ABSTRACT
This study evaluated different varicella vaccination strategies in Jiangsu province, China. A decision-tree Markov model was used to evaluate the cost effectiveness of various varicella vaccination strategies for children, including direct and selective vaccination (serotesting pre-vaccination). A cohort of one-year-old children was followed through 60 one-year Markov cycles. The parameter estimation was based on field work, the literature, and statistical yearbooks. We calculated the incremental cost-utility ratio (ICUR) using the saved quality-adjusted life year (QALY). One-way and probability sensitivity analyses were performed to assess uncertainty. Among 100,000 cohort members, one-dose and two-dose direct vaccination averted 8061 and 10,701 varicella cases, respectively, compared with no vaccination. Furthermore, compared with no vaccination, one-dose and two-dose direct vaccination saved one QALY at the ICUR of USD 21,401.33 and USD 35,420.81, respectively, at less than three times the per capita gross domestic product (USD 47,626.86) of Jiangsu. The ICURs of the one-dose and two-dose selective strategies versus no vaccination were USD 42,623.62 and USD 51,406.35 per QALY gained, respectively. The cost effectiveness results were most sensitive to the QALY loss of outpatients and vaccine prices. Thus, in Jiangsu, one-dose and two-dose direct varicella vaccination in children could be cost effective at the willingness to pay threshold of three times provincial GDP per capita from a societal perspective. The findings were sensitive to the vaccine price and health utility of varicella cases.
1. Introduction
Varicella, caused by the primary varicella zoster virus infection, is a ubiquitous acute infectious disease in children.Citation1 Although it is mild and self-limited, it can result in severe complications, such as bacterial skin infections, encephalitis, pneumonia, and even death.1,Citation2 Varicella vaccination provides effective protection against varicella zoster virus infection and significantly reduces the associated disease burden.Citation3–6 After the vaccine’s introduction, more than 70% of varicella cases have been averted in the United States.Citation5 To date, varicella vaccination has been adopted in routine vaccination programs for children in 36 countries and regions, including the United States, Australia, and Germany.Citation3,Citation7
In China, the varicella vaccine (category B vaccine) is included in the non‐Expanded Program on Immunization (non‐EPI), which is optional and self‐paid.Citation8 A systematic review showed that varicella vaccination coverage among children varied from 20.84% to 97.83% in China.Citation9 Recently, several Chinese cities, including Shanghai, Tianjin, and Suzhou, have added the vaccine to the local EPI and are providing free vaccination for children.Citation10,Citation11
The immunization policy decision was based on an economic evaluation of varicella vaccination. Reviews have confirmed its cost effectiveness in some high-income countries, including the United States, France, and the United Kingdom,Citation12,Citation13 and studies have reported its cost effectiveness in China.Citation14–16 However, the accumulated evidence on China is insufficient. Most of these studies conducted evaluations using the benefit–cost or cost–benefit ratio based on averted varicella cases, ignoring the health utility lost due to varicella cases. In terms of health technology assessment, cost utility analysis was conducted in more countries than cost effectiveness analysis.Citation17 Additionally, compared with direct vaccination, some studies suggested vaccination with prior screening (serotesting before vaccination) as the optimal option; however, the evidence on the cost effectiveness of prior screening is mixed,Citation18,Citation19 and it is thus unclear whether serotesting before vaccination (screen strategy) is cost effective in China. Similarly, the evidence on cost effectiveness of one-dose versus two-dose varicella vaccination in China is also insufficient.
Jiangsu, located in eastern China, has a high incidence of varicella as well as the highest economic development level among Chinese provinces.Citation20 In October 2018, Wuxi City in Jiangsu approved the provision of free two-dose varicella vaccines for children.Citation21 The purpose of our study was to conduct a cost effectiveness and cost utility analysis of different varicella vaccination strategies, including different number of doses and serotesting before vaccination. The findings provide suggestions and evidence for varicella vaccination policy decision-making in Jiangsu.
2. Materials and methods
2.1. Model
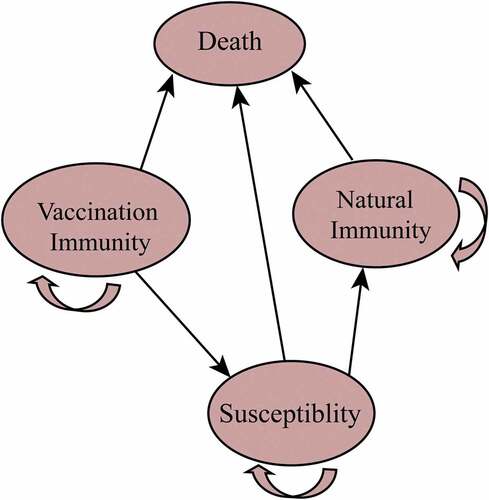
From a societal perspective, the decision-tree Markov model was used to evaluate various varicella vaccination strategies for children in Jiangsu. Five possible immunization strategies for different doses of varicella vaccine and serotesting before vaccination were considered ().
