ABSTRACT
Background
Individuals with type 1 diabetes (T1D) are at increased risk of infections from vaccine-preventable diseases. This study focuses on compliance of T1D patients to the recommended vaccination schedule, vaccination of their close contacts for influenza and on factors potentially contributing to vaccination program deviations.
Methods
The study population comprised children, adolescents and adults with T1D under follow-up at the Department of Pediatrics University Hospital and the Diabetic Center General Hospital, Heraklion, Crete-Greece. Data were extracted, following informed consent, from individual’s vaccination booklet, medical files and telephone interview. Vaccination records, demographic parameters, glycemic control and influenza vaccination of close contacts were assessed.
Results
The study included 258 participants (111 children/adolescents, 147 adults). Vaccination coverage for influenza was 76.7% for children, 64.4% for adults, for PCV 90.9% for children, but only 10.8% for the 23-valent, for hepatitis B 99% for children and 78.2% for adults. Youngsters were vaccinated against Hib 91.9%, meningococcus C 98.2%, measles-mumps-rubella 90.3%, chickenpox 86.4%, hepatitis A 76.5% and HPV 42.5%. Less than 65% of all individuals were fully vaccinated for diphtheria-tetanus-pertussis and meningococcus ACWY. Approximately 50% of the 605 close contacts were not vaccinated against influenza. Individuals with better glycemic status seemed to adhere to the recommended schedule and had a better vaccinated family environment.
Conclusions
Vaccination coverage for T1D individuals was sufficient regarding the majority of routine childhood vaccines, but less for adolescence and group-specific vaccines. Their family contacts were not sufficiently vaccinated for influenza. Targeted interventions are required in order to increase vaccination rates.
Introduction
Individuals with type 1 diabetes are a key target population of vaccination strategies, as they are at increased risk of developing complications from vaccine-preventable diseases, which may include prolonged hospitalization or even death.Citation1,Citation2
Type 1 diabetes mellitus, the most common type of diabetes in young people, affects about 1 in 400 children.Citation3,Citation4 High blood glucose levels can affect the immune responses and result in increased susceptibility to infections, morbidity and mortality.Citation5 Thus, vaccination is a critical strategy for individuals with T1D.
Current recommendations for diabetic individuals include vaccination against influenza, pneumococcus, diphtheria, tetanus and pertussis, hepatitis B and varicella.Citation6 The most common respiratory infections associated with diabetes are caused by Streptococcus pneumoniae and influenza. Diabetic individuals carry a 6 times higher risk of influenza-related hospitalization and a 4.4 times higher risk for hospitalization due to pneumonia compared to healthy individuals.Citation7,Citation8,Citation9 According to the Greek National Immunization Schedule, annual vaccination against influenza is recommended for children, adolescents and adults with T1D, and the 23-valent pneumococcal polysaccharide vaccine (PPSV) is recommended in addition to the 13-valent conjugate vaccine (PCV13) for individuals >2 y of age at increased risk of pneumococcal infections. Despite recommendations, coverage rates with PPSV remain poor.Citation10 Diabetic individuals are at risk of exposure to the hepatitis B virus as a result of procedures related to the management of diabetesCitation11 and at higher risk of complications caused by tetanus disease.Citation12
Vaccination of close contacts (parents, family members, caregivers and those living together) of high-risk patients for vaccine-preventable infections can limit their exposure to infectious diseases.Citation13–17 However, vaccination rates remain low, while published data regarding this strategy is limited both for adults and children with diabetes.
The aim of this study was to investigate the vaccination rates of patients, both children and adults, with T1D for the recommended vaccines. Influenza vaccination coverage rate of their household contacts and its effect on diabetic individuals was assessed. Correlation of demographic data, glycemic control and the close contacts’ influenza immunization status with the vaccination coverage of children and adults with T1D were also evaluated.
Patients and methods
Study population
This is a cross-sectional study conducted between September and December 2020. The study population included children, adolescents and adults with T1D, who were followed up at the Pediatric Outpatient Clinic of the University Hospital of Heraklion and the Diabetic Center of the Venizeleio General Hospital, Crete, Greece, respectively, the two major outpatient facilities for individuals with T1D in Crete. Demographic and vaccination coverage data were extracted via interview during the regular visit at the outpatient clinic or via telephone interview or review of vaccination records following informed consent. Demographics included age, residence, date and age of TD1 diagnosis, the more recent HbA1c value, type of insulin treatment (multiple-dose injection regimen/insulin pump) and any documented history of comorbidities. Individuals were also interviewed about the vaccination coverage against influenza of their family members/close contacts.
