ABSTRACT
This research aims to understand the level and determinants of people’s willingness to participate in a vaccine trial for COVID-19 in Senegal. We conducted a telephone survey among a marginal quota sample of 607 people over 18 years of age. Only 44.3% of the participants wanted to participate in a vaccine trial for COVID-19, with females intending to participate more than males (AOR = 1.82, 95% CI [1.22–2.72]). Participants who intended to be vaccinated against COVID-19 (AOR = 6.48, 95% CI [4.12–10.4]) and who thought that being infected with the coronavirus would have a significant impact on their health (AOR = 2.34, 95% CI [1.57, 3.51]) were more likely to agree to take part in the COVID-19 vaccine trial. Confidence in the vaccine, health personnel, and the government in the fight against the pandemic are key factors in participants’ willingness to participate in a vaccine trial in Senegal.
Introduction
Africa has long been the site of biomedical research, whether for drug testing or vaccines.Citation1–5 Moreover, social scientists have shown the need for a complex and nuanced view beyond the ethical challenges and power issues.Citation2,Citation4 However, we are in the process of “globalizing human subjects research”Citation6 after the scientific imperialism of the Pasteur Institutes.Citation7
In francophone Africa, particularly in Senegal, the COVID-19 pandemic has opened a new window of opportunity for these debates. Contrary to what is imagined about infectious diseases, after EbolaCitation8,Citation9 and Lassa,Citation10 Africa was neither the first continent to be affected nor the one that, thus far, suffered the most consequences.Citation11 However, a televised interview with two French doctors set off the “April Fool’s prank” on April 1st, 2020.Citation12 In a context where debates on the decolonization of global health are numerous,Citation13,Citation14 Africans heard these two Frenchmen say that it was necessary to test the BCG vaccine for prevention against COVID-19 in Africa. Academic reactions on the ethical issues this raises and reactions in Africa’s (social) media were numerous.Citation12,Citation15 Rumors about Africa as a vaccine testing ground continued to grow significantly.Citation16
At the end of the 1970s in Senegal, the first vaccine trials against hepatitis B gave rise to numerous ethical debates.Citation3 The 2007 meningitis vaccine trial also led to numerous controversies, showing the lack of journalist’s scientific culture and an attempt at political instrumentalization.Citation17 These stories were brought back to the forefront by the national media in April 2020 with the French doctors’ speech. Yet Africa is by far one of the continents where biomedical research is least carried out, with COVID-19 being no exception.Citation18 A May 2020 analysis showed that of the 1002 therapeutic clinical trials for COVID-19 worldwide, only 32 (3.2%) were conducted in Africa.Citation19 As of June 2021, of the 474 ongoing vaccine trials against COVID-19, only 19 are taking place in Africa (South Africa: 12; Kenya = 3; Egypt 3; Morocco 1Footnote1).
However, like other continents,Citation20 Africa needs to conduct research and trials to test drugs and vaccines against COVID-19.Citation21 Not only is this essential to adapt biomedical products and vaccines to national contexts and populations, but countries now have research centers and ethics committees competent to carry out these trials under good ethical conditions.Citation18 Thus, in a context where vaccines are the common solution to fight against the COVID-19 pandemic and other epidemics to come, it is important to understand the willingness of Senegalese to participate in a vaccine trial. Qualitative research in Senegal has shown that Senegalese people do not wish to be “exploited” by COVID-19 vaccine trials, but noted an ambiguous relationship and significant ignorance about how biomedical research works.Citation22 Thus, the objective of this article is to understand the level and determinants of Senegal’s population’s willingness to participate in a COVID-19 vaccine trial.
Materials and methods
Design
We conducted a cross-sectional, descriptive, and analytical study.
Population
In June 2020, we conducted a national telephone survey of 813 people to measure the social acceptability of government measures to combat COVID-19.Citation23 We used the marginal quota sampling strategyCitation23 to have a representative sample of the national population stratified by population weight by region, gender, and age group. This method is relevant in emergency situations such as the COVID-19 pandemic with sample sizes below 3,000.Citation23 Appropriate choice of quotas can reduce the variance of the estimate and the magnitude of its confidence interval. The quota sampling method can be as accurate as random sampling, if not better if the sample size is small.Citation24 We used the last general population census (2013) as a reference.
