ABSTRACT
Introduction
COVID-19 pandemic public health emergency is one of the worse disease outbreaks in the history of infectious disease. The consequence has resulted in over 4 million deaths globally. Therefore, a more in-depth understanding of the dynamics of the disease, vaccine development, and safety has become crucial for the disease eradication.
Objective
The study adopted bibliometric analysis to identify the global contribution in COVID-19 and Vaccine Safety and analyzed the current status, development, and research hotspots to reference for future research directions.
Methods
Studies published between January 1, 2019 and July 11, 2021 were retrieved from the Scopus database. Data analysis and visualization were conducted using VOSviewer ver 1.6.6, Bibliometrix app. (Using R).
Results
A total of 1827 publications with 12.14 average citations per document were identified. These publications were published in 796 journals by 10,243 authors (with 5.61 authors per document) from 80 countries/regions. About 33.75% of the researches were from the developed countries. The USA, China, and India were top contributors for scientific research on COVID-19 and vaccine safety. The “Vaccine” is the most productive journal with 58 articles. Li Y, NA NA, and Liu X were the top three prolific authors. Furthermore, “Human,” “Coronavirus disease 2019,” and “Drug safety,” were the most common frontier topics.
Conclusions
Our analysis highlights the characteristics of the most influential articles on COVID-19 related to vaccine safety. The findings provided valuable insight into the scientific research progress in this domain and suggest scaling-up research and information dissemination on COVID-19 and vaccine safety.
Introduction
Immunity in the human body is a strong defense against potential diseases and viruses. Therefore, outbreaks of infectious diseases and viruses of a novel attribute such as COVID-19 necessitate fast-track immune response in the human body, one of such is vaccine.Citation1 There are significant concerns among scientists and vaccine recipients about vaccine safety. These concerns cut across clinical trials, policies, costs, and acceptance from medical, psychosocial, cultural, and political factors.Citation2 Vaccine safety is a complex term that includes safety during clinical trialsCitation3,Citation4 and the general perception among recipients.Citation5 In the last decade, there has been a rapid improvement in vaccines against diseases supported by the improved health of millions of people. Nevertheless, information about vaccine safety still eludes people and has intensified the need to research and disseminate vaccines’ safety to the general population.Citation6
From several indications, humans desire freedom from life-threatening contingencies, which include viruses, ailments, and diseases. However, in human history, very few phenomena get to redefine societal setup and human interaction as pandemics.Citation7 In recent decades, globalization has brought about socio-economic changes that have promoted the threat of disease outbreaks and accelerated novel viruses. However, at the same time, globalization has facilitated international collaboration, enabling advances in disease research and surveillance.Citation8 Given the circumstance of oneness created by globalization, it is easy to find answers to why it has become so easy for COVID-19 to sweep across most human-inhabited continents.
When the first human cases of COVID-19 were reported [10], the World Health Organization (WHO) declared it a pandemic of global consequence. The only safety measures adopted were social distancing, quarantine, and lockdown of non-pharmaceutical actions advanced to curb the rate at which the virus was spreading.Citation9 Since its emergence in 2019, SARS-CoV-2, the virus that causes COVID-19, has infected over 83 million people and caused over 4 million deaths worldwide as of July 19, 2021.Citation10
The effective treatment strategy mostly adopted for COVID-19 has been the treatment of symptoms in patients. Consequently, several drugs have been used with varying levels of therapeutic effects. However, the actual drugs to cure COVID-19 are still quite elusive.Citation11 Nevertheless, vaccines could play an essential role in increasing population immunity, preventing severe disease, and reducing the ongoing health crisis.Citation3 Because of this, pharmaceutical and vaccine-related research has experienced an unprecedented surge. This surge has also led to the proliferation of research articles on related subjects.Citation11 However, there are concerns about whether an accelerated vaccine development can be accomplished safely, preventing potential adverse vaccine effects in the short-term and mid- and long-term.Citation12,Citation13 Thus, numerous studies have used various research approaches to explore COVID-19 and vaccine safety.
