ABSTRACT
Objectives
To evaluate severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine acceptance among patients with rheumatic diseases (RMD).
Methods
All rheumatology patients attending a large suburban health network were invited to participate in an anonymized online survey. The primary outcome of interest was SARS-COV-2 vaccine acceptance.
Results
The mean (SD) age of respondents (n = 641) was 52.7 (15.1) years and 74.4% (n = 474) were female. Sixty-five percent were willing to have a SARS-COV-2 vaccine, while 34.4% were vaccine-hesitant (unwilling or undecided). On multivariate analysis, vaccine acceptance was associated with smoking (OR: 2.25 [95% CI: 1.22–4.15; p = .009]), history of malignancy (OR: 2.51 [95% CI: 1.19–5.26; p = .015]), influenza or pneumococcal vaccination in the preceding year (OR: 2.69 [95% CI: 1.78–4.05; p < .001]) and number of COVID-Safe measures practiced (OR: 1.54 [95% CI: 1.05–2.26; p = .027]). Vaccine acceptance correlated with positive beliefs regarding vaccine efficacy (r = 0.40; p < .001) and safety (r = 0.36; p < .001). Vaccine acceptance correlated negatively with concerns regarding side-effects (r = −0.30; p < .001) and vaccine-associated RMD flare (r = −0.21; p < .001). In vaccine-hesitant respondents, 39.2% were more likely to accept vaccination if given a choice of which vaccine they receive and 54.5% if their rheumatologist recommended it. Twenty-seven percent of patients on immunomodulators were willing to withhold medications while 42.1% were willing if advised by their rheumatologist.
Conclusion
SARS-COV-2 vaccine hesitancy is prevalent amongst RMD patients and associated with concerns regarding vaccine safety, efficacy, side effects and RMD flare. Clinician recommendation, vaccine choice and communications targeting patient concerns could facilitate vaccine acceptance.
Significance and Innovations
Vaccine hesitancy is prevalent in RMD patients
Vaccine acceptance is associated with beliefs regarding vaccine safety and efficacy and concerns regarding RMD flare and vaccine-associated side effects
Vaccine choice and clinician recommendation have the potential to improve vaccine acceptance in patients who are hesitant
Introduction
Patients with rheumatic diseases (RMD) have faced significant challenges during the coronavirus disease 2019 (COVID-19) pandemic, including concerns regarding their risks of acquiring SARS-COV-2 infection, having a poorer outcome from SARS-COV-2 infection, disruptions to their usual care, and medication shortages.Citation1–3
The epidemiology of SARS-COV-2 infections in RMD patients has been the subject of extensive research.Citation4,Citation5 The varied findings of these studies are a reflection of heterogeneity in study designs and patient populations, and are confounded by risk mitigation strategies instituted by patients and clinicians. While some data have been reassuring, concerns remain regarding the risks posed by RMD and associated immunomodulatory therapies, given the wealth of historical data to support such concerns.Citation6,Citation7 Further uncertainty relates to the risk:benefit of continuation vs. interruption of immunomodulatory therapies at the time of vaccination.Citation8,Citation9
The rapid development of effective SARS-CoV-2 vaccines have brought with it the possibility of a return to normality through case prevention, countered by the challenge of vaccine hesitancy. SARS-COV-2 vaccine acceptance in the general population has previously been reported at 71.5% in one multinational survey and at 85% in an Australian survey conducted during the first wave of the pandemic in 2020.Citation10–12 In patients with RMDs, SARS-COV-2 vaccine acceptance has been reported between 54–82%.Citation13,Citation14
The vaccine hesitancy model is described as being influenced by confidence, complacency and convenience.Citation15–17 Assessing the factors that influence vaccine hesitancy in specific populations is fundamental for developing a targeted communication strategy to encourage vaccine acceptance. In a multinational survey of RMD patients, SARS-COV-2 vaccine acceptance was associated with age, fear, previous vaccine acceptance and specialist advice.Citation13 The impact of these and other factors may differ across countries that have had varying success in controlling the pandemic, and this data is lacking in RMD patients in Australia, where public health measures have resulted in low national case burden and mortality, permitting late commencement of national vaccine rollout.