For the selection of telephone numbers nationwide, we used the Random Digit Dialing (RDD) method. A computer program randomly generated a list of unique telephone numbers (n = 30,603) following the national numbering plan and respecting the market shares of the telephone operators. A second computer program sent an SMS to the previous list to provide information about the research and notify subscribers that they may be called. This program identified valid numbers by the delivery status of the SMS. This process allowed for a new list of numbers assumed to be valid (n = 10,931; 35.72%). We inserted the list into a Reactive Auto Dialer (RAD) to trigger calls in an automatic and optimized way. When the outgoing call was picked up (n = 6,576; 60.16%), the platform automatically detected whether it was a human, an answering machine, or if there was any doubt. An audio greeting in the national language (Wolof) was played to the phone call recipient, and that person was transferred to an interviewer (n = 1441; 21.91%) based in Dakar, who explained the research and asked for consent to participate. From the 1441 completed forms (including refusals and out of quota persons), 813 persons made up the final sample. Based on this first survey, which did not concern vaccine trials, we organized a second survey of these same people from December 24, 2020, to January 16, 2021. The final sample size was 607 (74.6%). A comparison of the characteristics of the quotas chosen for the sample between the two surveys showed a statistical difference for gender (p = .04), but not for region (p = .99) or age (p = .08).
Data collection and quality control
Five female interviewers speaking six languages (French, Diola, Wolof, Sérére, Pulaar, Soninké) carried out data collection through a telephone survey. We used tablets equipped with Open Data Kit (ODK) software to administer the questionnaire. We carried out data quality control by training enumerators, pre-testing tools, digitalizing, collecting data on a tablet, and recruiting a supervisor to monitor data collection daily in real-time.
Data collection instruments
The variables in our study were based on acceptability models.Citation25,Citation26 They included socio-demographic characteristics (age, gender, education, region of residence, wealth tercile, chronic medical conditions), vaccination history, attitude toward the COVID-19 vaccine measured by five items in the form of a five-point Likert scale, intention to be vaccinated against COVID-19, fear of the coronavirus, concern for serious health consequences if infected by the coronavirus, trust in the government to fight the COVID-19 epidemic, willingness to participate in COVID-19 a vaccine trial, and reasons for refusing or agreeing to participate in this trial.
Data analysis
We used R software version 4.0.3 for data analysis. We described the quantitative variables by the mean ± standard deviation and the qualitative variables by their frequencies. Then, we used a chi-squared (Ch2) test to compare two qualitative variables and a student’s t test, a qualitative and a quantitative variable. Finally, we used multivariate logistic regression to determine the factors associated with the willingness of respondents to participate in the COVID-19 vaccine trial. All variables with p-values less than 0.25 in the comparisons were retained for the full model construction.Citation27 We used the stepwise top-down selection procedure to build a more parsimonious reduced model.Citation28 Significance was considered at a p-value < 0.05.
Ethics
Senegal’s national health research ethics committee (SEN/20/23) approved this research. All individuals agreed to participate, were informed of the ethical issues, and were able to withdraw from the study at any time.
Results
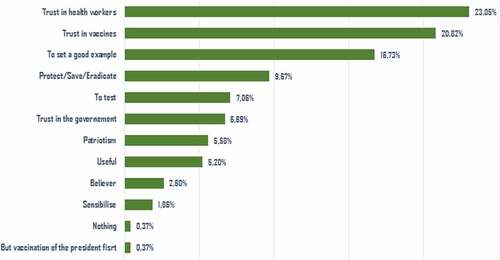
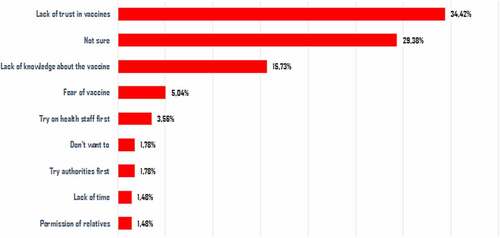
In our study, women represented 39.7% of the respondents. 15.5% of the respondents reported having a chronic disease. The average confidence in the Senegalese government in the fight against the epidemic was 6.6/10 ± 3.1. 54.4% of respondents expressed the intention to be vaccinated, and according to their statements, 44.3% wanted to participate in a vaccine trial for COVID-19 if it took place (). Of these, 23.0% (62/269) explained this willingness because they trusted the health workers (). Of those who refused to participate in the trial, 34.4% (116/337) expressed a lack of trust in vaccines, and 29.4% (99/337) perceived a lack of safety of the vaccine trial ().