Design and methods
Compliance with vaccination according to the Greek National Immunization Program for children, adolescents and adults regarding basic vaccination schedule was assessed for each individual. Vaccination against influenza and pneumococcus as recommended for people with chronic diseases was also evaluated. Analysis was performed considering the compliance of each individual to the age – appropriate recommended types and doses per vaccine. Three groups resulted, namely a) fully vaccinated, b) partially vaccinated, c) unvaccinated. As far as the annual influenza vaccine is concerned, patients were categorized as a) vaccinated once during the current or previous year, b) vaccinated for ≥2 consecutive years and c) unvaccinated. Another factor taken into account was the year that certain vaccines became available in Greece: 2000 for PCV and MCC, 2005 for varicella vaccine and 2012 for MenACWY.
The close contact vaccination effect was evaluated for two categories of the family members of diabetic individuals: a) parents, partners/spouses or other adult family member (grandparents and/or caregivers) and b) siblings, children of diabetic patients. Their vaccination coverage was analyzed in accordance with the national immunization guidelines for annual influenza vaccination for those who are in close contact with people with underlying diseases. Three levels of close contact vaccination for each participant were defined a) all close contacts of a diabetic patient were vaccinated for influenza, b) partially vaccinated family – i.e., some members only were vaccinated and c) no influenza vaccinated family members.
Statistical analysis
Data were expressed as median and range or means and standard deviation (SD). The chi-square test was used to compare categorical variables. Association between vaccination of patients with T1D and demographic data, glycemic control and close contact vaccination were determined by ordinal logistic regression in order to calculate the significance and probability level. The statistical significance was set at p 0.05. The Stata 13.1 statistics package was used.
The study was approved by the Ethics Committee of both hospitals and conforms to the Declaration of Helsinki.
Results
Participants’ characteristics
The study included 258 individuals with diabetes and 605 close contacts of 226/258 diabetic individuals. Regarding individuals with diabetes, 111 were children and adolescents (<18 y old) and 147 adults comprising almost 65.6% of the total T1D population of Crete. The median age was 20 y (3–70 y), with 53.1% being males. The median duration since diabetes diagnosis was 8 y (3–52 y), while the median value of the most recent HbA1c was 7.25% (5.3–13.5%). A percentage of 57.8% presented suboptimal glycemic control with HbA1c value ≥7%. In terms of diabetes management, 88% used the multiple-dose injection regimen (MDI) and 12% a continuous subcutaneous insulin infusion (CSII) system via a pump. Approximately 38% of the participants presented at least one comorbidity, including thyroid disorders, celiac disease and cardiovascular and kidney disease. Vaccination data was collected for 605 close contacts, of whom 65.3% were parents, partners/spouses or other adult family members and 34.7% siblings or children of diabetic individuals.
Coverage rates for the standard recommended vaccines
Complete vaccination against Hepatitis B was 87.2% for both children and adults, albeit 99% (110/111) for children/adolescents and 78.2% (115/147) for adults.
Regarding the DTaP/dTap vaccine, 145 of all study participants (56.2%) were fully vaccinated. In the group of individuals <18 y old, 64% (n = 71) were fully and 36% (n = 40) partially vaccinated, while 50.3% (n = 74) and 27.9% (n = 41) of adults were fully and partially vaccinated, respectively. A percentage of 12.2% (n = 18) of adults had unknown vaccination history.
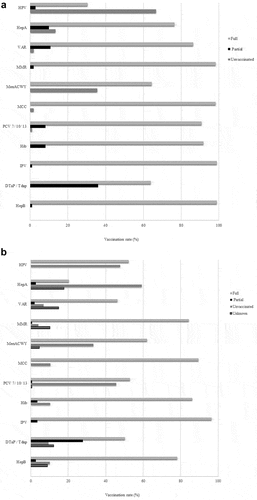
Data for vaccination rates against polio and Hib were extracted for 198 diabetic individuals. According to the records, 97.8% (n = 194) were fully vaccinated against polio and 89.3% (n = 177) against Hib. In the group of younger patients, 99% (n = 110) were fully immunized for polio and 91.9% (n = 102) for Hib. Data on vaccination rates of routine vaccines for children and adults is depicted in .
