?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In Japan, the herd immunity effect of rotavirus vaccine has not yet been proven. Here, we conducted active surveillance for hospitalization due to rotavirus acute gastroenteritis (AGE) among children under 5 years of age in pre-rotavirus vaccination years and self-financed rotavirus vaccination years to clarify the rotavirus vaccine effectiveness (VE) in reducing hospitalization rates. A time-series analysis showed that the monthly hospitalization rates observed after vaccine introduction were significantly lower than the forecasted hospitalization rates (p < .001, Mann-Whitney U test). In the third year after vaccine introduction, the hospitalization rate declined despite the low vaccination rate of 27–50% for the two preceding years. We estimated four types of VE, namely direct, indirect, total, and overall. The direct VE was calculated from the relative risk ratio of hospitalizations between vaccinated and unvaccinated children. The indirect VE was defined as the population-level effects of vaccination on children not receiving the vaccine. The total VE was defined as the combination of the direct and indirect VE on children receiving the vaccine. The overall VE was determined by the weighted average of indirect VE on the children not receiving the vaccine and the total VE on the children receiving the vaccine. The direct, indirect, total, and overall VE values were calculated as 82% (95% confidence interval, 52–93), 70% (51–82), 95% (87–98), and 86% (77–91), respectively. The high values of indirect, total, and overall VE indicate that the rotavirus vaccine produces a herd immunity effect.
Introduction
In both developing and developed nations, rotavirus is a major cause of gastroenteritis in young children that requires hospitalization.Citation1,Citation2 As self-financed rotavirus vaccines, Rotarix (GlaxoSmithKline), a monovalent vaccine (RV1), and RotaTeq (Merck), a pentavalent vaccine (RV5), became available in Japan in November 2011 and July 2012, respectively.Citation3,Citation4 Both rotavirus vaccines were introduced into the national immunization program in October 2020, based on reports showing their safety and effectiveness in Japan,Citation5–10 where it was reported that the rotavirus genotype G1P[8] was predominant during the pre-vaccine period.Citation11 It is unclear whether genotype changes after the introduction of rotavirus vaccine affect vaccine effectiveness (VE).
This study was conducted prior to the introduction of the rotavirus vaccine into the national immunization program. The purpose of this study was to examine the effectiveness of self-financed rotavirus vaccines using a time-series analysis and the risk ratio of hospitalization for rotavirus acute gastroenteritis (AGE).
Patients and methods
Study period and subjects
In Japan, the peak season of rotavirus AGE begins in November and ends in July of the following year. The period August-October is considered the off-season for rotavirus AGE. Therefore, we defined the period from November to October of the following year as one season. Since the 2006/2007 season, we have been conducting active surveillance for hospitalization by rotavirus AGE in children in Ise City, Mie Prefecture, Japan. The survey period in this study extended from the 2006/2007 to 2017/2018 seasons.
All patients under 5 years of age who were hospitalized with a diagnosis of AGE were tested for rotavirus. If the stool sample could not be collected at the time of admission, it was collected during hospitalization. We tested the stool samples by using a commercially available enzyme immunoassay kit (Rapidtesta; Sekisui Medical, Tokyo, Japan); the sensitivity and specificity of this kit were 92.6% and 97.1%, respectively, compared with those of the virus isolation culture method.Citation12 We assessed the rotavirus VE in pre-rotavirus vaccination years and self-financed rotavirus vaccination years.
Estimation of rotavirus vaccine coverage
The infant health examination rates in Ise City were as high as 96%–98% throughout the study period. In Japan, the local government provides opportunities for infant health examinations free of charge. In addition, vaccines incorporated into the national immunization program are free of charge. However, during the period of this study, the rotavirus vaccine was not included in the national immunization program, and hence, it was a self-financed vaccine. In each year, the vaccine coverage was confirmed by checking the immunization records in the mother and child health handbook during the health examinations performed at the ages of one and a half or three and a half years. Children were considered as vaccinated if they received an RV1 or an RV5 vaccine more than once.
Data analysis
The annual rotavirus AGE incidence rate in each year was estimated by calculations using the total number of cases of rotavirus AGE as the numerator and the population under the age of 5 years, which was obtained from the demographic data published by the city, as the denominator. The 95% confidence interval (CI) was calculated using the Poisson exact test. A Fisher’s exact test was used to compare the incidence rates of rotavirus hospitalization before and after the introduction of the rotavirus vaccine.