In this study, performed at the commencement of the national SARS-COV-2 vaccination rollout, we aimed to assess the acceptance of the SARS-COV-2 vaccination in Australian RMD patients, the factors contributing to vaccine acceptance, and the willingness to withhold immunomodulatory therapies to improve vaccine response. We also assessed the impact of the Australian Rheumatology Association’s SARS-COV-2 Vaccine Patient Information Sheet on vaccine acceptance.
Methods
Study design
Monash Health is a large health care network in the South East of Melbourne, Australia which serves a population of over 1.5 million people and provides >10,000 rheumatology outpatient services per year. All patients aged 18 years and over who were patients of rheumatology clinics at Monash Health over a 24-month period (n = 3451) were invited to participate in a de-identified cross-sectional survey regarding SARS-COV-2 vaccination (Supplementary material). The survey was reviewed and revised by four specialist clinicians and three patient research partners to optimize content validity and feasibility. The survey invitation was sent via short message service (SMS) in February 2021, coinciding with the commencement of the SARS-COV-2 vaccination rollout in Australia, and was conducted electronically via the Survey Monkey platform. Responses were collated over a one-week period.
Consenting patients completed an initial survey, followed by provision of the Australian Rheumatology Association (ARA) Patient Information Sheet (PIS) on SARS-COV-2 vaccination, and completion of a post-information survey. Following an introductory statement outlining the aims of the survey, patients were asked to proceed with the survey if they consented for their de-identified responses to be used for research purposes. All patients had the option of proceeding directly to the information sheet without participating in the survey. This study was approved by the Human Research Ethics Committee at Monash Health as a quality improvement project (Approval number: RES-21-0000126Q-73547).
Patient survey
The primary outcomes of interest were the prevalence of SARS-COV-2 vaccine acceptance and the factors influencing it. Analyses were conducted on respondents who answered this primary research question. Secondary outcomes were patient perceptions of withholding immunomodulatory therapy to optimize vaccine response, the impact of vaccine choice and clinician recommendation on vaccine acceptance in vaccine-hesitant patients, the percentage of patients who had received SARS-COV-2 vaccination information, and the impact of the SARS-COV-2 PIS on vaccine acceptance.
Exposures for the primary outcomes and patient willingness to withhold immunomodulatory therapy included age, sex, ethnicity, level of education, employment, RMD diagnosis and immunomodulatory therapies, disease duration, presence of poor prognostic factors for SARS-COV-2 infection, previous vaccine acceptance, adherence to COVID-Safe practices (i.e. hand hygiene, mask usage and social distancing) and patient beliefs. Patient beliefs regarding vaccine safety, vaccine efficacy, immunosuppressed status, concern regarding RMD flare and concern regarding SARS-COV-2 infection were assessed on a 5-point Likert scale of agreement (strongly disagree to strongly agree).
Patients who responded that they were undecided or unwilling to have a SARS-COV-2 vaccine were classified as vaccine-hesitant and were assessed on a 5-point Likert scale for the impact of factors that may optimize vaccine acceptance, namely vaccine choice and specialist or GP recommendations regarding vaccination.
Patients were asked if they had previously received information regarding the SARS-COV-2 vaccine and from which sources. A post-PIS survey assessed whether the information sheet was helpful, if it addressed all patient concerns, and whether it contributed to vaccine acceptance. Patients were invited to report unaddressed concerns in a free-text response.
Statistical analysis
Descriptive methods were used to report measures of central tendencies, distributions and frequencies for all exposures and outcomes.
Univariate analysis using chi-squared tests and odds ratios were used to assess associations between the exposures of interest (other than patient beliefs) and the primary outcome of vaccine acceptance (accepting vs. hesitant). Similar analysis was used to assess associations between the exposures of interest (other than patient beliefs) and the secondary outcome of willingness to withhold immunomodulatory therapy to improve vaccine response (willing or willing if advised vs. unwilling). Multivariate analysis was performed on exposures that were of statistical significance (p < .05). Pearson’s r was used to assess for correlations between the strength of patient beliefs and vaccine acceptance (accepting, undecided, or unwilling), and between the strength of patient beliefs and willingness to withhold immunomodulatory therapy (willing, willing if advised, or unwilling).