Table 1. Distribution of respondents by characteristics (N = 607)
The proportion of the men who agreed to participate in the COVID-19 vaccination trial (38.5%) was significantly lower than that of the women (53.1%, p = .001). People with a chronic disease have a higher intention to participate in the COVID-19 vaccination trial than others (55.3% vs 42.3%; p = .026) ().
Table 2. Distribution of respondents by characteristics and willingness to participate in COVID-19 vaccine research (N = 607)
The results of the multivariate analysis showed that participants who intended to be vaccinated against COVID-19 (AOR = 6.48, 95% CI [4.12–10.4]) and who thought that being infected with the coronavirus would have a significant impact on their health (AOR = 2.34, 95% CI [1.57, 3.51]) were more likely to agree to participate in a COVID-19 vaccination trial (). The other three factors positively associated with willingness to participate in a COVID-19 vaccination trial were being female (AOR = 1.82, 95% CI [1.22–2.72]), having a positive attitude toward the vaccine (AOR = 1.69, 95% CI [1.09, 2.62]), and having confidence in the Senegalese government to control the coronavirus epidemic (OR = 1.09, 95% CI [1.02, 1.16]).
Table 3. Results of the multivariable analysis
Discussion
While some studies on the subject exist in Northern countries,Citation29,Citation30 this research is one of the first in Africa. The results of this research are important in the context of the many controversies surrounding various vaccines, including AstraZeneca’s vaccine, which is at the heart of the COVAX initiative for Africa. As Senegal began administering the Sinopharm vaccine in early March 2021 and then its first doses of AstraZeneca, understanding people’s perceptions of vaccine trials are essential. Indeed, in a context where there are calls for the decolonization of global health research and for more vaccine trials to be conducted in Africa,Citation13,Citation18 obtaining the views of those affected is an essential ethical issue.Citation31,Citation32 Rumors about vaccines can influence people’s decisions, as was the case in the 1990s in CameroonCitation33 or its participation in a clinical trial in Gambia.Citation34 Anthropologists have shown extensively how participation in vaccine trials is complex and that local socio-cultural issues must be taken into account.Citation31
The proportion of people in Senegal intending to participate in a vaccine trial against COVID-19 (44.3%) is very similar to that in the UK (41.4%),Citation30 France (47.6%)Citation29 and Saudi Arabia (40.1%),Citation35 whose contexts are obviously very different. In the context of this study in Senegal, the most favorable factor for wanting to participate in a COVID-19 vaccine trial is having the intention to be vaccinated. Studies in France and the UK both confirm that the importance attached to COVID-19 vaccination is associated with the intention to participate in a vaccine trial.Citation29,Citation30 This finding is perfectly aligned with most conceptual and theoretical models in health education and promotion which show that intention is a key (but far from the only) determinant of behavior change.Citation36 While there is much work and debate on the concept of vaccine hesitancy,Citation37,Citation38 few studies have applied this concept to vaccination trials.Citation20,Citation30 Yet, conceptually, we might think that the conceptual models of vaccine hesitancy are applicable to vaccine trials since past experiences, perceived importance of vaccination or subjective norms also affect the intention to participate.Citation37 In a future study, it would be useful to adapt this conceptual model. It should be noted that we conducted this study before the vaccines arrived in Senegal and the context has since changed significantly. Indeed, at the time of writing this discussion (Mid-June 2021), the national vaccination rate is very low (2.7%) with very large regional disparities (https://www.covid19afrique.com/vaccination). It is possible that intention to participate in a vaccine trial will depend on the success of this vaccination campaign and the challenges of vaccine availability/accessibility, which future surveys will be able to tell us.
