ABSTRACT
This study aimed to determine social and behavioral predictors of completing a course of 4CMenB vaccine in adolescents in a parallel cluster randomized controlled trial enrolling secondary school students (approximately 15–18 years of age) in South Australia. Participating schools were randomized to vaccination at baseline (intervention) or 12 months (control). Students assigned to the intervention group were excluded because they have received the first dose of 4CMenB vaccine at baseline. Logistic regression models examined factors associated with non-vaccination or incomplete 4CMenB doses. The study population comprised 11391 students. Overall, 8.3% (n = 946) received no doses and 91.7% (n = 10445) at least one dose. Of 10445 students who initiated their primary dose, 1334 (12.8%) did not complete the two-dose course. The final adjusted model indicated factors associated with non-vaccination in school students were older age (adjusted odds ratio; aOR 7.83, 95% CI: 4.13–14.82), smoking cigarettes (aOR 3.24, 95% CI: 1.93–5.44), exposure to passive smoke (aOR 2.64, 95% CI: 1.48–4.71), Aboriginal or Torres Strait Islander (aOR 1.77, 95% CI: 1.23–2.55), smoking water pipes (aOR 1.94, 95% CI:1.28–2.92), low socioeconomic status (aOR 1.77, 95% CI:1.21–2.60), attending government schools (aOR 1.76, 95% CI: 1.28, 2.43) and participating in intimate kissing (aOR 1.40, 95% CI:1.10–1.79). Multivariable analysis for incomplete vaccination yielded similar findings. Social and behavioral predictors of non-vaccination or incomplete MenB doses were also known risk factors for carriage of Neisseria meningitidis. Immunization strategies to improve MenB vaccination completion need to be tailored to social behavior of adolescents.
Introduction
Invasive meningococcal disease caused by Neisseria meningitidis infection is associated with high morbidity and mortality, responsible for 1.2 million cases of infection per year, as well as approximately 135,000 deaths worldwide.Citation1,Citation2 The most important meningococcal disease-associated groups are A, B, C, W, X, and Y, serogroup B being one of the most predominant serogroups in Australia and other countries.Citation3,Citation4 Exposure to N. meningitidis is common in the general population, leading to asymptomatic pharyngeal carriage, which may be transient or long term.Citation5
Age influences carriage, with a rapid rise from 15 years of age to a peak at approximately 19 years in high-income countries, likely due to increases in the number and closeness of social contacts and exposure to risk factors in this age group.Citation6,Citation7 Although invasive meningococcal disease incidence is highest in infants, a second peak occurs in adolescents/young adults.Citation4 Several countries, including Australia, recommend the licensed 4CMenB vaccine to provide protection against meningococcal B (MenB) disease in adolescents aged 15–19 years due to their higher risk of group B meningococcal disease.Citation3
Despite the recommendation of MenB vaccination for adolescents, uptake of the vaccine in this age group has been suboptimal ranging between 17% to 22% for at least one dose of MenB in the USA.Citation8,Citation9 One reason for low MenB vaccine uptake among adolescents is lack of routine and publicly funded MenB vaccination programs.Citation10,Citation11 In the USA, MenB vaccine is recommended for adolescents or young adults aged 16–23 years (preferred age 16–18 years) on the basis of shared clinical decision-making.Citation12 In Australia, the MenB vaccine (4CMenB) is not funded in the National Immunization Program (NIP) for this age group, however South Australia has a state-funded MenB vaccine program for adolescents with 74% uptake for one dose and 66% for two doses in 15-year-olds in the first year of the program.Citation13 MenB vaccines are recently funded under the NIP in 2020 for people of all ages including adolescents with specified medical risk conditions, including defects in, or deficiency of, complement components, current or future treatment with eculizumab, or functional or anatomical asplenia. It is also funded for Aboriginal and Torres Strait Islander children ages less than 2 years of age.Citation14 While the recommendation for specific meningococcal vaccine use in each country depends on the predominant disease-causing serogroups,Citation15 low completion of multidose schedules is common among adolescents and young adults.Citation16 Although barriers and facilitators to vaccine uptake among adolescents have been extensively reviewed,Citation10,Citation17–20 there is no comprehensive assessment of social and behavioral factors influencing non-vaccination or incompletion of MenB vaccine dose series in this group. This study aimed to determine demographic, social and behavioral predictors of non-vaccination or non-completion of the recommended two-dose of MenB vaccine series among adolescents aged 15–19 years.