No power calculations were performed for this observational cross-sectional analysis. Statistical analyses were conducted using SPSS v.23.0 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
There was an 18.6% response rate to the survey, with 641/3451 patients addressing the primary outcome of interest (). The mean (SD) age of respondents was 52.7 (15.1) years and 74.4% (n = 474) were female. The most common ethnicities were White 71.4% (n = 457) and Asian 17.5% (n = 112). A third of respondents (n = 215) reported their highest level of education as high school, and 47.2% were currently employed (n = 301).
Table 1. Baseline demographics
The most common diagnoses were inflammatory arthritis (33.3%), systemic lupus erythematosus (18.0%), and systemic sclerosis (11.0%). Mean (SD) disease duration was 11.0 (±11.3) years. The most commonly reported immunomodulatory therapies were prednisolone (46.4%), hydroxychloroquine (30.6%), and methotrexate (23.8%). Twenty-one percent of respondents were using a biological disease modifying anti-rheumatic drug or a targeted synthetic disease modifying anti-rheumatic drug (b/tsDMARD).
Poor prognostic factors for SARS-COV-2 infection were reported by 55.1% (n = 353) of respondents, with the most common being hypertension (25.2%) and obesity (16.4%). The mean number of co-morbidities was 1.6 (±0.49).
Influenza or pneumococcal vaccination in the last 12 months was reported by 75.9% (n = 468) of respondents. Adherence to COVID-Safe practice including hand hygiene, mask wearing and social distancing was reported by 98.7% (n = 615) of participants.
The prevalence of missing data was <5% except for the following variables: patient diagnosis (5.3%), medication data (9.4%), willingness to withhold medications (5.3%), patient beliefs (6.6%), and previous receipt of vaccination information (8.9%). Missing data were not imputed.
In the pre-information survey, the Likert scale questions examining patient beliefs had a Cronbach-alpha value of 0.60 which suggests a low value for internal consistency. In comparison, the Likert scale questions in the post-information survey had a Cronbach-alpha value of 0.85 which had a higher level of internal consistency.
Factors associated with vaccine acceptance
Sixty-five percent of patients seen in the rheumatology clinic (n = 417) were willing to have the COVID-9 vaccine, while 34.4% (n = 221) were vaccine hesitant ().
On univariate analysis, acceptance was associated with age (OR: 1.01 [95% CI: 1.00–1.02; p = .045]), Asian ethnicity (OR: 1.74 [95% CI: 1.09–2.79; p = .020)], smoking (OR: 2.70 [95% CI: 1.54–4.73; p = .001]), past history of malignancy (OR: 2.10 [95% CI: 1.04–4.25; p = .039]), receipt of vaccination in the preceding year (OR: 3.45 [95% CI: 2.36–5.06; p < .001]), and the number of COVID-Safe measures practiced (OR: 1.84 [95% CI: 1.28–2.64; p = .001]). There were no statistically significant associations with gender, level of education, employment, RMD diagnosis or duration, comorbidities or the number or type of immunomodulators. Female respondents and respondents with a post-graduate education were numerically more likely to be vaccine hesitant, however this did not reach statistical significance ().
Following multivariate analysis, smoking (OR: 2.25 [95% CI: 1.22–4.15; p = .009]), history of malignancy (OR: 2.51 [1.19–5.26; p = .015]), vaccination in the preceding year (OR: 2.69 [95% CI: 1.78–4.05; p < .001]) and the number of COVID-Safe measures practiced (OR: 1.54 [95% CI: 1.05–2.26; p = .027]) remained statistically significant.
There was a strong correlation between beliefs regarding vaccine safety and vaccine efficacy (r = 0.82; p < .001). Vaccine acceptance correlated both with positive beliefs regarding vaccine safety (r = 0.36; p < .001) and vaccine efficacy (r = 0.40; p < .001). There was a weak negative correlation between vaccine acceptance and concerns regarding a vaccine-associated flare in RMD (r = −0.21; p < .001) and concerns regarding vaccine side effects (r = −0.30; p < .001). There were no correlations between vaccine acceptance and patient perception regarding their degree of immunosuppression (r = 0.06; p = .094) or concerns about risk of acquiring SARS-COV-2 (r = 0.31; p < .310).
