ABSTRACT
Chile has a passive surveillance system of adverse events following immunization (AEFI) that allows monitoring and evaluating the safety profile of the vaccines administered. Between 2018 and 2019, the National Immunization Program (NIP) changed from a pentavalent whole-cell pertussis vaccine (wP) to a hexavalent (DTaP-IPV-HepB-Hib) acellular pertussis vaccine (aP) for children <2 years.
Objectives
To describe the trend in the frequency of adverse events (AE) records associated to pertussis component vaccines between January 1st, 2015 and June 30th, 2020 in infants younger than 2-years-old in Chile, by reviewing the records submitted to the AEFI NIP, stratified by DTP-vaccine type, wP or aP.
Materials and methods
This was a retrospective observational study including all AEFI records of DTP (either aP or wP)-containing vaccines in the described sample. A descriptive analysis was performed according to vaccine type and AEFI, using MedDRA terminology.
Results
The total number of AEFI reports was 1,697: 815 corresponding to wP vaccines, 417 to aP vaccines, and 465 with unknown type. The reporting rates for the years 2015 to 2020 were 40.1, 56.2, 37.1, 24.7, 19.1, and 12.2 per 100,000 doses administered, respectively. The most reported AEFI were injection site erythema (42.9%), pyrexia (35.7%), and pain at the injection site (29.2%). Among all cases, 5.8% were SAEs (n = 98), 5.9% were SAEs for wP vaccines (n = 48) and 5.3% were for aP vaccines (n = 22).
Discussion
A significant decrease in AEFI reports was observed as of 2018, the year that the DTaP-IPV-HepB-Hib was introduced in the NIP.
Introduction
Vaccination is one of the most effective strategies to prevent diseases caused by pathogens such as viruses or bacteria. However, adverse events (AE) may follow the use of any vaccine. They are usually mild and self-resolving in nature, and while the most severe are extremely rare, the World Health Organization (WHO) suggests that countries should have in place an effective spontaneous AE reporting program (or passive safety surveillance) in order assess the safety profile and proper administration of vaccines, especially when new vaccines are introduced in any country.Citation1 Vaccines undergo extensive studies in thousands of subjects prior to licensure to ensure the assessment of the safety profile, but it is not until their widespread use that rare or very rare reactions are duly detected.Citation2
A spontaneous AEFI report is “an unsolicited communication by a healthcare professional or consumer to a manufacturer, regulatory authority or other organization that describes one or more adverse drug reactions in a patient who was given one or more medicinal products and that does not derive from a study or any organized data collection scheme.”(Citation3) The minimum data elements required include an identifiable reporter, an identifiable patient, an AE, a suspected product, as well as the possibility to add information whenever necessary.Citation3,Citation4
The reporter’s account must include the signs and symptoms of the AE. The dictionary of AE terms of the Medical Dictionary for Regulatory Activities (MedDRA) has become widespread and allows AE to be grouped together and analyzed to identify new risks related to the vaccine in question by applying statistical methods.Citation5 The reports cannot assess causality, but only association between the vaccine administration and the AE.Citation2 In this sense, it is preferred to present the reporting rate (cases/100,000 administered doses) over incidence in the population, although the results need to be carefully interpreted.Citation3
Chilean national pharmacovigilance system
In 2012, the Immunization Department of the Ministry of Health (MOH) and the Subdepartment of Pharmacovigilance (SDFV) of the Institute of Public Health (ISP) implemented a passive notification system to register, classify, follow, and evaluate post-immunization events. Healthcare professionals from the private or public sectors have the duty (although spontaneous) to report every AE following immunization (AEFI) or any event related to misuse temporarily associated with the vaccine administered, regardless of whether the vaccine is part of the National Immunization Program (NIP) or otherwise.Citation6,Citation7 The information collected through a specific form is entered into a database, where it is validated and coded by ISP SDFV personnel.Citation6,Citation8
Hexavalent vaccine pharmacovigilance
In Chile, before 2018, vaccination against diphtheria, tetanus, pertussis, polio, hepatitis B and H. influenzae type B was done using a pentavalent vaccine (DTwP-HepB-Hib) with a whole-cell pertussis component (wP) plus an oral polio vaccine, administrated at 2, 4, 6 and 18 months of age. Between 2018 and 2019, the vaccination schedule changed in a staggered way to a fully liquid hexavalent vaccine (DTaP-IPV-HepB-Hib; Hexaxim, Sanofi Pasteur) with an acellular pertussis (aP) component.Citation5,Citation9 The change in vaccine was triggered by different factors, including the new strategy for polio eradication: incorporating at least one dose of inactivated polio vaccine (IPV), cessation of production of the wP vaccine and an increased number of AEFI, particularly in preterms, when another wP pentavalent vaccine was used.Citation5 During the study period, pertussis component vaccine was co-administered with pneumococcal vaccine at 2 and 4 months of age, and for preterm infants also at 6 months of age.