A time-series analysis was performed using the monthly incidence rates of rotavirus AGE hospitalization from October 2006 to October 2011, before the introduction of the rotavirus vaccine. We applied the Holt-Winters additive seasonal smoothing method to predict the incidence rates of hospitalization from November 2011 to October 2018, assuming that the vaccine was not introduced, and compared the predicted data with the monthly hospitalization incidence rates after the introduction of rotavirus vaccine. A Mann-Whitney U test was used to investigate the significant difference between the forecasted and the observed incidence rates. We used Crystal Ball predictor (Kozo Keikaku Engineering Inc., Tokyo, Japan) software for time-series analysis. Other statistical analyses were performed using StatFlex version 7 (Artec Ltd, Osaka, Japan) and R version 4.0.5. Statistical significance was defined as p < .05.
Evaluation of VE
Using the measure of the relative risk (RR) in the vaccinated group compared with that in the unvaccinated group, VE is calculated by the formula (1 – RR) × 100.Citation13,Citation14 Since the hospitalization rate for rotavirus AGE after vaccination was small enough, the RR and the odds ratio were considered to be similar. Therefore, we used Fisher’s exact test to estimate the odds ratio.
We categorized VE into direct, indirect, total, and overall, as reported by Halloran et al.Citation13,Citation14 The hazard of disease before and after the introduction of vaccine is defined as follows: 1A, hazard in unvaccinated individuals in an unvaccinated population before the introduction of the vaccine; 2A, hazard in unvaccinated individuals in a vaccinated population after the introduction of the vaccine; 2B, hazard in vaccinated individuals in a vaccinated population after the introduction of the vaccine. The direct, indirect, total, and overall VE were calculated by using the following formula:
The direct VE of rotavirus vaccine was determined from the odds ratio of hospitalizations due to rotavirus AGE in the vaccinated and the unvaccinated groups of children under the age of 5 years from 2011/2012 to 2017/2018. The indirect VE was defined as the population-level effects of vaccination on people who did not receive the vaccine. The total VE was defined as the combination of the direct and indirect VE in children who received the vaccine. The overall VE was determined by the weighted average of the indirect VE in the children who did not receive the vaccine and the total VE in the children who received the vaccine. Indirect, total, and overall VE were calculated using the number of patients hospitalized with rotavirus AGE in the population under 5 years of age from 2006/2007 to 2010/2011, before the introduction of the vaccine.
Ethics
This study was conducted in accordance with the ethical guidelines for epidemiological research and the ethical guidelines for medical and health research involving human subjects. The study was approved by the ethics committee of the Japanese Red Cross Ise Hospital, Japan (No. 30–9). Since the database used for the study was fully anonymized, the requirement for written informed consent was waived by our ethics committee; however, the study was announced on our webpage, and the patients were given the opportunity to opt out.
Results
Characteristics of participants
shows the characteristics of the subjects in this study. This study included 544 (male, 337) patients with AGE who were admitted to our hospital during the 2006/2007–2017/2018 seasons. Because the R1 vaccine was introduced in November 2011, the periods from the 2006/2007 to 2010/2011 seasons and the 2011/2012 to 2017/2018 seasons were defined as pre-rotavirus vaccination years and self-financed rotavirus vaccination years, respectively.
Table 1. Age distribution of children hospitalized for rotavirus or non-rotavirus acute gastroenteritis
During the pre-rotavirus vaccination years, 300 (male, 190) patients with AGE were hospitalized. Of the 300 patients, the numbers of rotavirus AGE and non-rotavirus AGE patients were 91 (male, 53) and 209 (male, 137), respectively.
During the rotavirus vaccination years from 2011/2012 to 2017/2018, 244 (male, 147) patients with AGE were hospitalized. Of the 244 patients, 74 (male, 35) had rotavirus AGE, of which the numbers of vaccinated and unvaccinated patients were 5 (male, 2) and 69 (male, 33), respectively.
The ratio of rotavirus AGE hospitalizations to total AGE was not significantly different between the pre-rotavirus vaccination and rotavirus vaccination years (Fisher’s exact test, p = .722). Additionally, there was no significant difference between the ratio of hospitalizations of individuals under the age of 5 years between pre-rotavirus vaccination and rotavirus vaccination years (Fisher’s exact test, p = .848). The most common age of hospitalization for rotavirus AGE was 12 to 23 months of age both in pre-rotavirus vaccination and rotavirus vaccination years. The hospitalization rates of individuals under 36 months of age in pre-rotavirus vaccination and rotavirus vaccination years were 78% (71 of 91 patients) and 85% (63 of 74 patients), respectively.
