ABSTRACT
Objectives: This study summarizes passive surveillance data for adverse events following immunization (AEFI) in Zhejiang province.
Methods: The AEFI reports and number of doses on all vaccines used were extracted from the national AEFI surveillance system and the immunization information system of Zhejiang province (ZJIIS). Reporting rates of AEFI were calculated by age, city, severity of AEFI, categories of AEFI, vaccine types, and reaction categories.
Results: A total of 13,079 AEFI records were reported and 23,091,401 vaccine doses were administered, with a reporting rate of 56.64/100,000 doses for AEFI. The highest reporting rate of AEFI was observed among the infants <1 year of age (108.61/100,000 doses) and the lowest rate was observed among recipients aged ≥60 years. Most of the AEFI reports were vaccine product-related reactions (48.81/100,000 doses), and the lowest was vaccination errors (0.02/100,000 doses). The most frequently reported individual vaccine was DTP and Hib combined vaccine, with a reporting rate of 426.62/100,000 doses. The most frequently reported AEFI was fever/redness/induration (48.82/100,000 doses).
Conclusion: Our findings illustrated the high level of vaccine safety since the majority of those reported were not serious, or coincidentally associated with vaccination. Furthermore, the national AEFI surveillance system should be continuously used as a surveillance tool for monitoring of AEFI.
Introduction
The World Health Organization (WHO) recommends the systematic collection, analysis and evaluation of medical adverse events following immunization (AEFI) for expanded program on immunization (EPI) for all countries.Citation1,Citation2 The primary aim of the vaccination safety surveillance is the “early detection and analysis of adverse events to allow for appropriate and quick responses to emerging AEFI issues in order to decrease the negative impact on the health of individuals and the immunization program.”Citation1 Additionally, vaccination safety surveillance enables the detection of signals that will generate hypothesis, as well as the identification and rectification of gaps in this program to strengthen the EPI or routine vaccination.
Continuous assessment of the safety analysis of the post-licensure vaccines can provide a tool to evaluate the benefit-risk profiles of a specific vaccine which cannot be evaluated in pre-licensure clinical trials due to sample size limitations.Citation3–5 It also provides a communication channel to disseminate the up-to-date information to the public and helps to counteract the negative perceptions on vaccines and vaccination. It also reduces vaccine hesitancy by improving EPI information transparency. There is a good example from the AEFI surveillance system in Australia, which collects, collates and reviews the AEFI data submitted to the Therapeutic Goods Administration (TGA), the medicine and therapeutic regulatory agency of the Australian Government.Citation6 By efficiently running this system, Australia updates its vaccination schedules regularly, thereby maximizing the benefit–risk balance for the registered vaccines. However, the pharmacovigilance infrastructure of vaccines is always limited or even missing in most developing countries. This subsequently reduces the capacity for continuous review of AEFI subsequently.Citation7–9
China Ministry of Health (MOH) issued guidance for handling vaccine adverse reactions in 1980, but nationwide AEFI surveillance was not implemented until 2005. In March, 2005, with the technical support of the World Health Organization (WHO) and the experience from other countries, China established the passive surveillance system for AEFI, which was a passively collected spontaneous database, in 10 of China’s 31 provinces. In 2009, Zhejiang province joined in the national AEFI surveillance system.Citation10 The national AEFI surveillance system was upgraded in 2012, with adding variables of the case reporting form and improving the logic control of data entry and statistical functions.Citation11
The introduction of new vaccines such as human papilloma virus vaccine (HPV) or pentavalent rotavirus vaccine (RV5) needs strengthening of the AEFI surveillance systems in China. In addition, there is limited information on the performance, quality, responses to serious AEFI issues and the characteristics of the reported AEFIs in China. The aim of this study was to provide an initial evaluation of the performance and quality of the AEFI surveillance system in Zhejiang province by conducting a retrospective cross-sectional study and using the passive surveillance data in 2019. We provided a detailed analysis of the passively reported AEFIs through the demographic distribution, severity, type and classification of the AEFIs, and the reporting trends at monthly scale.
