ABSTRACT
Rotavirus (RV) causes up to half of hospital and community acute gastroenteritis (AGE) cases in young children in Italy. Two RV vaccines, available since 2006, are human RV (HRV) and human bovine RV (HBRV). This report looks back at the implementation of RV vaccination with HRV in Italy, and at HRV current and future perspectives. Initial regional policies led to national implementation by 2018, after scientific societies’ disease awareness efforts. Following vaccination, RV hospitalizations declined significantly, and cost savings were observed. The two-dose HRV vaccine is easily administered during compulsory vaccine visits, helping increase coverage. Intussusception, a serious event in children <1 year, was reported in Italy with a rate of 33–40 per 100,000 infants. RV vaccination presents a low increased risk of intussusception after the first dose, estimated at 0.6 cases per 100,000 doses in Italy in 2019. Parents should be aware of the intussusception risk and symptoms to ensure prompt treatment. It is widely recognized that the vaccination benefits (large numbers of RV hospitalizations prevented) outweigh the risk. HRV introduction in Italy was supported by epidemiologic burden studies, healthcare provider opinions, and congress debates, which significantly contributed to implementation of RV universal routine infant vaccination in Italy.
Introduction
Rotavirus (RV) is the most common cause of gastroenteritis in children under 5 years, with a full range of severity of clinical presentations, which may lead to emergency department visits and hospitalizations, even in industrialized countries. Highly contagious RV gastroenteritis (RVGE) also leads to nosocomial infections.Citation1
Since the availability in 2006 of two RV vaccines to protect infants and young children against RVGE, and the 2009 World Health Organization (WHO) recommendation that all countries should implement RV vaccination, the response in countries has been varied. Few European countries, the United States (US) and Australia rapidly adopted RV vaccination while other countries delayed the decision to consider RV universal routine vaccination (URV).Citation1
RV vaccination in Italy has evolved. Two RV vaccines were made commercially available since 2006, but only 10,000 babies were vaccinated in Italy in 2009, less than 2% of estimated newborns.Citation1 However, supported by the growing international body of evidence and the scientific societies positions, RV URV in Italy was first implemented at the regional level and finally at the national level in 2018. During this timeframe, human live-attenuated RV vaccine (HRV; Rotarix, GSK) was introduced into clinical use and followed by a number of publications, which supported healthcare providers in recommending the vaccine. Up to 2020, approximately 1.2 million babies were vaccinated with HRV in Italy (data on file). This report looks back at the implementation of RV vaccination with HRV in Italy and at its current and future perspectives.
Epidemiology
Before RV vaccination was implemented in Italy, studies estimated that a third to half of acute gastroenteritis (AGE) seen in the hospital was due to RV infection (e.g., 50.8% in the Lombardy regionCitation2 and 28.6% in the Veneto regionCitation3), while the incidence of nosocomial RV infection was around 5%.Citation4 RV hospitalization incidence was found to be highest in younger childrenCitation5 (e.g., 255 vs 177 per 100,000 for children aged <1 year vs <5 yearsCitation6). Similarly, around a third to half of community AGE cases were due to RV infection (e.g., at peaks, 49.1% in winter 2004–2005 and 53.9% in spring 2005), with the highest incidence in children <2 years old.Citation7,Citation8 A family pediatrician reported that the incidence of AGE in his practice was, surprisingly, as high as that of otitis and cough (5%), confirming the RV burden of disease (BoD) for the family pediatrician and the families.Citation9
Design and development of RV vaccines
Mechanisms eliciting protection, following natural RV infection, or live RV vaccination, have not been clearly defined. The two principal hypotheses on protection mechanisms differ on one key point: the role of serotype-specific neutralizing antibody.
Multicomponent vaccines, such as Rotashield (Wyeth Lederle Vaccines SA) or reassortant human bovine RV vaccine (HBRV; RotaTeq, Merck & Co), have been developed to stimulate neutralizing antibody against all major RV serotypes, based on the idea that protection comes from antibody that recognizes serotype-specific neutralization epitopes.Citation10 However, following RV infection, effectors that may elicit protection include non-neutralizing antibodies and T cells.Citation11 Therefore, the development of single-component RV vaccines, like Rotarix (HRV), was based on the idea that protection is multifactorial, elicited by immune effectors like cytotoxic T lymphocytes other than just neutralizing antibody.Citation10,Citation11 Following vaccination, cluster of differentiation 4 (CD4)-bearing T cells may produce antiviral cytokines earlier or in greater quantities than following primary infection; in vitro, RV replication is blocked by several cytokines.Citation12 Plotkin et al. (2017)Citation12 report several possible mechanisms for heterotypic protection including “antibodies against cross-reactive epitopes on outer capsid proteins VP4 and VP7, antigenically conserved inner capsid proteins that are actively transported through rotavirus-infected villous epithelial cells, rotavirus specific cytotoxic T lymphocytes that broadly cross-react with cells infected with different rotavirus serotypes, or antiviral cytokines generated by activated CD4-bearing T cells.”Citation12 Post-marketing surveillance studies have confirmed that HRV induces heterotypic protection, as reported in the label (Summary of Product Characteristics [SmPC] Rotarix).Citation13
The development of HRV began in Cincinnati in 1988, during a clinical trial of a bovine-derived RV strain that proved to be ineffective.Citation14 Serendipitously, in that season in Cincinnati, only one RV serotype, G1P8, was circulating. At the end of the trial, it was observed that natural infection with G1P8 could provide protection, even after asymptomatic infection, and that this strain induced neutralizing antibodies at least for G1–G4 serotypes. Thus, it was decided to use the clinical isolate named 89–12 for a human derived, live-attenuated oral vaccine development.Citation14 The strategy to attenuate the wild strain was to passage it multiple times in cell culture, the same technique used by Sabin for the production of the polio oral vaccine. The 89–12 strain was passed 33 times in African Green Monkey kidney cells after which it proved to be safe and efficacious in a two-dose schedule in a double-blind, placebo-controlled, multicenter, randomized efficacy trial enrolling 213 healthy infants.Citation14 Since then, the final vaccine formulation, named RIX4414, entered the development phase with large clinical trials in many countries around the world.Citation14 Clinical studies from Europe and Latin America showed a favorable benefit-risk profile, clearly demonstrating the vaccine’s efficacy and with no increased risk of intussusception observed.Citation13,Citation15
In 2006, the European Medicines Agency approved HRV for the active immunization of infants for prevention of gastroenteritis due to RV infection.Citation16 The two-dose oral HRV vaccine can be given from 6 weeks of age, with at least 4 weeks between doses, and the course must be completed by 24 weeks of age. Later that year, a second RV vaccine, HBRV,Citation17 was also approved in Europe. Both HRV and HBRV are effective in preventing RV, with good safety profiles, HBRV is administered in a three-dose schedule.Citation18,Citation19
Assessing and building disease awareness in Italy
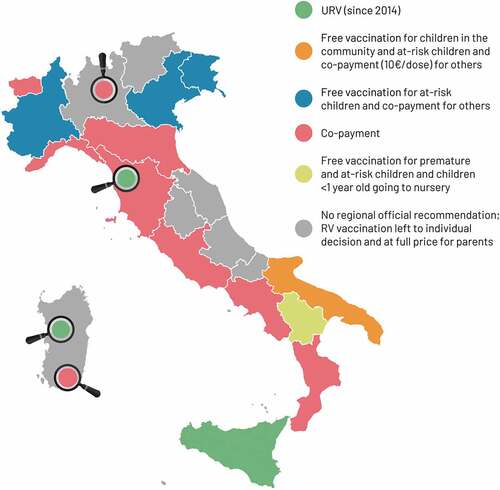
At first, the RV BoD and value of RV vaccination was under-recognized by pediatricians or parents. Regions in Italy had different vaccination policies in place (), and vaccination acceptance and coverage was heavily influenced by whether the vaccines were introduced in the Regional Calendar and if they were offered free of charge and promoted by healthcare providers. A survey carried out in 2009 assessing the views of public health officials found that only 52.4% would recommend adding RV vaccination to the National Immunization Program (NIP) free of charge.Citation20
Figure 1. Introduction of rotavirus (RV) vaccination in different regions of Italy (modified with permission fromCitation22). In 2014, only one Italian region (Sicily) and some health authorities actively offered free RV vaccination. The Puglia region introduced RV vaccination for all newborns at a discounted co-payment of €10.00/dose for families. Other regions offered free vaccination to defined categories of infants.Citation22 Magnifiers highlight municipality-specific type of rotavirus vaccination offer. RV, rotavirus; URV, universal routine vaccination.