Materials and methods
Study design and participants
This nested cohort study draws on data collected as part of a parallel cluster randomized controlled trial (RCT) enrolling secondary school students (approximately 15–18 years of age) throughout South Australia, in metropolitan and rural/remote areas from 2017 to 2018 to examine the impact of a two-dose 4CMenB vaccine series administered at least one month apart on the carriage of disease-associated meningococci in adolescent school students (ClinicalTrials.gov number, NCT03089086).Citation21 Students in the three final years of school were enrolled in the study. The trial’s exclusion criteria were known pregnancy, previous anaphylactic reaction to 4CMenB, and receipt of a previous dose of 4CMenB. Over 90% (n = 237) of secondary schools in South Australia were randomized to intervention (4CMenB vaccination at baseline in 2017) or control (4CMenB vaccination at study 12 months in 2018) with randomization stratified by school size and socioeconomic status as measured by the Index of Community Socio-Educational Advantage (ICSEA). Approximately 62% of year 10 and 11 students in South Australia enrolled in the trial. Study details are described elsewhere.Citation21,Citation22 In this analysis, only students from schools randomized to the control group were included as these students had not received the vaccine at baseline and therefore this allowed a comparison between students who declined offer/accepted offer at study completion. We cannot use baseline data as predictors to determine receipt of first dose for students assigned to the intervention group, since vaccination and baseline questionnaire were administered at the same visit for this group. Whereas, students assigned to the control group received their first dose at 12 months follow-up and second dose approximately 2-month after the first dose. Sociodemographic characteristics, health behaviors and social factors reported by students (control group) from their baseline questionnaire were assessed to examine factors associated with completing a two-dose course of 4CMenB vaccine in 2018. Students assigned to the control group that left school prior (mostly year 12 students in 2017) to either the first or second vaccination visit, were able to obtain their two-dose course of 4CMenB vaccine from their local immunization provider/council. Enough vaccine was provided through the study for all enrolled participants to receive 4CMenB vaccine free either at study enrollment or at study completion. Therefore, all control participants had access to free vaccine. Additionally, the State Government funded adolescent Meningococcal B (MenB) Immunization Program commenced on 1st of October 2018.Citation14 However, most school students from the control group who participated in the RCT received their second dose prior to commencement of the state funded program. Year 10 and 11 students were followed up in schools at 12 months, whereas the year 12 students only provided baseline data for the trial and were not in school at the end of the follow up period. To minimize the bias that might occur due to the difference in access to vaccination in this school year group, only students who were in years 10 and 11 at the first study visit in 2017 were included in this study.
Outcomes
The primary outcome was the proportion of adolescents in year 10 and 11 who did not receive two doses of the 4CMenB vaccine series. The secondary outcome was the proportion of adolescents in year 10 and 11 who received one vaccine dose but did not complete the two-dose course of 4CMenB vaccine series. Vaccination status was considered ‘non-vaccination’ if the adolescent did not receive any doses (zero doses) and “incomplete” if they did not receive the second dose of MenB vaccine with an interval of approximately 2 months (range 1–3 months).
Explanatory variables
Explanatory variables were selected based on the published literature, prior knowledge and variables included in the “B Part of It” study dataset. The students completed the baseline questionnaires on a separate form that only contained their study ID. No names were included on the forms to promote honest answers. Nurses provided hard copies of the questionnaires to students who were asked to complete it by themselves and advised that privacy would be maintained as the form only included a linking number. The questionnaire was later re-identified by subject number to link questionnaire data with carriage and demographic data. Internal quality checks, such as automatic range checks, were performed to identify data that appeared to be inconsistent, incomplete, or inaccurate. Factors measured at baseline that can potentially predict vaccination receipt at the study follow-up period were included. Baseline information on participants, age, gender, ethnicity, school characteristics, current smoking habit, number of people currently residing in their household, number of people in their house who smoke, recent partner, recent kissing and attendance at a party, pub, hotel were selected as exploratory variables predicting non-completion of MenB vaccine series among adolescents.