Methods
Study area
Zhejiang is a developed province with a large population of 70 million people in eastern areas of China. Of the total population, 7.23% children were under 7 years of age, 7.43% were 7–15 years old and 84.34% were above 15 years of age. Zhejiang province launched the EPI since 1978 with four vaccines and it continued to increase the number of vaccines up to 11 to date and with the administration of 20 million doses of vaccines each year.
According to the vaccination schedule of the EPI recommended by the health commission of China, children under 7 years were stipulated to receive the following vaccines free of charge: Calmette-Guérin vaccine (BCG) at birth; diphtheria-tetanus combined vaccine (DT) at 6 years of age; diphtheria-tetanus-pertussis combined vaccine (DTP) at 3,4,5 and 18 months of age; bivalent oral polio live-attenuated vaccine (bOPV) at 4 months and 4 years of age; inactivated polio vaccine (IPV) at 2 and 3 months of age; measles-mumps-rubella combined live attenuated vaccine (MMR) at 8 and 18 months of age; hepatitis B vaccine (HepB) at birth, 1 and 6 months of age; hepatitis A live attenuated vaccine (HepA-l) at 18 months of age; meningococcal polysaccharide vaccine type a (MPV-a) at 6 and 9 months of age; meningococcal polysaccharide vaccine types a and c (MPV-ac) at 3 and 6 years of age; Japanese encephalitis live-attenuated vaccine (JEV-l) at 8 and 24 months of age.
The BCG, the 1–3 doses of DTP, the 1–2 doses of IPV, the first dose of bOPV, the first dose of MMR, the 1–3 doses of HepB, the 1–2 doses of MPV-a and the first dose of JEV-I should be administered before 12 months of age. The fourth dose of DTP, the second dose of MMR, the first dose of HepA-l should be administered before 2 years of age. The DT, second dose of bOPV, the second dose of JEV-I, the 1–2 doses of MPV-ac should be administered before 7 years of age.
Additionally, the health commission of China also recommends children, adolescents or even adults to get the following self-paid vaccines: inactivated hepatitis A vaccine (HepA-i); inactivated Japanese encephalitis vaccine (JEV-i); meningococcal polysaccharide vaccine types a, c, y and w135 (MenV-acyw135); trivalent influenza vaccine (TIV); quadrivalent influenza vaccine (QIV); varicella-zoster live attenuated vaccine (VZV); Haemophilus influenzae type b vaccine (Hib); RV5; 23-valent pneumonia polysaccharide vaccine (PPV23); 13-valent pneumonia polysaccharide conjugate vaccine (PCV13); rabies virus vaccine (RRV); diphtheria-tetanus-pertussis-inactivated polio-Haemophilus influenzae type b combined vaccine (DTP-IPV-Hib); diphtheria-tetanus-pertussis-Haemophilus influenzae type b combined vaccine (DTP-Hib); meningococcal polysaccharide conjugate vaccine types a and c (MCV-ac); meningococcal types a and c- Haemophilus influenzae type b combined vaccine (Hib-MCV-ac); enterovirus vaccine type 71 (EV71); bivalent human papillomavirus vaccine (HPV2); quadrivalent human papillomavirus vaccine (HPV4); 9-valent human papillomavirus vaccine (HPV9); oral cholera vaccine (OCV).