A survey of family pediatricians carried out in 2013 showed that half of the respondents recommended RV vaccination and thought it should be offered to all children for free by the Local Health Unit (Azienda Sanitaria Locale, ASL), and 35% thought that the ASL should offer it with co-payment by the family.Citation21
A study in 2015, in Italian healthcare providers attending educational courses, found that 57.4% routinely recommended RV vaccination, but most reported that fewer than a quarter of vaccination attendees adhered to RV vaccination, due to skepticism about the vaccine (60.4%) or because cost was a barrier (34.1%). If the vaccine was free, 81.1% of the healthcare providers would recommend it.Citation23
A primary care study using a validated quality of life questionnaire reported that acute RVGE caused parents worry and distress and had a negative impact on their daily lives.Citation24 The majority (93.6%) of parents who had a child hospitalized for RVGE experienced high/medium stress, 74.5% were not aware of RV vaccination and nearly 80% would, after this experience, strongly recommend it to other parents.Citation25 In 2018, in the Naples area, RV vaccination was recommended and provided free of charge, yet only 15.3% of parents surveyed had vaccinated their child, while more than half of the other parents wanted to, but did not, due to lack of knowledge (77.9%) and because it had not been recommended by their family pediatrician (31.6%). Public education programs were found to be needed to increase coverage of RV vaccination.Citation26
Despite strong evidence on the vaccine’s efficacy and safety, and on the cost impact in other countries, it was not until 2017 that RV vaccination was included in the NIP and started to be increasingly known and accepted throughout Italy. In 2018 compared with 2017, knowledge about vaccine recommendations in a sample of healthcare providers attending educational courses had increased (95% vs 90%), more participants recommended the vaccine routinely (82% vs 76%), and more participants said >75% of their patients chose to get vaccinated (33% vs 11%). Parents’ acceptance of vaccination was driven by fear of severe gastroenteritis (50%) and by the national recommendation to vaccinate (24%). Parents’ reasons for refusal to vaccinate were skepticism (23% vs 55%), because it was not mandatory (34%), fear of intussusception (21%), and not recommended by a health professional (18%). Overall, RV coverage was increasing and may be due to health professional education, which may have increased recommendations for non-mandatory recommended vaccines.Citation27
A second survey carried out in 2017 showed that 96.7% of family pediatricians were aware of the actively recommended and non-obligatory status of RV vaccination. Compared to the 2013 survey,Citation21 there was an improvement in scientific knowledge on RV vaccines and a consistent increase (85.8% vs 48.4%) in sharing the opportunity of the free and active offer of RV vaccination.Citation28
In 2012, Calendario della Vita (CdV), a committee including four scientific societies: Italian Society of Preventive Medicine and Hygiene; Italian Federation of General Practitioners; Italian Society of Pediatrics; and Italian Family Pediatrician Association,Citation29 provided vaccine recommendations with supporting evidence. The objective was to help improve regional vaccination policies. In 2012, the CdV recommended RV vaccination with a co-payment by the family to cover part of the vaccine and administration cost.Citation29 From 2014, CdV recommended universal RV vaccination free of charge, and advised family pediatricians to help implement it in 2019.Citation30–32
Different Italian regions took different approaches to providing access to RV vaccination. Sicily was the first region in 2013 to implement universal RV vaccination actively offered and free of charge.Citation25 In February 2017, the Triennial National Vaccination Plan (PNPV 2017–2019)Citation33 aimed to overcome regional differences by recommending universal RV vaccination to all children over 6 weeks of age, free of charge, based on the CdV recommendations. The objective was to increase coverage to ≥60% (2017), ≥75% (2018), and ≥95% (2019).Citation33 In June 2017, the mandatory vaccination law was introduced. However, RV vaccination was not listed as a mandatory vaccination but was strongly recommended, like meningococcal and pneumococcal vaccines, and included in the Essential Levels of Assistance list (Livelli Essenziali di Assistenza).Citation34 Regional decision-makers’ knowledge, attitude and beliefs about vaccination were therefore crucial for vaccine implementation.Citation23
The Ministry of Health advises that recommended vaccines (such as RV) should be actively promoted through post, e-mail and short message service (SMS), and that vaccination services should ensure adherence to both mandatory and recommended vaccination, through informative interviews during each visit. Family pediatricians play a key role and must get involved in promoting vaccination.Citation34
Impact of regional URV implementation
In the first year after RV vaccination (with 35% coverage in 2013), the mean number of children hospitalized for RVGE in Sicily dropped by 39.3% and 48.3% (in children <5 years and <1 year, respectively).Citation35 The number of hospitalizations for intussusception did not change (15 cases in 2013 vs 15.4 per year between 2003 and 2012, for children <1 year).Citation20
In the first 5 years of vaccination (with an average coverage across Local Health Units in Sicily of 38.2%), RVGE hospitalization rates continued to decrease in the post- versus pre-vaccination periods; by 61.4% (aged <1 year), by 51.2% (aged 12–23 months), and by 48.8% (aged 24–35 months) with smaller decreases (around 25%) in ages 36–59 months.Citation36 The highest coverage (58.6%) was achieved in Trapani, which had a 56.5% reduction in hospitalization, while the lowest coverage (19.1%) was in Messina, which had a 15.7% decrease in hospitalization.Citation36
Despite low coverage (peak of 45% in 2016), there was a decline in average RVGE hospitalization costs and numbers of cases hospitalized in Sicily, resulting in cost savings of €1,134,056 per year (for direct medical, non-medical, and indirect costs) following RV vaccination.Citation37
Health economics
Studies found that AGE due to RV was typically more severe than other causesCitation38–42 resulting in high costs, for example, €1,536 (interquartile range [IQR] 1,279–1,608) per hospital case in Sicily,Citation43 nosocomial case costs of around €8.02 million per year in Italy,Citation4 and regional costs of up to €700,000 (Emilia Romagna region),Citation44 or over €1 million (Veneto region)Citation3 per year (Supplementary Table 1).