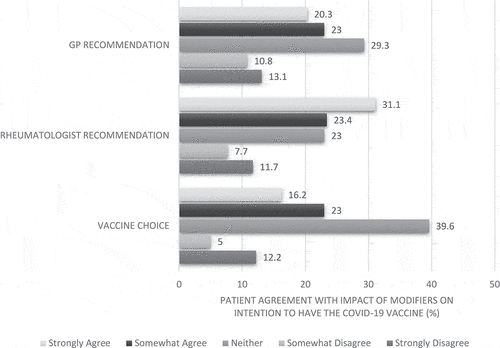
Potential modifiers for vaccine acceptance in hesitant patients
Among vaccine-hesitant respondents (n = 222), 39.2% strongly or somewhat agreed that being given a choice regarding which vaccine they received would increase their vaccine acceptance. () The impact of clinician recommendation was larger, with 54.5% and 43.4% of respondents respectively strongly or somewhat agreeing that a recommendation to have the vaccine from their rheumatologist or GP would increase their vaccine acceptance.
Patient willingness to withhold immunomodulators to optimize vaccine response
Of the patients who were on immunomodulators and responded to this question (n = 523), 27.0% were willing to withhold medications and accept the risk of a flare of their RMD while a further 42.1% were willing to withhold their immunomodulators if advised to do so by their rheumatologist. Thirty one percent of patients were unwilling to withhold their immunomodulators. () Retired and unemployed respondents were numerically more likely to be willing to withhold their immunomodulatory therapy, but this was not statistically significant.
Table 2. Patient willingness to withhold immunomodulatory therapy
Statistically significant associations with willingness to withhold immunomodulators were the use of any b/tsDMARD, SARS-COV-2 vaccine acceptance, and adherence to COVID-Safe practices (p < .05). () On multivariate analysis, the odds ratios for willingness to withhold immunomodulators was 1.78 [95% CI: 1.07–2.97; p = .030]) in patients on b/tsDMARDs and 1.78 [95% CI: 1.18–2.67; p = .006]) in patients who were accepting of the SARS-COV-2 vaccine. There was no significant association with methotrexate use (p = .377). There were no significant correlations between patient beliefs and willingness to withhold immunomodulators.
SARS-COV-2 vaccine patient information sources
82.8% (n = 519) of respondents had not received any formal information about the SARS-COV-2 vaccine at the time of survey. The most common sources of information regarding SARS-COV-2 vaccination were the news (56.3%), government (52.4%), social media (35.9%), and internet searches (29.1%).
Australian rheumatology association SARS-COV-2 patient information sheet (PIS)
The response rate for the post-PIS survey was 52.1% (n = 360/691). Of the respondents, 78.6% agreed/strongly agreed that the PIS was helpful, and 66.4% agreed/strongly agreed that the PIS addressed all their concerns. In addition, 53.6% reported they were more likely to get the vaccine as a result of receiving the PIS.
Patient concerns that were not adequately addressed by the PIS were summarized into themes and included: further information on safety (n = 67), interactions with medications (n = 14), further information on efficacy (n = 12), the logistics of the vaccine rollout (n = 11), information regarding vaccine choice (n = 10), information regarding RMD flare (n = 8) and safety in pregnancy (n = 3).
Discussion
The key findings of this study of vaccine acceptance in RMD patients, undertaken in a region with low community transmission, are a high prevalence of SARS-COV-2 vaccine hesitancy. Increased SARS-COV-2 vaccine acceptance in patients who received the influenza or pneumococcal vaccine in the preceding 12 months, practiced other COVID-Safe practices, and believed that the SARS-COV-2 vaccine is safe and effective, provides pointers to addressable factors through which to increase vaccine acceptance. Importantly, advice from clinicians and being offered vaccine choice also have the potential to increase vaccine acceptance. While it is not yet clear that such advice will be necessary, a majority of RMD patients were willing to withhold their immunomodulatory therapy in order to enhance vaccine response, particularly if advised by their treating rheumatologist.
