ABSTRACT
In France, for many vaccines, vaccine coverage is below the 95% cutoff. One reason is mistrust from a growing proportion of the general population toward vaccination. In 2017, a new law was promulgated, extending the number of mandatory vaccines from 3 to 11. Our objectives were twofold: to assess the population’s perception of the extension of mandatory vaccination (MV) 1 year after its introduction, and to evaluate how it affected their vaccine confidence. We performed a descriptive cross-sectional study using a questionnaire for adults who consulted a family physician in south-east France. Overall, 453 questionnaires were analyzed. The median age of respondents (female 78.4%) was 43 years; 85% had children. On a 0 to 100 scale, respondents evaluated their confidence in vaccination at a median of 75 (IQR 50–90). For 60% of respondents, MV was a good public health measure; for 47%, it was poorly explained by the Ministry of Health; for 46%, it was a violation of personal freedom; and for 49%, it resulted from pharmaceutical industry lobbying. When asked about the influence of the extension of MV, only 26.2% declared that it had changed (a little/a lot) their opinion, and this change was for the majority (74.7%) toward less confidence. Respondents who declared an increased level of confidence already had a better perception of vaccination (and inversely). In conclusion, our results show that MV only changed the perception of vaccination among a small proportion of respondents. For most respondents, MV reinforced their initial views about vaccination.
Introduction
In France, for many vaccines, vaccine coverage is below the 95% cutoff recommended by the World Health Organization (WHO) to maintain collective protection against targeted diseases.Citation1 For instance, in 2017, vaccination coverage at 24 months was 91.3% for hepatitis B vaccine, 80.3% for the second dose of measles-mumps-rubella (MMR) vaccine, 72.6% for meningococcus C vaccine, and 92.2% for the third dose of pneumococcus vaccine.Citation2 Furthermore, vaccine coverage is heterogeneous across the different French regions; for instance, it is lower in the Provence-Alpes-Côte d’Azur region (south-east) than the national level, especially in the Hautes-Alpes department: in this area, the vaccine coverage at 24 months of age is 68.9% for hepatitis B vaccine, 69.6% for the second dose of MMR vaccine, 43.1% for meningococcus C vaccine, and 80.3% for the third dose of pneumococcus vaccine.Citation2
The reasons for the low vaccination uptake are numerous. One major reason is mistrust in vaccination from a growing proportion of the general population. In 2016, Larson et al. assessed vaccine confidence among people from 67 countries; 46% of French respondents disagreed with the statement “Overall, vaccines are safe,” which was the highest figure among the surveyed countries.Citation3 This negative perception may originate from vaccine controversies such as the alleged association between the hepatitis B vaccine and multiple sclerosisCitation4 or the measles vaccine and autism,Citation5 which contributed to damaging the French population’s confidence in vaccination safety. Even physicians are not immune to these false beliefs regarding vaccines.Citation6 In this context, a public consultation on vaccination was organized in 2016, and one of the proposed improvements to the French vaccination policy was the extension of mandatory vaccination (MV).Citation7 Based on these conclusions, a new law was promulgated in 2017, extending the number of mandatory vaccines for all children born after January 1, 2018, from 3 (diphtheria, poliomyelitis, tetanus) to 11 vaccines (adding Haemophilus influenzae b, hepatitis B, measles, meningococcus C, mumps, pneumococcus, rubella, and pertussis).Citation8
The national French data for 2018 and 2019 showed that this policy was associated with better vaccine coverage in infants.Citation9 However, little is known about the perception of the French population regarding the extension of MV and its impact on the level of trust in vaccines. Our objectives were therefore to assess the population’s perception of the extension of MV 1 year after its introduction and to determine how this change impacted vaccine confidence.
Methods
Questionnaire
We performed a descriptive cross-sectional study based on an anonymous self-administered questionnaire.
The questionnaire was designed on the basis of a literature review, with 18 questions organized into four sections: 1) description of the main study objective; 2) demographic data of participants; 3) questions about vaccine status as well as vaccine confidence and perceptions; and 4) knowledge and perceptions of the extension of MV.
Questionnaire diffusion and participants
The study population consisted of adult patients coming for a consultation with a family physician in the Gapençais area (south-east France). The paper questionnaire was made available in three family physician clinics in the city of Gap as well as four clinics around Gap (Saint Bonnet en Champsaur, Ancelle, Pont du Fossé, and Veynes), accounting for a total of 33 family physicians. A billboard in the waiting room informed patients coming for a consultation about the study. Any adult willing to complete the questionnaire could participate.