Early costing studies of the outpatient costs of AGE in children in Italy found that costs were higher for younger children (mean cost of €116 vs €72 for <36 months vs >36 months) and that around 75% of this cost was due to lost productivity in family members.Citation45
Subsequent economic analyses of RV vaccination with HRV confirmed the impact of societal costs and consistently found vaccination to be cost-saving from a societal perspective.Citation46–49 In addition, HRV was found to be cost-effective from a health payer’s perspective (i.e., cost per quality-adjusted life-year [QALY] of €14,829), as it prevented a significant number of cases, hospitalizations and medical visits.Citation47 When adding the impact of herd immunity provided by RV vaccination, HRV was also found to be cost-saving from a health payer’s perspective. The model predicted vaccination would result in 71% fewer RVGE cases and 86% fewer hospitalizations, with an impact on quality of life and mortality, saving the National Health System over €14 million in costs.Citation48 An economic analysis of RV vaccination in Italy estimated that RVGE with no vaccination would cost €48.2 million (€90.8 per patient) with a QALY loss per patient of 0.0006.Citation49
Implementation of the two-dose HRV schedule
The Ministry of Health’s aim was to increase RV vaccination coverage to over 95% by 2019,Citation33 acknowledging that the mean national coverage is lower than expected to date, for instance, 26.15% in 2019 (birth cohort 2017) with consistent differences across regions.Citation50 However, in the Lombardy region, where RV was actively offered, coverage exceeded the 60% target in 2018 in the first year of URV implementation. The key drivers of this rapid increase in coverage were: a) good collaboration between vaccine services and pediatricians; b) the information interviews clearly discussing the risk-benefit profile; and c) from a practical viewpoint, the ease of administering two-dose HRV with other vaccines given at 3 and 5 months of age.Citation51
Studies from Italy and other countries have shown good coverage, compliance and adherence to vaccination with the two-dose HRV vaccine.Citation19 Two years after universal RV vaccination was introduced in the Lazio region, there was encouraging coverage (i.e., 28.5% in 2018 and 43.9% in 2019) and compliance (vaccinated infants received both doses) with HRV (i.e., 87.7% in 2018 and 83.2% in 2019).Citation52 Similarly, in the Veneto region, coverage among the 35,393 newborns in 2018 was 84.0% and 81.6% for the first and second dose, respectively, with a compliance rate of 97.1%.Citation53,Citation54
The NIP strongly recommends RV vaccination, starting in the third month of life (ages 8–12 weeks). HRV can be administered from 6 weeks of age, according to its SmPC. The interval between first and second dose should be at least 4 weeks, with HRV schedule completion ideally by 16 weeks (maximum 24 weeks of age).Citation13,Citation55 According to recently published estimates, delay in completing vaccination and achieving protection could result in around 120 preventable RVGE hospitalizations, costing the health system around €175,000 per year (based on the Lombardy region data).Citation19 Additionally, if the HRV schedule is completed within the ideal completion times (16 weeks of age), infants can be protected before they reach the peak age of hospitalization for intussusception (occurring around 16–36 weeks of age).Citation19
Safety
Intussusception is one of the most discussed potential serious adverse events following RV vaccine administration. In Europe, the rate of intussusception without vaccination is about 20–72/100,000 children under 1 year of age, depending on the country.Citation56
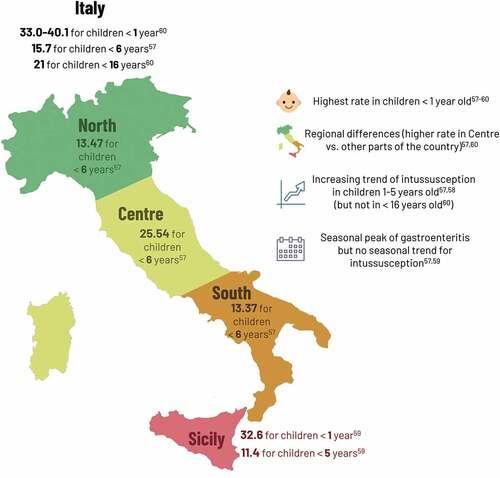
Several studies assessed the pre-vaccination burden of intussusception in Italy (). Higher intussusception hospitalization rates were reported in the Central regionCitation57 and 7.7% of intussusception cases had previous or concomitant AGE.Citation58 Intussusception cases were equally distributed across the year, whereas AGE cases had a seasonal peak in late winter/spring.Citation5,Citation59 The risk of intussusception remained highest in children <1 year, however, there was a significant increasing trend of intussusception hospitalizations in some older age groups (1–2 years and 2–6 years) between 2005 and 2014,Citation57 with another study reporting increasing incidence rates of 18% overall and 40% in ages 1–5 years.Citation58 From the national hospital discharge database, overall incidence in children <16 years of age, however, was stable from 2005 to 2012.Citation60
The reported increased post-vaccination risk is low, at around 1–6 additional cases per 100,000, and occurs mostly within 7 days of the first dose.Citation13,Citation56 The outcome of studies investigating intussusception post-RV vaccination continue to provide evidence that strongly supports continued vaccination.Citation56
In the US, the national passive surveillance system identified a low but significant risk (1.6 excess events per 100,000 vaccinations; 95% confidence interval [CI] 0.3–5.8) of intussusception 3–6 days after dose 1 of HRV. The risk was outweighed by vaccination benefits as there were 68 excess intussusception hospitalizations for 40,000 avoided RVGE hospitalizations per year.Citation61
The first study in a European setting (England) that showed a low increased risk of intussusception (1.68 per 100,000 doses) following HRV also found the risk mostly occurred in the first week after the first dose.Citation62 A subsequent retrospective ecological study in England for children under 3 years of age, encompassing 20,143,062 person-years, found a post-vaccination increase in intussusception hospital admission rates among infants of vaccination age, which was compensated for by a reduction among older infants. There was no overall change in hospital admission rates or clinical severity of intussusception (i.e., requiring surgery) before and after URV implementation.Citation63 The mechanisms following vaccination impacting on intussusception are not known. One hypothesis to explain the increase in risk in young infants is that children who are susceptible may experience intussusception at an earlier age, triggered by vaccination. Another hypothesis, which could explain reductions in overall cases of intussusception in the post-vaccination period, from this and other studies, is that RV vaccination may reduce the risk, as yet unproven, of intussusception from wild-type RV infection in older infants.Citation63 However, both hypotheses rely on a direct role of RV on intussusception, which is not sustained by the epidemiologic curve of RVGE and intussusception.Citation63
The results of the ecological study carried out in England are in line with a retrospective study carried out at a large pediatric hospital in Florence from 2009 to 2013, which investigated 222 intussusception cases in RV-vaccinated and non-vaccinated children.Citation64 Most subjects (84%) presented one time to hospital for intussusception, of which spontaneous resolution occurred in 16% of cases, while 7.4% required an enema, 61% needed hospitalization, and 14.8% underwent surgery. The most frequent symptoms were abdominal pain (41%), vomiting (35%), crying (19%), diarrhea (16%), and blood/mucus in the stool (14%). There were no differences in clinical severity and outcome of intussusception between RV-vaccinated and non-vaccinated children.Citation64