Reporting and investigation procedures
Based on the guidelines for AEFI surveillance issued by the health commission of China, the AEFI is defined as a reaction or an event following vaccination that is suspected to be related to the vaccination. AEFI surveillance covers all vaccines marketed in mainland China. All AEFI cases should be mandatorily reported to the center for disease control and prevention (CDC) at county level by health centers, physicians, vaccine manufacturers, and members of the public if (i) the AEFI occurred with a reasonable temporal association (i.e., within 3 months after vaccination), (ii) no other plausible cause explained the event, and (iii) the AEFI fulfilled one or more of the following criteria: it is serious, previously unknown to occur after vaccination, or the main cause for a physician visit or hospitalization.Citation11
Each reported AEFI should be investigated by the CDC at different administrative levels. The variables included in the case-reporting form were date of report, age and sex of patient, kind and lot and manufacture of suspect vaccine(s), description of the AEFI, time interval after vaccination, duration of the event, final outcomes of AEFI, qualifications of vaccination clinic or vaccinator, cold chain management, vaccination site, route, dose number, dosage and any other additional remarks from the reporter. All the data should be entered into the online national AEFI surveillance system, which is operated in accordance with China’s national AEFI guidelines. These guidelines are supported by the Law on the Prevention and Treatment of Infectious Diseases of the People’s Republic of China,Citation11 the Pharmaceutical Administration Law of the People’s Republic of China, and other laws and regulations. Expert committees are organized to review the reported AEFIs and evaluate the vaccine safety profiles. Expert committees are composed of independent experts from clinical medicine, epidemiology, laboratory practices, pharmacy, vaccinology, vaccine regulation, and other relevant fields. In cases of co-administration of two or more vaccines in an individual, we attributed the reported AEFI to the reporter suspected vaccine.
Category of AEFI
All AEFI records were divided into five categories according to the guidelines issued by the health commission of China:Citation10 (1) vaccine product-related reaction (minor reaction and severe reaction); (2) vaccination error; (3) vaccine quality defect-related reaction; (4) coincidental event; (3) anxiety reaction.
Definition of severity
All AEFI were assessed as non-serious or serious and further subdivided into the following categories of severity including the definition for “serious” AEFI according to the guidelines issued by health commission of China: (1) non-serious, with no intervention necessary or with physician visit or event interfering with daily activities or loss of working hours; (2) serious, with any untoward medical occurrence that results in death, hospitalization, prolongation of hospitalization, persistent or significant disability/incapacity, life-threatening or birth defect.Citation12
Data extraction
The adverse events reported in this study covered the period of January 1 to December 31, 2019. The AEFI was extracted from the national AEFI surveillance system on February 1, 2020, when all the revision or modification of each case report had been done. The number of various vaccines doses in Zhejiang province in 2019 was obtained from the online individual immunization information system of Zhejiang province (ZJIIS), which was established in 2005. Average annual population data to calculate reporting rates were obtained from the Zhejiang provincial Bureau of Statistics.
Descriptive analysis
A database was organized as an Excel file (Microsoft Office Excel 2020). Reporting rates of AEFI were calculated by use of the Excel program. Reporting rates of AEFI (per 100,000 distributed doses) were calculated by vaccine categories and reaction categories. The injection site reaction could be determined by the record of vaccination but the systematic reactions could not be determined which vaccine was to be suspected when the co-administration occurred. In that case, we attributed the reported AEFI to all vaccines co-administered. Patients were categorized by the following age groups: 0 to 1 year, 2 to 4 years, 5 to 7 years, 8 to 14 years, and ≥15 years. The difference of AEFI reporting rates between gender, age group, city and type of vaccine were assessed by chi-square test, with a P-value of 0.05 or less was considered to be significant.
Results
A total of 13,079 AEFI records were reported in the national AEFI surveillance system between January 1 to December 31, 2019 in Zhejiang province and there were 23,091,401 vaccine doses administered (7,335,023 persons included) during the same time period, with a reporting rate of 56.64/100,000 doses for AEFI. Of the reported AEFI records, 390 (2.98%) were reported by health centers, 12689 (97.02%) were reported by county-level CDC, and there were no AEFI records reported by municipal-level CDC or vaccine manufacturers. Of these reported AEFI records, 127 (0.97%) records were serious and the other 12952 (99.03%) were non-serious, with the reporting rate of 0.55/100,000 doses for serious AEFI and 56.09/100,000 doses for non-serious AEFI, respectively.
The reporting rate of AEFI was 56.44/100,000 doses for male (6512/11,537,533) and 56.84/100,000 doses for female (6567/11,553,868), with no significant difference between genders (χ2 = 3.26, P> .05). For the total records of AEFI, the highest reporting rate was observed in May (77.70/100,000 doses) and the lowest reporting rate was observed in January (34.79/100,000 doses). For the serious AEFI, the highest reporting rate was observed in March (0.82/100,000 doses) and the lowest reporting rate was observed in February (0.28/100,000 doses) ().