As Chile has a vaccination registry, it presented a unique opportunity to assess the post-marketing safety profile of DTP-containing vaccines used in the NIP in children <2 years of age, including preterm infants switching from a wP to an aP vaccine. The objective of this study was to describe the AEFI of DTP-containing vaccines in infants <2 years of age in Chile by reviewing spontaneous reports following immunization submitted to the national AEFI reporting system in Chile, stratified by vaccine type (pentavalent wP and hexavalent aP), and secondarily, the safety profile in preterm infants from 2015 to 2020.
“This research used information obtained from the Institute of Public Health (ISP) of Chile. The authors are grateful to the ISP of Chile for making these data available. All the results of the study are the responsibility of the authors and in no way commit the Institute.”
A table with the abbreviations used can be found in appendix 2.
Materials and methods
This was an observational, retrospective database study in infants <2 years old who received any DTP-containing vaccine between January 2015 and June 2020 in Chile.
Data sources
For this study, the Vaccine Pharmacovigilance Section of the SDFV of the Agencia Nacional de Medicamentos (ANAMED) Department of the ISP of Chile provided a database with all AEFI reports related to vaccinees who received the wP pentavalent or the aP hexavalent vaccines between January 1st 2015 to June 30th 2020 in Chile.
The database includes the vaccinee’s demographic data, information about the vaccine, concurrent medication and data on AEFI. An event is considered serious if the AE results in death, life-threatening situations, hospitalization or prolongation of existing hospitalization, or permanent disability.Citation7 The follow-up and clinical management of case is carried out by the pharmacovigilance team, comprised by professionals of the SDFV and the Immunization Department of the MOH, who run an analysis to determine the probability of an association between the event and the administered vaccine. Causality is evaluated according to the WHO’s causality criteria.Citation10 AEFI are described in the ISP database using the WHO Adverse Reactions Terminology (WHO-ART) dictionary. As MedDRA is currently the most common standard, a “bridge” was used to make the transfer from one terminology to another.
To determine the number of administered doses, data from the National Immunization Registry (known as RNI) was obtained. The number of pentavalent or hexavalent doses administered by region in children between 0 and 23 months and 29 days is available in this registry.Citation9
Statistical analysis
All analyses in this study were descriptive. Categorical variables were described as the total number and relative percentage per category, and continuous variables were described by the number of events, mean, standard deviation, median, Q1, Q3, minimum, and maximum, including missing data. The annual reporting rate of total AEFI records was calculated on the number of doses administered x 100,000 doses of each kind of vaccine. The analyses for all AEFI reports regardless of vaccine type were sub-stratified by gender, age at vaccination, reporter’sprofile, vaccine brand name, seriousness, no. of doses and vaccination year. The most commonly reported AE and Serious AE (SAE) per vaccine type were determined for the overall period, which are presented using the preferred term of the MedDRA classification system. The same analyses were furthermore conducted for preterm children.
The following was done to mitigate the missing data from the reports: i) only the reports that presented all minimum data elements required were included in the analysis; ii) if the vaccine brand or name was not indicated but the lot number was available, the missing information was found using that variable, and iii) all reports without a vaccine name as of 2019 were imputed to the aP hexavalent vaccine (Hexaxim®), because it was the only one that was administered in the public and private markets. Finally, in the AEFI reports submitted to the ISP there is an item in which the reporter describes the AE that contained data that had not been declared for other requested variables, thus helping to obtain lost data. In other cases, when the record presented missing relevant data, the report was omitted.