The safety of HRV vaccine is further supported by a clinical study in Sicily, where six Neonatal Intensive Care Units enrolled pre-term newborns for RV vaccination. Overall, 449 pre-term newborns, with an average gestational age of 31.4 weeks (standard deviation [SD] 2.7), received HRV at age 6.3 weeks (SD 0.6). Only 8% and 2% of vaccinated newborns reported abdominal colic and fever >38.5°C in the 15 days after the first dose, respectively. No serious adverse events were observed in the 30 days follow up.Citation65
Bonanni and Signorelli (2015)Citation56 published a report on the serious side effects of RV vaccine, with particular regard to intussusception. This risk was assessed, considering available scientific evidence and other European guidelines, but was not considered an impediment to recommending universal vaccination, as the benefits of vaccination far outweighed the risks.
The Italian National Drug Agency (AIFA) released a safety communication (in 2017) reconfirming the favorable benefit-risk profile of HRV and the positive impact on public health, in line with European-level conclusions, based on review of the evidence including an English study.Citation62 However, AIFA recommended that parents must be informed that there is a risk of intussusception within 30 days after RV vaccination, and in the case of specified signs and symptoms (i.e., severe vomiting, diarrhea, blood in feces, abdominal pain, etc.) parents need to seek medical assistance, and the consulted physician would be expected to assess the clinical picture in depth, and the RV vaccine schedule should be completed as per the recommendations and the SmPCs.Citation62
In 2017 and 2018, AIFA published an annual report on the vaccine adverse events that were spontaneously reported to the National Surveillance System for Vaccines. In both reports, no deaths were recorded following RV vaccination in Italy, and 2 and 8 cases of intussusception (2017 and 2018) had a possible link to vaccination. The resulting rate of intussusception was 1.5 per 100,000 doses administered, in line with other European countries.Citation66,Citation67 In the 2019 report,Citation68 when all regions fully implemented RV URV, no specific frequencies were reported for intussusception in the adverse events section for RV vaccines; however, based on reported figures, a rate of 0.6 cases per 100,000 administered doses can be calculated.
Finally, although intussusception, if it occurs in vaccinated babies, is generally referred to RV vaccination, it should be noted that other factors may have contributed to the intussusception event. RVGE itself can be a risk factor to trigger intussusception, as reported in different studies.Citation69–72 A case–control study (n = 125 cases and n = 190 controls) of risk factors for intussusception in children aged 0–5 years in Sicily (2009–2015) identified previous AGE (odds ratio [OR] 11.55 [95% CI 3.23–41.23], p < .001) and antibiotic use in the 30 days before hospitalization (OR 3.09 [95% CI 1.17–8.12], p = .009) as significant risk factors, while infants who had been exclusively breastfed for at least 2 months were at lower risk (OR 0.48 [95% CI 0.23–0.99], p = .009). For children born after December 2012, when RV vaccination became available, there was no association between intussusception and vaccination (OR 0.96 [95% CI 0.41–2.25], non-significant p = .92).Citation70
Current and future perspectives
Since RV vaccination has been implemented, a number of positive and unexpected findings have been reported, in addition to the effect on RV disease.
Austria was the first European country to implement universal infant RV vaccination in 2007, achieving 87% coverage by 2008. By the end of 2008, hospitalization had already decreased by 74% in the age group eligible for vaccination compared to pre-vaccination rates. In 2009, a further 22% decrease in hospitalization was noted among children aged 32–60 months of age, who were not eligible for the URV program due to their age. The high coverage achieved with URV is expected to have resulted in this indirect herd protection effect, by reducing RV circulation and virus shed from vaccinated children.Citation73 Similarly, based on a meta-analysis of studies in children <1 year of age from five countries, the European Center for Disease Prevention and Control reported a median herd effect of 22% on RVGE morbidity (19 − 25%) across 12 study years.Citation74 Nevertheless, the mechanism by which the herd effect is generated, for example, through transmission of vaccine virus conferring protection, or through reduced circulation of virus, or reduced numbers of carriers, has not yet been clarified, and further knowledge on RV disease and vaccine effects needs to be accumulated.Citation74
Increasing research shows that RV infection is systemic and not confined to the gastrointestinal tract.Citation75 Infection can present without diarrhea and may trigger symptoms including neurologic symptoms (e.g., seizures and epilepsy), neonatal complications and autoimmune diseases (e.g., diabetes mellitus and celiac disease), among others. These findings need further investigation, offer new clinical perspectives on RV vaccination, and new opportunities for public health.Citation76
Since the implementation of RV vaccination programs, countries have observed reductions in seizure hospitalizations in vaccinated children (e.g., a significant 20% reduction in seizures requiring emergency care or hospitalization in the year following the last RV vaccination).Citation76 Seizure hospitalization trends in a US study found the greatest reduction in rates was among children 0–2 months old and 12–23 months old (i.e., a 14–16% reduction in 2013 compared to the pre-vaccination period 2000–2006), and there was a seasonal trend with the largest decreases observed during the RV season.Citation77
Similarly, the incidence of type 1 diabetes (T1D) appears to be significantly lower in children who received RV vaccination.Citation75,Citation78,Citation79 In children <5 years of age in Australia, there was a 14% reduction in T1D incidence in children born after the introduction of RV vaccination in 2007 (i.e., rate reduction of 0.86 [95% CI, 0.74–0.99], p = .04 between 2000–2007 and 2008–2015).)Citation78 A cohort study in children in the US compared T1D incidence before (2001–2005) and after (2006–2017) RV vaccination was introduced. Only children who received the full course of vaccination appeared to have a significantly reduced risk of T1D (33% [95% CI 17–46] reduction) and T1D hospitalization (31% [95% CI 27–35] reduction) compared to partially vaccinated and unvaccinated children. The authors conclude that RV vaccination may be “the first practical measure” that could help with the prevention of T1D.Citation79
RV vaccination may also help in the prevention of celiac disease – two long-term follow-up studies in Finland found children vaccinated against RV had a lower risk of developing the autoimmune disease than unvaccinated children.Citation75
Conclusions
RV vaccination is highly recommended in Italy. Its benefits have been widely demonstrated in terms of vaccine efficacy and safety, reducing gastroenteritis morbidity, and providing cost savings to the National Health System and society.Citation1 This favorable benefit-risk profile and positive experience in Italy to date should support improved coverage and help reach the 95% coverage target.