Figure 1. AEFI records in the national AEFI surveillance system database, 2019, by month of onset of AEFI. (a): the total number of AEFI reported in 2019 with its reporting rate; (b): the number of serious AEFI reported in 2019 with its reporting rate
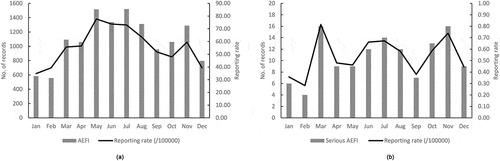
The highest AEFI reporting rate was observed among infants <1 year of age (108.61/100,000 doses), and the reporting rate of serious AEFI was 1.14/100,000 doses which was also the highest among all age group (χ2 = 355.86, P< .001). The sharp decrease in the reporting rate was also found in the elder age group, and the lowest reporting rate was observed among recipients aged ≥60 years ().
Table 1. Serious AEFI and non-serious AEFI records in the national AEFI surveillance system database, 2019, by age group
represents that most AEFI (3059, 23.39%) had been reported by Hangzhou, followed by Wenzhou (1850, 14.14%). The difference in AEFI reporting rate between cities was significant (χ2 = 51.30, P< .05). For the total AFEI records, Hangzhou had the highest reporting rate (66.68/100,000 doses), while Zhoushan had the lowest reporting rate (35.92/100,000 doses). For the serious AEFI records, Zhoushan had the highest reporting rate (1.05/100,000 doses), while Lishui had the lowest reporting rate (0.11/100,000 doses).
Table 2. Serious AEFI and non-serious AEFI records in the national AEFI surveillance system database, 2019, by city
Of the total AEFI reports, there were 11271 minor vaccine product-related reactions (48.81/100,000 doses), 1476 severe vaccine product-related reactions (6.39/100,000 doses), 4 vaccination errors (0.02/100,000 doses), 297 coincidental events (1.29/100,000 doses), 31 anxiety reactions (0.13/100,000 doses) ().
Table 3. Category of AEFI records in the national AEFI surveillance system database, 2019, by city
Thirty-one different vaccines were included in the 13079 AEFI records received during the study period (). The difference in AEFI reporting rate among vaccine types was significant (χ2 = 109.25, P< .001). The most frequently reported individual vaccine was DTP-Hib, with a reporting rate of 426.62/100,000 doses for the total AEFI and 2.72/100,000 doses for the severe AEFI. Furthermore, DTP-Hib was also the most frequent vaccine listed as suspected of being involved in a reported AEFI, which only one vaccine was listed as being suspected. The lowest frequently reported individual vaccine was IPV, with a reporting rate of 13.89/100,000 doses for the total AEFI and 0.20/100,000 doses for the severe AEFI. The highest reporting rate of vaccine product-related reaction was observed in MMR (43.57/100,000 doses) while the lowest reporting rate was observed in QIV (1.72/100,000 doses). The highest reporting rate of severe vaccine product-related reaction was observed in PPV23 (1.21/100,000 doses).
Table 4. Vaccine types listed as “suspected” in records of AEFI in the national AEFI surveillance system database, 2019
The distribution and frequency of clinical diagnosis of AEFI are shown in . The most frequently reported AEFI was fever/redness/induration (48.82/100,000 doses), followed by allergic rash (3.38/100,000), urticarial (1.26/100,000 doses), maculopapular rash (0.64/100,000 doses), other allergic reactions (0.19/100,000 doses), angioedema (0.14/100,000 doses), febrile convulsions (0.12/100,000 doses) and thrombocytopenic purpura (0.12/100,000 doses). The reporting rates of other reactions were under 0.1/100,000 doses.
Table 5. Clinical diagnosis of AEFI in the national AEFI surveillance system database, 2019
Most reported AEFI (90.52%) occurred in the first day after vaccination and 66.93% of the severe AEFI occurred in the first day after vaccination, and 87.26% of severe vaccine product-related reaction occurred in the first day after vaccination either ().