Statistical analysis
As this analysis involves the secondary use of data already collected in the high school RCT, no prespecified sample size calculation was undertaken. We first examined if demographic, school characteristics, behavioral variables measured at baseline were associated with receipt of two doses of 4CMenB vaccine at the 15-month follow-up. Logistic regression models with generalized estimating equations (GEEs) were used to estimate both the crude and adjusted odds ratios (aOR) and their corresponding 95% confidence intervals to determine factors associated with non-vaccination or incomplete two-dose course of 4CMenB vaccine among adolescents. To accommodate the potential correlations in vaccine receipt among students from the same school, the GEEs were used to account for clustering at the school level. In the mutually adjusted models, we included all covariates (i.e. age, gender, ethnicity/race, smoking status, relationship status, socioeconomic status (SES)) that were known potential confounders associated with vaccination uptake among adolescents based on the published literature.Citation10,Citation19,Citation20,Citation23 Given the relatively high follow-up rate and high adherence to the trial protocol in year 10 and 11 students, the overall missing information in either outcomes (vaccination status) or baseline (predictors) was minimal ranging between 0.1% to 6.9%. Therefore, all available data were used in the analyses. For all analyses, p values < .05 were considered statistically significant. All statistical analyses were performed using Stata version 15 (Stata Corp, College Station, Texas, USA). The protocol was approved by the Women’s and Children’s Health Network Human Research Ethics Committee.Citation21,Citation22
Results
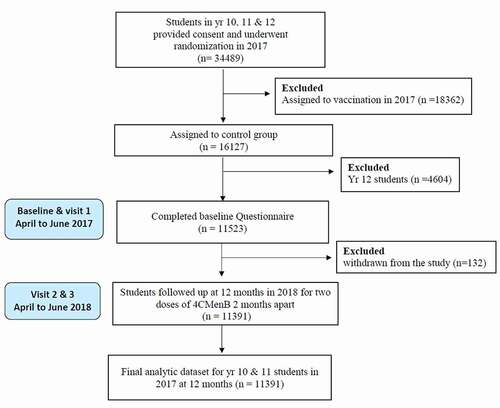
A total of 34489 students were enrolled between April 1 and June 30, 2017. Overall, 11391 students in years 10 (n = 6117) and 11 (n = 5274) were included in our analysis, after excluding 18632 students assigned to receive 4CMenB vaccination at baseline, 4604 students in year 12 and 132 participants who withdrew from the study at any stage (). The analysis included 5664 (49.72%) male students and 5727 (50.28%) female students from 112 participating secondary high schools in South Australia. Almost half of the participants (49.56%, n = 5649) at the baseline visit were 15 years old or younger (mean age, 15.61 ± 1.21 years). Students were predominantly Caucasian (69.15%, n = 7877) and from a low household overcrowding index (80.97%, n = 9223). Most (70.74%, n = 8058) of the students attended schools in metropolitan locations and just under half were from high Index of Relative Socioeconomic Disadvantage (IRSD) quintile (ICSEA) schools (47.23%, n = 5380). Baseline characteristics of the students are described in .
Table 1. Baseline characteristics of year 10 and 11 students participating in school RCT, 2017–2018, South Australia
The overall percentage of students who received the first dose of 4CMenB at the 12 months follow up was 91.62% (n = 10,436) and 80.06% (n = 9,120) received 4CMenB vaccine at the second vaccination visit approximately spaced 2 months apart. The median time from first-dose vaccination to second-dose vaccination was 64 days (Interquartile range [IQR], 57–77 days). Of the 955 students who did not receive the vaccine at the first vaccination visit, only nine received the 4CMenB vaccine at a subsequent vaccination visit. Of the final 11391 students, 8.30% (n = 946) received no doses and 91.69% (n = 10445) at least one dose with an overall two-dose completion rate of 79.98% (n = 9111). Of the 10445 students who initiated their primary dose at the first or second vaccination visits, 1334 (12.77%) did not complete the two-dose course of 4CMenB vaccine series. The prevalence of disease-associated (A, B, C, W, X, Y) N. meningitidis carriage detected at baseline among adolescents who did not receive any doses 4CMenB vaccine (25/946, 2.64%) was significantly higher compared to those who received at least one dose of 4CMenB vaccine during the study period (135/10445, 1.29%; p < .001).