In addition, high coverage is expected to have generated herd immunity, and new evidence suggests the vaccine may have a positive indirect impact against other diseases such as epilepsy, diabetes and celiac disease.
Rotarix introduction in Italy in 2006 was supported by evidence including: clinical trials in Europe, Latin America, Africa, and Asia; epidemiologic burden studies (13 in Italy); healthcare providers opinions (7 studies in Italy); and congress debates. Thus, the value of universal RV vaccination was demonstrated for various stakeholders including payers, healthcare professionals, and parents, which in turn significantly contributed to the implementation of RV universal mass vaccination in Italy, and to the vaccination of more than 1 million babies.
Disclosure of potential conflicts of interest
Paolo Bonanni reports personal fees outside of the submitted work, from the GSK group of companies, Sanofi Pasteur, MSD Merck, Pfizer, Seqirus, AstraZeneca, and Janssen for participation in advisory boards and meetings. Giorgio Conforti declares no financial and non-financial relationships and activities and no conflicts of interest. Elisabetta Franco reports reimbursement for participating in meetings and advisory boards from the GSK group of companies, MSD Merck, Pfizer, Sanofi Pasteur, and Seqirus, without personal fees. Giovanni Gabutti reports personal fees outside of the submitted work, from the GSK group of companies and Sanofi Pasteur Italy for advisory board membership and consultancy; and from MSD Merck, Pfizer, Seqirus, and Emergent Biosolutions for advisory board membership, consultancy, and lectures. Federico Marchetti is employed by and holds shares in the GSK group of companies. Antonella Mattei declares no financial and non-financial relationships and activities and no conflicts of interest. Rosa Prato reports, outside the submitted work, grants, personal fees, and non-financial support from the GSK group of companies and Pfizer; and personal fees and non-financial support from MSD Merck and Sanofi. Francesco Vitale reports, outside the submitted work, grants from the GSK group of companies, MSD Merck, Sanofi, and Pfizer. These authors declare no other financial and non-financial relationships and activities. Giorgio Conforti and Giovanni Vitali Rosati declare no financial and non-financial relationships and activities and no conflicts of interest.
Authors’ contributions
All authors (PB, GC, EF, GG, FM, AM, RP, GVR, and FV) have made substantial contributions to the study, drafting or reviewing of the article critically for intellectual content, and have approved the final version of the manuscript.
Trademark statement
Rotashield is a trademark owned by or licensed to Wyeth Lederle Vaccines SA. RotaTeq is a trademark owned by or licensed to Merck & Co, USA. Rotarix is a trademark owned by or licensed to the GSK group of companies.
Supplemental Material
Download MS Word (79.3 KB)Acknowledgments
The authors would like to thank Business & Decision Life Sciences platform for editorial assistance, manuscript coordination and design support for the digital illustrations, on behalf of GSK. Aurélie Roth (Business & Decision Life Sciences, on behalf of GSK) coordinated manuscript development and editorial support. Kavi Littlewood (Littlewood Writing Solutions, on behalf of GSK) provided medical writing support.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this research.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1955611.
Additional information
Funding
References
- Bartolozzi G. I vaccini contro i rotavirus (Vaccines against rotavirus). Area Pediatrica. 2011;12:1–9.
- Pellegrinelli L, Bubba L, Primache V, Chiaramonte I, Ruggeri FM, Fiore L, Binda S. Burden of pediatrics hospitalizations associated with rotavirus gastroenteritis in Lombardy (Northern Italy) before immunization program. Ann Ist Super Sanita. 2015;51:346–51. doi:10.4415/ann_15_04_16.
- Saia M, Giliberti A, Callegaro G, Baldovin T, Busana MC, Pietrobon F, Bertoncello C, Baldo V. Hospitalisation for rotavirus gastroenteritis in the paediatric population in the Veneto Region, Italy. BMC Public Health. 2010;10:636. doi:10.1186/1471-2458-10-636.
- Festini F, Cocchi P, Mambretti D, Tagliabue B, Carotti M, Ciofi D, Biermann KP, Schiatti R, Ruggeri FM, De Benedictis FM, et al. Nosocomial rotavirus gastroenteritis in pediatric patients: a multi-center prospective cohort study. BMC Infect Dis. 2010;10:235. doi:10.1186/1471-2334-10-235.
- Mattei A, Sbarbati M, Fiasca F, Angelone AM, Mazzei MC, di Orio F. Temporal trends in hospitalization for rotavirus gastroenteritis: a nationwide study in Italy, 2005–2012. Hum Vaccin Immunother. 2016;12(2):534–39. doi:10.1080/21645515.2015.1081726.
- Marocco A, Assael B, Gabutti G, Guarino A, Lopalco PL, Marchetti F, Ruggeri FM, Titone L, Tozzi AE, Vitali Rosati G, et al. [Hospitalisation associated with rotavirus gastroenteritis in Italy, 2001-2003, evaluated by means of ICD9-CM diagnostic codes]. Ig Sanita Pubbl. 2006;62:215–44.
- Ansaldi F, Lai P, Valle L, Riente R, Durando P, Sticchi L, Tucci P, Biasci P, Crovari P, Gasparini R, et al. Burden of rotavirus-associated and non-rotavirus-associated diarrhea among nonhospitalized individuals in central Italy: a 1-year sentinel-based epidemiological and virological surveillance. Clin Infect Dis. 2008;46(6):e51–55. doi:10.1086/527449.
- Van Damme P, Giaquinto C, Huet F, Gothefors L, Maxwell M, Van der Wielen M. Multicenter Prospective Study of the Burden of Rotavirus Acute Gastroenteritis in Europe, 2004–2005: the REVEAL Study. J Infect Dis. 2007;195(Suppl 1):s4–s16. doi:10.1086/516714.
- Gambotto S. Il vaccino anti-rotavirus una opportunità per il pediatra di famiglia. Il Giornale della vaccinazione. 2010;Anno II (2):3–6.
