involving 18 different groups of vaccines and 61 pairwise comparisons. ChAd3 at dose of 1010 VP was associated with a lower risk of myalgia (P-score 0.94). A total of 26 indirect comparisons were significant, with a 40% to 96% risk reduction of myalgia (Table S6 in the Supplement). These specific risk reductions were more important for ChAd3 vaccine (1010 VP) compared with rVSVΔG-ZEBOV-GP: 92% (0.08, 0.01–0.95) for 2 × 107 PFU, 94% (0.06, 0.00–0.98) for 107 PFU, 94% (0.06, 0.01–0.73) for 106 PFU, 94% (0.06, 0.00–0.65) for 108 PFU, and 96% (0.04, 0.00–0.53) for 5 × 107 PFU ().
For fever, data were available in 19 RCTs with 17 different groups vaccines yielding 57 pairwise comparisons. Ad5-EBOV at dose of ≤ 8 × 1010 was ranked as giving the best results (P-score 0.80). This vaccine was associated with a lower risk of fever (0.19, 0.06–0.57) compared with ChAd3 vaccine (1011). The corresponding specific risk reduction of fever for Ad5-EBOV vaccine (8 × 1010) compared with rVSVΔG-ZEBOV-GP were 82% (0.18, 0.06–0.52) for 106 PFU, 88% (0.12, 0.05–0.30) for 2 × 107 PFU, 90% (0.10, 0.03–0.28) for 108 PFU, and 91% (0.09, 0.02–0.37) for 5 × 107 PFU. In addition, ChAd3 (1011) and rVSVΔG-ZEBOV-GP vaccines (106, 2 × 107, 5 × 107, and 108) were associated with more fever than placebo, a lower dose of rVSVΔG-ZEBOV-GP vaccine (≤ 105), and Ad5-EBOV vaccine (≤ 8 × 1010 and 1.6 × 1011) (Table S7 in the Supplement). The remaining significant comparisons for headache, myalgia, and fever were showed in the Supplement (Table S5 to Table S7).
Heterogeneity, consistency, and sensitivity
Except for injection-site pain, fatigue, and fever, no general heterogeneity or inconsistency of vaccine effect was found (P value greater than 0.05; I2 ranging from 0% to 18%). These finding were supported by the heat plot displayed in the Supplemental Figures S4 to S9. In sensitivity analysis, after excluding the nine studiesCitation12–14,Citation19,Citation21,Citation24,Citation27–30 sponsored by the Industrial companies or the seven ones conducted in Africa,Citation15,Citation17,Citation20,Citation21,Citation26–28 the Ad5.ZEBOV (1.6 × 1011) became the most immunogenic vaccine (P-score 0.91 and 0.88, respectively). Finally, after considering only the nine studies with a lower risk of bias,Citation4,Citation13,Citation15,Citation17,Citation19,Citation21,Citation22,Citation26,Citation31 the rVSVΔG-ZEBOV-GP (108) became the most immunogenic vaccine (P-score 0.76).
Discussion
This study, based on 21 RCTs involving 5,275 healthy volunteers randomly assigned to 18 different groups of candidate vaccines, is the first network meta-analysis of vaccines against Ebola virus. Considering immunogenicity, we found that the rVSVΔG-ZEBOV-GP vaccine at the dose of 2 × 107 PFU was the most effective available option. These findings support the high protective role of this treatment option to prevent Ebola virus disease, as previously reported from individual phase 1 and/or 2 studies, and data from phase 3 conducted in contacts and contacts of contacts in Guinea (Conakry) and Sierra-Leonne.Citation3 We found a good overall consistency of the network meta-analysis for immunogenicity.
Despite a rapid immune response, the safety profile of the rVSVΔG-ZEBOV-GP vaccine (2 × 107) would be questionable for mass vaccination in the absence of immediate risk of exposure. Compared with others vaccines, we found increased injection site-pain and fever reported by patients who received a single dose of this vaccine. These findings are in lines with those reported in Ebola ça Suffit trial where 80 serious adverse events were identified, of which two were judged to be related to vaccination (one febrile reaction and one anaphylaxis). A high reactogenicity may increases vaccine hesitancy, especially in Africa where unfavorable socioeconomic factors, low level of health education, lack of disease awareness, religious and cultural beliefs may decrease vaccination uptake. Future studies should be conducted in African populations that have experienced Ebola disease to investigate risk factors and barriers to vaccination.