This study also shows that other factors related to the health of individuals affect the intention to participate in a trial, such as gender, perceived health status, belief in the consequences of vaccination on health, and positive attitude toward vaccination. This finding largely corresponds to the variables most often put forward to explain changes in health education behavior or vaccine hesitancy.Citation39 Indeed, several authors have pointed out the importance of the health status and risk perception of the people concerned in the context of controlled human infection studies like experimental vaccines for COVID-19.Citation32 Unlike France,Citation29 the fact that women seem to be more inclined to participate in a trial could be explained by the fact that they are often more involved with vaccinations, especially taking care of their children. A systematic review has recently shown that gender has a significant influence on the decision to vaccinate a child in Africa and that women were facilitators.Citation40 Even though in Senegal, 72.5% of deaths related to COVID-19 were men, women have indeed suffered more indirect impacts (economic, empowerment, education, violence, etc.) of the pandemic than men, as has been discussed for West Africa.Citation41 In addition, our previous work has shown that women had more trust in the government in the fight against the COVID-19 pandemic than men.Citation23 That being said, it seems that the gender issue has not yet been sufficiently taken into account in the reflections on trial vaccines in Africa,Citation42 like against meningitis.Citation43
It is not only individual factors that seem to influence the intention of study participants. The research confirms the importance of the notion of trust, which is an essential value for the effectiveness of health systemsCitation29 and for the intention to adopt health-promoting behaviors. Research on vaccine hesitancy highlighted the importance of trust in the decision toward health professionals and the vaccine itself.Citation37 However, trust in the media, politicians, and the State is also important: “hesitation to vaccinate can, in many cases, be evidence of a critical attitude towards the public authorities, and corresponds to an expectation, a legitimate demand, to which respectful attention must indeed be paid.”Citation38 With the acceptability of government measures to control COVID-19Citation30 or the intention to be vaccinated against COVID-19,Citation44 trust is an important determinant of willingness to participate in research.Citation20 A recent study in the UK confirms that “mistrust is a key factor in non-uptake for vaccination trials.”Citation30 A qualitative study in the US shows the importance of trust in science and a Saudi Arabia study of the importance of contributing to science for participation in the COVID-19 vaccine trial.Citation20,Citation35 Moreover, research in the United States of America has shown how racialized history affects African Americans’ trust in government, influenza vaccination,Citation45 or COVID-19 vaccine trials.Citation46 COVID-19 was a reminder that democracy, health, and pandemic are interrelated.Citation47 In Senegal, this trust seems to be multifaceted since it is demanded not only for the vaccine product but also for the health personnel and the government.
Due to financial constraints, we could not conduct a nationally representative survey for a house-to-house survey strategy which would have allowed us to have more household variables for the analyses. It would be useful to organize a qualitative study in the future to understand our findings, including the emic interrelationships between the role of trust and the interpretation of disease and vaccination trials. We will also try to see whether these declarations of intent to participate in a vaccine trial are likely to materialize. As we know, declarations in a cross-sectional survey are sometimes different from behaviors. Therefore, this in-depth understanding of the context and the perception of social actors must involve qualitative approaches, which we will mobilize in a second phase. It will also be interesting to compare the quantitative results of the intention to participate in a vaccine trial a few months after the vaccination campaign starts to see if the intentions and determinants have changed according to the relative effectiveness of the campaign. Finally, it would be useful to see whether these perceptions change depending on the type of vaccine available, since, at the moment, Senegal relies mainly on Sinopharm for its vaccine campaign.