- Ward RL. Possible mechanisms of protection elicited by candidate rotavirus vaccines as determined with the adult mouse model. Viral Immunol. 2003;16(1):17–24. doi:10.1089/088282403763635410.
- Ward R. Mechanisms of protection against rotavirus infection and disease. Pediatr Infect Dis J. 2009;28(3 Suppl):S57–59. doi:10.1097/INF.0b013e3181967c16.
- Parashar UD, Cortese MM, Offit PA. Rotavirus Vaccines. Plotkin’s Vaccines. 2018;950-969.e911.
- European Medicines Agency (EMA). Rotarix - Summary of Product Characteristics. 2020 [ accessed 2020 Oct 09] https://www.ema.europa.eu/en/documents/product-information/rotarix-epar-product-information_en.pdf
- Bernstein DI. Live Attenuated Human Rotavirus Vaccine, Rotarix™. Semin Pediatr Infect Dis. 2006;17(4):188–94. doi:10.1053/j.spid.2006.08.006.
- European Medicines Agency (EMA). Rotarix - Scientific discussion. 2006 [ accessed 2020 Oct 09] https://www.ema.europa.eu/en/documents/scientific-discussion/rotarix-epar-scientific-discussion_en.pdf
- European Medicines Agency (EMA). Rotarix EPAR. 2006 [ accessed 2021 Jan 06] https://www.ema.europa.eu/en/medicines/human/EPAR/rotarix
- European Medicines Agency (EMA). RotaTeq EPAR. 2006 [ accessed 2021 Jan 06] https://www.ema.europa.eu/en/medicines/human/EPAR/rotateq
- Burnett E, Parashar U, Tate J. Rotavirus vaccines: effectiveness, safety, and future directions. Paediatr Drugs. 2018;20:223–33. doi:10.1007/s40272-018-0283-3.
- Martinelli D, Fortunato F, Marchetti F, Prato R. Rotavirus vaccine administration patterns in Italy: potential impact on vaccine coverage, compliance and adherence. Hum Vaccin Immunother. 2020;17(5):1546–51. doi:10.1080/21645515.2020.1816109.
- Marchetti F, Morelli P. Measuring public health officers‘ opinions on new vaccination programs by means of the WHO Vaccine Introduction Guidelines. Hum Vaccin. 2009;5(9):640–44. doi:10.4161/hv.9092.
- Cantarutti A, Cantarutti L, Conforti G, Marchetti F, Sciolla N. La percezione dei pediatri di famiglia associati FIMP sulla vaccinazione contro le gastroenteriti acute da rotavirus. Presented at: 70° Congresso Italiano di Pediatria - SIP Palermo 11-14 giugno 2014. Il Medico Pediatra 1. 2014:32–33. [ accessed 2021 Apr 29]. https://www.ilmedicopediatra-rivistafimp.it/wp-content/uploads/2019/03/05_ATTIVITA-PROFESSIONALE_Rotavirus-1.pdf
- Amodio E, Costantino C, Cracchiolo M, Sciuto V, Vitale F. Health Technology Assessment Rotarix: L’esperienza della Sicilia quale regione capofila nella introduzione della vaccinazione universale contro i rotavirus. Ital J Public Health. 2014;3:28–37.
- Mita V, Arigliani M, Zaratti L, Arigliani R, Franco E. Italian physicians’ opinions on rotavirus vaccine implementation. Pathogens. 2017;6(4):56. doi:10.3390/pathogens6040056.
- Diez Domingo J, Patrzalek M, Cantarutti L, Arnould B, Meunier J, Soriano-Gabarro M, Meyer N, Pirçon J-Y, Holl K. The impact of childhood acute rotavirus gastroenteritis on the parents’ quality of life: prospective observational study in European primary care medical practices. BMC Pediatr. 2012;12:58. doi:10.1186/1471-2431-12-58.
- Marchetti F, Vetter V, Conforti G, Esposito S, Bonanni P. Parents‘ insights after pediatric hospitalization due to rotavirus gastroenteritis in Italy. Hum Vaccin Immunother. 2017;13(9):2155–59. doi:10.1080/21645515.2017.1336271.
- Napolitano F, Ali Adou A, Vastola A, Angelillo IF. Rotavirus infection and vaccination: knowledge, beliefs, and behaviors among parents in Italy. Int J Environ Res Public Health. 2019;16(10):1807. doi:10.3390/ijerph16101807.
- Amadori F, Terracciano E, Gennaio I, Mita V, Gargano D, Zaratti L, Franco E, Arigliani R. Opinions and attitudes of Italian healthcare workers towards recommended but not compulsory rotavirus vaccination. Hum Vaccin Immunother. 2021;17(2):497–502. doi:10.1080/21645515.2020.1776546.
- Marchetti F, Conforti G. Seconda indagine conoscitiva sull’opinione dei pediatri di famiglia italiani in merito alla vaccinazione contro i rotavirus. Presented at: 51 Congresso Nazionale - Societa Italiana di Igiene (SITI) 17-20 ottobre. Riva del Garda, Italy; 2018. p. 225. [accessed 2021 Apr 29]. http://www.igienistionline.it/docs/2018/41abstract.pdf .
- Societa Italiana di Igiene (SItI), Federazione Italiana Medici Pediatri (FIMP), (FIMMG). FIMdMG. Calendario Vaccinale Per La Vita 2012 [ accessed 2020 Oct 07] https://docplayer.it/4948666-Calendario-vaccinale-per-la-vita.html
- Societa Italiana di Pediatria (SIP), Societa Italiana di Igiene (SItI), Federazione Italiana Medici Pediatri (FIMP), (FIMMG). FIMdMG. Calendario Vaccinale Per La Vita 2° Edizione 2014 [ accessed 2020 Oct 07] http://www.asl5.liguria.it/Portals/0/Comunicati/19022015_Calendario-per-la-vita-2014-1.pdf
- Societa Italiana di Pediatria (SIP), Societa Italiana di Igiene (SItI), Federazione Italiana Medici Pediatri (FIMP), (FIMMG). FIMdMG. Calendario Vaccinale Per La Vita 3° Edizione 2016 [ accessed 2020 Oct 07] http://www.apel-pediatri.org/attachments/794_Calendario_vaccini_16.pdf
- Societa Italiana di Pediatria (SIP), Societa Italiana di Igiene (SItI), Federazione Italiana Medici Pediatri (FIMP), (FIMMG). FIMdMG. Calendario Vaccinale Per La Vita 4° Edizione 2019 [ accessed 2020 Oct 07] http://www.igienistionline.it/docs/2019/21cvplv.pdf
- Government of Italy. Piano Nazionale Prevenzione Vaccinale: PNPV 2017-2019. 2017 [ accessed 2020 Oct 09] http://www.salute.gov.it/imgs/C_17_pubblicazioni_2571_allegato.pdf
- Ministero della Salute. Ufficio V – prevenzione delle malattie trasmissibili e profilassi internazionale. 2017 [ accessed 2021 Mar 19] https://www.ordfarmbo.it/multimedia/allegati/10585-all1.PDF
- Costantino C, Amodio E, Vitale F. Impact on rotavirus gastro-enteritis hospitalisation during the first year of universal vaccination in Sicily. Paediatrics and International Child Health. 2015;35(4):342–43. doi:10.1080/20469047.2015.1109240.
