?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Health-care professionals in Ghana were among the prioritized high-risk groups to be administered with the initial supply of Oxford/AstraZeneca vaccine procured by the Government of Ghana. This study sought to assess and identify the determinants of COVID-19 vaccine short-term side effects among health-care workers. A cross-sectional study was conducted on 654 Ghanaian healthcare workers between 16th March and 5th May 2021. The study included health-care workers in registered health settings, who had been vaccinated against COVID-19 and consented to participate in the survey. Descriptive statistics, binary and multivariable logistic regression analyses were executed using SPSS version 22 at p < .05. The findings revealed that, 528 (80.7%) of the participants experienced adverse reactions, which lasted between 0 and 2 days among, 347 (53.1%) of the study participants. The most reported adverse reactions were general body weakness, 434 (32.0%), headache 371 (27.3%), and fever, 257 (19.1%). Health workers aged 35–39 and 40–44 years had lower odds of adverse reactions compared with those aged 25–29 years (aOR: 0.34, 95% C.I. 0.186,0.621, p < .001) and (aOR: 0.42, 95% C.I. 0.201,0.890, p = .023). Taking analgesics before vaccination decreased the likelihood of adverse reactions (aOR: 0.28, 95% C.I. 0.185,0.427, p < .001). High prevalence of adverse reactions was found among the healthcare workers, however short-lived. The most reported systemic adverse reactions were general body weakness, headache, and fever. We recommend intensification of campaigns on COVID-19 vaccines and their associated adverse effects to avoid the negative implication on uptake among the healthcare workers and the general population.
Introduction
The novel Corona Virus disease (COVID-19), an infectious respiratory virus, was discovered in Wuhan, China, claiming several millions of live globally. According to the available situational reports, Africa recorded over 2 million COVID-19 cases and over 49,000 deaths.Citation1 COVID-19 overwhelmed the health-care systems worldwide and in the case of Africa, resource constraints led to comparatively lower access to COVID-19 diagnostic testing, resulting in underdiagnoses, especially in asymptomatic or pauci-symptomatic individuals, and restricted basic and advanced treatment to prevent morbidity and mortality.Citation2–4
Governments worldwide have imposed several measures and protocols such as “travel bans, the mandatory wearing of nose masks, lockdowns, social distance, and frequent washing of hands with soap and under running water” to help halt the spread of COVID-19.Citation5 Researchers and scientists from China, the United Kingdom, the United States and other developed countries also embarked on the quest to produce several vaccines to curb the further spread, deaths and the devastating socioeconomic impact of COVID-19. Over 200 vaccine candidates are pursued globally; however, there is still uncertainty about the production of a stable and highly immunogenic COVID-19 vaccine.Citation6 The Food and Drug Administration (FDA) of the United States, Canada, and the United Kingdom granted authorization for Pfizer/BioNTech, Moderna, and AstraZenecca administration following successful clinical trials.Citation7–9
On 20th December, 2020, the Government of Ghana (GoG) had publicly stated its intention to commence procurement of the Oxford/AstraZeneca and Sputnik V COVID-19 vaccines for use in the region. AstraZenecca is shown to have 94.1% efficacy in prevention of COVID‐19 illness, including severe disease in persons aged 18 years or older.Citation10 On February 24, 2021, Ghana received 600,000 doses of AstraZenecca and due to a global limited supply of COVID-19 vaccines, the government gave priority to high-risk groups for the initial supply of vaccines.Citation5 Healthcare worker (HCWs), the elderly, especially those with chronic co-morbid disorders, and those in critical services are among the high-risk categories. HCWs are at a heightened risk of contracting COVID-19 due to direct or indirect contact with bodily secretions of COVID-19 patients/clients and visitors.Citation11,Citation12 In Ghana, as of March 12, 2020, nearly 1,629 nurses and midwives, had been infected with the virus, with 4 deaths and more than 450 doctors and dentists had been contaminated with COVID-19, with 7 deaths.Citation5
Available data from COVID-19 vaccines clinical trials revealed that side effects such as redness, fever, nausea, vomiting, dizziness, general body weakness, headache, anaphylaxis, and swelling at the site of vaccination injection were reported among some of the participants.Citation13 A recent research on self-reported adverse effects with the mRNA-1273 vaccine among HCWs revealed a wide range of symptomatology, with the majority of the symptoms being non-life threatening.Citation14 In some instances, side effects were significantly more common in women than in men, in individuals aged 55 years or younger than in those older than 55 years.Citation15
It is important to identify and track vaccine adverse effects – to increase vaccine safety and uptake. Since there are few studies on the experience and adverse effects/reactions of COVID-19 vaccines in Ghana and even globally, this study sought to ascertain the incidence of short-term adverse reactions from COVID-19 vaccination among health care professionals who had already taken the first dose of the vaccine in Ghana.