The research results seem logical in a global context where fake news is widespread about vaccines, where Africa has been the scene of numerous medical experimentation abuses, Citation1,Citation2 and where the current mistrust of the Senegalese state apparatus is significant, including in the context of the COVID-19 pandemic.Citation48 In terms of the implications of the results of this study, we believe that it is essential to increase this multifaceted trust to improve the willingness of Senegalese to participate in vaccine trials and to better understand with qualitative research the place of the perception of the disease and vaccinations in the declared intention.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
Notes
References
- Chabrol F. Biomedicine, public health, and citizenship in the advent of antiretrovirals in Botswana: the advent of antiretrovirals in Botswana. Dev World Bioeth. 2014;14:75–82. doi:10.1111/dewb.12051.
- Lachenal G. Le médicament qui devait sauver l’Afrique: un scandale pharmaceutique aux colonies. Paris (France): La Découverte; 2014.
- Moulin AM, Chabrol F, Ouvrier A. Chapitre 24. Histoire d’un vaccin pas comme les autres : les premiers pas du vaccin contre l’hépatite B au Sénégal. In: Delaunay V, Desclaux A, Sokhna C, editors. Niakhar, mémoires et perspectives. Montpellier (France): IRD Éditions;2018. p. 489–510. doi:10.4000/books.irdeditions.31872.
- Koster W, Ndione AG, Adama M, Guindo I, Sow I, Diallo S, Sakandé J, Ondoa P. An oral history of medical laboratory development in francophone West African countries. Af J Lab Med. 2021;10. doi:10.4102/ajlm.v10i1.1157.
- Ouvrier A. La recherche médicale en Afrique est un moyen pour l’Occident de tester des médicaments dangereux. Des idées reçues en santé mondiale. Montréal (Canada): Presses de l’Université de Montréal. p. 215–18; 2015. doi:10.4000/books.pum.3607
- Petryna A. Globalizing human subjects research. In: Petryna A, Lakoff A, Kleinman A, editors. Global pharmaceuticals. Durham: Duke University Press; 2006. p. 33–60. doi:10.1215/9780822387916-002.
- Moulin A-M. Patriarchal science: the network of the overseas pasteur institutes. Science and empires historical studies about scientific development and European expansion; Dordrecht: Springer Science + Business Media; 1992. p. 307–22.
- Holding M, Ihekweazu C, Stuart JM, Oliver I. Learning from the epidemiological response to the 2014/15 ebola virus disease outbreak. JEGH. 2019; [cited 16] Feb 2020. doi:10.2991/jegh.k.190808.002.
- Desclaux A, Badji D, Ndione AG, Sow K. Accepted monitoring or endured quarantine? Ebola contacts’ perceptions in Senegal. Soc Sci Med. 2017;178:38–45. doi:10.1016/j.socscimed.2017.02.009.
- N’koué Sambiéni E, Danko N, Ridde V. La Fièvre Hémorragique à Virus Lassa au Bénin en 2014 en contexte d’Ebola : une épidémie révélatrice de la faiblesse du système sanitaire. Anthropologie Et Santé. 2015 [cited 2017 Jan 11]. doi:10.4000/anthropologiesante.1772.
- Salyer SJ, Maeda J, Sembuche S, Kebede Y, Tshangela A, Moussif M, Ihekweazu C, Mayet N, Abate E, Ouma A.O. et al. The first and second waves of the COVID-19 pandemic in Africa: a cross-sectional study. The Lancet. 2021;S0140673621006322. doi:10.1016/S0140-6736(21)00632-2.
- Tilley. COVID-19 across Africa: colonial hangovers, racial hierarchies, and medical histories. J West Afr His. 2020;6:155. doi:10.14321/jwestafrihist.6.2.0155.
- Ridde V, Ouedraogo S, Yaya S. Closing the diversity and inclusion gaps in francophone public health: a wake-up call. BMJ Glob Health. 2021;6:e005231. doi:10.1136/bmjgh-2021-005231.
- Büyüm AM, Kenney C, Koris A, Mkumba L, Raveendran Y. Decolonising global health: if not now, when? BMJ Glob Health. 2020;5:e003394. doi:10.1136/bmjgh-2020-003394.
- Hellmann F, Williams-Jones B, Garrafa V. COVID-19 and moral imperialism in multinational clinical research. Arch Med Res. 2020;51:572–73. doi:10.1016/j.arcmed.2020.04.017.