The uptake of vaccinations in RMD cohorts has historically been suboptimal.Citation15,Citation18–20 There is data to suggest that acceptance of the influenza vaccine may have increased during the COVID-19 pandemic.Citation16 In this study, we found that two-thirds of RMD patients were willing to have the SARS-COV-2 vaccine while one third were hesitant. These findings are in stark contrast to the 85–90% acceptance reported in surveys of the general Australian population in 2020, a finding which may reflect specific concerns of RMD patients and the impact of evolving perceptions regarding vaccine safety.Citation12
There has been some variability in reports of vaccine acceptance in other RMD cohorts. The VAXICOV survey of 1,266 RMD patients found that only 54.2% of patients were willing to have SARS-COV-2 vaccination, while 32.2% were uncertain, and 13.6% were unwilling.Citation13 Similar to the VAXICOV study, we did not find a clear association between the presence of co-morbidities associated with a poor prognostic outcome with SARS-COV-2 and vaccine acceptance. There were however novel associations, with smokers and patients with a history of malignancy being more than twice as likely to be willing to have the SARS-COV-2 vaccine. In a smaller Italian survey, 44% of RMD patients were willing, 37% willing if advised, and 17% were unwilling to have the SARS-COV-2 vaccine.Citation14 The Italian cohort had an unusually low proportion of female respondents for an RMD cohort (39%); we did observe a trend toward increased vaccine hesitancy amongst women in our study. An important factor to consider in evaluating these data is the difference in SARS-COV-2 impact between countries, with Italy reporting a 45-fold cumulative risk of death per capita relative to Australia.Citation21
The prevalence of influenza and pneumococcal vaccination in the preceding 12 months was higher than SARS-COV-2 vaccination acceptance. We found that the likelihood of SARS-COV-2 vaccine acceptance was nearly three-fold higher in this sub-group, similar to findings in VAXICOV. The vast majority of our cohort (98.7%) reported adherence to COVID-Safe practices such as hand hygiene, mask wearing and social distancing, and there was a significant association between vaccine acceptance and the number of COVID-Safe measures practiced.
We also found that SARS-COV-2 vaccine acceptance increased with increasing age and in respondents who identified as Asian, however these findings were attenuated on multivariate analysis. The association with age has been reported in larger cohorts of the general population, while a higher vaccine acceptance has been seen in Asian countries.Citation10 We did not observe a significant impact of education on vaccine acceptance.
There were moderate correlations between the likelihood of vaccine acceptance and patient perceptions regarding vaccine efficacy and safety. These findings are consistent with previous literature regarding vaccine hesitancy, and are unsurprising given the accelerated timeline of SARS-COV-2 vaccine development and media reporting of safety and efficacy data.Citation11,Citation13,Citation22,Citation23 There was a weaker but significant correlation with concerns regarding a vaccine-associated flare of RMD and vaccine side effects. The lack of correlation with patient perception of immunosuppression and concerns regarding the risk of acquiring SARS-COV-2 may well relate to the relatively successful suppression of SARS-COV-2 community transmission in Australia.
Among vaccine-hesitant patients, who comprised a third of our cohort, we observed a substantial potential impact on acceptance of physicians recommending vaccination, similar to previous reports.Citation13 This highlights the key role of the RMD patient’s physician in supporting SARS-COV-2 vaccination. Further, we report a novel finding of the impact of vaccine choice in RMD patients, with 39.2% of vaccine-hesitant patients being more likely to have a SARS-COV-2 vaccine if they were given a choice. This survey took place during a period in which mainstream media discussion of safety concerns regarding some SARS-COV-2 vaccinations was prevalent.
Potential methods through which to communicate information intended to increase vaccine acceptance are many. The mass dissemination of a PIS is more feasible than an individual discussion with all patients, and nearly 4/5 patients found the PIS used here to be helpful. However, a number of respondents felt that their concerns had not been adequately addressed by the PIS and were no more likely to have the vaccine as a result. This in part reflects the complexity of the information that needs to be communicated and lingering patient concerns regarding vaccine safety, and highlights the role of individualized advice.