Methods
Study settings
Ghana, a west African country in Sub-Saharan Africa, is the only English-speaking country bordered by three French-speaking nations, Burkina Faso on the north, Togo on the east, Cote d’Ivoire on the west and the Gulf of Guinea by the south. Ghana is categorized as a lower-middle-income country, with a population of about 30.9 million people and 16 administrative regions. A total of 357 hospitals, 1004 health centers, 140 district hospitals, 998 clinics, and 5,421 Community-based Health Planning and Services (CHPS) were established in Ghana as of 2017. Similar to most African countries, Ghana health care systems, infrastructures and human resources are not equitably distributed as most of the health facilities and health workers are overwhelmingly concentrated in the Ashanti and Greater Accra regions, especially in urban areas. For example, of Ghana’s 357 hospitals, 128 (35.9%) are located in the Ashanti region, 99 (27.7%) in the Greater Accra region and 9 (2.5%) are located in the Western Region. In addition, Ghana has 4,016 doctors as of 2017, with almost 39.4% located in the Greater Accra region and 20.5% located in the Ashanti region.Citation5,Citation16
Study design
A cross-sectional study was conducted among Ghanaian healthcare workers between 16th March and 5th May 2021. This survey adopted both convenient and snowballing sampling techniques due to the existing nature of the pandemic. This method was chosen because it provides sufficient social distancing, limit physical contact with respondents and eliminates movements of researchers or volunteers and get responses quickly as far as possible amidst the outbreak. A simple close and open-ended questionnaire tool was developed on google form and shared via WhatsApp platforms of Healthcare workers and to reach out to more health professionals, a snowball approach was used, in which respondents were encouraged to forward or share the online survey contact with other health workers.
Eligibility criteria
The study included clinicians, nurses, midwives, pharmacists, biomedical scientists, community health works, and other healthcare providers practising in both registered private and public health settings in Ghana, who have vaccinated against COVID-19 and consented to participate in the survey. Healthcare workers who had not taken at least one COVID-19 vaccine shot, who refused to consent and those with incomplete data were excluded. Participants aged below 18 years were also excluded because of their vulnerability as minors.
Sample size estimation
The minimum sample size was estimated using the Cochrane formula ; given that Z (at 95% confidence interval) = 1.96, the prevalence was set at 50% (to assume an equal distribution or variability), hence p = .50, q(1-p) = 0.50, and e (margin of error) = 0.05, 380 participants were required. Factoring in the 10% non-response rate during the study, at least 418 participants were needed for the analysis. However, this study recruited 654 healthcare professionals.
Data collection
The developed questionnaire covered two sections, sociodemographic characteristics of the study participants and COVID-19 related adverse reaction questions. The demographic information such as age, gender, marital status, education, and profession were solicited from the participants. In addition, information on preexisting medical conditions and whether or not participants took pain medications before the administration of the COVID-19 vaccine were collected. Participants were also asked questions on the experience of adverse reaction(s) from the vaccine, type of adverse reaction, and duration of reaction (s).
Measurement and variables
Adverse side effects in this study were defined as non-life threatening symptoms within 0–8 days after vaccination, this was the dependent variable and it was assessed using the question “experienced an adverse reaction/effects after receiving the first shot of vaccine” and the responses were “Yes” and “No.” The independent variables were gender, preexisting medical condition, types of adverse reaction and duration of reaction in days.