Generally, vaccines have been widely lauded as the most outstanding achievement of modern medicine , and vaccine safety evidence has been subjected to a series of scrutiny centered on the risks and benefits of vaccination.Citation14 These risks assessments of vaccines revolve around not only COVID-19 vaccine safety but rather encompasses all aspects of human known infectious diseases that require vaccines for immune support for morbidity and mortality prevention.Citation15 Consequently, there are mixed concerns and conclusions on whether vaccines have adverse effects (AEs) post-administration.Citation16 Therefore, experts suggested that it is crucial to intensify the post-licensing surveillance systems for monitoring and evaluation through various means available to monitors AEs by adopting surveillance systems like Vaccine Safety Datalink, Clinical Immunization Safety Assessment Project, and Vaccine Adverse Reporting System.Citation17 The WHO-funded projects that backed these surveillances and many other programs systematically assess the AEs of vaccines when they occur, in most cases rarely.Citation18
Therefore, vaccine safety is a binding domain directly associated with recipients’ negative perception (Hesitancy/Rejection) and positive perception (acceptance/confidence).Citation14 In reality, the massive COVID-19 vaccination of the global population comes with significant challenges based on the scale of implementation.Citation19 In the United States and many other regions, there are controversies on vaccine-triggered autoimmune diseases, the safety of human papillomavirus vaccine, among others that has raised vaccine safety concern,Citation20–22 and a surveillance system was set up for monitoring of these concerns.Citation23–25 Thus, a systematic analysis of recipients’ voices and concerns is essential in the quest to increase global awareness of vaccine safety and ensure acceptance.Citation26 In the wake of evidence that a substantial part of vaccine safety concern evolved from misinformation,Citation27 reports from the Global Advisory Committee on Vaccine Safety highlight some of the challenges faced in appraising practical recommendable scientific evidence to support vaccine safety.Citation28
The evidence on the ongoing global COVID-19 vaccination shows that as of July 12, 2021, roughly 3.5 billion people have been vaccinated,Citation10 and there are multilevel to the AEs among different groups.Citation12 Among the global concerns on COVID-19 vaccine safety, it has been articulated that AE evidence should be estimated and the rates of the occurence.Citation29 Consequently, there are updates from the Center for Disease Control (CDC) on some of the controversies surrounding the concerns for the COVID-19 vaccine.Citation30 In response to the glaring societal divides concerning vaccines, it becomes imperative to communicate and disseminate vaccine safety information by adopting result-oriented means when possible.Citation31 Thus, this current study attempts to aggregate progress in specifics on the COVID-19 and Vaccine safety research to support the growing need to disseminate information on COVID-19 vaccine safety globally.
The current study presents a bibliometric novel approach to explore global research output on COVID-19 and Vaccine safety. Bibliometric analyses provide objective information through the comprehensive assessment of a specific scientific research trend by identifying the number and distribution of publications related to the direction, authorship, co-authorship, and the most cited articles.Citation32–37 The bibliometric approach to studying COVID-19 has been adopted by several researchers in different areas, including a general overview of COVID-19 research,Citation38 the comparative method, gender distribution of authors on medical publications on COVID-19.Citation39 Bibliometric evidence demonstrates that China and the United States have contributed the most significant literature volume to scientific publications on COVID-19.Citation40 This work is a comprehensive analysis of the general body of literature on COVID-19 related to vaccine safety. Most COVID-19 related bibliometric studies have not focused on this area exclusively despite the massive global attention to vaccine development, testing, and safety.
Therefore, the current study attempt to provides a template to access research progress on COVID-19 vaccine-related topics to give researchers and global health policymakers a general overview of the milestone on the theme to facilitate future research directions. Therefore, the study objectives are:
To examines COVID-19 and Vaccine safety research trends, identifying the top contributing authors and the most cited articles in the research domain.
The study aims to provide extensive evidence on the hot topics, funding agencies, country contribution in COVID-19, and vaccine safety research.
Methods and materials
Key research focus
The primary objective of this study is to use bibliometric analysis to identify the global research contributions on COVID-19 and Vaccine Safety and analyze the current status, development, and research hotspots to reference future research directions. Overall, the study attempted to understand research milestones and focus on COVID-19 and Vaccine safety to encourage further future research and information dissemination.
Study design
The study adopted a bibliometric analysis to explore COVID-19 and Vaccine safety publications indexed in the Scopus database. A bibliometric analytical technique is an approach that has been progressively supporting the monitoring and evaluation of research in various empirical disciplines to guide future research direction, policy frameworks, and actions.
Data sources
The data for this study were retrieved from the Scopus database (https://www.scopus.com/). The Scopus database is an international repository purposely selected as it covers a significant number of global journals. This database is publicly accessible with a university subscription and network; thus, no ethical consideration is necessary to extract and publish data retrieved.
Literature search strategy
The following search terms were used to identify articles relevant to documents: TITLE-ABS-KEY (2019-Coronavirus* or 2019-CoV* or 2019-nCoV* or 2019-New Coronavirus* or 2019-Novel Coronavirus* or Corona Virus Disease-2019* or Coronavirus 2019* or Coronavirus Disease 2019* or Coronavirus Disease-19* or Coronavirus-2019* COVID19* COVID-19* or COVID-2019* or nCoV2019* or nCoV-2019* Novel Coronavirus 2019* or SARS coronavirus 2* or SARS-CoV2* or SARS-CoV-2* or Severe Acute Respiratory Syndrome Coronavirus 2* or Wuhan AND Coronavirus* or Hubei AND Coronavirus* or Corona Virus* or Coronavirus* or vaccine* and vaccine safety* vacc*). Information on the search strategy is shown in the flowchart .
Figure 1. Flow chart showing the selection of documents focusing on COVID-19 and vaccine safety research.