Ethical considerations
The Scientific Ethical Committee (CEC) of the Universidad de los Andes, Chile, granted authorization to conduct the study on March 24, 2020, under folio CEC202013. At the same time, a Non-Disclosure Agreement was signed for the data provided by the ISP to the Universidad de los Andes in Santiago, Chile.
Results
General results in children under 2 years of age
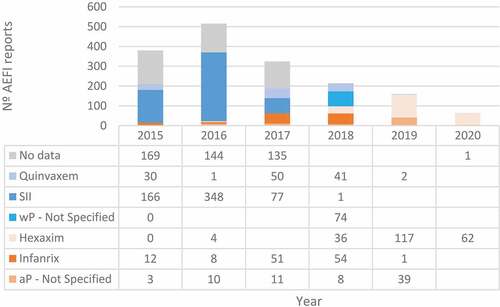
During the study period, several DTP-containing vaccines were associated with AEFI between January 1st, 2015 and June 30th, 2020, as shown in .
Table 1. Vaccine brand of reported AEFI
A total of 1,697 AEFI reports were reported after immunization with DTP-containing vaccines, and 4,951,556 doses of pertussis-containing vaccines were administered in infants <2 years in Chile during the study period, with an overall reporting rate of 34.27 per 100,000 administered doses.
As shown in , the reporting rates varied significantly by year, with the highest reporting rate in 2016 (56.23 per 100,000; n = 515) and the lowest in the first half (1 H) of 2020 (12.23 per 100,000; n = 63). There was a continuous decrease in AEFI reports rates and events starting in 2017 which continued until 1H2020, with a year-to-year percentage change of between −23% and −36%. The lowest rate and number of events in the study period were reported as of 2018, which coincides with the introduction of the acellular hexavalent vaccine to the NIP.
Table 2. Number of AEFI reports by year of immunization in infants < 2 years for pertussis component vaccines, according to Vaccine Type. Chile Jan 2015–June 2020
Most AEFI reports were reported in children under 12 months (68%; n = 1,158) and in males (54.1%; n = 918). AEFI reports were more reported after the first dose (37.3% of total AEFI reports; n = 633) for both wP (39.4%; n = 321) and aP (31.4%; n = 131) vaccines. The median age of children experiencing AEFI was well aligned with the recommended schedule of 2, 4, 6, and 18 months. The most frequent reporter were Nurses (69%), and a large percentage of AEFI reports were from public institutions (80.8%).
AEFI reports by vaccine brand and type are shown in . Between 2015 and 2017, there was a significant number of AEFI reports for which neither data on the vaccine brand nor vaccine type was available, which hindered the analysis. In the study period, SII’s wP pentavalent vaccine was associated with the highest number of reports, particularly between 2015 and 2016. Due to the lack of data on specific doses administered per vaccine brand and the large number of missing data between 2015 and 2017, comparison of rates of AEFI reports between vaccines before 2018 could not be done.
Of the total reports, 5.8% were considered serious AEFI reports (n = 98): for wP it was 5.9% (n = 48), for aP 5.3% (n = 22), and 6% (n = 28) for unspecified DTP-vaccine type . According to WHO seriousness criteria, the main cause of seriousness was requiring patient hospitalization (64%).
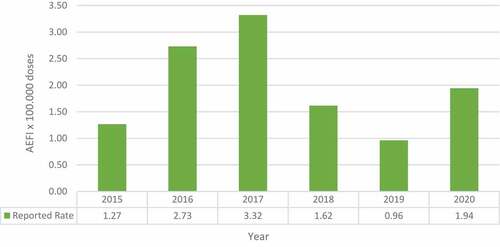
The reporting rate for SAE increased from 2015 to 2017 and then decreased in 2018 and 2019 (). In the first half of 2020, there was an increase in SAE. The SAEs per year from 2015 to 2020 were 12, 25, 29, 14, 8 and 10 respectively.