Only a few studies use self-evaluation to assess the impact of mandatory vaccine policies on people’s trust in vaccination. Therefore, to determine the size of the population required for this study, we relied on the self-evaluated perception of vaccine safety, which, according to some of our previous studies, has a notable variance; for example, in midwives, the median self-evaluated safety is 80/100 with interquartile ranges [IQR] of 65 and 90;Citation10 in first-year medical students, it is 75/100 with IQR of 50 and 90.Citation11 To observe a 10% shift in these perceptions with an α risk of 0.05 and a 1-β value of 0.9, respectively, 200 subjects would be needed; we therefore chose to include a minimum of 400 respondents so as not to overlook unexpected effects.
Ethical statement
In accordance with French laws, the protocol and questionnaire were submitted to the Grenoble-Alpes University Hospital ethics committee; it received a favorable opinion on February 13, 2019. Participation in the survey was voluntary, anonymous, and without any financial compensation.
Data analyses
For descriptive analysis, continuous variables were presented as means (and standard deviation) or as medians (and interquartile ranges [IQR]) depending on the distribution of variables. Categorical variables were described as absolute numbers and frequencies.
For univariate analysis, we used Student’s t test (normal distribution) or the Mann-Whitney U test (skewed distribution). Spearman’s rank correlation coefficient was used for correlations between variables. Categorical variables were compared using a chi-square test. Statview software 5.0 (SAS Institute Inc.) was used to perform these analyses.
Results
Baseline characteristics of respondents
A total of 1,000 questionnaires were made available in the seven participating family physician clinics for a period of 2 months from March 5, 2019. A total of 552 questionnaires were completed; after the exclusion of 3 minors and 96 people not living in the study area, 453 questionnaires were used for the final analysis.
The median age of respondents was 43 years (IQR 35–57), with 78.4% (355/453) being women. The majority of respondents had children (382/449; 85%), with a median of two children (IQR 1–2). Parents with children aged 3 years or under (and thus impacted by MV) represented 15.8% (63/398) of respondents. Overall, 82% of parents believed that their child(ren) had completed their vaccinations (308/377).
Confidence in vaccination
On a 0 to 100 scale, respondents evaluated their vaccine confidence at a median of 75 (IQR 50–90) (N = 435). When comparing the vaccine confidence of parents of child(ren) aged 3 years or under, parents of child(ren) between 4 and 15 years, and parents of older child(ren) or childless participants, the median was respectively 60 (IQR 40–85), 75 (IQR 50–85), and 80 (IQR 50–95). The answers to the different questions regarding vaccination in general are reported in .
Table 1. Answers to the questions regarding vaccination in general
Perception of mandatory vaccination
The answers to the questions regarding the MV policy are detailed in . For all the questions except for the one about the Ministry of Health’s adequate explanation of the policy, parents of child(ren) under the age of 3 had a more negative perception of MV than other participants. The participants whose answers reflected a more negative perception of MV tended to be significantly younger ().
Table 2. Answers to the questions regarding mandatory vaccination based on the parenthood and age of respondents
Influence of mandatory vaccination
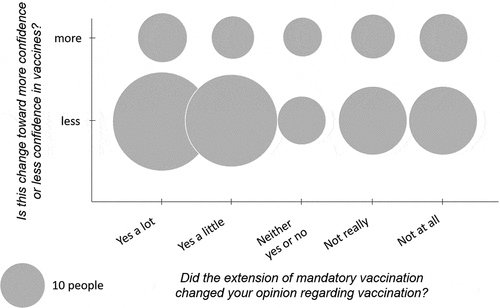
To the question “Did the extension of mandatory vaccination change your opinion regarding vaccination?,” 61/438 (13.9%) answered “yes a lot,” 54 (12.3%) “yes a little,” 85 (19.4%) “neither yes or no,” 99 (22.6%) “not really,” and 139 (31.8%) “not at all.” Participants were then asked whether they had more or less confidence in vaccination, with 202 participants responding to the question (including those who had answered “not really” or “not at all” to the previous question, and whose answers were also analyzed). Among them, 51 (25.3%) had “more confidence” and 151 (74.7%) “less confidence” in vaccination. This asymmetry was observed when classifying participants according to their answer to the previous question “Did the extension of mandatory vaccination change your opinion regarding vaccination?” shows that a negative perception (“less confidence”) was predominant in all answers to this question.