There is overwhelming medical consensus that RMD patients should receive SARS-COV-2 vaccination. There is, however, variation in guidance regarding the timing of vaccinations relative to the use of immunomodulatory therapy, in the absence of robust data.Citation7–9,Citation24–27 We found that 27.6% of respondents were willing to withhold their immunomodulatory therapy and potentially risk a flare of their underlying RMD in order to boost their vaccine response. A further 42% of patients were willing to do so if advised by their rheumatologist, highlighting again the impact that the treating physician could have on facilitating this intervention. The predictors of willingness to withhold immunomodulatory therapy to augment vaccine response on multivariate analysis were b/tsDMARD use and SARS-COV-2 vaccine acceptance. There was a trend toward unemployed and retired respondents being more likely to be willing to withhold their immunomodulatory therapy, potentially reflecting the increased impact that a flare may have on the ability to attend work or school. A third of patients were unwilling to withhold their immunomodulatory therapy.
Rates of COVID-19 infection have differed across the world, potentially contributing to a variation in risk perception and vaccine hesitancy. An international study conducted by Lazarus et al. looked at vaccine acceptance across 19 different countries.Citation28 In China, respondents <50 years old were more likely to accept vaccination than those >50 years old, in contrast to trends in Europe. Higher education increased acceptance of vaccination in India, Ecuador, France, Germany and US, but not in Canada, Spain, and the UK. In India where there have been high rates of infection, a vaccination survey conducted in February 2021 amongst medical students showed 10.6% vaccine hesitancy, which was linked to concerns regarding vaccine safety and efficacy, and lack of trust in government agencies.Citation29 A social media analytics study conducted in September to December of 2020 looked at COVID-19 vaccine sentiments in Indian citizens. They found only 35% positive sentiment during this time, with a correlation between an increase in positive sentiments and an increase in number of COVID-19 cases throughout the months.Citation30 Taiwan has one of the lowest rates of COVID-19 infection, reflected in 63.5% of people perceiving COVID-19 as “not serious,” and only 52.7% of people willing to receive the vaccine.Citation31 Australia’s similarly low rates of COVID-19 infection is a probable contributor to the complacency in the uptake of vaccines in the first half of 2021.
This the first large study of vaccine acceptance in RMD patients in a location with low community transmission of SARS-COV-2, and the findings are particularly relevant for other countries in which low community transmission has been achieved. A key strength of this study is the standardization of recruitment, with all patients seen at our center invited to participate. While we did not impute missing data, the prevalence of missing data was low for most variables. Limitations of the study design and the resulting potential for response bias should be recognized in interpreting the findings. Although our survey was reviewed by specialist clinicians and patient research partners, no formal validation of measurement properties was conducted when creating the survey. Responses may have been biased toward English-speaking patients, or those with higher medical or technological literacy. Survey responses regarding the impact of a PIS are subject to additional response bias. We also relied on self-reported data for all exposures and outcomes, which may have resulted in misclassification bias. Finally, the non-significance of some associations should be interpreted in the context of a lack of sample size calculation. Larger studies would be important to confirm the significance of our findings and our findings may not be relevant to countries with a high incidence of SARS-COV-2 community transmission.
Conclusion
SARS-COV-2 vaccine hesitancy is prevalent amongst RMD patients in a community with low rates of SARS-COV-2 transmission. There is a significant need for increasing levels of education regarding vaccine safety, vaccine efficacy and the risk of RMD flare. Clinician recommendation and vaccine choice have the potential to increase vaccination uptake in hesitant patients.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Key indexing terms
Coronavirus disease 2019 (COVID-19), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), vaccination, rheumatic disease (RMD), vaccine acceptance, vaccine hesitancy
Supplemental Material
Download MS Word (32.3 KB)Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1958611.
Additional information
Funding
References
- Dejaco C, Alunno A, Bijlsma JW, Boonen A, Combe B, Finckh A, Machado PM, Padjen I, Sivera F, Stamm TA, et al. Influence of COVID-19 pandemic on decisions for the management of people with inflammatory rheumatic and musculoskeletal diseases: a survey among EULAR countries. Ann Rheum Dis. 2020:1–9.