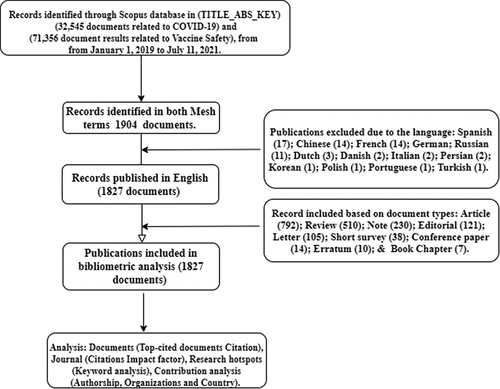
The dates of publications on COVID-19 and vaccine safety retreived also ranged from January 1, 2019 to July 11, 2021. Only documents published in English were included in the study. The database search was performed on a single day to avoid the possibility of introducing unfairness due to the daily citation updates. Two members of our research team (THM and TYA) independently assessed the retrieved documents.
Eligibility criteria (inclusion and exclusion)
Based on the Mesh output retrieved; only documents that directly mentioned the search terms in the TITLE_ABS_KEY were included, and publications in English were included in the bibliometric analysis. Other languages such as German and Russian were excluded to remove language mix-ups in the descriptive analysis and visualization. Bibliometric indicators such as document type, citations, authors, research category, keywords, country of origin, most frequently cited papers, productive journals, and affiliation were analyzed. The data extraction process is presented in the flow chart for the bibliometric analysis (). Lastly, the impact factor (IF) for journals was obtained from the Journal Citation Report (JCR) database for the year 2020.
Bibliometric analysis
The bibliometric analysis primarily reports descriptive statistics of retrieved documents data by visualization and ranking. Information on the most productive authors, corresponding authors’ countries, journals, and articles were appraised by accessing the top 10 in the categories using Number of Publications (NP), Total Citations (TC), h_index, criteria for ranking performances in each domain. The co-occurrence of the keyword reported in Scopus were assessed and visualized using the Bibliometrix package in R,Citation41 and VOSviewer version 6.6 software (Leiden University, Leiden, The Netherlands) was employed to show the core authors, organizations, countries,Citation42 for descriptive purposes only.
Results
General information and annual trend
The number of publications distribution shows the direction of research on COVID-19 and vaccine safety. The publication record was estimated at 14 documents reported in 2019, followed by 730 documents in 2020, and 1083 documents as at July 11, 2021. Thus, the result indicated an increase in the scientific growth of COVID-19 Vaccine safety-related publications. The search protocol adopted resulted in 1904 documents after synchronizing the Mesh terms in COVID-19 and Vaccine Safety. Documents were further screened based on “Language” as only English published articles were considered in the study.
The study identified 1827 documents from January 1, 2019 to July 11, 2021 published in Scopus database search. About 10243 authors, approximately 12.14 per document, contributed to the COVID-19 and Vaccine Safety research papers. Among the total, 792 (43.35%) were full research articles, and 510 (27.91%) were review papers. The general characteristics of the metadata are presented in Supplementary Table S 1.
Top 10 most cited documents
The top 10 most cited documents in COVID-19 and vaccine safety are presented in . Overall, high-quality articles based on the citation score explore the core of research in COVID-19 and Vaccine safety which was published by Polack et al., 2020 under the title “Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine” with received total citation (TC) = 1229 citations scores, followed by an article published by Jackson et al., 2020 under the title “An mRNA vaccine against SARS-COV-2- Preliminary report Drug treatment options for the 2019-new coronavirus (2019-nCoV)” with received (TC = 704) citations scores, both these articles were published in New England Journal of Medicine with Impact factor (IF = 91.245).
Table 1. Top 10 cited article on Covid-19 and vaccine safety research
Top 10 most active authors
A total of 10243 authors contributed 1827 documents. The top 10 authors on COVID-19 and vaccine safety are presented in . Li Y from the United Kingdom was a top-ranked author with the number of publications (NP) = 14 articles and total citations of (TC = 959), followed by NA NA from the London School of Hygiene USA, with (NP = 14) and total citations (TC = 57), and Liu X, from UK, with (NP = 12) articles and total citations (TC = 1582).
Table 2. Top 10 productive author’s in Covid-19 and vaccine safety research
Productive corresponding author’s country
In , about 80 countries generated the total number of COVID-19 and vaccine safety research retreived from the Scopus database. The USA was the most productive country based on the number of publications (NP = 380), followed by China (NP = 121) and India (NP = 101). Furthermore, the USA was the most influential country based on Total Citations (TC = 7677). The USA has a robust national collaboration in COVID-19 and Vaccine safety research with reported intra-country collaboration/Single Country Publications SCP = 307 and inter-country collaboration/Multiple Country Publication (MCP = 73) documents. Overall top 10 listed countries has Lower International Collaboration and Multiple Country Publications ratio (MCP_Ratio) was less 0,50).
Table 3. Top 10 productive corresponding author country in Covid-19 and vaccine safety research
Distribution of most productive journals
A total of 1827 documents in COVID-19 and vaccine safety articles were published in 796 academic journals. The top 10 productive journals are shown in . Journal of “Vaccine” was the most influential journal with the number of publications and total citation (NP = 58, TC = 577), followed by “Vaccines” (NP = 41, TC = 485), “JAMA-Journal of the American Medical Association” (NP = 39 = TC = 696), “The Lancet” (NP = 33, TC = 2725), Science (NP = 30, TC = 609), and “Human Vaccines &Immunotherapeutics” (NP = 16, TC = 83). Other information related to Journal h_index, Journal impact factor, quartile range, and active years of publication are presented in .