Figure 2. Reported Rate of Serious AEFI from pertussis component vaccine in infants < 2 years x 100,000 doses administered. Chile, Jan 2015–June 2020.
Following MedDRA classification, the most common AEFI were injection site erythema (42.9%), pyrexia (35.7%), pain at the injection site (29.2%), application site inflammation (19.6%), crying (16.1%), and irritability (10.5%).
Regarding SAEs, the most common ones were pyrexia (n = 36), seizure (n = 31), apnea (n = 16), and pallor (n = 15). Of the AEFI reports considered serious, there was a higher number of AEs per wP vaccine report than in aP reports.; nonetheless, there was a significant number of events for which the vaccine type was unknown. A list with the 10 most reported is shown in and a complete list is provided in Appendix 1. It should be noted that the most frequent concomitant vaccines are the pneumococcal vaccine and the IPV/OPV.
Table 3. Frequency of occurrence of the 10 most reported AEFI and serious AEFI by vaccine type, classified according to the MedDRA Preferred Term (PT). Chile, Jan 2015–June 2020
Results in preterm children
In Chile, between 2015 and 2020, out of 1697 AEFI reports, 41 were recorded for premature children. Sixteen were recorded each for wP and for aP vaccines, and 9 for an unknown vaccine type. There were AEFI in preterms of all gestational ages, although for aP vaccines AEFI reports were in extremely preterm (n = 10; 63%) and very preterm (n = 5; 31%) ().
Table 4. AEFI reports in preterms, sub-classified by gestational age and vaccine type. Chile, Jan 2015–June 2020
The year with the most reports was 2017, with 17 reports, followed by 2018 with 9, and in 2019 and 2020 they dropped to 3 (). Nonetheless, until 2017 information on gestational age was not routinely informed; hence, data on this population is most likely underestimated.
Table 5. Preterm AEFI reports per vaccine type, brand, and year. Chile, Jan 2015–June 2020
AEFI reports in preterm infants were highest after the first dose with 34 reports, then after the second with 5, and after the third only 2. No reports were found for the toddler booster.
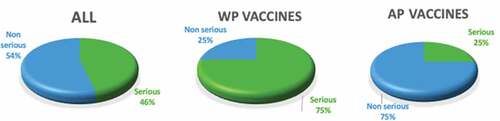
A total of 46.3% of AEFI reports in preterm infants were considered serious. Of the wP vaccine reports, 75% were considered serious, while of the aP vaccine reports, 25% were considered serious ()
Figure 3. Serious and non-serious EAFI reports by vaccine type in preterm infants. Chile, Jan 2015–June 2020.
According to the MedDRA classification, the most common PT in preterms is apnea (n = 28), then followed by pyrexia (n = 8), cyanosis (n = 6), and decreased oxygen saturation and pain at the injection site (n = 6 each) (). Most serious AEFI were related to wP vaccines while most non-serious AEFI were associated with aP vaccines.
Table 6. Most frequent Preferred Terms in AEFI reports by seriousness associated to wP and aP vaccines in preterms, according to the MedDRA. Chile, Jan 2015–June 2020
Discussion
This study presents the AEFI reported after vaccination with DTP-containing vaccines in infants younger than 2 years between 2015 and 2020 in Chile. Since 2017, reported AEFI have been decreasing in Chile, which is related to the change of wP vaccine manufacturer and the subsequent, staggered introduction of aP hexavalent vaccines, reaching the lowest frequency and rate when the aP hexavalent vaccine was used exclusively.
This is the first study of its kind in Latin America and, to the best of our knowledge, in the world, leveraging routinely collected AEFI reports to assess the changes in AEFI rates after the switch from wP pentavalent to aP hexavalent vaccines.
In the literature, we have only found studies examining AE differences between wP and aP vaccines by analyzing data obtained from emergency department admissions, hospitalizations, clinical trials and systematic reviews including clinical trials. We have also found AE analyses of passive surveillance systems but without making the comparison between pertussis vaccines with different components (wP and aP). The importance of this new study is that it collects all AEFI data of pertussis vaccination from a country like Chile where we can find one of the strongest reporting systems in Latin America & The Caribbean as the notification of AEFI is mandatory by law since 2012Citation11 that also includes all diseases preventable by vaccines included in the NIP. Additionally, Chile has a high vaccination rate from where we were able to study all reports from the target population under 2 years of age over a period of 5.5 years: where a wP was predominantly used and then switched to an aP vaccine.