Table 6. The interval between AEFI onset and immunization in the national AEFI surveillance system database, 2019
Discussion
Clinical vaccine trials usually involve a limited number of study subjects and may not allow for the detection of the rare adverse events. One of the primary goals of AEFI passive surveillance is to detect vaccine safety signals and generate hypotheses for further studies.Citation13 Thus, post-licensure surveillance of AEFI is a necessary integral part of a vaccination program to continuously monitor the safety of vaccines when routinely used. To our knowledge, studies on AEFI were still very little from China and our reports could add the baseline data in this field. Another advantage was that we provided a detailed analysis of the characteristics of AEFI, as well as gave some clues on the reasons for AEFI occurrence and its frequency over time, by seriousness of AEFI, age group, city.
We found that there were disparities in the reporting rates of the general AEFI and the serious AEFI between 2019 and the time period of 2008–2011, while the vaccines used in Zhejiang province had not been changed since 2007. For example, the reporting rate of the general AEFI was 56.64/100,000 doses in 2019, which was six times more than the reporting rate of 9.2/100,000 doses for the time period of 2008–2011. The reporting rate of the serious AEFI was 0.55/100,000 doses in 2019, which was half of the reporting rate of 0.94/100,000 for the time period of 2008–2011.Citation12 The reporting rate of AEFI differed markedly from the findings from Zimbabwe during the 1997 to 2017 period (0.58/100,000 doses). Approximately 5 to 7 AEFI reports per 100,000 doses were received by the Vaccine Adverse Event Reporting System (VAERS) in the United States.Citation14 In Australia in 2009,Citation15 a rate of 14.1 AEFI per 100,000 doses was reported. Variable reporting requirements, case definitions, and settings as well as variable compliance with reporting were the main reasons for the disparities in reporting rate of AEFI among different countries. However, the remarkable increase in the reporting rate of AEFI in Zhejiang province could be due to the enhanced vaccine safety awareness among both vaccination providers and recipients in recent years. Especially, the annual training programs had been used to build reporting and analytic capacity of AEFI staff, healthcare providers, and diagnostic AEFI expert panels, in order to enhance AEFI surveillance development. Our findings were consistent with those at the national level. For example, the number of AEFI cases reported to the surveillance system has increased by approximately 30% year by year since 2005. This increase was likely indicative of improved case ascertainment and detection rather than the true increase in vaccine reactogenicity. This conclusion was supported by the decreasing reporting rate deemed serious from the 2008 to 2011 period (1/100,000 doses) to 2019 (0.55/100,000 doses). Unfortunately, the reporting rates of AEFI from other countries like Japan or Germany were not comparable with that from this study as they used the total population as the denominator but not the vaccination doses.
In this study, we found some cities had a high reporting rate of serious AEFI but had a low reporting rate of non-serious AEFI (e.g. Zhoushan). It also could be observed in the AEFI passive surveillance system from other countries.Citation16–18 This pattern suggested the sensitivity of the AEFI surveillance system in the individual city was different and some cities had a lower sensitivity. It was likely to be related, to some extent, to known the disparities in the notification or investigation procedures of AEFI. Further study to evaluate and compare AEFI surveillance sensitivity across cities would help to elucidate this.
The vaccine product-related reaction was the most common AEFI observed in this study. These reactions were associated with the route and/or site of administration of the vaccine product, or caused by the immune-mediated process. Our finding was similar with the reports from other countriesCitation19–21 and we indicated that all vaccination providers should conduct the medical screening for contraindications carefully before giving a shot to minimize these reactions. Vaccination error was very rare in Zhejiang province because of the strengthened routine vaccination service since 2008 through the provincial vaccination staff training program and the skill competition in every 2–3 years. Identifying a coincidental AEFI that was falsely attributed to a vaccine product was vital as otherwise the coincidence might result in loss of public confidence in the vaccine, with the consequent reemergence of vaccine-preventable disease. Although the reporting rate of coincident event was low in this study, cause-specific categorization was still important as it would enable the differentiation of coincidental events from vaccine reactions especially for events such as death whose occurrence and miscommunication with the community could disrupt the EPI service.