Table 4. Top 10 productive journals in Covid-19 and vaccine safety research 2021
Top 10 subject categories and funding organization for COVID-19 and vaccine safety
The top 10 Subject categories and funding organization COVID-19 and Vaccine Safety research are listed in Supplementary Table S2. The majority of the documents with the significant numbers of published articles were in the field of Medicine (NP = 1317), followed by Immunology and Microbiology (NP = 449), and Biochemistry, Genetics and Molecular Biology (NP = 343). National Institutes of Health were amongst the top-funding organization for research in COVID-19 and Vaccine Safety with Number of Publication (NP = 114), followed by The US Department of Health and Human Services (NP = 106, National Institute of Allergy and Infectious Diseases (NP = 41), and National Natural Science Foundation of China (NP = 41).
Word cloud analysis of top 100 keywords plus
To understand the co-occurrence of keywords, we listed the top 10 keywords that appeared most frequently in COVID-19 and Vaccine Safety publications (). The most commonly encountered Keywords Plus terms (derived from the titles, abstract, and Keywords) in the retrieved literature were “human” (1458), “coronavirus disease 2019” (1156) “humans” (1037), ”’Vaccination” (906), pandemic” (853), ““drug safety” (841), “covid 19” (682), “sars_cov-2vaccine” (628), “drug efficacy” (591), and “priority journal” (583).
Network visualization analysis
In the network analysis between co-authorship and authors’ published documents, a minimum of three instances of authorship were required to meet the inclusion criteria, and 221 authors reached this threshold. Only 171 were presented in 9 clusters with Links (L) and Total Link Strength (TLS) of coauthors (L = 802, TLS = 1275). Chen E.T (L = 20, TLS = 71) and Drew S. (L = 13, TLS = 39) among reported authors (Supplementary Figure S 1A). According to published documents 80 countries and regions contributed to research productivity on COVID-19 and Vaccine Safety.
For co-authorship analysis and countries, a minimum of 10 instances of authorship were required to meet the inclusion criteria, and 40 countries who reached this threshold were presented in 5 clusters with different colors. As a result, the top authorship countries based on the number of lengths and total links strength (L = 459, TLS = 1857). The USA (L = 530, TLS = 657), followed by Germany (L = 33, TLS = 200), and Australia (L = 34, TLS = 179) among reported countries (Supplementary Figure S 1B). In addition, the co-authorship analysis in the documents published based on organization affiliation shows a minimum of three organizations instances of authorship were required to meet the inclusion criteria. About57 organizations who met this threshold were presented in four clusters with different colors based on the links (L = 96) and Total links strength between them (TLS = 283) as shown in Supplementary Figure S 1C.
Conceptual structure and collaborative countries analysis
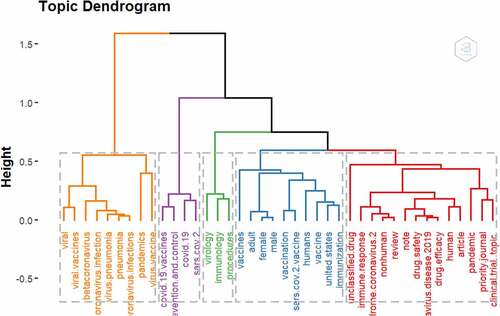
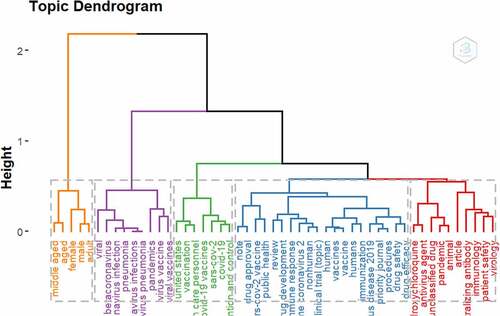
shows the standard and prominent conceptual frameworks of retrieved articles using a Factorial Analysis and Multiple Correspondence Analysis (MCA) for 75 Keywords terms distributed in 5 clusters. In addition, Correspondence analysis (CA) for 75 Keyword terms distributed in 5 clusters are presented in .
Discussion
The bibliometric analysis offers additional empirical supports for vaccine research, which may facilitate understanding other vaccine-related issues, such as vaccine development, acceptance, and hesitancy among the global population. Thus, the objective of the current study is to provide empirical evidence on global research milestones and performances on COVID-19 and vaccine safety. There is remarkable progress in extending COVID-19 and Vaccine safety research. The aggregate research publication was extracted from the Scopus database from January 1, 2019 to July 11, 2021, a total of 1827 documents were published in the database and contributed by 10,243 authors. The developed countries contributed about 33.75% of the COVID-19 and Vaccine Safety-related research retrieved. The search in the Scopus database provides a single assessment of one repository, which may have limited the coverage of the current study. However, the Scopus database is a large database for many global journals and provides a vast platform for researchers to present their scholarly work, especially research centering on COVID-19 and Vaccine safety.