Univariate and multivariate associations among demographic, behavioral, and social predictors of non-vaccination (compared to receiving at least one 4CMenB vaccine dose) are shown in . In the multivariable logistic regression analyses, factors that increased the likelihood of non-vaccination included, being an older adolescent or young adult (age ≥18) (aOR 7.83, 95% CI: 4.13–14.83), smoking cigarettes (aOR 3.24, 95% CI: 1.93–5.44), exposure to passive smoke at home and outside the house (aOR 2.64, 95% CI: 1.48–4.71), smoking water pipes (aOR 1.94, 95% CI: 1.28–2.92), identifying as Aboriginal or Torres Strait Islander (aOR 1.77, 95% CI: 1.23–2.55), low ICSEA quintile (aOR 1.77, 95% CI: 1.21–2.60), attending Government schools (aOR 1.76, 95% CI: 1.28–2.43), participating in intimate kissing (aOR 1.40, 95% CI: 1.10–1.79), attending schools in metropolitan locations (aOR 1.31, 95% CI: 1.00–1.71) and having a partner (aOR 1.26, 95% CI: 1.02–1.57) ().
Table 2. Factors associated with non-vaccination (zero doses) of the two-dose schedule of 4CMenB vaccine among adolescents in year 10 and 11
In the univariate analysis, smoking cigarettes, having a partner, participating in intimate kissing and attending government schools were associated with an increased likelihood of non-completion of the 4CMenB vaccine series, but these associations were not significant in the final adjusted model (). In the final adjusted model examining factors contributing to incomplete 4CMenB vaccination (compared to receiving two doses) similar associations of sociodemographic, social, and behavioral predictors of non-completion of the 4CMenB vaccine series were identified (). Additional significant predictors of incomplete 4CMenB vaccination status that were not associated with non-vaccination included living in overcrowded house (aOR 1.72, 95% CI: 1.06–2.78) and attendance at pubs or clubs (aOR 1.19, 95% CI: 1.00–1.42) ().
Table 3. Factors associated with receiving only one dose of 4CMenB vaccine among Adolescents in year 10 and 11
Discussion
This is the first study to evaluate social and behavioral factors associated with 4CMenB vaccine series completion among adolescents. Our analysis is based on a state-wide sample derived from the largest RCT cohort of adolescents vaccinated with 4CMenB to date as part of the school immunization program in South Australia.Citation21 Among approximately 11,000 adolescents included in our study, we observed 80% completion rate of the two-dose regimen within three months of the first dose. This suggests that a significant proportion of adolescents have missed opportunities for vaccination or initiate the series but do not complete the recommended two-dose of 4CMenB vaccine series. However, completion of the 4CMenB vaccine series in our study is impressively higher than a previous study in USA, with a completion rate of 58%.Citation23 A similar trend has been reported in 2017 for MenACWY vaccine uptake among adolescents in the USA where a coverage of at least one dose of MenACWY among adolescents age 13–17 years was estimated to be 85%, yet uptake of a booster dose at 16 years of age remains suboptimal at 44%.Citation24 On the basis of immunogenicity responses, completion of the two-dose 4CMenB vaccine course in adolescents is necessary for maximal protection against meningococcal B infection.Citation25 Focused efforts are required to overcome the drop off between the first and subsequent doses in school delivered immunization programs or in community or health facilities.