Table 1. Description of different varicella vaccination strategies*
We assumed that a cohort of 100,000 newborns entered the model and followed the strategies mentioned above. The Markov model cycle was one year for each stage, and the end point of circulation was sixty years. The Markov model included four states: susceptibility, natural immunity, vaccine immunity, and death ( and Supplementary Material 1). The vaccine immunity state was classified into I and II according to the doses received: these states represented people receiving one-dose and two-dose vaccination with a protective antibody, respectively.
Symptomatic and asymptomatic infections can occur in susceptible populations infected with the varicella zoster virus. The medical treatment for symptomatic cases consists of ambulatory treatment and hospitalization. Vaccinated persons may become susceptible to infection due to the waning of protective antibodies. This study was subject to some conditions: (1) all people with symptomatic infections were assumed to have received medical treatment; (2) herpes zoster in adults was not considered; (3) the herd immunity effect was not considered; and (4) infected patients, including symptomatic and asymptomatic cases, were assumed to develop natural immunity, who would then have persistent protective antibodies.Citation16
2.2. Model parameters
We collected model parameters using two methods. The epidemiological and economic parameters were collected through field work as base case values. We obtained a range of parameters (e.g., the economic cost of varicella patients) from previous studies conducted in Jiangsu and other regions of China. The minimum and maximum values were used as the lower and upper limits of the range, respectively. Additionally, systematic reviews provided certain parameters (e.g., vaccine efficacy and vaccine immunity waning rates), which were not directly available in China.
2.2.1. Field work
The field investigation was carried out in Wuxi city in southern Jiangsu. From 2011 to 2018, Wuxi was among the top three cities in Jiangsu in the incidence of varicella,Citation11 and the vaccination rate was low (approximately 0.54% in 2016).Citation22
We sampled the costs of varicella cases reported by the China Disease Prevention and Control Information System for Wuxi in 2017. The sample size was calculated using the following parameters: 4% varicella incidence, 1.8% precision, and 5% type I error.Citation23 We increased the sample size by 10% (to 499) to ensure quality of the survey.
The stratified sampling method was adopted. We divided Wuxi into three levels based on economic characteristics (per capita gross domestic product [GDP])Citation24 and selected one area from each level and two clinics from each area (Supplementary Material 2: Figures S1-S2). We then sampled 167 cases at each level.
The field work was conducted from January to February 2018. We used unified questionnaires to obtain relevant information regarding the sampled varicella cases through face-to-face or telephonic interviews. All research team members were trained prior to the investigation. We contacted the participants personally and communicated the purpose of the study. Interviews were performed after obtaining consent. The questionnaire elicited information on respondent characteristics, method of treatment (outpatient or inpatient), direct cost (direct medical and non-medical expenses), and days of work missed by family caregivers. After completion, the questionnaire was checked for unanswered questions or unclear expressions, which were then clarified with the participants.
2.2.2. Epidemiologic variables
The mortality rate was obtained from the Jiangsu 2018 Statistical Yearbook.Citation25 The infection, case fatality, complication, and hospitalization rates of varicella were derived through field work and using surveillance data. The results of the model parameters are presented in . Previous studies provided the range of probabilities mentioned above.Citation26–32 The natural immunity rate was taken from previous studies.Citation33 According to our estimation, the range of the natural immunity rate was ±20% of the base value.Citation34,Citation35 We used data from European studies as a range due to insufficient available data on the incidence of complications from varicella infection in China.Citation36,Citation37
Table 2. Parameters of the varicella vaccine in the model
2.2.3. Vaccination and screen variables
The baseline value and range of different doses of vaccine efficacies were adopted from a meta-analysis of 42 studies.Citation3 People with vaccine immunity can become susceptible to infection because of the decay of protective antibodies. Cases that occurred more than 42 days after vaccination were defined as breakthrough varicella.Citation38 In this study, the waning rates for one-dose and two-dose vaccine immunity were found to be 0.02 and 0.00, respectively,Citation39 and the range was derived from a systematic review.Citation40 In the model, the coverage was set to 60% in the direct vaccination strategy,Citation9 and selective vaccination was assumed to cover all children without antibodies after the serologic test. Reports published by the Centers for Disease Control and Prevention (CDC) provided the sensitivity and specificity of serotesting.Citation41
2.2.4. Cost
The model included the costs of vaccination, serotesting, and the disease. The cost of vaccination consisted of vaccine and administration costs, including transportation, cold chain operations, management training, personnel labor, and fixed assets depreciation. We obtained the data through face-to-face interviews with local CDC staff. The range of vaccine prices and administration costs were derived from the literature.Citation14–16,Citation42
The cost of the disease consisted of both direct and indirect costs. Direct costs included direct medical and non-medical expenses; indirect costs were the cost of work missed by family caregivers [Indirect disease cost = time of work missed by family caregivers (day)× per capita disposable income/365.2543]. For outpatients, the time of work missed was calculated as the number of visits to the clinic (one visit counted as one day); for inpatients, the time of work missed was calculated as the number of days of hospitalization. The range of cost of disease was derived from studies.Citation14–16,Citation32,Citation43
We consulted local health providers in Wuxi about the price of serotesting and borrowed the range from previous studies.Citation44,Citation45 All costs (RMB) were converted to 2017 USD (USD 1 = RMB 6.7518). (http://data.stats.gov.cn/easyquery.htm?cn=C01).