The current evidence shows that the top author contributing research investigation on COVID-19 and Vaccine safety was LI Y from the United Kingdom with an h-index 8 and a total citation of 959. Among the top 10 productive authors ranking, the United Kingdom has five authors’ representations, China had three representations, and the United States had two representatives in the top 10. This evidence contrasts with other bibliometrics that has found the authors from the United States as the most contributors in most research domains.Citation33,Citation43 While this evidence is valid for the first authorship, the corresponding author analysis presents the United States as the topmost cited corresponding author country representation with up to 7677 total citations, followed by China, India, and the United Kingdom. The journal result offers an insight into the journals supporting research on COVID-19 and vaccine safety. The top journal publisher was Vaccine, Vaccines, JAMA, The Lancet, and Science.
The top-cited documents offer more in terms of the general interest of research based on the research focus and the volume of citation amassed. For instance, the topmost cited article titled Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine published by the New England Journal of Medicine had up to 1229 citations since publication in 2020. The research expanded empirical evidence on the safety of BNT162b2 mRNA Covid-19 Vaccine through a randomized trial of 43,548 study recipients of placebo and BNT162b2 where there was a 95% efficacy of the vaccine in preventing COVID-19. The study also reported evidence of vaccine efficacy across subgroups of different diversity. In addition, the study reported a safety profile of minimal pain at the injection site, fatigue, and headache.Citation44 Other prominently cited articles focused on the safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2,Citation4 and possible maternal and infant outcomes from coronavirus 2019-NCOV (SARS-CoV-2) infecting pregnant women,Citation45 among others focusing on safety research.
The subject area on COVID-19 and Vaccine Safety was “Medicine” as the Top research Domain with about 816 estimated publications closely followed by “Immunology and Microbiology and “Biochemistry, Genetics, and Molecular Biology among other top domains. The leading funding institution was the National Institutes of Health, United States. Also, featuring in the top-ten funding institution was National Natural Science Foundation of China, Bill and Melinda Gates Foundation, and Pfizers-BioNTech Research.
The wordcloud analysis shows the most prominent keyword in COVID-19 and Vaccine Safety research published in the Scopus database. The most occurring word was “Human,” which is most common in titles, abstracts, and keywords on research centered on COVID-19 Vaccine Safety and shows that special attention has been placed on COVID-19 Vaccine safety on humans. Similarly, “pandemic,” “COVID-19,” and “coronavirus disease 2019” are some of the top-occurring keywords in the wordcloud. In the conceptual mapping of the top 75 keywords distributed across five clusters, the most common conceptual frameworks focus on COVID-19 Vaccine, Sars Cov2, COVID-19, and epidemiology. Vaccine and vaccination-related terms were prominent in all the five clusters derived from the factorial analysis, which further presents evidence that researchers are particularly interested in developing safe and effective vaccines for use and protection against COVID-19.
The outcome of this study acclimatizes the need to improve and increase research on COVID-19 and vaccine safety. The research approach adopted in this study limited the data analyzed to the Scopus database with a shortage of research on COVID-19 and vaccine safety in the web of science. Therefore, there is an urgent need to scale up the study on COVID-19 vaccine safety to fast-track the eradication of the infection globally. Similarly, research contributions are not evenly distributed globally as most top research and citations were clustered in the developed countries with the United Kingdom, the United States, and except for China, which had the leading authorship and citations in COVID-19 and vaccine safety research. The USA has been a global scientific leader because of the scale of its economy and level of research effort.Citation46 Given that there is substantial authorship from the developing countries, more attention should be accorded to publications coming from the region to increase their research influence and citation.
Implication for future research and practice
The evidence in the publications analyzed shows remarkable research efforts to explore empirical evidence on COVID-19 and Vaccine safety cutting across clinical and psychosocial research. With over 3.5 billion COVID-19 vaccine recipients globally,Citation10 significant intervention is still needed to ensure a reduction in poor awareness of vaccine safety. However, with recent evidence showing the magnitude of vaccine hesitancy as it is ranked as one of the top 10 threats to global health,Citation27 there is room for improvement on vaccine safety research, especially in regions that may have low productivity in research and dissemination of clinical information. Vaccine Safety Datalink, Clinical Immunization Safety Assessment Projects, and Vaccine Adverse Reporting System, among other projects, have been enhancing the dissemination of vaccine safety information globally and monitoring reported cases of AEs; thus, it is crucial to intensify research effort to support these projects in achieving significant and evidenced-based results. More specifically, it has been suggested that there is a need to formulate a two-way partnership to support physician and patients communication.Citation26 Such an approach can be adopted for vaccine safety research whereby programs and platforms are organized to educate the global population about clinical trials on vaccines to boost confidence among the recipients. Similarly, these platforms should provide the opportunity to receive vaccine recipients’ concerns and ways to improve their experiences.