- Restivo V, Caracci F, Sannasardo CE, Scarpitta F, Vella C, Ventura G, Tramuto F, Costantino C. Rotavirus gastroenteritis hospitalization rates and correlation with rotavirus vaccination coverage in Sicily. Acta Biomed. 2018;89:437–42. doi:10.23750/abm.v89i3.7578.
- Costantino C, Restivo V, Tramuto F, Casuccio A, Vitale F. Universal rotavirus vaccination program in Sicily: reduction in health burden and cost despite low vaccination coverage. Hum Vaccin Immunother. 2018;14(9):2297–302. doi:10.1080/21645515.2018.1471306.
- Zuccotti G, Meneghin F, Dilillo D, Romanò L, Bottone R, Mantegazza C, Giacchino R, Besana R, Ricciardi G, Sterpa A, et al. Epidemiological and clinical features of rotavirus among children younger than 5 years of age hospitalized with acute gastroenteritis in Northern Italy. BMC Infect Dis. 2010;10:218. doi:10.1186/1471-2334-10-218.
- Panatto D, Amicizia D, Giacchino R, Tacchella A, Natalizia AR, Melioli G, B andettini R, Di Pietro P, Diana MC, Gasparini R. Burden of rotavirus infections in Liguria, Northern Italy: hospitalisations and potential savings by vaccination. Eur J Clin Microbiol Infect Dis. 2011;30(8):957–64. doi:10.1007/s10096-011-1180-7.
- Forster J, Guarino A, Parez N, Moraga F, Román E, Mory O, Tozzi AE, de Aguileta AL, Wahn U, Graham C, et al. Hospital-based surveillance to estimate the burden of rotavirus gastroenteritis among European children younger than 5 years of age. Pediatrics. 2009;123(3):e393–400. doi:10.1542/peds.2008-2088.
- Albano F, Bruzzese E, Bella A, Cascio A, Titone L, Arista S, Izzi G, Virdis R, Pecco P, Principi N, et al. Rotavirus and not age determines gastroenteritis severity in children: a hospital-based study. Eur J Pediatr. 2007;166(3):241–47. doi:10.1007/s00431-006-0237-6.
- Diez-Domingo J, Baldo J-M, Patrzalek M, Pazdiora P, Forster J, Cantarutti L, Pirçon J-Y, Soriano-Gabarró M, Meyer N. Primary care-based surveillance to estimate the burden of rotavirus gastroenteritis among children aged less than 5 years in six European countries. Eur J Pediatr. 2011;170(2):213–22. doi:10.1007/s00431-010-1289-1.
- Amodio E, Tabacchi G, Cracchiolo M, Sciuto V, Vitale F. Hospitalisation of children aged 0–59 months with rotavirus gastro-enteritis before the introduction of routine vaccination (Sicily 2003–2012). Paediatr Int Child Health. 2015;35(4):319–23. doi:10.1080/20469047.2015.1109228.
- Gabutti G, Lazzara C, Marsella M, Bergamini M, Malaventura C, Borgna-Pignatti C. Burden of hospitalizations due to Rotavirus infection in Emilia Romagna, Italy. Acta Biomed. 2007;78:176–81.
- Fontana M, Zuin G, Pancheri P, Fusco FC, Lambertini A, Berni Canani R. Costs associated with outpatient diarrhoea in infants and toddlers: a nationwide study of the Italian Society of Paediatric Gastroenterology and Hepatology (SIGEP). Dig Liver Dis. 2004;36(8):523–27. doi:10.1016/j.dld.2004.03.012.
- Giammanco MD, Coniglio MA, Pignato S, Giammanco G. An economic analysis of rotavirus vaccination in Italy. Vaccine. 2009;27(29):3904–11. doi:10.1016/j.vaccine.2009.04.002.
- Standaert B, Marocco A, Assael B, Gabutti G, Guarino A, Lopalco PL, Marchetti F, Ruggeri F, Titone L, Tozzi A, et al. [Cost-effectiveness of universal rotavirus vaccination with Rix4414 in Italy]. Pharmacoeconomics-Italian Research Articles. 2008;10(1):23–35. doi:10.1007/BF03320638.
- Vitale F, Barbieri M, Dirodi B, Vitali Rosati G, Franco E. [A full economic evaluation of extensive vaccination against rotavirus with RIX4414 vaccine at National and Regional level in Italy]. Ann Ig. 2013;25:43–56. doi:10.7416/ai.2013.1905.
- Capri S, Veneziano MA. Health Technology Assessment Rotarix: la vaccinazione anti-rotavirus in Italia: valutazione economica. QIJPH. 2014;3:55–67.
- Ministero della Salute. Vaccinazioni dell’età pediatrica. Anno 2019 (coorte 2017). 2019 [ accessed 2021 Jan 26] http://www.salute.gov.it/imgs/C_17_tavole_20_8_1_file.pdf
- Barbato C, Cattaneo EE. Efficace introduzione della vaccinazione contro i rotavirus nella Regione Lombardia. Il Giornale della vaccinazione. 2018; Anno X:4–6.
- Aquilani S, Dari S, Fiasca F. Assessing rotavirus vaccination coverage and compliance after two years of local experience in Italy. Ann Ig. 2020;32:433–35. doi:10.7416/ai.2020.2367.
- Regiona de Veneto. Attività vaccinale 2019: aggiornamento delle coperture vaccinali in età pediatria. 2019 [accessed 2021 Feb 03] http://repository.regione.veneto.it/public/282de3d2c92fff99f414f9496a3cca9d.php?lang=it&dl=true
- TuttiItalia.it. Andamento demografico della popolazione residente in Veneto dal 2001 al 2019. 2019 [ accessed 2021 Feb 03] https://www.tuttitalia.it/veneto/statistiche/popolazione-andamento-demografico/
- Regione Piemonte - Direzione Sanità e Welfare - Settore Prevenzione e Veterinaria. Vaccinazioni – recupero attività e aggiornamenti (Classificazione 14.130.30;1/2014C;4). 2014.
- Bonanni P, Signorelli C. [Anti-rotavirus and intussusception: no evidence to discontinue the universal vaccination policy]. Ig Sanita Pubbl. 2015;71:549–57.
- Mattei A, Fiasca F, Mazzei M, Sbarbati M. Unparalleled patterns of intussusception and rotavirus gastroenteritis hospitalization rates among children younger than six years in Italy. Ann Ig. 2017;29:38–45. doi:10.7416/ai.2017.2130.