The rate of AEFI reports was the highest in 2016, which related to the year when there was a change in the wP pentavalent vaccine manufacturer. This supports the earlier findings by Thomsen et al. (2019), who described the rate of AEFI notified in Chile between 2014 and 2016. In that study, the overall reporting rate was 44.30 per 100,000 doses for wP pentavalent vaccines, slightly higher than the one seen in this study (34.27 per 100,000 administered dose), which presents both wP and aP vaccines together. The reported rates since the exclusive use of the aP hexavalent vaccine are even lower than those reported by Thomsen when wP vaccines were most often used.Citation8
Despite the fact that reporting systems may vary by country and that situational and community factors may influence over- or underreporting, the reporting rates observed in our study are similar to those observed in North American and European countries: in the USA, the decrease in the reporting rate reported by Zhou when switching from DTwP to DTaP vaccines was from 26.2 to 12.5 reports per 100,000 doses administered, similar to the present study, which reaches a rate in 2020 of 12.2 per 100,000 doses administered;Citation12 similarly, the rates reported by countries using aP vaccines are 19.8 for Poland, 8.9 for Italy, 3.8 for the Netherlands, and 1.1 for France per 100,000 doses.Citation13
Although causality cannot be determined in this study, the results suggests that wP vaccines are associated with more AEFI than aP hexavalent vaccines, and that the introduction of the latter could decrease the number of events.Citation14 In this study, a lower reporting rates of AEFI related to DTP-containing vaccines since the switch to an acellular hexavalent vaccine from a wP pentavalent vaccine was observed.
In 2017, Chile changed the wP pentavalent vaccine, which related to a first decrease in the frequency and rate of AEFI reports. In 2018, a second change was implemented, which consisted of administering an aP hexavalent vaccine in the first two vaccination doses (2 and 4 months) and a wP pentavalent + OPV vaccine in the last two doses (6 and 18 months). In this period of concomitant use, a lower rate of AEFI reports was observed; however, this direct comparison between aP (first and second doses) and wP vaccines (third and booster doses) should be avoided, since clinical trials have shown a higher reactogenicity in the first doses.Citation15–17
Several studies on vaccine hesitancy have shown that the safety profile and AEs can be a barrier and discourage vaccination and increase hesitancy.Citation18 Various studies, including in Chile, have shown that wP pentavalent vaccines are responsible for most AEFI in children and that the group with the highest frequency of reported AE are infants under 1 year of age, due to the fact that they receive the greatest number of vaccines.Citation16,Citation19,Citation20 Hence, the fact that hexavalent aP is associated with lower rates of AEFI, being a vaccine frequently administered in children under 2 years of age, aP hexavalent could help to increase confidence toward vaccination.
Various studies have shown that acellular pertussis vaccines have a more tolerable safety profile than whole-cell vaccines.Citation12,Citation14,Citation21,Citation22 The rate of SAE has been shown to decrease after switching to an acellular vaccine during the period evaluated. In the same sense, in the USA, serious reports, as well as non-serious ones, decreased by 50%.Citation12
There are differences in the estimated rates of serious AEFI reports in this study compared to those of Thomsen et al. (2019). Overall, they estimated reporting rates between 5.58 and 10.27 per 100,000 doses, while in our study there were found to be lower than 3.5 per 100,000 doses. Although rates by vaccine type could not be determined, the type of events were aligned with what has been reported elsewhere for another aP hexavalent vaccine.Citation13 In this sense, Patel et al. reported that SAEs in their research were rare in the hexavalent vaccine, probably due to its acellular component.Citation19 On the other hand, in the USA, Klein et al. (2019) followed a cohort of children under 2 years of age divided into three groups with different aP vaccine schedules with different concomitant vaccines. In this study, the active reporting of SAE was between 0.5 and 3.6%.Citation15 These differences may be due, as mentioned above, to the different reporting systems as well as corresponding to an active and a passive AEFI surveillance.