Figure 1. Distribution of the 202 participants who answered the two questions: “Did the extension of mandatory vaccination change your opinion regarding vaccination?” (X axis) and “Is this change toward more confidence or less confidence in vaccines?” (Y axis). The size of the discs shows the distribution of participants when self-evaluating both the intensity of the impact of the mandatory vaccination and the polarization (more or less confidence) of this impact. (The figure does not include the 244 participants who answered “Neither yes or no,” “Not really,” or “Not at all” to the question “Did the extension of mandatory vaccination change your opinion regarding vaccination?” and who then did not answer the question “Is this change toward more confidence or less confidence in vaccines?”).
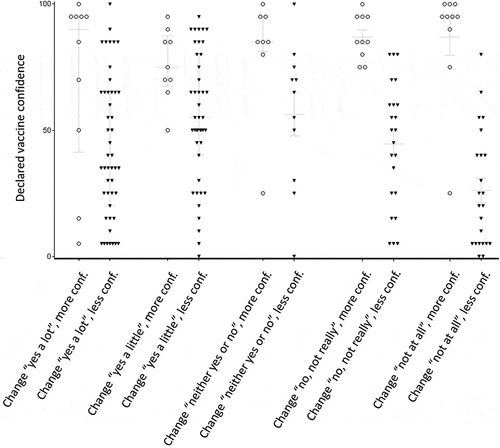
Participants who declared a change toward less confidence already had a lower median confidence in vaccination (50 [25–70]) than those who declared a change toward more confidence (85 [70–95]) (p < .0001); this difference was observed when considering the different responses to the question “Did the extension of mandatory vaccination change your opinion regarding vaccination?” ().
Figure 2. Vaccine confidence according to the answers to two questions: “Did the extension of mandatory vaccination change your opinion regarding vaccination?” and “Is this change toward more confidence or less confidence in vaccines?” (more or less confidence). Bars are for median and interquartile range. (Again, the figure does not include the 244 participants who answered “Neither yes or no,” “Not really,” or “Not at all” to the question “Did the extension of mandatory vaccination change your opinion regarding vaccination?” and who then did not answer the question “Is this change toward more confidence or less confidence in vaccines?”).
Moreover, participants declaring a change toward more confidence were significantly older than those declaring a change toward less confidence (median age 53 [38–64] vs 38 [33–50] years, p = .0002). There was no difference based on the gender of respondents or whether they had young children under the age of 3 years.
Discussion
Due to the low level of confidence in vaccines, concerns have arisen in France regarding the risk of lower vaccine coverage, notably in infants. Therefore, the number of mandatory vaccines for children under 2 years was extended in 2018. This was immediately associated with a better vaccine coverage in this population, although the impact of the policy in terms of vaccine confidence is unknown.
Main results regarding vaccine confidence
The declared level (on a 0–100 scale) of confidence in vaccination was heterogeneous, being less than 75 for 50% of participants and less than 50 for 25%. Overall, respondents considered vaccination to be effective, although 32% believed that vaccines were dangerous. Other studies have already highlighted this difference in age, with 25–34 year-old being the least favorable to vaccination.Citation12 We observed that confidence in vaccination was lower among parents with younger child(ren) and younger participants. This association with parenthood has already been noted,Citation13,Citation14 and may reflect the view, among others, that the regular vaccination of one’s infant enhances the negative perceptions of vaccines.
Main results regarding the perception of mandatory vaccination
The perception of MV is contrasting and frequently negative. Although 60% of participants considered it to be a good public health measure, nearly half declared that it had rather not or not at all been explained. Nearly half of respondents believed that it violated their personal freedom and resulted from pharmaceutical industry lobbying. Before 2018, infant vaccines were partly mandatory and partly recommended. It was thus expected that MV would bring more clarity around the issue; however, 40% of participants had the opposite view. These different results suggest that 18 months after the promulgation of this measure, people still have concerns regarding its efficacy and legitimacy. Once again, the parents of younger children have a more negative perception, which may reflect the fact that they are the most impacted by the extension of MV, thus triggering stronger feelings.