2.2.5. Utility weights
Quality adjusted life years (QALYs) were used to measure the quality of life of varicella patients. The baseline value and range of health utility were borrowed from a study in Hong Kong.Citation46 We assumed that the utility of the health state was one and that of the asymptomatic state was 0.9995. Additionally, we assumed that the QALY loss for infections with complications was ten times that for infections without complications.
2.2.6. Discounting
Outcomes and costs were discounted at 3% per year.Citation47
2.3. Cost effectiveness analysis
We performed the analysis using Treeage Pro 2011 software (Williamstown, MA). The incremental cost-utility ratio (ICUR) was used to evaluate the different vaccination strategies. ICUR is the difference in total cost among various strategies, divided by the difference in total QALYs. The strategy was considered cost effective if the ICUR was lower than a threshold of three times the per capita GDP. The per capita GDP in Jiangsu was USD 15,875.62 in 2017.Citation25 The Consolidated Health Economic Evaluation Reporting Standards checklist is provided in Supplementary Material 3.
2.4. Sensitivity analysis
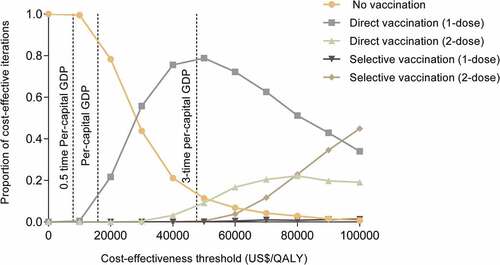
We implemented one-way sensitivity analyses for all parameter ranges and described the results using a tornado diagram to show the eight parameters that the ICUR was most sensitive to. A probability sensitivity analysis of 10,000 simulations was performed to understand the influence of parameter uncertainties on the model results. We assigned the distribution of parameters based on their properties and the literature.Citation35,Citation48 We used a willingness to pay (WTP) threshold of three times the per capita GDP of Jiangsu province in the base case analysis and a more stringent threshold of 0.5 to 1 times the GDP when constructing cost-effectiveness acceptability curves.
3. Results
3.1. Characteristics of participants
A total of 540 varicella patients were contacted; 528 (97.78%) agreed to participate in the field work. These included 265 men (50.19%) and 263 women (49.81%), with 520 (98.48%) outpatients and eight (1.52%) inpatients. The mean age of the patients was 8.28 years. The median medical costs of outpatients and inpatients in Wuxi were USD 68.48 and 581.41, respectively. No varicella infections that resulted in complications were noted in our study. We assumed that the medical cost of infections with complications is twice that of infections without complications.Citation36 This assumption was derived from a study in Poland, which reported that the mean cost of hospitalization of varicella cases with complications was twice that of hospitalization of varicella cases without complications.Citation36
3.2. Base case results
In our model, direct varicella vaccination, both one-dose and two-dose, was cost effective compared with no vaccination. The ICUR of one-dose and two-dose direct vaccination versus no vaccination was less than three times the per capita GDP of Jiangsu in 2017 (USD 47,626.86). Direct varicella vaccination strategies with additional costs can significantly reduce the number of clinical varicella cases (). One-dose and two-dose direct vaccination saved one QALY at the ICURs of USD 21,401.33 and USD 35,420.81, respectively. The ICUR of two-dose versus one-dose vaccination (USD 78,216.24/QALY) was higher than the threshold.
Table 3. Cost-utility and cost-effectiveness outcomes for different varicella vaccination strategies
Direct vaccination was a more cost-effective strategy than selective vaccination. The ICURs of the selective vaccination strategy (two-dose) versus no vaccination were higher than three times the per capita GDP in 2017. Compared with one-dose or two-dose direct vaccination, selective vaccination required an additional USD 2,974,494.22 or USD 4,762,603.387, respectively.
3.3. Sensitivity analysis
The QALY loss (utility weights) of varicella outpatients and vaccine price were the main variables influencing the dominant strategy in the model (). Threshold analysis showed that if the QALY loss of varicella outpatients was lower than 0.0041, the dominant strategy would be no vaccination, and if the QALY loss was higher than 0.0168, the dominant strategy would be two-dose direct vaccination; otherwise, one-dose direct vaccination was the most cost-effective. If the vaccine price was lower than USD 11.67, two-dose direct vaccination would be dominant; otherwise, one-dose direct vaccination was the most cost-effective.
Figure 2. Tornado diagram of parameters in models*.