Ethical consideration
According to the GHS-ERC guidelines/standard operating procedures; research studies that are considered for exemption are minimal risk research studies that conform to one or more of the following categories of research. Research involving the use of educational tests, survey procedures, interview procedures or observation of public behavior EXCEPT if information obtained is recorded in such a manner that human subjects can be identified, directly or through identifiers linked to the subjects.Citation17
Data used was obtained in adherence to the principles of the declaration of Helsinki and local regulatory requirements. Participants aged below 18 years were excluded because of their vulnerability as minors. Prior to the participation, the purpose of study, the confidential and voluntary nature of the survey and the estimated time it will take to complete the questionnaire were explained to potential respondents. Respondents were also informed that by choosing to access the survey link, they are providing their consent to participate.
Data analysis
All statistical analyses were executed using Statistical Package for the Social Science (SPSS; IBM, USA), Version 22. Descriptive statistics were estimated for all variables and presented as percentage (%) or number (n). To identify the factors associated with the short-term adverse reaction, a binary logistic regression analysis was conducted at p < .10. The variables significant at p < .10 were further analyzed using multivariable logistic regression model, adjusted for confounding factors using stepwise method of forward likelihood ratio (Entry p < .05, Removal p > .1). .Statistical significance was set at p < .05.
Results
Sociodemographic characteristics of healthcare workers
presents the sociodemographic characteristics of the study participants. The age ranged from 25 to 44 years, mean age of 32.24 ± 4.30 years and the most aged 30–34 (48.5%). A significant number of the healthcare professionals were males, 335 (51.2%), single, 324 (49.5%), attained degree certificate, 337 (51.5%) and nurses by profession, 269 (41.1%).
Table 1. Sociodemographic characteristics of healthcare workers
Short-term adverse reaction after COVID-19 vaccine administration
The majority of the healthcare workers had no underlying medical condition, 563 (86.1%) and 271 (41.4%) took medication, paracetamol, before the vaccine administration to avoid unwanted adverse reactions. The majority experienced an adverse reaction, 528 (80.7%) which lasted between 0 and 2 days among most of the participants, 347 (53.1%). The most reported short-term adverse reactions were general body weakness, 434 (32.0%), headache 371 (27.3%), and fever, 257 (19.1%) ().
Table 2. Short-term adverse reaction after COVID-19 vaccine administration
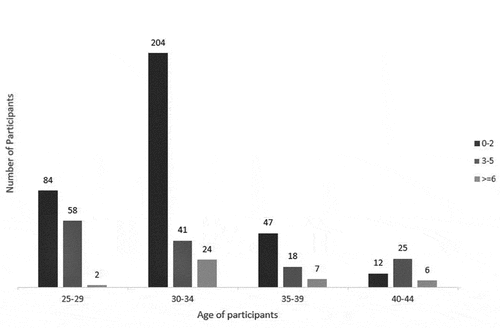
Association between the length of adverse reaction by age of participants
depicts that the ages of participants were significantly associated with the duration of adverse reactions. The most frequent duration of adverse reactions reported among the age groups was 0–2 days, however, the adverse reactions lasted for 3–5 days among the participants aged 40–44 years. No significant association was observed between gender, preexisting medical conditions, pain medication and length of adverse reactions among the healthcare workers.
Factors associated with short-term adverse reaction among the healthcare professionals
The regression depicted age and medication use were significantly associated with the risk of experiencing short-term adverse reaction both in the bivariate and multivariate analyses. Health worker aged 35–39 years had lower odds of adverse reactions from COVID-19 vaccine administration compared with those aged 25–29 years (aOR: 0.34, 95% C.I. 0.186,0.621, p < .001). Also, the likelihood of experiencing adverse reactions was lower in participants aged 40–44 years compared with those aged 25–29 years (aOR: 0.42, 95% C.I. 0.201, 0.890, p = .023). Participants who administered medication, thus paracetamol before COVID-19 vaccine injection had less likelihood of experiencing adverse reactions compared to those who took no pain medication (aOR: 0.28, 95% C.I. 0.185,0.427, p < .001) ().