- Desclaux A Covid-19: en Afrique de l’Ouest, le vaccin n’est pas le nouveau «magic bullet»; 2021 [accessed 2021 June 11]. https://vih.org/20210202/la-mondialisation-des-informations-et-la-fabrique-des-opinions-sur-les-traitements-du-covid-en-afrique/.
- Ouvrier M-A, Geissler W, Moulin A-M. Faire de la recherche médicale en Afrique: ethnographie d’un village-laboratoire sénégalais. Paris (France): IRD; 2015. p. 228.
- Kana MA, LaPorte R, Jaye A. Africa’s contribution to the science of the COVID-19/SARS-CoV-2 pandemic. BMJ Glob Health. 2021;6:e004059. doi:10.1136/bmjgh-2020-004059.
- Nkeck JR, Ndoadoumgue AL, Temgoua MN. COVID 19 pandemic, status of clinical trials in Africa on May 2020: need to reinforce. Pan Afr Med J. 2020:35. doi:10.11604/pamj.supp.2020.35.2.24349.
- Wentzell E, Racila A-M. The social experience of participation in a COVID-19 vaccine trial: subjects’ motivations, others’ concerns, and insights for vaccine promotion. Vaccine. 2021:S0264410X21003170. doi:10.1016/j.vaccine.2021.03.036.
- Brotherton H, Usuf E, Nadjm B, Forrest K, Bojang K, Samateh AL, Bittaye M, Roberts CA, d’Alessandro U, Roca A, et al. Dexamethasone for COVID-19: data needed from randomised clinical trials in Africa. The Lancet Global Health. 2020;8:e1125–e1126. doi:10.1016/S2214-109X(20)30318-1.
- Desclaux A. L’acceptabilité du vaccin anti-COVID en AOC. Dakar (Sénégal); CRCF/IRD; 2020.
- Ridde V, Kane B, Gaye I, Ba F, Diallo A, Bonnet E, Traoré Z, Faye A Acceptability of government measures against Covid-19 pandemic in Senegal: a mixed methods study. In Review. Dec 2020. doi:10.21203/rs.3.rs-131071/v1.
- Riandey B, Blöss-Widmer I. Introduction aux sondages à l’usage du plus grand nombre. 2009 [accessed 2021 June 11]. https://hal.archives-ouvertes.fr/hal-01272371.
- Huijts NMA, Molin EJE, Steg L. Psychological factors influencing sustainable energy technology acceptance: a review-based comprehensive framework. Renew Sustain Energy Rev. 2012;16:525–31. doi:10.1016/j.rser.2011.08.018.
- Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res. 2017;17:88. doi:10.1186/s12913-017-2031-8.
- Hosmer DW, Lemeshow S. Applied logistic regression. 2nd ed. New York (USA): Wiley-Interscience Publication; 2000.
- Zhang Z. Model building strategy for logistic regression: purposeful selection. Annal Trans Med. 2016;4:4–10. doi:10.21037/atm.2016.02.15.
- Detoc M, Bruel S, Frappe P, Tardy B, Botelho-Nevers E, Gagneux-Brunon A. Intention to participate in a COVID-19 vaccine clinical trial and to get vaccinated against COVID-19 in France during the pandemic. Vaccine. 2020;38:7002–06. doi:10.1016/j.vaccine.2020.09.041.
- Sethi S, Kumar A, Mandal A, Shaikh M, Hall CA, Kirk JMW, Moss P, Brookes MJ, Basu S. The UPTAKE study: implications for the future of COVID-19 vaccination trial recruitment in UK and beyond. Trials. 2021;22:296. doi:10.1186/s13063-021-05250-4.
- Kaljee L, Pach A, Stanton B. Applied anthropology, vaccine trials and feasibility studies: intersections of local knowledge, biomedicine, and policy. Pract Anthropol. 2011;33:39–43. doi:10.17730/praa.33.4.j14752n270j1w761.