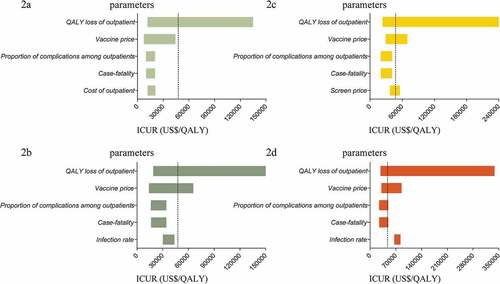
Other variables, including the proportion of complications among outpatients, case fatality of varicella, and direct vaccination (1-dose) coverage could also influence the dominant strategy. If the proportion of complications was lower than 0.0751, the dominant strategy would be one-dose direct vaccination, and if this proportion was higher than 0.1134, the dominant strategy would be two-dose selective vaccination; otherwise, two-dose direct vaccination was most cost-effective. If the proportion of complications was lower than 0.0110, the dominant strategy would be one-dose direct vaccination, and if the proportion was higher than 0.0156, the dominant strategy would be two-dose selective vaccination; otherwise, two-dose direct vaccination was the most cost-effective.
The probability sensitivity analysis compared five possible strategies (). At a cost-effectiveness threshold of USD 15,875.62/QALY (once per capita GDP), there is a 9.49% probability of one-dose direct vaccination being cost-effective. At a cost-effectiveness threshold of USD 47,626.86/QALY (three times the per capita GDP), there is a 77.17% probability of one-dose direct vaccination and an 8.85% probability of two-dose direct vaccination being cost effective. There is only a 0.1% probability of one-dose direct vaccination being cost effective at a threshold of USD 7937.81/QALY (0.5 times the per capita GDP).
4. Discussion
Our model study showed that, compared with no vaccination, the one-dose and two-dose direct varicella vaccination strategies in Jiangsu were cost effective from a societal perspective, under the assumption that WTP is three times the provincial GDP. Compared with both the one- and two-dose direct vaccination strategies, selective vaccination that includes serologic tests before vaccination would not be cost-saving. Selective vaccination requires additional cost relative to direct vaccination, but does not avert more clinical varicella cases or QALY loss.
Our findings showed that one-dose and two-dose direct vaccination are likely to be cost effective in Jiangsu Province, which is consistent with some studies.Citation12,Citation40,Citation48,Citation49 The findings also supported the current evidence that direct varicella vaccination was cost effective.Citation14,Citation15 The evaluation index of our study was ICUR considering the health utility of varicella patients. Cost utility analysis can capture more damaging health outcomes and clinical consequences.Citation17,Citation50
In our study, the ICURs of the two-dose vaccination strategy were higher than those of the one-dose vaccination strategy. The two-dose strategy was less cost-effective than the one-dose strategy, which is consistent with previous studies conducted in the United States, Colombia, and Iran.Citation48,Citation49,Citation51 However, the implementation of health decision-making in China requires the consideration of evidence from different perspectives, not only cost-effectiveness analysis. Given the efficacy and immunity sustainability of the one-dose vaccine and the heavy burden of varicella outbreaks in school settings, the Advisory Committee on Immunization Practices in the United States recommended a routine two-dose varicella vaccination program.Citation49 A retrospective study conducted in Ningbo, eastern China, showed that the breakthrough varicella infection rate of one-dose vaccination was much higher than that of two-dose vaccination.Citation52 Moreover, the quality of vaccines attracted public attention in China after the Changchun Changsheng vaccine incident in 2018.Citation53,Citation54 The emergence of breakthrough varicella cases after one-dose vaccination could cause public distrust in the vaccine. Based on the evidence mentioned above, we recommend a two-dose vaccine program in Jiangsu.
We found that cost-effectiveness outcomes were sensitive to the QALY loss of varicella outpatients. Lower QALY loss of outpatients was associated with higher ICURs of vaccination strategies, both in the one-dose-and two-dose strategies. This highly influences the cost effectiveness of the vaccination strategy. Previous studies showed that the loss of health utility in varicella cases was low.Citation45,Citation55 However, given that few studies report the loss of health utility for varicella cases in China, future research should investigate this topic further. A previous review and some studies reported that the varicella vaccine price is an important parameter for cost-effectiveness outcomes.Citation12,Citation48 Our tornado modeling also suggests that cost-effectiveness outcomes are highly sensitive to the vaccine cost.
Our study suggests that, compared with direct vaccination, the selective vaccination strategy (serotesting before vaccination) was less cost-effective. These results did not indicate the need for evaluation of serotesting before varicella vaccination in children younger than five years, as mentioned in previous articles.Citation18 Another study showed that direct vaccination in school-age children was more cost-effective.Citation19 However, some studies suggested that serotesting before vaccination is more cost-saving than direct vaccination, which was conducted in special populations, including pregnant women, healthcare workers, and adults.Citation44,Citation56,Citation57 The inoculated population could explain the difference between these results. In this study, the cost effectiveness of varicella vaccination was evaluated in newborns with low varicella seroprevalence (natural immunity rate). Pregnant women, healthcare workers, adolescents, and adults mostly achieve immunity as they contract varicella at a young age. The high seropositivity rate in this population makes it more cost-efficient to perform serological tests before vaccination. Therefore, we suggest that direct varicella vaccination is more cost-effective than selective vaccination for younger children.