Table 3. Factors associated with short-term adverse reaction among the healthcare professionals
Discussion
Healthcare professionals in Ghana were among the prioritized high-risk groups to be administered with the initial supply of Oxford/AstraZeneca vaccine procured by the Government of Ghana. There is paucity of data on non-life-threatening side effect of the Oxford/AstraZeneca vaccine within the high-risk group of Ghanaian and healthcare professionals to have received the first shot. This study sought to assess and identify the determinants of COVID-19 vaccine short-term side effects among healthcare workers in Ghana. We found that significant number of the healthcare professionals who had been vaccinated were males. This finding is confirmed by a study conducted on the acceptability of COVID-19 Vaccination among healthcare workers in Ghana prior to the procurement and importation into the country. The study reported that the probability of female health care workers receiving the COVID-19 vaccines if available was less compared with males.Citation5 This result supports other observational findings that show male healthcare workers are more likely than the female to consider COVID-19 vaccines.Citation18,Citation19
This gender disparity in the vaccination may be attributable to the perception that men have higher risk of infection and death from the disease than women. Also, men are naturally bold to receive the vaccine as a sign of the inherent nature of how they were created as courageous beings. We also observed that nurses formed a greater proportion of our study participants compared to the other professionals. However, Agyekum et al.,Citation5 reported a high likelihood of COVID-19 vaccine acceptance in medical doctors compared to nurses and other health workers. According to Ghana Health Service, 2017 facts and figures report, nurses to medical doctors ratio was 68,493: 4,016, thus 17:1,Citation16 this could explain why nurses formed a greater proportion of who had vaccinated.
Few of the healthcare workers had an underlying medical condition and the probable explanation is that healthcare workers are more educated, have gained more knowledge and understand the relevance and practice of a healthy lifestyle on human health and consequently less likely to suffer from chronic illness. Similarly, we observed few of the healthcare workers took pain medications like paracetamol, before the vaccine administration to avoid unwanted adverse reactions. This could be explained by the array of information available to health workers on the avoidance of pain killers before or after COVID-19 vaccination as it may theoretically reduce the immune response to the vaccine.
The majority of our study participants experienced mild adverse reactions from the vaccine, which lasted mostly between 0 and 2 days. Much of this was expected because, since the rolling in of the COVID-19 vaccination, people around the world have reported temporary side effects.Citation7,Citation14 This observation may have a critical implication on the willingness of these individuals to accept the second dose of the vaccine. As a result, it is critical for the government to continue to sensitize the healthcare workers that the protection conferred by the COVID-19 vaccine far outweighs the risk of adverse reactions. This probably will strengthen their zeal to take the second shot. Since healthcare workers are a credible source of knowledge on vaccination to patients, their negative response to the COVID-19 vaccines can affect the COVID-19 vaccine’s adoption in the general population.
The most reported systemic adverse reactions among our study participants were general body weakness, headache, and fever. Similarly, a report from a clinical trial in the UK found that systemic adverse reactions such as headache and fatigue, injection-site pain were frequently reported after the first dose.Citation7 Also, Kadali et al.,Citation14 found that their health workers reported symptoms such as regional pain, fatigue, headache among others during the initial stages of the post vaccination period. These findings were much anticipated since data from clinical trials on the vaccines authorized so far suggested the plausible reactions the general populace must expect.
A significant association was found between age and the duration of reported adverse reactions. Older individuals are more susceptible to pain and poorly managed compared to younger persons as reported by Schofield, Pat,Citation20 and this could explain why pains in the older healthcare workers lasted longer than that of the younger workers.