Figure 1. (a). Vaccination coverage for standard recommended vaccines of children and adolescents with type 1 diabetes. (b). Vaccination coverage for standard recommended vaccines of adults with type 1 diabetes.
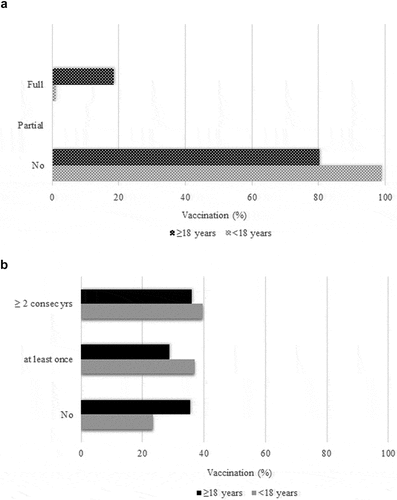
Vaccination against pneumococcus (conjugated pneumococcal vaccine PCV7/10/13) was less satisfactory, as 69.4% (n = 179) of the participants were fully vaccinated. This lack of adherence to guidelines is attributed to older patients of whom 53% (n = 78) were vaccinated, while the corresponding rate for those <18 y was as high as 90.9% (n = 101). On the other hand, the coverage of both groups with the additional 23-valent vaccine was at remarkably low levels, as only an estimated 10.8% of all the participants were found to be vaccinated. Younger patients were almost totally unvaccinated (99%). Data is shown in ).
Figure 2. (a). 23-valent polysaccharide pneumococcal vaccine coverage for people with type 1 diabetes. (b). Influenza vaccine coverage for people with type 1 diabetes mellitus.
Younger diabetic individuals were almost fully covered with the meningococcal vaccine against meningitis C (98.2%). Data for the older patients were very limited, probably due to the rather recent introduction of this particular vaccine to the national immunization program. Vaccination coverage against serogroups A, C, W and Y was 63.1% (n = 103) for both groups and 64.4% (n = 49) for those <18 y of age. Meningococcal B vaccine, which is not recommended and not recompensated by the national insurance, presented a poor 8.1% (n = 16) for all doses.
Individuals with T1D are adequately vaccinated against measles, mumps and rubella as 90.3% (n = 233) of both age groups and 98.2% (n = 109) of the group <18 y had received the vaccine. As for varicella vaccine, a percentage of 86.5% of the group <18 y were completely covered (). Regarding the older patient group 29.9% had natural immunity to varicella and an additional 46.2% had received the vaccine.
Hepatitis A vaccine, which is also included in the national immunization program, had been received by 76.6% of the younger group of diabetic individuals. Study female participants, for whom HPV vaccine is recommended, were fully vaccinated at a percentage of 42.5% (n = 34). Vaccination coverage of patients <18 y and ≥18 y is presented in . For Influenza, 37.6% (n = 97) of participants were vaccinated ≥2 consecutive years, an additional 32.2% (n = 83) at least once (current or previous year) and 30.2% (n = 78) were never vaccinated. ) depicts influenza vaccination coverage for both age groups.
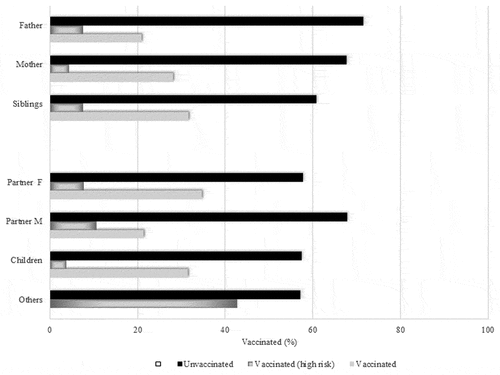
Regarding vaccination of close contacts vaccination status against influenza was assessed for a total number of 605 family members of diabetic individuals. It seems that among close contacts accounting for 226 families of people with T1D only 23.5% (n = 53) were all vaccinated against influenza, while 25.2% (n = 57) report that “some members in the family” had the vaccine and finally 51.3% (n = 116) were families with not any member vaccinated. Influenza vaccination of family members, per category, is shown in .
Figure 3. Close contacts per age group: vaccination coverage of close contacts for children and adults with type 1 diabetes. The upper part (father, mother, sibling) depicts contacts of younger patients (<18 y), the lower that of older individuals.