The reporting rates of AEFI associated with pneumonia-containing vaccines, pertussis-containing vaccines and measles containing vaccine were higher than those associated with other vaccines. Our results were in line with the national surveillance results in recent years,Citation11,Citation22 furthermore, fever, redness and induration at the injection site were the most frequent reported AEFI followed receipts of pneumococcal bacteria-containing vaccines, pertussis bacteria-containing vaccines and measles virus-containing vaccine were higher than those for other vaccines. We assumed that it was associated with the nature of the agents in these vaccines. It indicated that we should continue to pay close attention to these vaccines in the future surveillance work. Besides, the mechanism researches on understanding the development of adverse reactions should be implemented to improve the vaccine antigen components, production process and additives.
In this study, the reporting rates of clinical diagnosis of AEFI were similar with the results from other countries, indicating the safety profiles of these vaccines used in Zhejiang province. For example, the reporting rate of vaccine-associated paralytic polio in our study, which was induced by the oral live attenuated polio vaccine, was similar with the average estimate of 1 per million doses.Citation23,Citation24 The reporting rate of Guillain Barre Syndrome was 0.01/100,000 doses in this study, which was lower than those findings from UK (4.57/100,000 doses),Citation25 Finland (0.18–10.3/100,000 doses),Citation26 and Canada (1.0–2.3/100,000 doses).Citation27 We found the acute disseminated encephalomyelitis was rare, which was lower than the incidence rate among general population (0.4–0.8/100,000).Citation28 The reporting rate of anaphylactic shock in this study was very similar to those found in the USA (0.65/100,000 doses) and Canada (0.2–2.6/100,000 doses), but lower than that from Finland (1.69–4.47/100,000 doses).Citation26 The reporting rate of thrombocytopenic purpura in Zhejiang was also lower than the incidence of thrombocytopenic purpura among the general population (4.8/100,000).Citation26
There are still several limitations and advantages regarding this study. As an inherent weakness of passive reporting systems, there was significant variability in reporting quality, potential for biased reporting (leading to overall underreporting), limited power to establish or disprove the causal relationship with vaccination in the individual report, and lack of control group. Our study also has a few advantages. First, data had been obtained widely from an entire province and over a year period. Second, all AEFI reports were scrutinized in a standardized fashion according to the guidelines for AEFI surveillance. Third, the number of distributed vaccine doses was available and allowed calculation of AEFI reporting rates per distributed vaccine doses.
Conclusion
The regular analysis and publication of AEFI surveillance data collated in the national AEFI surveillance system remained as the important aspects of EPI, and this would serve as a baseline for repeated analyses of the ongoing surveillance in the future. The data presented here illustrated the high level of vaccine safety. The benefits of vaccination far outweighed the risks of AEFI, particularly since the majority of those reported were not serious, or coincidentally associated with vaccination. Our findings could be used to implement the health education to the general population to enhance the confidence of vaccine or reduce the hesitation of vaccination, resulting a high vaccination coverage. In future, the quality of the AEFI surveillance system should be improved through collecting more detailed individual clinical data, making the standardized case definitions, enhancing the follow-up of patients, and establishing of a sentinel system for active surveillance.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Ethics approval and consent to participate
This study was approved by the ethical review board of Zhejiang provincial CDC. All the data were anonymous when we exported them from ZJIIS and kept confidential without individual identifiers.
Author contributions
Y.H. and XJ. P conceived and designed the experiments; H.L. and Y.C. performed the experiments; HK. L. and Y.W. analyzed the data; LZ.S. and FX. C. contributed reagents/materials/analysis tools; XJ. P and Y.H. wrote the paper.
Acknowledgments
We are grateful to all physicians in all health centers for providing AEFI reports in Zhejiang province. We thank our colleagues of CDCs at municipal and county levels for collecting and collating data.
Additional information
Funding
References
- Global Advisory Committee on Vaccine Safety. 6-7 December 2017. Releve epidemiologique hebdomadaire / Section d’hygiene du Secretariat de la Societe des Nations = Weekly epidemiological record / Health Section of the Secretariat of the League of Nations . 2018;93:17–30.