Population-based hospitalization rate
The rates of hospitalization due to rotavirus AGE in the pre-rotavirus vaccination and rotavirus vaccination years were compared using Fisher’s exact test. There was no decrease in the hospitalization rate for 2 years after the introduction of the rotavirus vaccine. Conversely, the 2012/2013 season showed the highest incidence rate throughout the seasons. However, in the third year after the introduction of the rotavirus vaccine, the hospitalization rate declined despite the low vaccination rate of 27%–50% for the preceding two years ().
Table 2. Population-based figures of hospitalizations for rotavirus acute gastroenteritis less than 5 years
Distribution of rotavirus AGE cases by month of hospitalization
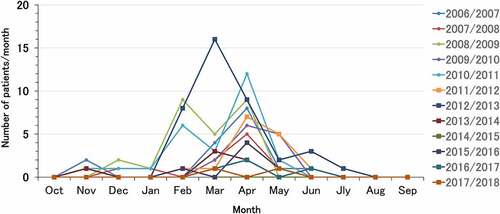
shows the distribution of rotavirus AGE cases by the month of hospitalization. The pre-rotavirus vaccination years are indicated by small circles, and the self-financed rotavirus vaccination years are indicated by squares. The peak season for hospitalization for rotavirus AGE was observed from November to July of the following year in both the pre-rotavirus vaccination and rotavirus vaccination years. Although the number of hospitalizations decreased from the third year after the introduction of the vaccine, there was no change in the peak seasons in the pre-rotavirus vaccination and rotavirus vaccination years.
Time-series analysis
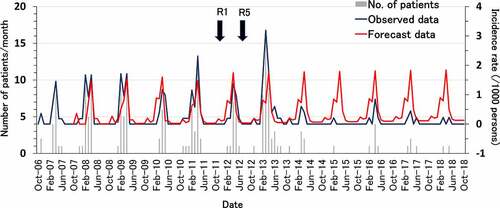
shows the results of a time-series analysis. Assuming the rotavirus vaccine was not introduced, the monthly incidence rate of rotavirus AGE would be forecasted to peak at approximately 1.8/1000 persons/month in each year. In contrast, the observed monthly hospitalization rate was 3.2/1000 persons/month in the 2012/2013 season, the second year after the introduction of the vaccine, and this rate was the highest throughout the study period. However, after the third year, hospitalization rates began to decline. Even the peak hospitalization rate of 0.85/1000 persons/month in the 2015/2016 season was lower than that in the pre-vaccine period. Comparing the hospitalization rates for rotavirus AGE in the pre-rotavirus vaccination and rotavirus vaccination years, the monthly hospitalization rates observed after the introduction of the vaccine were significantly lower than the forecasted hospitalization rates (p < .001, Mann-Whitney U test).
Figure 2. Forecast of hospitalization incidence rate for rotavirus acute gastroenteritis in patients under 5 years of age by time-series analysis. R1, monovalent vaccine; R5, pentavalent vaccine. Blue line shows observed incidence rates, and red line shows forecasted incidence rates obtained by the Holt-Winters additive seasonal smoothing method.
VE
Between 2012 and 2018, 3980 children were vaccinated with rotavirus vaccine, and 5 of them were hospitalized for rotavirus AGE, whereas 17 out of 2422 unvaccinated children were hospitalized for rotavirus AGE. During the pre-rotavirus vaccination years (2007–2011), there were 143 hospitalizations among 6161 children. The values of direct, indirect, total, and overall VE in the vaccination period were 82% (95% CI, 52–93), 70 (51–82), 95% (87–98), and 86% (77–91), respectively ().
Table 3. Rotavirus vaccine effectiveness estimates against rotavirus acute gastroenteritis hospitalization in post-vaccine period
Discussion
To evaluate the efficacy of a vaccine against rotavirus AGE, it is necessary to accurately survey the vaccine coverage and the number of patients hospitalized with rotavirus AGE before and after the introduction of the vaccine. In this study, we set up a cohort in Ise City from November 2006 to October 2018. Active surveillance in the cohort revealed how the hospitalization rate for rotavirus AGE of individuals under 5 years of age changed during the pre-rotavirus vaccination years and the self-financed rotavirus vaccination years. A time-series analysis showed that vaccination significantly reduced hospitalization due to rotavirus AGE. In addition, by examining the indirect, total, and overall VE, it was confirmed that the rotavirus vaccine not only exerts direct effects but also a herd immunity effect.