Association of diabetic individuals’ characteristics to vaccination compliance
Age appears to be an important factor contributing to vaccination for a number of vaccines, such as hepatitis B (p = .001), DTaP/dTap (p = .002), varicella (p < .001), hepatitis A (p < .001) and pneumococcus (p < .001). The lowest vaccination rates were found in adult study participants. There is no association between age and coverage with IPV (p = .239), MMR (p = .088) and influenza vaccine (p = .101). Regarding the special vaccine recommendations for diabetic individuals, PPSV23 vaccine shows a positive correlation with age (p < .001), as an extremely high percentage of unvaccinated individuals is observed in the group of those <18 y. Adherence to the yearly administered influenza vaccine had no significant association with sex (OR 0.90, 95% CI, 0.56–1.43, p = .664), diabetes-related variables such as years since diabetes diagnosis (OR 1.00, 95% CI 0.96–1.04, p = .789) and insulin regimen (OR 1.23, 95% CI 0.59–2.54, p = .569).
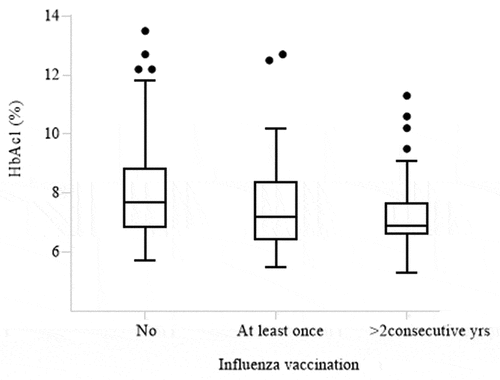
Glycemic control was the only factor associated with vaccination coverage, since individuals with poor glycemic control appear to have lower rates of vaccination against influenza (). In particular, for any 1% increase of HbA1c, there appears to be a 29% decrease of the possibility for higher vaccination coverage (OR 0.71, 95% CI, 0.60–0.85, p = .0001).
Association between vaccination of diabetic individuals and vaccination of close contacts
Compliance of diabetic individuals with influenza vaccination seems to be related to influenza vaccination rates of their close contacts. More specifically, when a patient with diabetes is being vaccinated even once (current or previous year), it is 10.4 times more likely to belong to a better vaccinated family, compared to the non-vaccinated individuals (OR 10.4, 95% CI, 4.4–24.7, p < .001). In fact, when a patient is vaccinated against influenza for ≥2 consecutive years, it is 17.8 times more likely to belong to a better vaccinated environment against influenza (OR 17.8, 95% CI, 7.58–41.9, p < .001). While 86% of diabetics who were found to be unvaccinated for influenza also had poorly vaccinated environment/family, individuals with better vaccination rates for influenza had their close contacts vaccinated too.
No association was found between vaccination rates of close contacts of diabetic individuals and years from diagnosis (OR 1.03, 95% CI, 0.98–1.08, p = .138), HbA1c value (OR 1.05, 95% CI, 0.85–1.29, p = .642), insulin regimen (MDI/CSII) (OR 0.99, 95% CI, 0.49–20.2, p = 1.000/OR 0.95, 95% CI, 0.42–21.1, p = .974) and other characteristics, such as sex (OR 0.96, 95% CI, 0.55–1.66, p = .887), age (OR 0.99, 95% CI, 0.96–1.02, p = .769) and place of residence (OR 0.84, 95% CI, 0.35–1.98, p = .693).
Association between family members’ characteristics to vaccination compliance
Compliance with the annual influenza vaccination of close contacts of diabetic individuals seems to have no significant association with age (OR 0.74, 95% CI, 0.52–1.05, p = .096), when comparing coverage against influenza between adult family members (parents, partners/spouses) of people with T1D and children/siblings of diabetic individuals. No association was observed in the vaccination of adult family members with gender, when checking compliance of mothers as opposed to fathers (OR 0.83, 95% CI 0.51–1.34, p = .45) and female versus male partners/spouses (OR 0.64, 95% CI 0.21–1.96, p = .44).