Although recent studies have demonstrated no discernible effect of recombinant MenB vaccines on disease-associated carriage,Citation21,Citation26 there is still a need for direct protection of adolescents and young adults at an age of greater risk of invasive meningococcal disease.Citation7 Interestingly, it was found that groups of adolescents with the highest risk of carriage in the original RCTCitation21 were least likely to be vaccinated or complete a two-dose series of 4CMenB in this study. The present study suggests that older adolescents, who are an important risk age-group for serogroup B meningococcal disease were more likely to be unvaccinated or have an incomplete course of the recommended two doses 4CMenB, consistent with previous studies of other meningococcal vaccines.Citation23,Citation27 Although all students in our study were offered 4CMenB vaccine, school absenteeism in older adolescents is common which may contribute to non-vaccination or under vaccination in this age group. Additionally, parents are likely to be the primary decision-makers for early adolescent’s vaccination compared to older adolescents who may be more participatory in vaccination decisions.Citation28 Interventions aimed at improving completion of 4CMenB series among older adolescents are needed and catch-up immunization campaigns should include sufficient information about and better access to the vaccine to help older adolescents make informed decisions about vaccination.Citation18 Furthermore, it is important to maintain high MenB vaccine series completion rates in younger adolescents before they enter the highest age-based risk period. This may maximize the likelihood of protection prior to entering the age group at highest risk of invasive meningococcal disease.Citation29
Other sociodemographic factors associated with low completion of 4CMenB vaccine were male gender, although findings from a systematic review of studies of multi-dose vaccination in adolescents reported inconsistent results in relation to gender.Citation20 The RCT in South Australia demonstrated that students who identify as Aboriginal or Torres Strait Islander had almost double the carriage prevalence compared to students that identified as CaucasianCitation21 and they were twice more likely not to complete the 4CMenB vaccine series in this study. It is widely recognized that Aboriginal peoples in Australia have higher invasive meningococcal disease notification rates than non-Aboriginal peoplesCitation30 with disease due to serogroup B being four times higher for Aboriginal people in 2017 (2.0/100 000) compared to non-Aboriginal people (0.5/100 000).Citation25 This suggests the need to improve strategies that are culturally appropriate, including active communication and better access in targeted campaigns for Aboriginal adolescents. For adolescents who missed the MenB vaccine doses delivered in school-based programs, catch up vaccine programs could incorporate Aboriginal Community Controlled Health Organization services to deliver culturally appropriate immunization resources and interventions for Aboriginal adolescents.
Social and behavioral factors, rather than age or gender, can explain the higher prevalence of meningococcal carriage among adolescents and young adults.Citation6 Importantly, our study demonstrates that social and behavioral factors such as active and passive cigarette smoking, waterpipe tobacco smoking, being in a relationship, intimate kissing, attending pubs and clubs, and household crowding were all strongly and independently associated with either non-vaccination or non-completion of the recommended two doses of MenB vaccine series, which are known predisposing risk factors for meningococcal carriage in adolescents and young adults.Citation21,Citation31 Cigarette smoking,Citation32 passive smoking,Citation32 intimate kissing,Citation33 low socio-economic status,Citation34 overcrowded living,Citation34–36 identifying as Aboriginal or Torres Strait Islander,Citation37 attending nightclubs,Citation33,Citation36 and have also inconsistently been found to be associated with developing invasive meningococcal disease. This highlights that targeted interventions to improve Men B vaccine coverage gap in adolescents with risk factors may also provide protection for those at highest risk of developing invasive meningococcal disease.Citation33 Administering meningococcal vaccine programs through schools provides an opportunity for parental participation in vaccination decisions and normalization of the process for adolescents. The school program also improves equitable access for adolescents from all socio-economic groups, particularly lower socio-economic groups where risk factors for carriage including smoking and household crowding are likely to be more prevalent.
The major strength of this study is the large sample size, representative of the adolescent population in South Australia. Another strength of the present study is the inclusion of extensive data on known risk factors for meningococcal carriage and disease. This enabled us to explore if predisposing factors for meningococcal carriage are also associated with vaccination behavior among adolescents. To improve retention rates, 20 USD iTunes cards were provided at the first study visit and 12-month follow up visit and text message reminders were sent prior to the school visits to notify parent/participants of when their first and second dose of vaccination will occur. There is evidence that financial incentives improve uptake of vaccines compared to standard practice.Citation18 There is also a risk the financial incentives may introduce bias, by being more appealing to adolescents with lower SES to complete the two-dose regimen. However, this is unlikely as students from low SES areas were less likely to have complete course of two-doses 4CMenB vaccine series. Another potential limitation is students that left school could not be identified. Furthermore, the fact that adolescents were all part of a study means that their social behavior might be different to adolescents partaking in the state government funded MenB immunization program,Citation14 although uptake in the study was almost as high as the state funded program.Citation13 Therefore, the estimated vaccine uptake and social and behavioral factors may differ for students receiving the vaccine via the standard school immunization program and the year 12 students that were excluded from our study.