In the sensitivity analysis, we found a substantial change in vaccines effect estimates from those seen in the overall network meta-analysis for immunogenicity. When excluding studies sponsored by the industry companiesCitation12–14,Citation19,Citation21,Citation24,Citation27–30 or those conducted elsewhere other than Africa area,Citation15,Citation17,Citation20,Citation21,Citation26–28 the Ad5.ZEBOV (1.6 × 1011 VP) became the most immunogenic vaccine, suggesting that Ad26.ZEBOV might be a possible alternative vaccine. Compared to placebo, the Ad26.ZEBOV vaccine gives the highest immunogenic level, and was associated with a lower rate of reactogenicity. Moreover, no difference in terms of risk difference (0.04 [95% CI; −0.13 to 0.20]) were observed as compared to the rVSVΔG-ZEBOV-GP (2 × 107) vaccine. Pending the results of a large randomized trial between these two vaccines, we recommend to use the Ad26.ZEBOV (5 × 1010 VP) vaccine with an MVA boost in cases of contraindication or limited availability of the rVSVΔG-ZEBOV-GP (2 × 107) vaccine to stop future outbreak of Ebola. However, in both indirect comparisons, the precision (95% CI) of vaccine effect estimates for Ad26.ZEBOV (1.6 × 1011) compared with rVSVΔG-ZEBOV-GP (2 × 107) is high, reflecting the relatively small number of participants contributing to the network meta-analysis. The rVSVΔG-ZEBOV-GP vaccine (2 × 107) ranked 4th out of 15 groups of vaccines and 5th out of 17 groups of vaccines, respectively (sensitives analyses). In addition, after taking into account only studies with a low risk of bias, the rVSVΔG-ZEBOV-GP (108) become the most immunogenic vaccine, while the same vaccine at dose of 2 × 107 was ranked 3th out of 14 groups of treatments. Nevertheless, these sensitivity analyses were performed on a small number of RCTs with few participants which may have reduced the power of the test.
Some limitations were present in this study. First, the classification used to define these 18 groups of vaccines for comparisons is disputable, and possibly other categorization would result in different conclusions. In addition, the conclusions of the overall network analysis differ substantially from those of the sensitivity analyses, mainly after exclusion of studies sponsored by industrial companies or those conducted outside Africa, suggesting caution in interpretating the data. Second, the different ELISA assays methods and the different thresholds used to define seroconversion rates for immunogenicity may influence the results of the efficacy analysis. Standardized methods would be preferable in order to improve the conclusion in future studies.Citation32 An alternative approach to the use of a single time point of ELISA data would be to focus on peak titers regardless of time point, although neglecting the value of the onset of protection is a problem with this method. Third, the use as a single time-point of the outcome analysis, 28-day after single immunization which limits the ability to assess immunogenicity of Ebola vaccines of more than one dose regimen. Moreover, the methods of comparing vaccines with different immunization regimens in a network meta-analysis remains an open question due to transitivity requirement. Finally, as the search was restricted to English language trials, a residual publication bias is possible despite our effort to locate unpublished trials through ClinicalTrial.gov. Nevertheless, Jüni Peter et al. suggest that bias induces by excluding non-language English trials has only modest effects on aggregated treatment effect estimates.Citation33
Our findings suggest that the rVSVΔG-ZEBOV-GP vaccine with dose of 2 × 107 has the strongest evidence for being the most effective vaccine in terms of immunogenicity to prevent the next outbreak of Ebola virus disease. These findings have implications on the design of future clinical trials, and management of the next outbreak of Ebola Virus Disease. Future large prospective RCTs are needed to draw final conclusions.
Abbreviations
Disclosure of potential conflicts of interest
We declare no competing interests in relation to this work.