This study evidence suggests there is still room for improving global contribution to vaccine safety and reduce reliance on resource-rich countries for scientific support. At country levels, there should be accountability and a framework for information dissemination about vaccine safety. Hence, there is a need for worldwide redress that will facilitate countries’ involvement in research. In summary, intensifying research efforts will improve the overall achievement of vaccine safety based on outcome of clinical trials. In all, the intervention measure engaged during the COVID-19 public health emergency and information dissemination approach on the safety of the COVID-19 vaccine can channel future vaccine intervention by reducing vaccine hesitancy and negative perception globally. Therefore, funding support is encouraged for more research from global health stakeholders. Funding support should also be accorded to social scientists to explore the salient psychosocial experience of vaccine recipients to further consolidate and support intervention for the international community to improve vaccine acceptance in combating infectious disease outbreaks. The study evidence also gives credit to research advancement and funding from the developed countries, non-governmental organizations, and China for funding research on COVID-19 and vaccine safety researches. Thus, while the world continues to steer away from the impact of COVID-19, efforts must continue to ensure that vaccines are safe through research, clinical trials, and other indicators that will reduce the poor perception of the safety of vaccines globally.
This current research show novelty as the first bibliometric analysis of COVID-19 and vaccine safety as a wholesome topic on published articles in the Scopus database. However, a few limitations should be considered when interpreting the study findings. First, we only used the Scopus database, one of the largest repositories, to obtain publications spanning the COVID-19 outbreak. Therefore, this single database included in the study must be considered when interpreting the current study evidence. We, however, believe that the use of the Scopus database for analysis provides a robust insight for understanding research performance focusing on COVID-19 and Vaccine Safety. Second and equally vital was excluding the web of science database as the search result produced too few publications on COVID-19 and Vaccine safety necessary for analysis. Lastly, the exclusion of other databases for comparisons, such as PubMed, Google Scholar, and other non-English databases published, is acknowledged. The implication of excluding other databases may have limited the scope of the data analyzed on the conclusions presented in the study is solely based on the Scopus database. These omissions should be considered, and we encourage further future research exploring other databases.
Conclusion
In summary, there is a remarkable global effort toward research on COVID-19 and vaccine safety. The study shows the general increasing contribution on COVID-19 and vaccine safety from the United Kingdom, the United States, China, and India. Also, the United States and the United Kingdom remain instrumental in ensuring that clinical trials for vaccine safety are successful and the increasing financial support from the region. Generally, developed and developing countries have contributed a significant share of the authorship, organization, and publishing journals in the Scopus database, which engrave their effort to ensure COVID-19 Vaccine safety and improve public perception. Lastly, effort and research intervention should continually be intensified to increase evidence on COVID-19 and vaccine safety in clinical trials and psychosocial research and implementing a two-way communication approach to increase vaccine acceptance globally.
Authors’ contributions
TYA and THM Conceived the idea and designed the study; TYA and THM: Searched and collected the data; TYA, FOA, and THM: Wrote the first draft of the manuscript; TYA and THM: Software and formal analysis; TYA, THM, FOA, SC, AET, IHM, HHM, AA and EI: Reviewed and edited the final draft. All the authors read and approved the final manuscript for publication.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download TIFF Image (392.6 KB)Supplemental Material
Download TIFF Image (759.2 KB)Supplemental Material
Download TIFF Image (330.4 KB)Supplemental Material
Download MS Word (17 KB)Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1969851.
References
- Baxby D. Edward Jenner’s role in the introduction of smallpox vaccine. In: Plotkin S, editor. History of vaccine development. New York (NY): Springer; 2011. p. 48. doi:10.1007/978-1-4419-1339-5_3.
- Larson HJ, Cooper LZ, Eskola J, Katz SL, Ratzan S. New decade of vaccines 5 addressing the vaccine confi dence gap. Lancet. 2011;378:526–35. doi:10.1016/S0140-6736(11)60678-8.
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi:10.1016/S0140-6736(20)32661-1.
- Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Bellamy D, Bibi S, Bittaye M, Clutterbuck EA, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–78. doi:10.1016/S0140-6736(20)31604-4.
- Calina D, Docea AO, Petrakis D, Egorov AM, Ishmukhametov AA, Gabibov AG, Shtilman MI, Kostoff R, Carvalho F, Vinceti M, et al. Towards effective COVID‑19 vaccines: updates, perspectives and challenges (Review). Int J Mol Med. 2020;46:3–16. doi:10.3892/ijmm.2020.4596.
- Moxon ER, Siegrist C. New decade of vaccines 1 the next decade of vaccines : societal and scientifi c challenges. Lancet. 2011;378:348–59. [Internet]. [accessed 2011 July 20]. doi:10.1016/S0140-6736(11)60407-8.
- Huremovic D. Brief history of pandemics (pandemics throughout history). Psych Pandem. 2019. [accessed 2021 July 20]. doi:10.1007/978-3-030-15346-5_2.
- Saunders-Hastings PR, Krewski D. Reviewing the history of pandemic influenza: understanding patterns of emergence and transmission. Pathogens. 2016;5:66. PMID: 27929449; PMCID: PMC5198166. doi:10.3390/pathogens5040066.
- Zhang Y, Jiang B, Yuan J, Tao Y. The impact of social distancing and epicenter lockdown on the COVID-19 epidemic in mainland China: a data-driven SEIQR model study. medRxiv. 2020;2019.