Limited effect so far of MV policy on vaccine confidence
Our study showed that the extension of MV did not increase confidence in vaccination 18 months after its implementation. Indeed, only 26.2% of respondents answered that MV had changed (a lot/a little) their perception of vaccination, while 74.7% of participants who noted this change declared that they had less confidence in vaccination. Respondents who became less confident initially had a low level of confidence in vaccination. This suggests that for the majority of participants, the implementation of this policy reinforced their initial (positive or negative) perception of vaccines. Interestingly, as shown in , several participants had a low level of confidence but declared that the MV policy had improved their confidence. However, this is counterbalanced by the fact that a greater number of participants had a rather high level of confidence in vaccines but declared that the new policy had decreased their confidence. A similar study should be performed in the coming years to assess after a longer period of time whether vaccine confidence is reinforced by MV. In the general population, more people were skeptical about vaccination in 2016 than in 2014.Citation12,Citation15 In the Hautes-Alpes department studied here, confidence in vaccination is one of the lowest in France.Citation16
Our results are consistent with a study performed in France in 2017, which explored the perceptions of a general population sample in relation to a potential MV policy (not yet implemented at that time).Citation17 Among more than 3,000 participants, 64.5% agreed with the extension of MV and 68.7% considered it to be a necessary step, whereas 33.8% thought it unsafe for children and 56.9% viewed it as authoritarian. The similarity of our results suggests that the perception of MV has not substantially evolved during the first year of its implementation.
Italy implemented a policy of MV in 2017. A study performed in Tuscany observed that the rate of individuals expressing vaccine hesitancy decreased from 15.6% to 11.5% between 2017 and 2018,Citation18 with the authors interpreting this as a positive effect of MV. However, participants were not asked to specifically report the extent to which MV had influenced their trust in vaccines.
Interestingly, an online study performed in France in 2019 exploring the perceptions of 1,500 mothers of 0- to 17-month-old infants showed a progression of vaccine confidence between 2018 and 2019.Citation19 Once again, however, participants were not asked to specifically report whether MV had influenced their confidence in vaccines.
Strengths and limitations
Our study is the first to explore the perception of the French general population regarding MV 1 year after the enactment of the law. We focused on a French region where vaccine confidence was already low. The high number of respondents (N = 453) strengthens our results.
However, our study has several limitations. The response rate was 45% for the distributed questionnaires. Further, we studied MV in general without focusing on specific vaccines. Indeed, many respondents expressed that they were favorable to MV but not for all vaccines, especially the vaccine against hepatitis B, which has been the most controversial in France since the 1990s.
Conclusion
Our study showed that MV changed the perception of vaccination for only a small proportion of respondents. For the majority, MV simply reinforced their initial views about vaccination. More health communication campaigns on vaccines should be implemented, specifically targeting the parents of young children.
Authors contributions
O.E. initiated the study. A.M, L.G., and O.E. contributed to the study design. A.M., L.G., and O.E. contributed to the implementation and supervision of the study. O.E. analyzed the data and takes responsibility for the accuracy of the data analysis. M.L.M. and O.E. drafted the manuscript. All authors read and approved the final manuscript.
Consent to participate
All participants were informed of the anonymous nature of the study and their freedom to participate or not. Their participation had no influence on any medical treatment provided.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Ethical approval
The protocol and questionnaire were submitted to the Grenoble-Alpes University Hospital ethical committee and received a favorable opinion on February 13, 2019.
Availibility of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Santé Publique France. What is vaccine coverage?; 2019 [accessed 2021 Apr 14]. https://www.santepubliquefrance.fr/determinants-de-sante/vaccination/articles/qu-est-ce-que-la-couverture-vaccinale
- Santé Publique France. Data on French vaccine coverage; 2020 [accessed 2021 Apr 14]. https://www.santepubliquefrance.fr/determinants-de-sante/vaccination/donnees/#tabs
- Larson HJ, de Figueiredo A, Xiahong Z, Schulz WS, Verger P, Johnston IG, Cook AR, Jones NS. The state of vaccine confidence 2016: global insights through a 67-country survey. EBioMedicine. 2016;12:295–301. doi:10.1016/j.ebiom.2016.08.042. PMID: 27658738.
- Nau J-Y A court acknowledged the responsibility of hepatitis B vaccine in multiple sclerosis. Le Monde; 1998. [accessed 2021 April 10]. https://www.lemonde.fr/archives/article/1998/06/10/un-tribunal-reconnait-la-responsabilite-du-vaccin-anti-hepatite-b-dans-la-sclerose-en-plaques_3674138_1819218.html
- Wakefield AJ, Murch SH, Anthony A, LInnell J, Casson DM, Malik M, Berelowitz M, Dhillon AP, Thomson MA, Harvey P, et al. RETRACTED: ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. The Lancet. 1998;351(9103):637–41. doi:10.1016/s0140-6736(97)11096-0. PMID: 9500320.