Author contributions
ADi conceived the study design, analyzed the data, and drafted the manuscript, MCB supervision of data collection, and critical revision of the manuscript, VW collected data, MHD analyzed the data, BDD, AD, MCG, and FG interpreted and substantially revised the manuscript. All authors approved the final version of the manuscript.
Supplemental Material
Download Zip (103.8 MB)Availability of data and materials
The data are available upon request ([email protected]).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1932214.
References
- WHO: Ebola virus disease [situation report] Geneva, Switzerland: World Health Organization; 2016http://apps.who.int/ebola/ebola-situation-reports .
- Urbanowicz RA, McClure CP, Sakuntabhai A, Sall AA, Kobinger G, Müller MA, Holmes EC, Rey FA, Simon-Loriere E, Ball JK, et al. Human adaptation of Ebola virus during the West African outbreak. Cell. 2016;167:1079–1087.e5. doi:10.1016/j.cell.2016.10.013.
- Henao-Restrepo AM, Camacho A, Longini IM, Watson CH, Edmunds WJ, Egger M, Carroll MW, Dean NE, Diatta I, Doumbia M, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!). Lancet. 2017;389(10068):505–18. doi:10.1016/S0140-6736(16)32621-6.
- Heppner D, Kemp T, Martin B, Ramsey W, Nichols R, Dasen E, Link CJ, Das R, Xu ZJ, Sheldon EA, et al. Safety and immunogenicity of the rVSV∆G-ZEBOV-GP Ebola virus vaccine candidate in healthy adults: a phase 1b randomised, multicentre, double-blind, placebo-controlled, dose-response study. Lancet Infect Dis. 2017;17:854‐866. doi:10.1016/S1473-3099(17)30313-4.
- Mulangu S, Dodd LE, Davey RT, Tshiani Mbaya O, Proschan M, Mukadi D, Lusakibanza Manzo M, Nzolo D, Tshomba Oloma A, Ibanda A, et al. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381:2293–303. doi:10.1056/NEJMoa1910993.
- Martins KA, Jahrling PB, Bavari S, Kuhn JH. Ebola virus disease candidate vaccines under evaluation in clinical trials. Expert Rev Vaccines. 2016;15:1101–12. doi:10.1080/14760584.2016.1187566.
- Wang Y, Li J, Hu Y, Liang Q, Wei M, Zhu F. Ebola vaccines in clinical trial: the promising candidates. Hum Vaccines Immunother. 2017;13:153–68. doi:10.1080/21645515.2016.1225637.
- U.S. Department of Health and Human Services National Institutes of Health, National Cancer Institute. Common terminology criteria for adverse events (CTCAE) Version 5.0. 2017 Nov 27.
- Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JAC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928–d5928. doi:10.1136/bmj.d5928.
- Schwarzer G, Carpenter JR, Rücker G. Meta-analysis with R. Springer International Publishing; 2015. p. 252.
- Martin J, Sullivan N, Enama M, Gordon I, Roederer M, Koup R, Bailer RT, Chakrabarti BK, Bailey MA, Gomez PL, et al. A DNA vaccine for Ebola virus is safe and immunogenic in a phase I clinical trial. Clin Vaccine Immunol. 2006;13:1267‐1277. doi:10.1128/CVI.00162-06.
- Ledgerwood J, Costner P, Desai N, Holman L, Enama M, Yamshchikov G, Mulangu S, Hu Z, Andrews CA, Sheets RA, et al. A replication defective recombinant Ad5 vaccine expressing Ebola virus GP is safe and immunogenic in healthy adults. Vaccine. 2010;29:304‐313. doi:10.1016/j.vaccine.2010.10.037.
- Zhu F-C, Hou L-H, Li J-X, Wu S-P, Liu P, Zhang G-R, Hu Y-M, Meng F-Y, Xu -J-J, Tang R, et al. Safety and immunogenicity of a novel recombinant adenovirus type-5 vector-based Ebola vaccine in healthy adults in China: preliminary report of a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet. 2015;385:2272–79. doi:10.1016/S0140-6736(15)60553-0.