- Tomianovic D, Bauwens J, Heininger U, Bonhoeffer J. Global vaccine safety assessment: challenges and opportunities. Pediatr Infect Dis J. 2016;35:446–48. doi:10.1097/INF.0000000000000983.
- Yoon D, Kim JH, Lee H, Shin JY. Updates on vaccine safety and post-licensure surveillance for adverse events following immunization in South Korea, 2005-2017. Yonsei Med J. 2020;61:623–30. doi:10.3349/ymj.2020.61.7.623.
- Oliveira PMN, Lignani LK, Conceicao DAD, Farias P, Takey PRG, Maia MLS, Camacho LAB. Surveillance of adverse events following immunization in the late 2010s: an overview of the importance, tools, and challenges. Cad Saude Publica. 2020;36(Suppl 2):e00182019. doi:10.1590/0102-311x00182019.
- Sato APS, Ferreira VLR, Tauil MC, Rodrigues LC, Barros MB, Martineli E, Costa ÂA, Inenami M, Waldman EA. Use of electronic immunization registry in the surveillance of adverse events following immunization. Rev Saude Publica. 2018;52:4. doi:10.11606/S1518-8787.2018052000295.
- Dey A, Wang H, Quinn H, Pillsbury A, Glover C, Hickie M, Wood N, Beard F, Macartney K. Surveillance of adverse events following immunisation in Australia: annual report, 2018. Commun Dis Intell. 2018;2020:44.
- Ranganath BG, Hiremath SG. Adverse events following immunisation with SA 14-14-2 Japanese encephalitis vaccine in children of Kolar in Karnataka. J Indian Med Assoc. 2012;110:10–12.
- Lawrence G, Campbell-Lloyd S, Rixon G. Monitoring adverse events following immunisation. N S W Public Health Bull. 2003;14:21–24. doi:10.1071/NB03006.
- Al Awaidy S, Bawikar S, Prakash KR, Al Rawahi B, Mohammed AJ. Surveillance of adverse events following immunization: 10 years’ experience in Oman. Eastern Mediterranean health journal = La revue de sante de la Mediterranee orientale = al-Majallah al-sihhiyah li-sharq al-mutawassit. 2010;16:474–80.
- Yih WK, Lee GM, Lieu TA, Ball R, Kulldorff M, Rett M, Wahl PM, McMahill-Walraven CN, Platt R, Salmon DA, et al. Surveillance for adverse events following receipt of pandemic 2009 H1N1 vaccine in the Post-Licensure Rapid Immunization Safety Monitoring (PRISM) System, 2009-2010. Am J Epidemiol. 2012;175:1120–28. doi:10.1093/aje/kws197.
- Liu D, Wu W, Li K, Xu D, Ye J, Li L, Wang H. Surveillance of adverse events following immunization in China: past, present, and future. Vaccine. 2015;33:4041–46. doi:10.1016/j.vaccine.2015.04.060.
- Hu Y, Li Q, Lin L, Chen E, Chen Y, Qi X. Surveillance for adverse events following immunization from 2008 to 2011 in Zhejiang Province, China. Clin vacc immunol. 2013;20:211–17. doi:10.1128/CVI.00541-12.
- Choe YJ, Cho H, Song KM, Kim JH, Han OP, Kwon YH, Bae GR, Lee HJ, Lee J-K. Active surveillance of adverse events following immunization against pandemic influenza A (H1N1) in Korea. Jpn J Infect Dis. 2011;64:297–303.
- Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015;33:4398–405. doi:10.1016/j.vaccine.2015.07.035.
- Mahajan D, Roomiani I, Gold MS, Lawrence GL, McIntyre PB, Menzies RI. Annual report: surveillance of adverse events following immunisation in Australia, 2009. Commun Dis Intell Q Rep. 2010;34:259–76.
- Niu MT, Ball R, Woo EJ, Burwen DR, Knippen M, Braun MM, VAERS Working Group Adverse events after anthrax vaccination reported to the Vaccine Adverse Event Reporting System (VAERS), 1990-2007. Vaccine. 2009;27:290–97. doi:10.1016/j.vaccine.2008.10.044.