- Le Marechal M, Fressard L, Agrinier N, Verger P, Pulcini C. General practitioners’ perceptions of vaccination controversies: a French nationwide cross-sectional study. Clin Microbiol Infect. 2018;24(8):858–64. doi:10.1016/j.cmi.2017.10.021. PMID: 29104170.
- Citizen comity for the concertation regarding vaccination; 2016 [accessed 2021 Apr 14]. http://concertation-vaccination.fr/
- Légifrance. Décret n° 2018-42 du 25 janvier 2018 relatif à la vaccination obligatoire – légifrance; 2018. [accessed 2021 April 10]. https://www.legifrance.gouv.fr/jorf/id/JORFTEXT000036543886/
- Lévy-Bruhl D, Fonteneau L, Vaux S, Barret A-S, Antona D, Bonmarin I, Che D, Quelet S, Coignard B. Assessment of the impact of the extension of vaccination mandates on vaccine coverage after 1 year, France, 2019. Euro Surveill. 2019;24(25):pii=1900301. doi:10.2807/1560-7917.ES.2019.24.25.1900301. PMID: 31266592.
- Massot E, Epaulard O. Midwives’ perceptions of vaccines and their role as vaccinators: the emergence of a new immunization corps. Vaccine. 2018 Aug 16;36(34):5204–09. doi:10.1016/j.vaccine.2018.06.050.
- Daudel L, Mary J, Epaulard O. Perception of mandatory infant vaccines and trust in vaccination among first-year healthcare students: an opportunity window for the training of future healthcare workers. Vaccine. 2020 Jan 22;38(4):794–99. doi:10.1016/j.vaccine.2019.10.099.
- Gautier A, Chemlal K, Jestin C Adhesion to vaccination in France: results from the the Barometre Sante 2016; 2017 [accessed 2021 Apr 14]. https://www.santepubliquefrance.fr/determinants-de-sante/vaccination/documents/article/adhesion-a-la-vaccination-en-france-resultats-du-barometre-sante-2016
- Rozbroj T, Lyons A, Vaccine-Hesitant LJ. and vaccine-refusing parents’ reflections on the way parenthood changed their attitudes to vaccination. J Community Health. 2020;45(1):63–72. doi:10.1007/s10900-019-00723-9. PMID: 31392603.
- Funk C, Kennedy B, Efferon M (Pew Research Center). Vast majority of Americans say benefits of childhood vaccines outweigh risks; 2017. [accessed 2021 Apr 14]. https://www.pewresearch.org/internet/wp-content/uploads/sites/9/2017/02/PS_2017.02.02_Vaccines_FINAL.pdf
- Verger P, Fressard L, Collange F, Gautier A, Jestin C, Launay O, Raude J, Pulcini C, Peretti-Watel P. Vaccine hesitancy among general practitioners and its determinants during controversies: a national cross-sectional survey in France. EBioMedicine. 2015;2(8):891–97. doi:10.1016/j.ebiom.2015.06.018. PMID: 26425696.
- Agence Régionale de Santé (ARS) Provence Alpes Côte d’Azur. [Vaccine perception]; 2016. [accessed 2021 Apr 14]. https://www.paca.ars.sante.fr/system/files/2019-01/BS2016-130918_Final_0.pdf
- Mathieu P, Gautier A, Raude J, Goronflot T, Launay T, Debin M, Guerrisi C, Turbelin C, Hanslik T, Jestin C, et al. Population perception of mandatory childhood vaccination programme before its implementation, France, 2017. Eurosurveillance. 2019;24(25):1900053. doi:10.2807/1560-7917.ES.2019.24.25.1900053. PMID: 31241041.
- Tavoschi L, Quattrone F, De Vita E, Lopalco PL. Impact of mandatory law on vaccine hesitancy spectrum: the case of measles vaccine catch-up activities in Tuscany, Italy. Vaccine. 2019;37(49):7201–02. doi:10.1016/j.vaccine.2019.09.092. PMID: 31606250.
- Cohen R, Martinot A, Gaudelus J, Subtil D, Stahl JP, Pujol P, Picquet V, Lepetit H, Longfier L, Leboucher B, et al. Infant mandatory vaccinations: confirmation of a positive impact. Med Mal Infect. 2020;50(1):74–77. doi:10.1016/j.medmal.2019.11.007. PMID: 31843343.