- Huttner A, Dayer J, Yerly S, Combescure C, Auderset F, Desmeules J, Eickmann M, Finckh A, Goncalves AR, Hooper JW, et al. The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: a randomised double-blind, placebo-controlled phase 1/2 trial. Lancet Infect Dis. 2015;15:1156‐1166. doi:10.1016/S1473-3099(15)00154-1.
- Kibuuka H, Berkowitz N, Millard M, Enama M, Tindikahwa A, Sekiziyivu A, Costner P, Sitar S, Glover D, Hu Z, et al. Safety and immunogenicity of Ebola virus and Marburg virus glycoprotein DNA vaccines assessed separately and concomitantly in healthy Ugandan adults: a phase 1b, randomised, double-blind, placebo-controlled clinical trial. Lancet Lond Engl. 2015;385:1545‐1554.
- Agnandji S, Huttner A, Zinser M, Njuguna P, Dahlke C, Fernandes J, Yerly S, Dayer J-A, Kraehling V, Kasonta R, et al. Phase 1 trials of rVSV Ebola vaccine in Africa and Europe. N Engl J Med. 2016;374:1647‐1660. doi:10.1056/NEJMoa1502924.
- Tapia M, Sow S, Lyke K, Haidara F, Diallo F, Doumbia M, Traore A, Coulibaly F, Kodio M, Onwuchekwa U, et al. Use of ChAd3-EBO-Z Ebola virus vaccine in Malian and US adults, and boosting of Malian adults with MVA-BN-Filo: a phase 1, single-blind, randomised trial, a phase 1b, open-label and double-blind, dose-escalation trial, and a nested, randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2016;16:31‐42.
- De Santis O, Audran R, Pothin E, Warpelin-Decrausaz L, Vallotton L, Wuerzner G, Cochet C, Estoppey D, Steiner-Monard V, Lonchampt S, et al. Safety and immunogenicity of a chimpanzee adenovirus-vectored Ebola vaccine in healthy adults: a randomised, double-blind, placebo-controlled, dose-finding, phase 1/2a study. Lancet Infect Dis. 2016;16:311‐320. doi:10.1016/S1473-3099(15)00486-7.
- Milligan I, Gibani M, Sewell R, Clutterbuck E, Campbell D, Plested E, Nuthall E, Voysey M, Silva-Reyes L, McElrath MJ, et al. Safety and immunogenicity of novel adenovirus type 26- and modified vaccinia ankara-vectored Ebola vaccines: a randomized clinical trial. JAMA. 2016;315:1610‐1623. doi:10.1001/jama.2016.4218.
- Agnandji S, Fernandes J, Bache E, Obiang Mba R, Brosnahan J, Kabwende L, Pitzinger P, Staarink P, Massinga-Loembe M, Krähling V, et al. Safety and immunogenicity of rVSVDELTAG-ZEBOV-GP Ebola vaccine in adults and children in Lambarene, Gabon: a phase I randomised trial. Plos Med. 2017;14. doi:10.1371/journal.pmed.1002402.
- Zhu F, Wurie A, Hou L, Liang Q, Li Y, Russell J, Wu SP, Li JX, Hu YM, Guo Q, et al. Safety and immunogenicity of a recombinant adenovirus type-5 vector-based Ebola vaccine in healthy adults in Sierra Leone: a single-centre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Lond Engl. 2017;389:621‐628.
- Kennedy S, Bolay F, Kieh M, Grandits G, Badio M, Ballou R, Eckes R, Feinberg M, Follmann D, Grund B, et al. Phase 2 placebo-controlled trial of two vaccines to prevent Ebola in Liberia. N Engl J Med. 2017;377:1438‐1447. doi:10.1056/NEJMoa1614067.
- ElSherif M, Brown C, MacKinnon-Cameron D, Li L, Racine T, Alimonti J, Rudge TL, Sabourin C, Silvera P, Hooper JW, et al. Assessing the safety and immunogenicity of recombinant vesicular stomatitis virus Ebola vaccine in healthy adults: a randomized clinical trial. CMAJ Can Med Assoc J. 2017;189:E819‐E827. doi:10.1503/cmaj.170074.