- Nzolo D, Ntetani Aloni M, Mpiempie Ngamasata T, Mvete Luemba B, Bazundama Marfeza S, Bothale Ekila M, Nsibu CN, Tona NL. Adverse events following immunization with oral poliovirus in Kinshasa, Democratic Republic of Congo: preliminary results. Pathog Glob Health. 2013;107:381–84. doi:10.1179/2047773213Y.0000000113.
- Silva SS, Oliveira VC, Ribeiro HC, Alves TG, Cavalcante RB, Guimaraes EA. Analysis of adverse events following immunization in Minas Gerais, Brazil, 2011: a cross-sectional study. Epidemiologia e servicos de saude. 2016;25:45–54. doi:10.5123/S1679-49742016000100005.
- Clothier HJ, Lawrie J, Lewis G, Russell M, Crawford NW, Buttery JP. SAEFVIC: surveillance of adverse events following immunisation (AEFI) in Victoria, Australia, 2018. Commun Dis Intell. 2018;2020:44.
- Mentzer D, Oberle D, Keller-Stanislawski B. Adverse events following immunisation with a meningococcal serogroup B vaccine: report from post-marketing surveillance, Germany, 2013 to 2016. Euro Surveillance: Bulletin Europeen Sur Les Maladies Transmissibles = European Communicable Disease Bulletin. 2018;23. doi:10.2807/1560-7917.ES.2018.23.17.17-00468.
- Samuels S, Marijne Heeren A, Zijlmans H, Welters MJP, Van Den Berg JH, Philips D, Kvistborg P, Ehsan I, Scholl SME, Nuijen B, et al. HPV16 E7 DNA tattooing: safety, immunogenicity, and clinical response in patients with HPV-positive vulvar intraepithelial neoplasia. Cancer Immunol Immunother. 2017;66:1163–73. doi:10.1007/s00262-017-2006-y.
- Wu WD, Liu DW, Wu BB. [Analysis on the surveillance of adverse events following immunization in China, 2007-2008]. Zhongguo Yi Miao He Mian Yi. 2009;15:538.
- Yu L, Lu D, Yang H, Zou J, Wang H, Zheng M, Hu J. A comparative and correlative study of the Voice-Related Quality of Life (V-RQOL) and the Voice Activity and Participation Profile (VAPP) for voice-related quality of life among teachers with and without voice disorders. Medicine. 2019;98:e14491. doi:10.1097/MD.0000000000014491.
- Bermudez-de-Alvear RM, Galvez-Ruiz P, Martinez-Arquero AG, Rando-Marquez S, Fernandez-Contreras E. Evaluation of psychometric properties of voice activity and participation profile (VAPP): a Spanish version. J Voice. 2019;33:582 e15- e22. doi:10.1016/j.jvoice.2018.01.005.
- Stowe J, Andrews N, Wise L, Miller E. Investigation of the temporal association of Guillain-Barre syndrome with influenza vaccine and influenza like illness using the United Kingdom General Practice Research Database. Am J Epidemiol. 2009;169:382–88. doi:10.1093/aje/kwn310.
- Black S, Eskola J, Siegrist CA, Halsey N, MacDonald N, Law B, Miller E, Andrews N, Stowe J, Salmon D, et al. Importance of background rates of disease in assessment of vaccine safety during mass immunisation with pandemic H1N1 influenza vaccines. Lancet. 2009;374:2115–22. doi:10.1016/S0140-6736(09)61877-8.
- Hauck LJ, White C, Feasby TE, Zochodne DW, Svenson LW, Hill MD. Incidence of Guillain-Barre syndrome in Alberta, Canada: an administrative data study. J Neurol Neurosurg Psychiatry. 2008;79:318–20. doi:10.1136/jnnp.2007.118810.
- Chakravarty A. Neurologic illness following post-exposure prophylaxis with purified chick embryo cell antirabies vaccine. J Assoc Physicians India. 2001;49:927–28.