In China, the varicella vaccine is a type II vaccine that requires out-of-pocket payment. Our results demonstrated that the cost effectiveness of varicella vaccination was sensitive to the WTP threshold, which was three times the per capita GDP of Jiangsu, a province with top 3 GDP among provinces in China. Health authorities would face financial pressure if they included were to consider including the varicella vaccine in the routine vaccine program. Given the affordability and sustainability of health policy, routine varicella vaccinations can be considered for inclusion in childhood immunity scheduling in economically advanced cities. Additionally, the two-dose vaccination schedule, which is less cost-effective than the one-dose strategy, needs to be determined based on evidence from different perspectives.
Our study has several limitations. First, the varicella cases included in the retrospective survey did not have severe complications, which might lead to results of high cost-effectiveness for varicella vaccinations relative to no vaccination. The sensitive analyses showed that the proportion of complications among outpatients was also the main variable influencing the dominant strategy. A large sample size should be surveyed to estimate the proportion of varicella complications in China in future research. Second, our findings need to be generalized with caution as some of the data are local. Third, the analyses were not stratified by gender or region. Cost effectiveness analyses might be associated with differing economic levels in different areas. Fourth, we assumed the first dose of varicella vaccination was administered at 12 months and the second at 15 months of age.Citation48 However, evidence suggests that a shorter interval may be optimal for immunity maintenance and epidemiologic impact.Citation58–60 Finally, we did not include zoster in the model. A previous review did not find a conclusive association between varicella vaccination introduction and an increase in the incidence of herpes zoster.Citation7 Given the insufficient local data, it is difficult to make some assumptions about zoster. Besides, the end point of circulation was 60 years in our Markov model; however, herpes zoster infection usually occurs in people aged over 50 years.Citation61–63 Further economic evaluation of varicella vaccinations considering herpes zoster needs to be undertaken.
5. Conclusion
This study found that varicella vaccination in children was cost effective at the WTP threshold of three times provincial GDP per capita from the societal perspective in Jiangsu, China. Our findings are sensitive to the vaccine price and health utility of varicella cases. Varicella vaccination is more likely to be cost effective when the vaccine price is low and the loss of health utility for cases is high.
Disclosure of potential conflicts of interest
All authors: No reported conflicts of interest.
Author contributions
Hui Jin, Shixin Xiu, Qiang Wang, and Leesa Lin conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript; Qiang Wang, Shixin Xiu, Liuqing Yang, Jinxin Huang, Tingting Cui, Naiyang Shi, Xuwen Wang, Yuan Shen, Enpin Chen, Bing Lu, designed the data collection instruments and collected data; Qiang Wang and Shixin Xiu carried out the initial analyses. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Informed Consent Statement
All subjects gave their informed consent for inclusion before they participated in the study.
Ethics Statement
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Southeast University Zhongda Hospital (2017ZDKYSB033).
Supplemental Material
Download MS Word (316.3 KB)Supplemental Material
Download PDF (366.4 KB)Acknowledgments
We are grateful to all those who helped in the process of collecting data. We thank all the editors and reviewers for their suggestions.
Data Availability Statement
Some or all data, models, or code generated or used during the study are available from the corresponding author by request.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1958608.
Additional information
Funding
References
- Heininger U, Seward JF. Varicella. Lancet. 2006;368(9544):1365–76. doi:10.1016/S0140-6736(06)69561-5.
- Goh AEN, Choi EH, Chokephaibulkit K, Choudhury J, Kuter B, Lee PI, Marshall H, Kim JO, Wolfson LJ. Burden of varicella in the Asia-Pacific region: a systematic literature review. Expert Rev Vaccines. 2019;18(5):475–93. doi:10.1080/14760584.2019.1594781.
- Marin M, Marti M, Kambhampati A, Jeram SM, Seward JF. Global varicella vaccine effectiveness: a meta-analysis. Pediatrics. 2016;137(3):e20153741. doi:10.1542/peds.2015-3741.
- Hirose M, Gilio AE, Ferronato AE, Ragazzi SL. The impact of varicella vaccination on varicella-related hospitalization rates: global data review. Rev Paul Pediatr. 2016;34(3):359–66. doi:10.1016/j.rpped.2015.12.006.
- Seward JF, Watson BM, Peterson CL, Mascola L, Pelosi JW, Zhang JX, Maupin TJ, Goldman GS, Tabony LJ, Brodovicz KG, et al. Varicella disease after introduction of varicella vaccine in the United States, 1995-2000. Jama. 2002;287(5):606–11. doi:10.1001/jama.287.5.606.