- Li J, Hou L, Meng F, Wu S, Hu Y, Liang Q, Chu K, Zhang Z, Xu -J-J, Tang R, et al. Immunity duration of a recombinant adenovirus type-5 vector-based Ebola vaccine and a homologous prime-boost immunisation in healthy adults in China: final report of a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Glob Health. 2017;5:e324‐e334. doi:10.1016/S2214-109X(16)30367-9.
- Regules J, Beigel J, Paolino K, Voell J, Castellano A, Hu Z, Muñoz P, Moon JE, Ruck RC, Bennett JW, et al. A recombinant vesicular stomatitis virus Ebola vaccine. N Engl J Med. 2017;376:330‐341. doi:10.1056/NEJMoa1414216.
- Mutua G, Anzala O, Luhn K, Robinson C, Bockstal V, Anumendem D, Douoguih M. Safety and Immunogenicity of a 2-dose heterologous vaccine regimen with Ad26.ZEBOV and MVA-BN-filo Ebola vaccines: 12-month data from a phase 1 randomized clinical trial in Nairobi, Kenya. J Infect Dis. 2019;220:57‐67. doi:10.1093/infdis/jiz071.
- Anywaine Z, Whitworth H, Kaleebu P, Praygod G, Shukarev G, Manno D, Kapiga S, Grosskurth H, Kalluvya S, Bockstal V, et al. Safety and immunogenicity of a 2-dose heterologous vaccination regimen with Ad26.ZEBOV and MVA-BN-filo Ebola vaccines: 12-month data from a phase 1 randomized clinical trial in Uganda and Tanzania. J Infect Dis. 2019;220(1):46‐56. doi:10.1093/infdis/jiz070.
- Samai M, Seward JF, Goldstein ST, Mahon BE, Lisk DR, Widdowson MA, Jalloh MI, Schrag SJ, Idriss A, Carter RJ, et al. The Sierra Leone trial to induce a vaccine against Ebola: an evaluation of rVSVΔG-ZEBOV-GP vaccine tolerability and safety during the West Africa Ebola outbreak. J Infect Dis. 2018;217:S6‐15. doi:10.1093/infdis/jiy020.
- Halperin SA, Arribas JR, Andrews CP, Chu L, Das R, Simon JK, Simon JK, Onorato MT, Liu K, Martin J, et al. Six-month safety data of recombinant vesicular stomatitis virus ebola envelope glycoprotein vaccine in a phase 3 double-blind, placebo-controlled randomized study in healthy adults. J Infect Dis. 2017;215:1789‐98. doi:10.1093/infdis/jix189.
- Halperin SA, Das R, Onorato MT, Lui K, Martin J, Grant-Klein R, Nichols R, Coller B-A, Helmond FA, Simon JK, et al. Immunogenicity, lot consistency, and extended safety of rVSVΔG-ZEBOV-GP vaccine: a phase 3 double-blind, placebo-controlled randomized study in healthy adults. J Infect Dis. 2019;220:1127‐35. doi:10.1093/infdis/jiz241.
- Clark DK, Xu R, Matassov D, Latham TE, Ota-Setlik A, Gerardi C, Luckay A, Witko SE, Hermida L, Higgins T, et al. Safety and immunogenicity of a highly attenuated rVSVN4CT1-EBOVGP1 Ebola virus vaccines: a randomized double-blind, placebo-controlled, phase 1 clinical trial. Lancet Infect Dis. 2020;20:455‐66.
- Niemuth NA, Rudge TL Jr, Sankovich KA, Anderson MS, Skomrock ND, Badorrek CS, Sabourin CL. Method feasibility for cross-species testing, qualification, and validation of the Filovirus animal nonclinical group anti-Ebola virus glycoprotein immunoglobulin G enzyme-linked immunosorbent assay for non-human primate serum samples. Plos ONE. 2020;15(10):e0241016. doi:10.1371/journal.pone.0241016.
- Jüni P, Holenstein F, Sterne J, Bartlett C, Egger M. Direction and impact of language bias in meta-analysis of controlled trials: empirical study. Int J Epidemiol. 2002;31:115–23. doi:10.1093/ije/31.1.115.