Rotavirus vaccines have been reported to increase the incidence of intussusception in older infants.Citation10,Citation15,Citation16 Therefore, vaccination must be completed within 6–8 months after birth to reduce the development of intussusception. In Japan, health examinations of children aged 1.5 and 3.5 years old are the responsibilities of the local government of the region in which they live. In Ise City, the average consultation rate for health examinations is as high as 96% or more.
Since 2002, our hospital has been the only pediatric hospitalization facility in the region, and all rotavirus AGE cases requiring hospitalization are treated at our hospital. In addition, because the cohort is from a region of low social population change, we calculated the person-years assuming that the children born each year remain in the cohort during the observation period.Citation17 Thus, this study was able to accurately estimate the hospitalization-suppressing effect of rotavirus vaccines with accurate vaccination coverage and active surveillance of the disease.
As previously reported,Citation7,Citation18 in the third year after the introduction of the rotavirus vaccine, the hospitalization rate declined, despite the low vaccination rate of 27%–50% in the two preceding years. The two-year delay in the realization of rotavirus VE may be explained by the time taken by the vaccinated children to reach an age of 12 to 36 months, which is the age at which the majority of hospitalizations for rotavirus AGE occur ().
It has been reported that the vaccination against rotavirus has reduced the hospitalization rate due to rotavirus AGE in Japan.Citation5–9 Here, we showed the rotavirus VE clearly through a time-series analysis in Japan. Our data showed a decrease in the hospitalization rate of unvaccinated children after the 2013/2014 season, presumably because of the herd protection effect of the vaccine.Citation19 In addition, by examining four types of VE, namely direct, indirect, total, and overall VE, the herd immunity effect was clarified for the first time in Japan. A report from the United States showed that in a year with 65.9% rotavirus vaccination coverage in commercially insured children, the direct, indirect, total, and overall VE were 87%, 44%, 92%, and 83%, respectively. When the vaccination coverage reached 85.9%, those of VE were 90%, 82%, 98%, and 96%, respectively.Citation14 Our results showed similar tendency that the direct, indirect, total and overall VE throughout 2012–2018 in which vaccination coverage was 62% were 82%, 70%, 95% and 86%, respectively.
Group A rotavirus is classified into G and P types, according to the gene sequences of the two types of the outer capsid proteins VP7 and VP4 containing neutralizing antigens.Citation20 The incidence of the disease caused by the G-type and P-type strains of rotavirus differs depending on the country/region and varies from year to year. Before the introduction of the rotavirus vaccine, the most predominant genotype from 2007 to 2011 was G3P[8]. After the introduction of the rotavirus vaccine, the most predominant genotype was either G1P[8] or G2P[4] in alternate years.Citation7,Citation11 It is still unclear whether differences in gene lineages and mutations in antigenic sites are related to VE. Therefore, it is important to monitor the genotypic changes in epidemic strains and the VE in the region even after the introduction of the vaccine.Citation7,Citation21
In general, it is difficult to estimate the immunization rate of self-financed vaccines, albeit its necessity in understanding the impact of a vaccine. In this study, we were able to obtain an accurate history of vaccination by interviewing the parents and by checking the records in the mother and child health handbook at the time of the medical examinations. The limitations of this study are that it is a local study, and the observed population is small.
In conclusion, our data confirm that the rotavirus vaccine is effective in reducing hospitalizations for rotavirus AGE and elicits a herd immunity effect on the vaccinated and unvaccinated children under 5 years of age.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis. 2003;9(5):565–72. doi:10.3201/eid0905.020562.
- Kamiya H, Nakano T, Kamiya H, Yui A, Taniguchi K, Parashar U; Rotavirus Epidemiology Study Group. Rotavirus-associated acute gastroenteritis hospitalizations among Japanese children aged <5 years: active rotavirus surveillance in mie prefecture, Japan. Jpn J Infect Dis. 2011;64:482–87.
- Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, et al.; Human Rotavirus Vaccine Study Group. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354(1):11–22.
- Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354(1):23–33. doi:10.1056/NEJMoa052664.
- Iwata S, Nakata S, Ukae S, Koizumi Y, Morita Y, Kuroki H, Tanaka Y, Shizuya T, Schödel F, Brown ML, et al. Efficacy and safety of pentavalent rotavirus vaccine in Japan: a randomized, double-blind, placebo-controlled, multicenter trial. Hum Vaccin Immunother. 2013;9(8):1626–33. doi:10.4161/hv.24846.