- Marin M, Meer HC, Seward JF. Varicella prevention in the United States: a review of successes and challenges. Pediatrics. 2008;122:E744–E751. doi:10.1542/peds.2008-0567.
- Varela FH, Pinto LA, Scotta MC. Global impact of varicella vaccination programs. Hum Vaccin Immunother. 2019;15(3):645–57. doi:10.1080/21645515.2018.1546525.
- Chinese Center for Disease Control and Prevention. Varicella vaccine. Beijing (CN): National Immunization Program; 2014. [accessed 2019 Dec 26]. http://nip.chinacdc.cn/zstd/ymzs/201211/t20121130_72490.htm .
- Liu AP, Sun TT. Meta-analysis of varicella vaccine coverage among Chinese children. Chin J Vaccines Immun. 2017;23:698–704.
- Zhang L Study on epidemiological characteristics and epidemic factors of varicella in Jiangsu Province. [Master dissertation]. Nanjing(CN): Nanjing Medical University; 2019.
- Xinhua News Agency. Varicella vaccine was included in Shanghai immunization program; 2018 [accessed 2019 Dec 26]. http://m.xinhuanet.com/2018-08/01/c_129924748.htm .
- Rozenbaum MH, van Hoek AJ, Vegter S, Postma MJ. Cost-effectiveness of varicella vaccination programs: an update of the literature. Expert Rev Vaccines. 2008;7(6):753–82. doi:10.1586/14760584.7.6.753.
- Hodgkinson B, Wang T, Byrnes J, Scuffham P. Modelling a cost-effective vaccination strategy for the prevention of varicella and herpes zoster infection: a systematic review. Vaccine. 2021;39(9):1370–82. doi:10.1016/j.vaccine.2021.01.061.
- Tu ZB, Xu QY, Wan GF, Nie PY. Economic burden of varicella and cost -benefit analysis of vaccine immunization in Honggutan district of Nanchang. Chin J Public Health Manage. 2019;35:164–67.
- Deng X, He HQ, Zhou Y, Pan JR, Yan R, Tang XW, Fu J. Economic evaluation of different chickenpox vaccination strategies. J Zhejiang Univ (Med Sci). 2018;47:374–80.
- Yin DP The immunization strategy evaluation and cost-benefit analysis of varicella attenuated live vaccine In China. [Doctoral dissertation]. Beijing(CN): Chinese Center for Disease Control and Prevention; 2012.
- Jakubiak-Lasocka J, Jakubczyk M. Cost-effectiveness versus cost-utility analyses: what are the motives behind using each and how do their results differ?—A polish example. Value Health Reg Issues. 2014;4:66–74. doi:10.1016/j.vhri.2014.06.008.
- Figueira M, Christiansen D, Barnett ED. Cost-effectiveness of serotesting compared with universal immunization for varicella in refugee children from six geographic regions. J Travel Med. 2003;10(4):203–07. doi:10.2310/7060.2003.40545.
- Lieu TA, Finkler LJ, Sorel ME, Black SB, Shinefield HR. Cost-effectiveness of varicella serotesting versus presumptive vaccination of school-age children and adolescents. Pediatrics. 1995;95:632–38.
- Sui HT, Li JC, Wang M, Liu YM, Yin DP. Varicella epidemiology in China, 2005-2015. Chin J Vaccines Immun. 2019;25:155–59.
- Wuxi Municipal Center for Disease Control and Prevention. Free vaccine against varicella. Wuxi(CN); 2019 [accessed 2019 Dec 26]. http://wjw.wuxi.gov.cn/doc/2019/05/10/2477651.shtml .
- Xiu SX, Wang XW. Epidemiological characteristics of varicella in Wuxi, 2012-2016. Mod Preventive Med. 2017;44:3467–9,74.
- Sun ZQ, Xu YY. Medical Statistics. 4th ed. Beijing (CN): People's Medical Publishing House; 2014. p. 573–756.
- Wuxi Statistics Bureau. Wuxi statistical yearbook 2019. Wuxi(CN); 2020 [accessed 2019 Dec 26]. http://tj.wuxi.gov.cn/doc/2019/09/12/2655098.shtml .
- Jiangsu Statistics Bureau. Jiangsu statistical yearbook 2018. Nanjing(CN); 2020 [accessed 2019 Dec 26]. http://tj.jiangsu.gov.cn/2018/nj02.htm2019 .
- Mirinaviciute G, Kristensen E, Nakstad B, Flem E. Varicella-related primary health-care visits, hospitalizations and mortality in Norway, 2008-2014. Pediatr Infect Dis J. 2017;36(11):1032–38. doi:10.1097/INF.0000000000001656.
- Hussey H, Abdullahi L, Collins J, Muloiwa R, Hussey G, Kagina B. Varicella zoster virus-associated morbidity and mortality in Africa - a systematic review. BMC Infect Dis. 2017;17(1):717. doi:10.1186/s12879-017-2815-9.