Given the unique public health context seen in 2020 due to the COVID-19 pandemic, data recorded this year should be analyzed cautiously, as it is subject to bias. The low number of AEFI reported in 1H2020 could be related to less willingness by Health Care Professionals (HCP) to report them due to more pressing conditions, such as COVID-19 patients. On the other hand, the higher number and rate of SAE is most likely an outlier which could have been caused by a change in health reporting patterns and behavior by parents and HCPs, which could have contributed to a higher reporting of SAE. Due to the pandemic, parents may be more sensitive to possible AE and take them to the emergency department more frequently. Nonetheless, there was a very low number of events for the entire study period and even in 2020. When data for the entirety of 2020 and for other vaccines is available, it would be important to contrast them as well as trends in other countries. Even so, it was still lower than those reported for pentavalent vaccines currently manufactured in India.
We found that in Chile, the main AE related to vaccines, using MedDRA PTs, were injection site erythema, pyrexia, pain at the injection site, application site inflammation, and crying, and they were associated more frequently with wP vaccines, which is in line with the literature and product inserts. In the USA, the most frequent AE reports of pertussis-containing vaccines were local reactions and pyrexia, and AEs were milder than those found for the DTwP.Citation23 A global evaluation of AEs from an aP hexavalent vaccine published in 2020, mainly European countries, also confirms that the most frequent AE were fever, crying, and local adverse reaction.Citation13 The same was found in Oman after a 10-year analysis of AEs: the wP pentavalent vaccine reported 73.8% of local reactions and 5.6% of systemic reactions.Citation19 This was also observed by active surveillance in Germany and the Czech Republic, where pain, erythema, and swelling were the most frequent injection site reactions and fever, vomiting, and crying the most frequent systemic reactions.Citation17
As noted by Zhou (2003) after the change from wP to aP vaccines in the USA, serious AE decreased, as did systemic AE such as fever and neurological reactions such as seizures. This is in line with the results of our study; however, Zhou evidenced an increase of local reactions with the introduction of aP vaccines, mainly in children with the booster administration of the DTaP vaccine.Citation12 In our study, we identified no rare or serious AE that have not been previously described in the literature.
The AEs that generate the greatest concern in the population and that are traditionally associated with pertussis-containing vaccines are the neurological ones, in which we find apnea in preterm infants, seizures and hypotonic-hyperresponsive episode (HHE). These AEs have been reported with both vaccines (wP and aP), but with a lower frequency in aP, which we could also confirm in our study.Citation8,Citation13,Citation23 Given that it is contraindicated for a child to receive a second or a third dose of pertussis-containing vaccine after having had an episode of seizure or HHE with the previous dose,Citation24 what was found in our study is of real importance, since the aP vaccine provides confidence among the population resulting in a greater vaccine coverage.
In our study, apnea was the most frequent AE in preterms for both wP and aP vaccines. Occurrence of apnea could be triggered by several factors. In absence of medical history, it is not possible to evaluate and determine etiology of those post-immunization apnea cases. It is known that extreme preterm infants (born at less than 28 weeks of gestation) may be at risk for cardio-respiratory apnea given the presence of respiratory immaturity. Incidence of recurrent apnea increases inversely proportionally to the gestational age.Citation25
Limitations
A number of study limitations are inherent to the study design. Although reporting AEFI is an obligation, which is instructed through decree,Citation6 there is no control that ensures full compliance with the norm and underreporting, especially of mild AEs, is expected, as is over-reporting following the introduction of new vaccines or drugs (known as the Weber effect).Citation26 This study furthermore does not allow assessing causality, and many children receive other vaccines concomitantly. Nonetheless, other vaccines did not change during this period in Chile and the results showing higher reactogenicity associated with wP vaccines are well aligned with other publications. However, the data obtained from spontaneous reporting systems give us important vaccine safety information that could help identify signals of potential risks of rare or previously unknown AEFI.