- Restivo V, Costantino C, Tramuto F, Vitale F. Hospitalization rates for intussusception in children aged 0–59 months from 2009 to 2014 in Italy. Hum Vaccin Immunother. 2017;13(2):445–49. doi:10.1080/21645515.2017.1264784.
- Costantino C, Restivo V, Cuccia M, Furnari R, Amodio E, Vitale F. Analysis of hospitalizations due to intussusception in Sicily in the pre-rotavirus vaccination era (2003–2012). Ital J Pediat. 2015;41:52. doi:10.1186/s13052-015-0160-4.
- Trotta F, Da Cas R, Bella A, Santuccio C, Salmaso S. Intussusception hospitalizations incidence in the pediatric population in Italy: a nationwide cross-sectional study. Ital J Pediatr. 2016;42(1):89. doi:10.1186/s13052-016-0298-8.
- Haber P, Parashar UD, Haber M, DeStefano F. Intussusception after monovalent rotavirus vaccine—United States, Vaccine Adverse Event Reporting System (VAERS), 2008–2014. Vaccine. 2015;33(38):4873–77. doi:10.1016/j.vaccine.2015.07.054.
- Agenzia Italiana del Farmaco (AIFA). Comunicazione di sicurezza sui vaccini anti-rotavirus. 2017 [ accessed 2020 Oct 09] http://www.crfvcalabria.it/doc/Comunicazione_sicurezza_rotavirus_rev_22_05.pdf
- McGeoch LJ, Finn A, Marlow RD. Impact of rotavirus vaccination on intussusception hospital admissions in England. Vaccine. 2020;38(35):5618–26. doi:10.1016/j.vaccine.2020.06.078.
- Bianconi M, Adorni V, Nucci A, Ghiori F, Ricci S, Nieddu F, Lippi F. 558 - Gestione clinica di casi di invaginazione intestinale presso l’ospedale meyer negli anni 2009-2013 in bambini vaccinati o meno contro i rotavirus. Presented at: 48 Congresso Societa Italiana di Igiene (SItI) 14 – 17 Ottobre. Milan: Italy; 2015, p. 167. [accessed 2021 Apr 29]. http://www.sitinazionale.org/site/new/images/docs/congressi/2015/1014milanoatti.pdf .
- Costantino C, Restivo V, Tramuto F, Casuccio A, Palermo M, Vitale F. Safety of in-neonatal intensive care unit administration of rotavirus vaccination among preterms. European Journal of Public Health. 2020;32(1):167-91. doi:10.1093/eurpub/ckaa165.1255.
- Agenzia Italiana del Farmaco (AIFA). Comunicazione di sicurezza sui vaccini anti-rotavirus. 2017 [ accessed 2020 Oct 09] http://www.crfvcalabria.it/doc/Comunicazione_sicurezza_rotavirus_rev_22_05.pdf
- Agenzia Italiana del Farmaco (AIFA). Rapporto Vaccini 2018 - la sorveglianza postmarketing in Italia. 2018 [ accessed 2020 Oct 09] https://www.aifa.gov.it/documents/20142/241056/Rapporto+Vaccini+2018.pdf/62975a0e-a836-da32-f7b9-1e39196374dd
- Agenzia Italiana del Farmaco (AIFA). Rapporto Vaccini 2019 - la sorveglianza postmarketing in Italia. 2019 [ accessed 2021 Jan 18] https://www.aifa.gov.it/documents/20142/241052/Rapporto_Vaccini_2019.pdf/78c5e8ef-938d-add8-52b2-869e6dab323f
- Willame C, Cheuvart B, Aris E, Vetter V, Cohet C. Association between rotavirus gastroenteritis and intussusception: suggested evidence from a retrospective study in claims databases in the United States. Hum Vaccin Immunother. 2021;17(1):269–77. doi:10.1080/21645515.2020.1770514.
- Restivo V, Costantino C, Giorgianni G, Cuccia M, Tramuto F, Corsello G, Casuccio A, Vitale F. Case–control study on intestinal intussusception: implications for anti-rotavirus vaccination. Expert Rev Vaccines. 2018;17(12):1135–41. doi:10.1080/14760584.2018.1546122.
- Mansour AM, El Koutby M, El Barbary MM, Mohamed W, Shehata S, El Mohammady H, Mostafa M, Riddle MS, Sebeny PJ, Young SY, et al. Enteric viral infections as potential risk factors for intussusception. J Infect Dev Ctries. 2013;7(1):28–35. doi:10.3855/jidc.2321.
- Minney-Smith CA, Levy A, Hodge M, Jacoby P, Williams SH, Carcione D, Roczo-Farkas S, Kirkwood CD, Smith DW. Intussusception is associated with the detection of adenovirus C, enterovirus B and rotavirus in a rotavirus vaccinated population. J Clin Virol. 2014;61(4):579–84. doi:10.1016/j.jcv.2014.10.018.
- Paulke-Korinek M, Kundi M, Rendi-Wagner P, De Martin A, Eder G, Schmidle-Loss B, Vecsei A, Kollaritsch H. Herd immunity after two years of the universal mass vaccination program against rotavirus gastroenteritis in Austria. Vaccine. 2011;29(15):2791–96. doi:10.1016/j.vaccine.2011.01.104.
- European Centre for Disease Prevention and Control (ECDC). Scientific Advice - Expert opinion on rotavirus vaccination in infancy. 2017 [ accessed 2020 Oct 12]. https://www.ecdc.europa.eu/en/publications-data/expert-opinion-rotavirus-vaccination-infancy
- Gómez-Rial J, Rivero-Calle I, Salas A, Martinón-Torres F. Rotavirus and autoimmunity. J Infect. 2020;81(2):183–89. doi:10.1016/j.jinf.2020.04.041.
- Rivero-Calle I, Gómez-Rial J, Martinón-Torres F. Systemic features of rotavirus infection. J Infect. 2016;72(Suppl):s98–s105. doi:10.1016/j.jinf.2016.04.029.
- Pringle KD, Burke RM, Steiner CA, Parashar UD, Tate JE. Trends in rate of seizure-associated hospitalizations among children <5 years old before and after rotavirus vaccine introduction in the United Sates, 2000-2013. J Infect Dis. 2018;217:581–88. doi:10.1093/infdis/jix589.
- Perrett KP, Jachno K, Nolan TM, Harrison LC. Association of rotavirus vaccination with the incidence of type 1 diabetes in children. JAMA Pediatrics. 2019;173(3):280–82. doi:10.1001/jamapediatrics.2018.4578.
- Rogers MAM, Basu T, Kim C. Lower incidence rate of type 1 diabetes after receipt of the rotavirus vaccine in the United States, 2001–2017. Sci Rep. 2019;9(1):7727. doi:10.1038/s41598-019-44193-4.