- COVID-19 Map - Johns Hopkins Coronavirus resource center [Internet]. [accessed 2021 Jul 12]. https://coronavirus.jhu.edu/map.html.
- Chen Y, Cheng L, Lian R, Song Z, Tian J. COVID-19 vaccine research focusses on safety, efficacy, immunoinformatics, and vaccine production and delivery: a bibliometric analysis based on VOSviewer. Biosci Trends. 2021;15:64–73. Epub 2021 Mar 19. PMID: 33746182. doi:10.5582/bst.2021.01061.
- Kostoff RN, Briggs MB, Porter AL, Spandidos DA, Tsatsakis A. Comment: COVID-19 vaccine safety. Int J Mol Med. 2020;46:1599–602. Epub 2020 Sep 18. PMID: 33000193; PMCID: PMC7521561. doi:10.3892/ijmm.2020.4733.
- Caddy S. Developing a vaccine for covid-19 spike protein. BMJ. 2020;1790:1–2. [accessed on 2021 July 20]. doi:10.1136/bmj.m1790.
- Di Pasquale A, Bonanni P, Garçon N, Stanberry LR, El-Hodhod M, Tavares Da Silva F. Vaccine safety evaluation: practical aspects in assessing benefits and risks. Vaccine. 2016;34:6672–80. Epub 2016 Nov 8. PMID: 27836435. [Internet]. doi:10.1016/j.vaccine.2016.
- Vichnin M, Bonanni P, Klein NP, Garland SM, Block SL, Kjaer SK, Sings HL, Perez G, Haupt RM, Saah AJ, et al. An overview of quadrivalent human papillomavirus vaccine safety: 2006 to 2015. Pediatr Infect Dis J. 2015;34:983–91. doi:10.1016/j.vaccine.2016.
- Dudley MZ, Halsey NA, Omer SB, Orenstein WA, O’Leary ST, Limaye RJ, Salmon DA. The state of vaccine safety science: systematic reviews of the evidence. Lancet Infect Dis. 2020;20:e80–9. [Internet]. [accessed on 2021 July 20]. doi:10.1016/S1473-3099(20)30130-4.
- Moro PL, Haber P, McNeil MM. Challenges in evaluating post-licensure vaccine safety: observations from the centers for disease control and prevention. Expert Rev Vaccin. [Internet]. 2019;18:1091–101. doi:10.1080/14760584.2019.1676154.
- McClenathan BM, Edwards KM. Vaccine safety: an evolving evidence-based science. Br J Clin Pharmacol. 2019;85:2649–51. Epub 2019 Aug 19. PMID: 31373717; PMCID: PMC6955397. doi:10.1111/bcp.14080.
- Mellet J, Pepper MS. A covid-19 vaccine: big strides come with big challenges. Vaccines. 2021;9:39.1–14.39. PMID: 33440895; PMCID: PMC7827578. doi:10.3390/vaccines9010039.
- Destefano F, Bodenstab HM, Offit PA. Principal controversies in vaccine safety in the United States. Clin Infect Dis. 2019;69:726–31. PMID: 30753348. doi:10.1093/cid/ciz135.
- Chen RT, Shimabukuro TT, Martin DB, Zuber PLF, Weibel DM, Sturkenboom M. Enhancing vaccine safety capacity globally: a lifecycle perspective. Vaccine. 2015;33:D46–54. Epub 2015 Oct 1. PMID: 26433922; PMCID: PMC4663114. doi:10.1016/j.vaccine.2015.06.073.
- McCoy CA. The social characteristics of Americans opposed to vaccination: beliefs about vaccine safety versus views of U.S. vaccination policy. Crit Public Health. 2020;30:4–15. [Internet]. [accessed on 2021 July 20]. doi:10.1080/09581596.2018.1501467.
- Lee GM, Romero JR, Bell BP. Postapproval vaccine safety surveillance for covid-19 vaccines in the US. JAMA - J Am Med Assoc. 2020;324:1937–38. PMID: 33064152. doi:10.1001/jama.2020.19692.
- Julianne G, Marquez P, Su J, Calvert GM, Liu R, Myers T, Nair N, Martin S, Clark T, Markowitz L, et al. First month of COVID-19 vaccine safety monitoring — United States. US Dep Heal Hum Serv Dis Control Prev. 2021;70:283–888. PMID: 33630816. doi:10.15585/mmwr.mm7008e3.
- Haynes K. Preparing for COVID-19 vaccine safety surveillance: a United States perspective. Pharmacoepidemiol Drug Saf. 2020;29:1529–31.1529-1531. Epub 2020 Oct 2. PMID: 32978861; PMCID: PMC7537525. doi:10.1002/pds.5142.
- Holt D, Bouder F, Elemuwa C, Gaedicke G, Khamesipour A, Kisler B, Kochhar S, Kutalek R, Maurer W, Obermeier P, et al. The importance of the patient voice in vaccination and vaccine safety—are we listening? Clin Microbiol Infect. [Internet]. 2016;22:S146–53. A Suppl 5: S146-S153. Epub 2016 Dec 6. PMID: 27939015. doi:10.1016/j.cmi.2016.09.027.