Another limitation relates to incomplete information for the calculation of reporting rates, since the total number of doses administered per vaccine brand was not available. This was mostly the case before 2018, when wP pentavalent vaccines were used in the NIP in Chile.
In addition, information about preterm infants was not routinely collected before 2017. Moreover, as neither the number of preterm infants born every year nor the vaccines administered to them is available, it was not possible to determine rates.
Conclusion
This study presents a description of AEFI related to pertussis contained vaccines administered to infants under 2 years, reported to the Chilean National Pharmacovigilance System between January 2015 and June 2020, during which reports of whole-cell and acellular vaccines were found. In the analyzed period, a decrease in AEFI was observed as of 2018, the year of the introduction of the aP vaccine. The results of this study support the safety profile of the fully liquid hexavalent aP vaccine over pentavalent wP vaccines, even in preterm infants, due to the decrease of AEs. In this sense, Chile can be a model for countries in the region, promoting the switch to safer vaccines.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the Institute of Public Health and its employees from the Vaccine Pharmacovigilance Section of the Pharmacovigilance Subdepartment of the Studies and Evaluation of Projects Subdepartment of the ANAMED Scientific Affairs Department
Additional information
Funding
References
- World Health Organization Global Vaccine Safety. AEFI detection. Geneva (Switzerland): WHO; 2020 [accessed 2021 January 10]. Available from: https://www.who.int/vaccine_safety/initiative/detection/en/.
- European Medicines Agency. CH E2E Pharmacovigilance planning (Pvp). European Medicines Agency; 2004.
- CIOMS/WHO Working Group on Vaccine Pharmacovigilance. Definition and application of terms for vaccine pharmacovigilance. CIOMS/WHO, editor. Geneva; 2012. p. 193.
- World Health Organization Global Vaccine Safety. Core variables for AEFI Switzerland: WHO; 2020 [accessed 2021 January 10] https://www.who.int/vaccine_safety/news/HL_1/en/.
- Ministerio de Salud de Chile, Subsecretaría de Salud Pública. Inmunización programatica contra la Difteria, Tétanos, Tos convulsiva, Hepatitis B, Poliomelitis y las enfermedades invasoras causadas por Haemophilus Influenzae tipo B en niños y niñas menores de 2 años; 2018.
- Ministerio de Salud de Chile. Guía de Sistema de Notificación, Evaluación y Seguimiento de Eventos Supuestamente Atribuidos a Vacunación e Inmunización y Error Programático. Chile Subdepartamento de Farmacovigilancia. Agencia Nacional de Medicamentos. Instituto de Salud Pública de Chile. Departamento de Inmunizaciones. Subsecretaría de Salud Pública. División de Planificación Sanitaria, editors; 2013.
- Departamento Agencia Nacional de Medicamentos Instructivo Para La Notificación De Sospechas De Reacciones Adversas A Medicamentos 2020. ISP, editor; 2020.
- Thomsen O, Saldaña A, Cerda J, Abarca K. Seguridad en vacunas: descripción de los eventos adversos notificados al sistema de vigilancia en Chile, 2014 a 2016. Rev Chil Infectol. 2019;36(4):461–68. doi:10.4067/S0716-10182019000400461.
- Departamento de estadísticas e Información de Salud, Ministerio de Salud. Gobierno de Chile Cobertura de vacunación programática: departamento de estadísticas e Información de Salud. Ministerio de Salud. Gobierno de Chile; 2020 [accessed 2020 July 20] http://www.deis.cl/estadisticas-inmunizaciones/.
- World Health Organization. Causality assessment of an adverse event following immunization (AEFI): user manual for the revised WHO classification. Second ed. Geneva; 2018.
- Ministerio de Salud. Subsecreataría de Salud Pública. Decreto 7 Aprueba el Reglamento sobre Notificación de Enfermedades Transmisibles de Declaracion Obligatoria y su Vigilancia; 2019.
- Zhou W, Ellenberg S. Surveillance for safety after immunization: vaccine adverse event reporting system (VAERS). Morbidity and Mortality Weekly Report: MMWR. Surveillance Summaries; 2003.