Studies are needed to determine how to improve completion of vaccine doses in adolescents with consideration of co-designing strategies with young people. As behavioral risk factors were strongly associated with under-vaccination, strategies should focus on behavioral interventions or “nudges” to improve completion of two dose schedules, not only for meningococcal vaccines but also for human papillomavirus vaccine and COVID−19 vaccines when they become approved for young people.
Conclusions
The present study offers a robust evaluation of social and behavioral factors in predicting non-vaccination and completion of the two-dose 4CMenB vaccine series among adolescents. Many of the social and behavioral predictors of low 4CMenB vaccine uptake among adolescents identified in the current study were also known risk factors for carriage of N. meningitidis reflecting a higher risk population remaining unprotected. The study findings can be used to co-design with adolescents, targeted interventions to improve coverage alongside the highly effective school-based immunization programs to maximize the uptake of vaccines recommended for adolescents.
Disclosure of potential conflicts of interest
HSM is an investigator on vaccine trials sponsored by Industry (the GSK group of companies, Novavax, Pfizer). HSM’s, MMc’s and HM’s institution receives funding for investigator-led studies from Industry (Pfizer, Sanofi-Pasteur and the GSK group of companies). HSM, HM and MM receive no personal payments from Industry.
Contribution to authorship
HM, MM and HSM have all contributed to the planning and design of the study, and to the interpretations of the data. HM performed data analyses and prepared the first draft of the manuscript. All named authors were involved in critically reviewing the content, and have approved the final version for publication.
Acknowledgments
We thank the VIRTU team, SA Health, Adelaide Health Technology Assessment, SA Pathology, Local government immunization providers, and the Women’s and Children’s Hospital Foundation. HSM is supported by a NHMRC PF APP1155066.
Data availability statement
The datasets generated and/or analysed during the current study are available upon reasonable request to Prof. Helen Marshall ([email protected]) and subject to regulatory approvals.
Additional information
Funding
References
- Rouphael NG, Stephens DS. Neisseria meningitidis: biology, microbiology, and epidemiology. Methods Mol Biol. 2012;799:1–9.
- Chang Q, Tzeng Y-L, Stephens DS. Meningococcal disease: changes in epidemiology and prevention. Clin Epidemiol. 2012;4:237–45.
- Pelton SI. The global evolution of meningococcal epidemiology following the introduction of meningococcal vaccines. J Adolescent Health. 2016;59(2, Supplement):S3–S11. doi:10.1016/j.jadohealth.2016.04.012.
- Borrow R, Alarcón P, Carlos J, Caugant DA, Christensen H, Debbag R, De Wals P, Echániz-Aviles G, Findlow J, Head C, et al. The global meningococcal initiative: global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert Rev Vaccines. 2017;16(4):313–28. doi:10.1080/14760584.2017.1258308.
- Caugant DA, Tzanakaki G, Kriz P. Lessons from meningococcal carriage studies. FEMS Microbiol Rev. 2007;31(1):52–63. doi:10.1111/j.1574-6976.2006.00052.x.
- MacLennan J, Kafatos G, Neal K, Andrews N, Cameron JC, Roberts R, Evans MR, Cann K, Baxter DN, Maiden MCJ, et al. Social behavior and meningococcal carriage in British teenagers. Emerg Infect Dis. 2006;12(6):950–57. doi:10.3201/eid1206.051297.
- Christensen H, May M, Bowen L, Hickman M, Trotter CL. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(12):853–61. doi:10.1016/S1473-3099(10)70251-6.
- Elam-Evans LD, Yankey D, Singleton JA, Sterrett N, Markowitz LE, Williams CL, Fredua B, McNamara L, Stokley S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 Years - United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(33):1109–16. doi:10.15585/mmwr.mm6933a1.
- Walker TY, Elam-Evans LD, Yankey D, Markowitz LE, Williams CL, Mbaeyi SA, Fredua B, Stokley S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67(33):909–17.
- Niccolai LM, Hansen CE. Suboptimal uptake of meningococcal vaccines among older adolescents: barriers, solutions, and future research directions. Hum Vaccin Immunother. 2020;16(12):3208–12. doi:10.1080/21645515.2020.1754052.
- Kuhdari P, Stefanati A, Lupi S, Valente N, Meningococcal GG. B vaccination: real-world experience and future perspectives. Pathog Glob Health. 2016;110:148–56.