- Yoshikawa T, Matsuki T, Sato K, Mizuno M, Shibata M, Hasegawa S, Morita M, Iwasa M, Gopala K, Holl K. Impact of rotavirus vaccination on the burden of acute gastroenteritis in Nagoya city, Japan. Vaccine. 2018;36(4):527–34. doi:10.1016/j.vaccine.2017.12.006.
- Asada K, Kamiya H, Suga S, Nagao M, Ichimi R, Fujisawa T, Umemoto M, Tanaka T, Ito H, Tanaka S, et al. Rotavirus vaccine and health-care utilization for rotavirus gastroenteritis in Tsu City, Japan. Western Pac Surveill Response J. 2016;7(4):28–36. doi:10.5365/wpsar.2016.7.3.005.
- Kobayashi M, Miyazaki M, Ogawa A, Tatsumi M. Sustained reduction in rotavirus-coded hospitalizations in children aged <5 years after introduction of self-financed rotavirus vaccines in Japan. Hum Vaccin Immunother. 2020;16:132–37.
- Sato T, Nakagomi T, Nakagomi O. Cost-effectiveness analysis of a universal rotavirus immunization program in Japan. Jpn J Infect Dis. 2011;64:277–83.
- Ledent E, Lieftucht A, Buyse H, Sugiyama K, Mckenna M, Holl K. Post-marketing benefit-risk assessment of rotavirus vaccination in Japan: a simulation and modelling analysis. Drug Saf. 2016;39(3):219–30. doi:10.1007/s40264-015-0376-7.
- Dey SK, Ushijima H, Phathammavong O, Chanit W, Okitsu S, Mizuguchi M, Ota Y. Seasonal trend and serotype distribution of rotavirus infection in Japan, 1981-2008. Pediatr Infect Dis J. 2010;29:166–67. doi:10.1097/INF.0b013e3181b79460.
- Ishida E. Rapidtesta Rota-Adeno. Med Technol. 2008;36(13):1402. in Japanese.
- Halloran ME, Struchiner CJ, Longini IM Jr. Study designs for evaluating different efficacy and effectiveness aspects of vaccines. Am J Epidemiol. 1997;146(10):789–803. doi:10.1093/oxfordjournals.aje.a009196.
- Panozzo CA, Becker-Dreps S, Pate V, Weber DJ, Jonsson Funk M, Stürmer T, Brookhart MA. Direct, indirect, total, and overall effectiveness of the rotavirus vaccines for the prevention of gastroenteritis hospitalizations in privately insured US children, 2007–2010. Am J Epidemiol. 2014;179(7):895–909. doi:10.1093/aje/kwu001.
- Weintraub ES, Baggs J, Duffy J, Vellozzi C, Belongia EA, Irving S, Klein NP, Glanz JM, Jacobsen SJ, Naleway A, et al. Risk of intussusception after monovalent rotavirus vaccination. N Engl J Med. 2014;370(6):513–19. doi:10.1056/NEJMoa1311738.
- Yih WK, Lieu TA, Kulldorff M, Martin D, McMahill-Walraven CN, Platt R, Selvam N, Selvan M, Lee GM, Nguyen M. Intussusception risk after rotavirus vaccination in U.S. Infants. N Engl J Med. 2014;370(6):503–12. doi:10.1056/NEJMoa1303164.
- Higashigawa M, Maeyama T, Yoshino A, Matsuda K, Ito M, Maji T, Ichimi R. Incidence of childhood primary immune thrombocytopenic purpura. Pediatr Int. 2015;57(5):1041–43. doi:10.1111/ped.12788.
- Curns AT, Steiner CA, Barrett M, Hunter K, Wilson E, Parashar UD. Reduction in acute gastroenteritis hospitalizations among US children after introduction of rotavirus vaccine: analysis of hospital discharge data from 18 US states. J Infect Dis. 2010;201(11):1617–24. doi:10.1086/652403.
- John TJ, Samuel R. Herd immunity and herd effect: new insights and definitions. Eur J Epidemiol. 2000;16(7):601–06. doi:10.1023/A:1007626510002.
- Matthijnssens J, Ciarlet M, Rahman M, Attoui H, Bányai K, Estes MK, Gentsch JR, Iturriza-Gómara M, Kirkwood CD, Martella V, et al. Recommendations for the classification of group A rotaviruses using all 11 genomic RNA segments. Arch Virol. 2008;153(8):1621–29. doi:10.1007/s00705-008-0155-1.
- Jain S, Vashistt J, Changotra H. Rotaviruses: is their surveillance needed? Vaccine. 2014;32(27):3367–78. doi:10.1016/j.vaccine.2014.04.037.