- Antony A, Connelly K, De Silva T, Eades L, Tillett W, Ayoub S, Morand E. Perspectives of patients with rheumatic diseases in the early phase of COVID-19. Arthritis Care Res (Hoboken). 2020;72(9):1189–95. doi:10.1002/acr.24347.
- Romao VC, Cordeiro I, Macieira C, Oliveira-Ramos F, Romeu JC, Rosa CM, Saavedra MJ, Saraiva F, Vieira-Sousa E, Fonseca JE, et al. Rheumatology practice amidst the COVID-19 pandemic: a pragmatic view. RMD Open. 2020;6(2):e001314. doi:10.1136/rmdopen-2020-001314.
- Benucci M, Damiani A, Giannasi G, Li Gobbi F, Quartuccio L, Grossi V, Infantino M, Manfredi M. Serological tests confirm the low incidence of COVID-19 in chronic rheumatic inflammatory diseases treated with biological DMARD. Ann Rheum Dis. 2020. doi:10.1136/annrheumdis-2020-218214.
- Francesconi P, Cantini F, Profili F, Mannoni A, Bellini B, Benucci M. COVID-19 epidemiology in rheumatic diseases in Tuscany: a case-control study. Joint Bone Spine. 2021;88(3):105131. doi:10.1016/j.jbspin.2021.105131.
- Youssef J, Novosad SA, Winthrop KL. Infection risk and safety of corticosteroid use. Rheum Dis Clin North Am. 2016;42(1):157–76, ix–x. doi:10.1016/j.rdc.2015.08.004.
- Arnold J, Winthrop K, Emery P. COVID-19 vaccination and antirheumatic therapy. Rheumatology (Oxford). 2021. doi:10.1093/rheumatology/keab223.
- Force, A.C.-V.C.G.T. COVID-19 vaccine clinical guidance summary for patients with rheumatic and musculoskeletal diseases. A.C.o. Rheumatology, Editor; 2021.
- Landewe RB, Machado PM, Kroon F, Bijlsma HW, Burmester GR, Carmona L, Combe B, Galli M, Gossec L, Iagnocco A, et al. EULAR provisional recommendations for the management of rheumatic and musculoskeletal diseases in the context of SARS-CoV-2. Ann Rheum Dis. 2020;79(7):851–58. doi:10.1136/annrheumdis-2020-217877.
- Lazarus JV, Ratzan SC, Palayew A, Gostin LO, Larson HJ, Rabin K, Kimball S, El-Mohandes A. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. 2020;27:225–228.
- Dodd RH, Pickles K, Nickel B, Cvejic E, Ayre J, Batcup C, Bonner C, Copp T, Cornell S, Dakin T, et al. Concerns and motivations about COVID-19 vaccination. Lancet Infect Dis. 2021;21(2):161–63. doi:10.1016/S1473-3099(20)30926-9.
- Dodd RH, Cvejic E, Bonner C, Pickles K, McCaffery KJ, Ayre J, Batcup C, Copp T, Cornell S, Dakin T, et al. Willingness to vaccinate against COVID-19 in Australia. Lancet Infect Dis. 2021;21(3):318–19. doi:10.1016/S1473-3099(20)30559-4.
- Felten R, Dubois M, Ugarte-Gil MF, Chaudier A, Kawka L, Bergier H, Costecalde C, Pijnenburg L, Fort J, Chatelus E, et al. Vaccination against COVID-19: expectations and concerns of patients with autoimmune and rheumatic diseases. Lancet Rheumatol. 2021;3(4):e243–e245. doi:10.1016/S2665-9913(21)00039-4.
- Campochiaro C, Trignani G, Tomelleri A, Cascinu S, Dagna L. Potential acceptance of COVID-19 vaccine in rheumatological patients: a monocentric comparative survey. Ann Rheum Dis. 2021;80(6):816–17. doi:10.1136/annrheumdis-2020-219811.
- Michel M, Vincent FB, Rio S, Leon N, Marcelli C. Influenza vaccination status in rheumatoid arthritis and spondyloarthritis patients receiving biologic DMARDs. Joint Bone Spine. 2016;83(2):237–38. doi:10.1016/j.jbspin.2015.02.016.