- Arlant LHF, Garcia MCP, Avila Aguero ML, Cashat M, Parellada CI, Wolfson LJ. Burden of varicella in Latin America and the Caribbean: findings from a systematic literature review. BMC Public Health. 2019;19(1):528. doi:10.1186/s12889-019-6795-0.
- Neyro SE, Ferolla FM, Molise C, Stach P, Romano P, Marone S, de Mena A, Plat F, Voto C, Soto P, et al. Clinical and epidemiological impact of varicella infection in children prior to the introduction of the varicella vaccine in the national immunization schedule of Argentina. Arch Argent Pediatr. 2019;117(1):12–18. doi:10.5546/aap.2019.eng.12.
- Widgren K, Giesecke J, Lindquist L, Tegnell A. The burden of chickenpox disease in Sweden. BMC Infect Dis. 2016;16(1):666. doi:10.1186/s12879-016-1957-5.
- Singleton RJ, Holman RC, Person MK, Steiner CA, Redd JT, Hennessy TW, Groom A, Holve S, Seward JF. Impact of varicella vaccination on varicella-related hospitalizations among American Indian/Alaska Native people. Pediatr Infect Dis J. 2014;33(3):276–79. doi:10.1097/INF.0000000000000100.
- Yang J Epidemiological characteristics and medical expenses research of varicella in Karamay. [Master dissertation]. Beijing (CN): Chinese Center for Disease Control and Prevention); 2017.
- Devecioglu E, Gokcay G, Boran P, Eren T, Yilmaz G, Badur S. Prevalence of maternal measles, rubella, mumps and varicella antibodies in the first six months of life. Mikrobiyol Bul. 2018;52(3):324–27. doi:10.5578/mb.67169.
- Chen C, Cervero Liceras F, Flasche S, Sidharta S, Yoong J, Sundaram N, Jit M. Effect and cost-effectiveness of pneumococcal conjugate vaccination: a global modelling analysis. Lancet Glob Health. 2019;7(1):e58–e67. doi:10.1016/S2214-109X(18)30422-4.
- Nayagam S, Conteh L, Sicuri E, Shimakawa Y, Suso P, Tamba S, Njie R, Njai H, Lemoine M, Hallett TB, et al. Cost-effectiveness of community-based screening and treatment for chronic hepatitis B in The Gambia: an economic modelling analysis. Lancet Global Health. 2016;4(8):E568–E78. doi:10.1016/S2214-109X(16)30101-2.
- Wysocki J, Malecka I, Stryczynska-Kazubska J, Rampakakis E, Kuter B, Wolfson LJ. Varicella in Poland: economic burden in children 1-12 years of age in Poland, 2010-2015. BMC Public Health. 2018;18(1):410. doi:10.1186/s12889-018-5298-8.
- Bernal JL, Hobbelen P, Amirthalingam G. Burden of varicella complications in secondary care, England, 2004 to 2017. Euro Surveill. 2019;24(42):1900233. doi:10.2807/1560-7917.ES.2019.24.42.1900233.
- Chartrand SA. Varicella vaccine. Pediatr Clin North Am. 2000;47(2):373–94. doi:10.1016/s0031-3955(05)70212-1.
- Rafferty E, McDonald W, Qian WC, Osgood ND, Doroshenko A. Evaluation of the effect of chickenpox vaccination on shingles epidemiology using agent-based modeling. Peerj. 2018:6. doi:10.7717/peerj.5012.
- Damm O, Ultsch B, Horn J, Mikolajczyk RT, Greiner W, Wichmann O. Systematic review of models assessing the economic value of routine varicella and herpes zoster vaccination in high-income countries. BMC Public Health. 2015;15:533. doi:10.1186/s12889-015-1861-8.
- Prevention of varicella: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Centers for disease control and prevention. MMWR Recomm Rep. 1996;45(RR–11):1–36.
- Xiu SX Disease burden of hepatitis E and health economic evaluation of vaccine interventions in Dongtai area. [Master dissertation]. Nanjing (CN): Southeast University; 2011.
- Zhang XS, Wang XL, Li H, Li XB. Ecnomic burden of children outpatients aged 0-14 with varicella in 6 municipalities in Gansu. Mod Preventive Med. 2013;40:1063–4+7.
- Chodick G, Ashkenazi S, Livni G, Lerman Y. Cost-effectiveness of varicella vaccination of healthcare workers. Vaccine. 2005;23(43):5064–72. doi:10.1016/j.vaccine.2005.06.004.
- Merrett P, Schwartzman K, Rivest P, Greenaway C. Strategies to prevent varicella among newly arrived adult immigrants and refugees: a cost-effectiveness analysis. Clin Infect Dis. 2007;44(8):1040–48. doi:10.1086/512673.
- Chui KS, Wu HL, You JH. Cost-effectiveness analysis of varicella vaccine as post-exposure prophylaxis in Hong Kong. Scand J Infect Dis. 2014;46(1):27–33. doi:10.3109/00365548.2013.847529.