Discussion
Τhis study provides useful information on the vaccination coverage of children and adults with type 1 diabetes, as well as their close contacts’ influenza immunization status. The results underline that immunization with the primary series of vaccines is satisfactory apart from hepatitis A and varicella vaccine. Delays occur in booster doses for diphtheria-tetanus-pertussis and the vaccines in adolescence, i.e for HPV in girls and the meningococcal conjugate vaccine MCV4. This finding is in accordance with results of previous studies concerning the general population of children and adolescents in Greece.Citation18–20 Several studies have already underlined suboptimal coverage of adolescents with booster doses for diphtheria-tetanus-pertussis and HPV vaccine.Citation21,Citation22
Immunization of individuals with T1D is of great importance due to the susceptibility of these patients to vaccine-preventable infectious diseases. Data on this subject is scant, but vaccination coverage for this specific group is inadequate. A cross-sectional study, which assessed vaccination coverage of 275 children with chronic diseases, showed that children with T1D had the lowest vaccination coverage rates against diphtheria, tetanus, pertussis, hepatitis B, polio and Hib, and the highest rate of delayed doses as compared to other chronic disease groups. The same study showed influenza vaccination rate lower than 60%.Citation23 Non-adherence to influenza recommendation was due to lack of information in 45.5% of cases.
The same reason was recalled for inadequate immunization for hepatitis B (60%), MMR and Hib (50%). Delay in pneumococcal vaccination was attributed to fear for the vaccine in 33.3%.Citation24 In a similar study on vaccination rates of children with chronic diseases, influenza vaccine was not provided in children with T1D because of doctors’ misunderstanding of recommendations. Influenza vaccine coverage was estimated to be in the range of 21–61%, and pneumococcal vaccine rate was lower than 25%.Citation25 Influenza vaccination rate in patients with T1D has been reported to remain inadequate at levels that range from 30% in children to around 40% in adults.Citation9Citation26–27 More recent studies have shown rise in influenza vaccination rates in children with T1D up to 62.6%.Citation28
In the present study, better glycemic control as indicated by a level of HbA1c below 7% was found to be associated with better influenza vaccination. No other factor among those searched for was proved to affect influenza vaccination. This is in accordance with previous studiesCitation28 and implies that compliance with diabetic treatment and lifestyle leads to compliance with disease-associated vaccination recommendations as well.
Hepatitis B vaccination was suboptimal in the adult population in our study in contrast to high rates in children with T1D. This finding is in accordance with similar findings for the adult population with T1D in other studies that ascribe this to lack of information and physicians’ unawareness of this special recommendation.Citation26,Citation29 Discrepancy between vaccination rates in children and adults with T1D was also found for pneumococcal vaccine. This is the case for the 13-valent conjugate vaccine whereas for the 23-valent polysaccharide extremely low rates were recorded for both age groups. The latter is in accordance with published data for all diabetic individuals,Citation26,Citation27 especially for children with T1D.Citation10
Published data on the vaccination coverage of close contacts of diabetic patients in order to improve their protection against vaccine-preventable diseases, is limited. In our study, approximately 50% of the close contacts of diabetic individuals were not vaccinated against influenza. Interestingly, patients better vaccinated for influenza had better vaccinated close contacts. This study focused on influenza vaccination of 605 close contacts of 226 children, adolescents, and adults with T1D and found low rates. Even lower rates have been reported in one study that assessed close contacts of patients with T2D and found that around 30% of close contacts were vaccinated for influenza.Citation24 Further analysis of close contacts’ immunization status revealed no age or gender discrepancies. Reasons for lack of vaccination have included concerns about safety and efficacy as well as misinformation about the necessity of influenza vaccine.
A major advantage of the present study is the assessment of a large population of close contacts within families of individuals with T1D of different age groups and can give a good insight into the present situation. A limitation is that the studied population of adults and children with T1D comprises most of the people with T1D living in Crete (around 70%) but not all of them. Nevertheless, they represent the majority of individuals with T1D from the two major diabetic centers of Crete that were contacted in person or by phone with no biased eligibility criteria. In addition, information on close contacts’ vaccination was based on self-recall, but this limitation can be surpassed by the fact that the annual influenza vaccine can be easily recalled.
In conclusion, T1D individuals were sufficiently vaccinated regarding the basic vaccination schedule, but inadequately covered for adolescence and group-specific vaccines. Their family contacts were not sufficiently vaccinated for influenza. The results of the present study imply that indirect protection of individuals with chronic diseases and especially T1D can be maximized with better understanding of misconceptions. Strategies should focus on improved vaccine uptake of individuals with poor glycemic control and their close contacts. General practitioners along with pediatricians should be alert and inform on group-specific vaccines. Pediatric and adult endocrinologists can also play a crucial role for compliance of diabetic individuals with the standard and group-specific vaccine recommendations. This could, in turn, augment close contacts’ vaccination as shown by the association of the rate of vaccinations and compliance to insulin and lifestyle treatment for diabetes with better close contacts’ influenza immunization. Implementation of vaccine uptake strategies for special groups such as individuals with type 1 diabetes could improve vaccination compliance and contribute to public health issues related to vaccine-preventable diseases.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Diallo D, Santal C, Lagrée M, Martinot A, Dubos F. Vaccination coverage of children with chronic diseases is inadequate especially for specifically recommended vaccines. Acta Paediatr. 2020;109(12):2677–84. Epub 2020 Apr 27. PMID: 32239549. doi:10.1111/apa.15275.