- Al Nokhatha S, MacEoin N, Conway R. Correspondence on ‘Influence of COVID-19 pandemic on decisions for the management of people with inflammatory rheumatic and musculoskeletal diseases: a survey among EULAR countries.’ Ann Rheum Dis. 2021. doi:10.1136/annrheumdis-2021-219847.
- Byravan S, Fardanesh A, Tahir H, Moorthy A. Emerging COVID-19 vaccines: a rheumatology perspective. Int J Rheum Dis. 2021;24(2):144–46. doi:10.1111/1756-185X.14048.
- Loubet P, Kernéis S, Groh M, Loulergue P, Blanche P, Verger P, Launay O. Attitude, knowledge and factors associated with influenza and pneumococcal vaccine uptake in a large cohort of patients with secondary immune deficiency. Vaccine. 2015;33(31):3703–08. doi:10.1016/j.vaccine.2015.06.012.
- Hmamouchi I, Winthrop K, Launay O, Dougados M. Low rate of influenza and pneumococcal vaccine coverage in rheumatoid arthritis: data from the international COMORA cohort. Vaccine. 2015;33(12):1446–52. doi:10.1016/j.vaccine.2015.01.065.
- Figueroa-Parra G, Esquivel-Valerio JA, Santoyo-Fexas L, Moreno-Salinas A, Gamboa-Alonso CM, De Leon-Ibarra AL, Galarza-Delgado DA. Knowledge and attitudes about influenza vaccination in rheumatic diseases patients. Hum Vaccines Immunother. 2020;17:1–6.
- Organization, W.H. COVID-19 weekly epidemiological update. 2021 [accessed 2021 Mar 03]. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19—23-march-2021 .
- Lurie N, Saville M, Hatchett R, Halton J. Developing Covid-19 vaccines at pandemic speed. N Engl J Med. 2020;382(21):1969–73. doi:10.1056/NEJMp2005630.
- Borriello A, Master D, Pellegrini A, Rose JM. Preferences for a COVID-19 vaccine in Australia. Vaccine. 2021;39(3):473–79. doi:10.1016/j.vaccine.2020.12.032.
- Park JK, Lee YJ, Shin K, Ha YJ, Lee EY, Song YW, Choi Y, Winthrop KL, Lee EB. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2018;77(6):898–904.
- Association, A.R. COVID-19 vaccination for rheumatology patients. A.R. Association, Editor; 2021.
- Connolly CM, Ruddy JA, Boyarsky BJ, Avery RK, Werbel WA, Segev DL, Garonzik-Wang J, Paik JJ. Safety ofthefirstdose ofmRNA SARS-CoV-2vaccines inpatients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021;80:1100–01. doi:10.1136/annrheumdis-2021-220231.
- Geisen UM, Berner DK, Tran F, Sümbül M, Vullriede L, Ciripoi M, Reid HM, Schaffarzyk A, Longardt AC, Franzenburg J, et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis. 2021. doi:10.1136/annrheumdis-2021-220272.
- Lazarus JV, Wyka K, Rauh L, Rabin K, Ratzan S, Gostin LO, Larson HJ, El-Mohandes A. Hesitant or Not? The association of age, gender, and education with potential acceptance of a COVID-19 vaccine: a country-level analysis. J Health Commun. 2020;25(10):799–807. doi:10.1080/10810730.2020.1868630.
- Jain J, Saurabh S, Kumar P, Verma MK, Goel AD, Gupta MK, Bhardwaj P, Raghav PR. COVID-19 vaccine hesitancy among medical students in India. Epidemiol Infect. 2021;149:e132. doi:10.1017/S0950268821001205.
- Praveen SV, Ittamalla R, Deepak G. Analyzing the attitude of Indian citizens towards COVID-19 vaccine - A text analytics study. Diabetes Metab Syndr. 2021;15(2):595–99. doi:10.1016/j.dsx.2021.02.031.
- Tsai FJ, Yang H-W, Lin C-P, Liu JZ. Acceptability of COVID-19 vaccines and protective behavior among adults in Taiwan: associations between risk perception and willingness to vaccinate against COVID-19. Int J Environ Res Public Health. 2021;18(11):5579. doi:10.3390/ijerph18115579.