- Geoghegan S, O’Callaghan KP, Offit PA. Vaccine safety: myths and misinformation. Front Microbiol. 2020;11:1–7. doi:10.3389/fmicb.2020.00372.
- Asturias EJ, Wharton M, Pless R, MacDonald NE, Chen RT, Andrews N, Salisbury D, Dodoo AN, Hartigan-Go K, Zuber PLF. Contributions and challenges for worldwide vaccine safety: the global advisory committee on vaccine safety at 15 years. Vaccine. 2016;34:3342–49. doi:10.1016/j.vaccine.2016.05.018.
- Black SB, Law B, Chen RT, Dekker CL, Sturkenboom M, Huang WT, Gurwith M, Poland G. The critical role of background rates of possible adverse events in the assessment of COVID-19 vaccine safety. Vaccine. 2021;39:2712–8. [Internet]. [accessed 2021 July 20]. doi:10.1016/j.vaccine.2021.03.016.
- Centers for Disease Control and Prevention. COVID-19 vaccine safety update. 2021 [Internet] 1–4. [accessed 2021 July 20]. https://www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update/covid-19-vaccine-safety-update-comirnaty-january-2021_en.pdf.
- Seeber L, Michl B, Rundblad G, Trusko B, Schnjakin M, Meinel C, Weinberg U, Gaedicke G, Rath B. A design thinking approach to effective vaccine safety communication. Curr Drug Saf. 2015;10:31–40. PMID: 25859673. doi:10.2174/157488631001150407105400.
- Oh J, Kim A. A bibliometric analysis of COVID-19 research published in nursing journals. Sci Ed. 2020;7:118––124. doi:10.6087/kcse.205.
- Musa TH, Akintunde TY, Musa HH, Ghimire U, Gatasi G. Malnutrition research output: a bibliometric analysis for articles index in web of science between 1900 and 2020. Electron J Gen Med. 2021;18:em293. doi:10.29333/ejgm/10840.
- Musa HH, El-Sharief M, Musa IH, Musa TH, Akintunde TY. Global scientific research output on sickle cell disease: a comprehensive bibliometric analysis of web of science publication. Sci Afr. 2021;12:e00774.12: e00774. doi:10.1016/j.sciaf.2021.e00774.
- Akintunde TY, Musa TH, Musa HH, Musa IH, Shaojun C, Ibrahim E, Tassang AE, Helmy MSEDM. Bibliometric analysis of global scientific literature on effects of COVID-19 pandemic on mental health. Asian J Psychiatr. [Internet]. 2021;63:102753. doi:10.1016/j.ajp.2021.102753.
- Akintunde TY, Musa TH, Musa HH, Ibrahim E, Muhideen S, Kawuki J. Mapping the global research output on Ebola vaccine from research indexed in web of science and scopus : a comprehensive bibliometric analysis. Hum Vaccin Immunother. [Internet]. 2021;2021:1–13. doi:10.1080/21645515.2021.1948785.
- Musa HH, Musa TH, Musa IH, Musa IH. Global scientific research progress in mycetoma: a bibliometric analysis. Trans R Soc Trop Med Hyg Hyg. 2021:1–13. trab072. doi:10.1093/trstmh/trab072.
- El Mohadab M, Bouikhalene B, Safi S. Bibliometric method for mapping the state of the art of scientific production in Covid-19. Chaos Soliton Soliton Fract. 2020;139:110052. doi:10.1016/j.chaos.2020.110052.
- Andersen JP, Nielsen MW, Simone NL, Lewiss RE, Jagsi R. COVID-19 medical papers have fewer women first authors than expected. Elife. 2020;9:e58807:1–7. doi:10.7554/eLife.58807.
- Aristovnik A, Ravšelj D, Umek L. A bibliometric analysis of covid-19 across science and social science research landscape. Sustainability. 2020;12:9132. doi:10.3390/su12219132.
- Aria M, Cuccurullo C. Bibliometrix: an R-tool for comprehensive science mapping analysis. J Informetr. 2017;11:959–75. doi:10.1016/j.joi.2017.08.007.
- Van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84:523–38. Epub 2009 Dec 31. PMID: 20585380; PMCID: PMC2883932. doi:10.1007/s11192-009-0146-3.
- Kawuki J, Yu X, Musa TH. Bibliometric analysis of Ebola research indexed in web of science and Scopus (2010-2020). Biomed Res Int. 2020;5476567. PMID: 32964036; PMCID: PMC7486633. doi:10.1155/2020/5476567.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;5476567. PMID: 32964036; PMCID: PMC7486633. doi:10.1155/2020/5476567.
- Schwartz DA, Graham AL. Potential maternal and infant outcomes from coronavirus 2019-NCOV (SARS-CoV-2) infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses. 2020;12:1–16. doi:10.3390/v12020194.
- Patiño-Barbosa AM, Bedoya-Arias JE, Cardona-Ospina JA, Rodriguez-Morales AJ. Bibliometric assessment of the scientific production of literature regarding Mayaro. J Infect Public Health. 2016;9:532–34. doi:10.1016/j.jiph.2015.10.001.