- Mbaeyi SA, Bozio CH, Duffy J, Rubin LG, Hariri S, Stephens DS, MacNeil JR. Meningococcal Vaccination: recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR Recomm Rep. 2020;69(9):1–41. doi:10.15585/mmwr.rr6909a1.
- Marshall HS, Wang B, Andraweera P, McMillan M, Giles L, Almond S, A'Houre M, Lally N, Denehy E, Koehler A, et al. 4CMenB vaccine impact and effectiveness against meningococcal disease and gonorrhoea in a world first infant, child and adolescent program in South Australia. The 39th Annual Meeting of the European Society of Paediatric Infectious Diseases (ESPID): online meeting; 2021 May 4 to 29; Geneva, Switzerland. Available from: https://keneswp.azureedge.net/wp-content/uploads/sites/133/2021/05/ESPID-2021-Abstract-Book.pdf .
- National Centre for Immunisation Research and Surveillance. Meningococcal vaccines for Australians 2020 [cited 2021 June 04]. Available from: https://www.ncirs.org.au/sites/default/files/2020-06/Meningococcal%20vaccines%20for%20Australians%20fact%20sheet_1%20July%202020_Final_0.pdf.
- Ali A, Jafri RZ, Messonnier N, Tevi-Benissan C, Durrheim D, Eskola J, Fermon F, Klugman KP, Ramsay M, Sow S, et al. Global practices of meningococcal vaccine use and impact on invasive disease. Pathog Glob Health. 2014;108(1):11–20. doi:10.1179/2047773214Y.0000000126.
- Burman C, Serra L, Nuttens C, Presa J, Balmer P, York L. Meningococcal disease in adolescents and young adults: a review of the rationale for prevention through vaccination. Hum Vaccin Immunother. 2019;15(2):459–69. doi:10.1080/21645515.2018.1528831.
- kDas JK, Salam RA, Arshad A, Lassi ZS, Bhutta ZA. Systematic review and meta-analysis of interventions to improve access and coverage of adolescent immunizations. J Adolescent Health. 2016;59(4, Supplement):S40–S8. doi:10.1016/j.jadohealth.2016.07.005.
- Abdullahi LH, Kagina BM, Ndze VN, Hussey GD, Wiysonge CS. Improving vaccination uptake among adolescents. Cochrane Database Syst Rev. 2020;1:Cd011895.
- Sakou II, Tsitsika AK, Papaevangelou V, Tzavela EC, Greydanus DE, Tsolia MN. Vaccination coverage among adolescents and risk factors associated with incomplete immunization. Eur J Pediatr. 2011;170(11):1419–26. doi:10.1007/s00431-011-1456-z.
- Gallagher KE, Kadokura E, Eckert LO, Miyake S, Mounier-Jack S, Aldea M, Ross DA, Watson-Jones D. Factors influencing completion of multi-dose vaccine schedules in adolescents: a systematic review. BMC Public Health. 2016;16:172. doi:10.1186/s12889-016-2845-z.
- Marshall HS, McMillan M, Koehler AP, Lawrence A, Sullivan TR, MacLennan JM, Maiden MCJ, Ladhani SN, Ramsay ME, Trotter C, et al. Meningococcal B vaccine and meningococcal carriage in adolescents in Australia. N Engl J Med. 2020;382(4):318–27. doi:10.1056/NEJMoa1900236.
- Marshall HS, McMillan M, Koehler A, Lawrence A, MacLennan JM, Maiden MCJ, Ramsay M, Ladhani SN, Trotter C, Borrow R, et al. B part of it protocol: a cluster randomised controlled trial to assess the impact of 4CMenB vaccine on pharyngeal carriage of Neisseria meningitidis in adolescents. BMJ Open. 2018;8(7):e020988. doi:10.1136/bmjopen-2017-020988.
- Packnett E, Irwin DE, Novy P, Watson PS, Whelan J, Moore-Schiltz L, Lucci M, Hogea C. Meningococcal-group B (MenB) vaccine series completion and adherence to dosing schedule in the United States: a retrospective analysis by vaccine and payer type. Vaccine. 2019;37(39):5899–908. doi:10.1016/j.vaccine.2019.06.065.