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. 4th ed. Oxford: Oxford University Press; 2015.
- Paternina-Caicedo A, De la Hoz-Restrepo F, Gamboa-Garay O, Castaneda-Orjuela C, Velandia-Gonzalez M, Alvis-Guzman N. How cost effective is universal varicella vaccination in developing countries? A case-study from Colombia. Vaccine. 2013;31(2):402–09. doi:10.1016/j.vaccine.2012.10.100.
- Zhou F, Shefer A, Wenger J, Messonnier M, Wang LY, Lopez A, Moore M, Murphy TV, Cortese M, Rodewald L. Economic evaluation of the routine childhood immunization program in the united States, 2009. Pediatrics. 2014;133(4):577–85. doi:10.1542/peds.2013-0698.
- Drummond M, Sculpher M, Torrance G, O’Brien B, Stoddart G. Methods for the economic evaluation of health care programmes. 3rd ed. Oxford (UK): Oxford University Press; 2005.
- Esmaeeli S, Yaghoubi M, Nojomi M. Cost-effectiveness of varicella vaccination program in Iran. Int J Prev Med. 2017;8:103. doi:10.4103/ijpvm.IJPVM_295_16.
- Pan X, Shu M, Ma R, Fang T, Dong H, Sun Y, Xu G. Varicella breakthrough infection and effectiveness of 2-dose varicella vaccine in China. Vaccine. 2018;36(37):5665–70. doi:10.1016/j.vaccine.2018.05.025.
- Gan LQ, Wang X, Zhou YL. Knowledge attitude and its influencing factors of Shenzhen residents to preventive vaccination after Changchun Changsheng vaccine incident. China Trop Med. 2019;19:762–66.
- National Health Commission of the People’s Republic of China. Investigation of vaccine cases and related accountability in Changchun Changsheng Company, Jilin Province. Beijing(CN); 2018 [accessed 2019 Dec 26]. http://www.nhc.gov.cn/wjw/xwdt/201808/077c79823f22407ab6ee5d43b260ce76.shtml; .
- van Hoek AJ, Melegaro A, Gay N, Bilcke J, Edmunds WJ. The cost-effectiveness of varicella and combined varicella and herpes zoster vaccination programmes in the United Kingdom. Vaccine. 2012;30(6):1225–34. doi:10.1016/j.vaccine.2011.11.026 .
- Smith WJ, Jackson LA, Watts DH, Koepsell TD. Prevention of chickenpox in reproductive-age women: cost-effectiveness of routine prenatal screening with postpartum vaccination of susceptibles. Obstet Gynecol. 1998;92(4 Pt 1):535–45. doi:10.1016/s0029-7844(98)00221-x.
- Hanslik T, Boëlle P-Y, Schwarzinger M, Carrat F, Freedberg KA, Valleron A-J, Flahault A. Varicella in French adolescents and adults: individual risk assessment and cost-effectiveness of routine vaccination. Vaccine. 2003;21(25-26:3614–22. doi:10.1016/s0264-410x(03)00405-5.
- Wutzler P, Bonanni P, Burgess M, Gershon A, Sáfadi MA, Casabona G. Varicella vaccination - the global experience. Expert Rev Vaccines. 2017;16(8):833–43. doi:10.1080/14760584.2017.1343669.
- Rafferty ERS, McDonald W, Osgood ND, Qian W, Doroshenko A. Seeking the optimal schedule for chickenpox vaccination in Canada: using an agent-based model to explore the impact of dose timing, coverage and waning of immunity on disease outcomes. Vaccine. 2020;38(3):521–29. doi:10.1016/j.vaccine.2019.10.065.
- Bonanni P, Gershon A, Gershon M, Kulcsár A, Papaevangelou V, Rentier B, Sadzot-Delvaux C, Usonis V, Vesikari T, Weil-Olivier C, et al. Primary versus secondary failure after varicella vaccination: implications for interval between 2 doses. Pediatr Infect Dis J. 2013;32(7):e305–e313. doi:10.1097/INF.0b013e31828b7def.
- Hoshi SL, Kondo M, Okubo I. Cost-effectiveness of varicella vaccine against herpes zoster and post-herpetic neuralgia for elderly in Japan. Vaccine. 2017;35(24):3264–71. doi:10.1016/j.vaccine.2017.04.046.
- Curran D, Patterson B, Varghese L, Van Oorschot D, Buck P, Carrico J, Hicks K, Lee B, Yawn B. Cost-effectiveness of an adjuvanted recombinant zoster vaccine in older adults in the United States. Vaccine. 2018;36(33):5037–45. doi:10.1016/j.vaccine.2018.07.005.
- Sun X, Wei Z, Lin H, Jit M, Li Z, Fu C. Incidence and disease burden of herpes zoster in the population aged ≥50 years in China: data from an integrated health care network. J Infect. 2021;82(2):253–60. doi:10.1016/j.jinf.2020.12.013.