- PuenteGómez I, Verheust C, Hanssens L, Dolhain J. Safety profile of Infanrix hexa – 17 years of GSK’s passive post-marketing surveillance. Expert Review of Vaccines. 2020;19(8):771–79. doi:10.1080/14760584.2020.1800458.
- Hawken S, Manuel D, Deeks S, Kwong J, Crowcroft N, Wilson K. Underestimating the safety benefits of a new vaccine: the impact of acellular pertussis vaccine versus whole-cell pertussis vaccine on health services utilization. American Journal of Epidemiology. 2012;1(176(11)):1035–42. doi:10.1093/aje/kws167.
- Klein N, Abu-Elyazeed R, Cheuvart B, Janssens W, Mesaros N. Immunogenicity and safety following primary and booster vaccination with a hexavalent diphtheria, tetanus, acellular pertussis, hepatitis B, inactivated poliovirus and Haemophilus influenzae type b vaccine: a randomized trial in the United States. Human Vaccines & Immunotherapeutics. 2019;3(15):809–21. doi:10.1080/21645515.2018.1549449.
- Sebastian J, Gurumurthy P, Ravi M, Ramesh M. Active surveillance of adverse events following immunization (AEFI): a prospective 3-year vaccine safety study. Therapeutic Advances in Vaccines and Immunotherapy. 2019;7:251513551988900. doi:10.1177/2515135519889000.
- Prymula R, Kieninger D, Feroldi E, Jordanov E, B’Chir S, DaCosta X. Immunogenicity and safety of primary and booster vaccinations of a fully liquid DTaP-IPV-HB-PRP-T hexavalent vaccine in healthy infants and toddlers in Germany and the Czech Republic. The Pediatric Infectious Disease Journal. 2018;37(8):823–30. doi:10.1097/INF.0000000000002109.
- Gidengil C, Chen C, Parker A, Nowak S, Matthews L. Beliefs around childhood vaccines in the United States: a systematic review. Vaccine. 2019;37(3):6793–802. doi:10.1016/j.vaccine.2019.08.068.
- Patel P, Al-Rawahi B, Al-Jawari A, Al-Abaidani I, Al-Abri S. Surveillance of adverse events following immunization in Oman, 2006-2015. East Mediterr Health J. 2018;24(2):119–26. doi:10.26719/2018.24.2.119.
- Lopes S, Perin J, Prass T, Carvalho S, Lessa S, Dórea J. Adverse events following immunization in Brazil: age of child and vaccine-associated risk analysis using logistic regression. International Journal of Environmental Research and Public Health. 2018;15(6):1149. doi:10.3390/ijerph15061149.
- DuVernoy TS, Braun MM, Group tVW. Hypotonic–hyporesponsive episodes reported to the vaccine adverse event reporting system (VAERS), 1996–1998. Pediatrics. 2000;106(4):52. doi:10.1542/peds.106.4.e52.
- Sittlejohn L, Clothier H, Perrett K, Danchin M. Surveillance of adverse events following the introduction of 13-valent pneumococcal conjugate vaccine in infants, and comparison with adverse events following 7-valent pneumococcal conjugate vaccine, in Victoria, Australia. Human Vaccines & Immunotherapeutics. 2015;11(7):1828–35. doi:10.1080/21645515.2015.1048937.
- Moro P, Perez-Vilar S, Lewis P, Bryant-Genevier M, Kamiya H, Cano M. Safety surveillance of diphtheria and tetanus toxoids and acellular pertussis (DTaP) vaccines. Pediatrics. 2018;1:142.
- Department of Vaccines and Biologicals. Supplementary information on vaccine safety. Part 2 background rates of adverse events following immunization. WHO, editor; 2000.
- Eichenwald E. Apnea of prematurity. Pediatrics. 2016 Jan 1;137(1):e20153757. doi:10.1542/peds.2015-3757.
- Hoffman KB, Dimbil M, Erdman CB, Tatonetti NP, Overstreet BM, Weber T. Effect and the United States food and drug administration’s adverse event reporting system (FAERS): analysis of sixty-two drugs approved from 2006 to 2010. Drug Saf. 2014;37(4):283–94. doi:10.1007/s40264-014-0150-2.