- Shah SK, Miller FG, Darton TC, Duenas D, Emerson C, Lynch HF, Jamrozik E, Jecker NS, Kamuya D, Kapulu M, et al. Ethics of controlled human infection to address COVID-19. Science. 2020;368(6493):832–34. doi:10.1126/science.abc1076.
- Feldman-Savelsberg P, Ndonko FT, Sterilizing S-EB. Vaccines or the politics of the womb: retrospective study of a rumor in cameroon. Med Anthropol Q. 2000;14:159–79. doi:10.1525/maq.2000.14.2.159.
- O’Neill S, Dierickx S, Okebe J, Dabira E, Gryseels C, d’Alessandro U, Peeters Grietens K. The Importance of blood is infinite: conceptions of blood as life force, rumours and fear of trial participation in a fulani village in rural gambia. Gregson A, editor. PLoS ONE. 2016;11:e0160464. doi:10.1371/journal.pone.0160464.
- Felemban RM, Tashkandi EM, Mohorjy DK. The willingness of the Saudi Arabian population to participate in the COVID-19 vaccine trial: a case-control study. J Taibah Univ Med Sci. 2021. doi:10.1016/j.jtumed.2021.03.001.
- de Vries H. An integrated approach for understanding health behavior; The I-Change model as an example. PBSIJ. 2017:2. doi:10.19080/PBSIJ.2017.02.555585.
- Dubé E, Laberge C, Guay M, Bramadat P, Roy R, Bettinger JA. Vaccine hesitancy: an overview. Hum Vaccin Immunother. 2013;9:1763–73. doi:10.4161/hv.24657.
- Moulin A-M, Thomas G L’hésitation vaccinale, ou les impatiences de la santé mondiale. La vie des idées; 2021 [accessed 2021 June 11]. https://laviedesidees.fr/L-hesitation-vaccinale-ou-les-impatiences-de-la-sante-mondiale.html.
- Godin G, Kok G. The theory of planned behavior: a review of its applications to health-related behaviors. Am J Health Promot. 1996;11:87–98. doi:10.4278/0890-1171-11.2.87.
- Bangura JB, Xiao S, Qiu D, Ouyang F, Chen L. Barriers to childhood immunization in sub-Saharan Africa: a systematic review. BMC Public Health. 2020;20:1108. doi:10.1186/s12889-020-09169-4.
- Laouan FZ. West Africa rapid gender analysis for COVID-19. CARE, Bamako, Mali; 2020. p. 21.
- Wassenaar DR, Barsdorf NW. The ethical involvement of women in HIV vaccine trials in Africa: discussion paper developed for the African AIDS vaccine programme. Women Health. 2007;45:37–50. doi:10.1300/J013v45n01_03.
- Marchetti E, Mazarin-Diop V, Chaumont J, Martellet L, Makadi M-F, Viviani S, Kulkarni PS, Preziosi M-P. Conducting vaccine clinical trials in sub-Saharan Africa: operational challenges and lessons learned from the meningitis vaccine project. Vaccine. 2012;30:6859–63. doi:10.1016/j.vaccine.2012.09.008.
- Latkin C, Dayton L, Yi G, Konstantopoulos A, Park J, Maulsby C, Kong X. COVID-19 vaccine intentions in the United States, a social-ecological framework. Vaccine. 2021;S0264410X21002383. doi:10.1016/j.vaccine.2021.02.058.
- Jamison AM, Quinn SC, Freimuth VS. “You don’t trust a government vaccine”: narratives of institutional trust and influenza vaccination among African American and white adults. Soc Sci Med. 2019;221:87–94. doi:10.1016/j.socscimed.2018.12.020.
- Warren RC, Forrow L, Hodge DA, Truog RD. Trustworthiness before trust — covid-19 vaccine trials and the black community. N Engl J Med. 2020;383:e121. doi:10.1056/NEJMp2030033.
- Bollyky TJ, Kickbusch I. Preparing democracies for pandemics. BMJ. 2020:m4088. doi:10.1136/bmj.m4088.
- CIVICUS Monitor. National civic space ratings. Johannesburg (South Africa); 2021 [accessed 2021 June 11]. https://monitor.civicus.org.