- Walker TY, Elam-Evans LD, Yankey D, Markowitz LE, Williams CL, Mbaeyi SA, Fredua B, Stokley S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67(33):909–17. doi:10.15585/mmwr.mm6733a1.
- Invasive meningococcal disease national surveillance reports −1 January to 31 December 2017 Canberra Australia: australian government, department of health; 2017 [cited 2021 Feb 18]. Available from: https://www1.health.gov.au/internet/main/publishing.nsf/Content/ohp-meningococcal-W.htm.
- Read RC, Baxter D, Chadwick DR, Faust SN, Finn A, Gordon SB, Heath PT, Lewis DJM, Pollard AJ, Turner DPJ, et al. Effect of a quadrivalent meningococcal ACWY glycoconjugate or a serogroup B meningococcal vaccine on meningococcal carriage: an observer-blind, phase 3 randomised clinical trial. The Lancet. 2014;384(9960):2123–31. doi:10.1016/S0140-6736(14)60842-4.
- Kurosky SK, Esterberg E, Irwin DE, Trantham L, Packnett E, Novy P, Whelan J, Hogea C. Meningococcal vaccination among adolescents in the United States: a tale of two age platforms. J Adolesc Health. 2019;65(1):107–15. doi:10.1016/j.jadohealth.2019.02.014.
- Gowda C, Schaffer SE, Dombkowski KJ, Dempsey AF. Understanding attitudes toward adolescent vaccination and the decision-making dynamic among adolescents, parents and providers. BMC Public Health. 2012;12:509. doi:10.1186/1471-2458-12-509.
- MacNeil JR, Rubin L, Folaranmi T, Ortega-Sanchez IR, Patel M, Martin SW. Use of serogroup B meningococcal vaccines in adolescents and young adults: recommendations of the advisory committee on immunization practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(41):1171–76. doi:10.15585/mmwr.mm6441a3.
- Archer BN, Chiu CK, Jayasinghe SH, Richmond PC, McVernon J, Lahra MM, Andrews RM, McIntyre PB. Epidemiology of invasive meningococcal B disease in Australia, 1999-2015: priority populations for vaccination. Med J Aust. 2017;207(9):382–87. doi:10.5694/mja16.01340.
- Spyromitrou-Xioufi P, Tsirigotaki M, Ladomenou F. Risk factors for meningococcal disease in children and adolescents: a systematic review and META-analysis. Eur J Pediatr. 2020;179(7):1017–27. doi:10.1007/s00431-020-03658-9.
- Fischer M, Hedberg K, Cardosi P, Plikaytis BD, Hoesly FC, Steingart KR, Bell TA, Fleming DW, WENGER JD, Perkins BA, et al. Tobacco smoke as a risk factor for meningococcal disease. Pediatr Infect Dis J. 1997;16(10):979–83. doi:10.1097/00006454-199710000-00015.
- Tully J, Viner RM, Coen PG, Stuart JM, Zambon M, Peckham C, Booth C, Klein N, Kaczmarski E, Booy R, et al. Risk and protective factors for meningococcal disease in adolescents: matched cohort study. BMJ (Clinical Research Ed) 2006;332(7539):445–50. doi:10.1136/bmj.38725.728472.BE.
- Olea A, Matute I, González C, Delgado I, Poffald L, Pedroni E, Alfaro T, Hirmas M, Nájera M, Gormaz A, et al. Case-control study of risk factors for meningococcal disease in chile. Emerg Infect Dis. 2017;23(7):1070–78. doi:10.3201/eid2307.160129.
- Bruce MG, Rosenstein NE, Capparella JM, Shutt KA, Perkins BA, Collins M. Risk factors for meningococcal disease in college students. JAMA. 2001;286(6):688–93. doi:10.1001/jama.286.6.688.
- Harrison LH, Kreiner CJ, Shutt KA, Messonnier NE, O’Leary M, Stefonek KR, Lin H, Lynfield R, Barrett NL, Arnold KE, et al. Risk factors for meningococcal disease in students in grades 9–12. Pediatr Infect Dis J. 2008;27:3. doi:10.1097/INF.0b013e31815c1b3a.
- Massey P, Aboriginal DD. Torres Strait Islander peoples at higher risk of invasive meningococcal disease in NSW. N S W Public Health Bull. 2008;19(5–6):100–03. doi:10.1071/NB07047.