The regression depicted age and medication use were significantly linked to the risk of experiencing short-term adverse reaction(s) both in the bivariate and multivariate analyses. Health workers aged 35–39 years and 25–29 years had lower odds of adverse reaction from COVID-19 vaccine administration compared with those aged 25–29 years. Data from other community-based surveys provide evidence to support our reports of higher frequency of side-effects in younger than in older individuals.Citation10,Citation21 The possible explanation could be the increase in titer of post vaccine expression of counterbalance antibodies in younger people,Citation22 confirming the association between age and symptoms of adverse conditions in this study. Furthermore, it suggests that, immune- mediated mechanisms resulting in increased reactogenicity responsible of symptoms expressions.
According to AstraZeneca Company, prophylactic use of paracetamol can reduce some symptoms. In this study, HCWs who took medication, i.e., paracetamol before the COVID-19 vaccine injection had less likelihood of suffering from adverse reaction compared to those who took no pain medication. This finding was much expected because the analgesic blocks the pain receptors and numbness thereby experiencing minimal or no unpleasant side reactions compared with their counterparts.Citation20 However, it must be noted that the immune response in the healthcare workers who took the pain medications might be lesser than the other group as aforementioned. Overall, this recent study fills the knowledge gap of non-existing data on self-reported non-life threatening adverse effect of the COVID-19 vaccine from high-risk healthcare personnel in Ghana. It also delivers supplementary evidence to the general populace about the transient adverse effects of the vaccines to eschew negative publicity, myth and misinformation to increase population vaccine willingness.
Limitations of the study
This study was not without limitations, the findings from this study are self-reported and not verified or confirmed by the investigators. This study did not take into account the implication of the reported side-effects on the willingness to uptake the second dose. There is possibility of a recall bias, as those filling the questionnaire might be those experiencing symptoms more frequently. Also, because of the cross-sectional nature of the study, the investigators could not ascertain the cause and effect relationship between COVID-19 vaccination and post-vaccination adverse reaction.
Conclusion
A high prevalence of non-life threatening adverse reactions was found among the Ghanaian healthcare professionals, however short-lived. The most reported systemic adverse reactions among our study participants were general body weakness, headache, and fever and often lasted 0–2 days. Younger participants and individuals who refused administration of pain medication prior to the vaccination reported adverse reactions more frequently compared to their counterparts. This finding could inform stakeholders of the gender and pain medication variation in the development of the post-vaccination adverse reactions. We recommend intensification of campaigns on COVID-19 vaccines and their associated adverse effects. An educational strategy of this kind should include factual information, discuss the various vaccination issues, and answer questions regarding the COVID-19 vaccines misconception.
Authors’ contributions
Authors SD, FOB and CN conceived. Authors SD, SA, FOB and CN designed and participated in the data collection. Authors SD, RA, MHA EYW and EO analysed and interpreted the data. Authors FOB, CN, EL, MHA, TAB and MD conducted the literature search and wrote the first draft of the manuscript. All authors critically reviewed, revised and approved the final manuscript.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful for the immense contributions healthcare workers in Ghana for participating in this study.
References
- Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–34. doi:10.1016/S1473-3099(20)30120-1.
- El-Sadr WM, Justman J. Africa in the path of Covid-19. N Engl J Med. 2020;383(3):e11. doi:10.1056/NEJMp2008193.
- Gilbert M, Pullano G, Pinotti F, Valdano E, Poletto C, Boëlle PY, D’Ortenzio E, Yazdanpanah Y, Eholie SP, Altmann M, et al. Preparedness and vulnerability of African countries against importations of COVID-19: a modelling study. Lancet. 2020;395(10227):871–77. doi:10.1016/S0140-6736(20)30411-6.
- Paintsil E. COVID-19 threatens health systems in sub-Saharan Africa: the eye of the crocodile. J Clin Invest. 2020;130(6):2741–44. doi:10.1172/JCI138493.
- Agyekum MW, Frempong Afrifa-Anane G, Kyei-Arthur F, Addo B, Author C. Acceptability of COVID-19 vaccination among health care workers in Ghana. medRxiv [Internet]. 2021;2021. doi:10.1101/2021.03.11.21253374.
- Chung JY, Thone MN, Kwon YJ. COVID-19 vaccines: the status and perspectives in delivery points of view. Adv Drug Deliv Rev. 2020;170:1–25.
- Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P, Sudre CH, Nguyen LH, Drew DA, Merino J, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis [Internet]. 2021;3099(21):1–11. http://www.ncbi.nlm.nih.gov/pubmed/33930320.
- National Geographics.com N co. FDA grants emergency authorization of Moderna’s COVID-19 vaccine [Internet]; 2020. [accessed 2021 July 20]. https://www.nationalgeographic.com/science/health-and-human-body/human-diseases/coronavirus-vaccine-tracker-how-they-work-latest-developments-cvd/.
- Vasileiou E, Simpson CR, Shi T, Kerr S, Agrawal U, Akbari A, Bedston S, Beggs J, Bradley D, Chuter A, et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet (London, England). Available from 2021;397(10285):1646–57. [Internet] http://www.ncbi.nlm.nih.gov/pubmed/33901420%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC8064669.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–15. doi:10.1056/NEJMoa2034577.
- Chou R, Dana T, Buckley DI, Selph S, Fu R, Totten AM. Epidemiology of and risk factors for coronavirus infection in health care workers: a living rapid review. Ann Intern Med. 2020;173(2):120–36. doi:10.7326/M20-1632.
- Mahajan NN, Mathe A, Patokar GA, Bahirat S, Lokhande PD, Rakh V, Gajbhiye R, Rathi S, Tilve A, Mahajan K, et al. Prevalence, clinical presentations and treatment outcomes of COVID-19 among healthcare workers at a dedicated hospital in India. J Assoc Physicians India. 2020;68(12):16–21.
- Remmel A. COVID vaccines and safety: what the research says. Nature. 2021;590(7847):538–40. doi:10.1038/d41586-021-00290-x.
- Kadali RAK, Janagama R, Peruru S, Gajula V, Madathala RR, Chennaiahgari N, Malayala SV. Adverse effects of COVID‐19 mRNA‐1273 vaccine: a randomized, cross‐sectional study on healthcare workers with detailed self‐reported symptoms. J Med Virol. 2021;93(7):4420–29. doi:10.1002/jmv.26996.
- Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P, Sudre CH, Nguyen LH, Drew DA, Merino J, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21(7):939–49. doi:10.1016/S1473-3099(21)00224-3.
- Ghana Health Service. The health sector in Ghana: facts and Figures 2018. Minist Heal Ghana [Internet]. 2018;1–50. [accessed 2021 July 20]. https://ghanahealthservice.org/downloads/Facts+Figures_2018.pdf.
- Ghana Health Service Ethics review committee. Ghana Health Service Ethics review committee(GHS-ERC) guidelines/standard operating procedures. 2015.
- Shekhar R, Sheikh AB, Upadhyay S, Singh M, Kottewar S, Mir H, Barrett E, Pal S. COVID-19 vaccine acceptance among health care workers in the United States. Vaccines. 2021;9(2):119. doi:10.3390/vaccines9020119.
- Shaw J, Stewart T, Anderson KB, Hanley S, Thomas SJ, Salmon DA, Morley C. Assessment of US health care personnel (HCP) attitudes towards COVID-19 vaccination in a large university health care system. Clin Infect Dis An Off Publ Infect Dis Soc Am. 2021. doi:10.1093/cid/ciab054.
- Schofield P. Pain in older adults: epidemiology, impact and barriers to management. Rev Pain. 2007;1(1):12–14. doi:10.1177/204946370700100104.
- Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, Voysey M, Aley PK, Angus B, Babbage G, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396(10267):1979–93. doi:10.1016/S0140-6736(20)32466-1.
- Jabal KA, Ben-Amram H, Beiruti K, Batheesh Y, Sussan C, Zarka S, Edelstein M. Impact of age, gender, ethnicity and prior disease status on immunogenicity following administration of a single dose of the BNT162b2 mRNA Covid-19 Vaccine: real-world evidence from Israeli healthcare workers, December-January 2020. medRxiv. 2021. doi:10.1101/2021.01.27.21250567.