- Wang Y, Cheng M, Wang S, Wu F, Yan Q, Yang Q, Li Y, Guo X, Fu C, Shi Y, et al. Vaccination coverage with the pneumococcal and influenza vaccine among persons with chronic diseases in Shanghai, China, 2017. BMC Public Health. 2020;20(1):359. PMID: 32188428. doi:10.1186/s12889-020-8388-3.
- Streisand R, Monaghan M. Young children with type 1 diabetes: challenges, research, and future directions. Curr Diab Rep. 2014;14(9):520. PMID: 25009119. doi:10.1007/s11892-014-0520-2.
- DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet. 2018;391(10138):2449–62. doi:10.1016/S0140-6736(18)31320-5.
- Carey IM, Critchley JA, DeWilde S, Harris T, Hosking FJ, Cook DG. Risk of infection in type 1 and type 2 diabetes compared with the general population: a matched Cohort study. Diab Care. 2018;41(3):513–21. Epub 2018 Jan 12. PMID: 29330152. doi:10.2337/dc17-2131.
- Centers for Disease Control and Prevention. Vaccinations for adults with diabetes 2020. Saint Paul (Minnesota): Immunization action coalition [accessed 2020 Nov 10]. https://www.immunize.org/catg.d/p4043.pdf.
- Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: a review of pathogenesis. Indian J Endocrinol Metab. 2012;16(Suppl1):S27–S36. PMID: 22701840. doi:10.4103/2230-8210.94253.
- Kornum JB, Thomsen RW, Riis A, Lervang HH, Schønheyder HC, Sørensen HT. Diabetes, glycemic control, and risk of hospitalization with pneumonia: a population-based case-control study. Diab Care. 2008;31(8):1541–45. Epub 2008 May 16. PMID: 18487479. doi:10.2337/dc08-0138.
- Rabbone I, Scaramuzza AE, Iafusco D, Bonfanti R, Lombardo F, Cherubini V, Toni S, Cerutti F, Zuccotti GV. Pandemic influenza vaccination coverage in children with type 1 diabetes: analysis from seven Italian centers. Hum Vaccin. 2011;7(12):1291–92. Epub 2011 Dec 1. PMID: 22108031. doi:10.4161/hv.7.12.18335.
- Mambwe T, Janardhan S, Quattrocchi E, Sheffer-Babila S. SUN-139 pneumococcal vaccination rates among pediatric diabetics. J Endocr Soc. 2019;3(Suppl 1):139. doi:10.1210/js.2019-SUN-139.
- Calliari LE, Almeida FJ, Noronha RM. Infections in children with diabetes. J Pediatr (Rio J). 2020;96(Suppl 1):39–46. Epub 2019 Oct 27. PMID: 31666181. doi:10.1016/j.jped.2019.09.004.
- Streamline MCN. Diabetics at an increased risk for tetanus infections 2005. Austin (TX): PerfectCube, LCC [accessed 2020 Nov 10]. https://www.migrantclinician.org/files/streamline/20050708_mcn_streamline.pdf.
- Guzman – Cottril JA, Phillipi CA, Dolan SA, Nyquist AC, Win A, Siegel J. Free vaccine programs to cocoon high-risk infants and children against influenza and pertussis. Am J Infect Control. 2012;40(9):872–76. PMID: 23116758. doi:10.1016/j.ajic.2012.05.028.
- Nitsch-Osuch A. Cocoon strategy of vaccinations: benefits and limitations. In: Farhat A, Hassan H, Hani O, editors. Vaccines. London (UK): IntechOpen Limited; 2017. p. 3–22.
- Fiore AE, Shay DK, Broder K, Iskander J, Uyeki T, Mootrey G, Bresee J, Cox N. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58(RR–8):1–52. PMID: 19644442.
- Glezen WP. Emerging infections: pandemic influenza. Epidemiol Rev. 1996;18(1):64–76. PMID: 8877331. doi:10.1093/oxfordjournals.epirev.a017917.
- Chi RC, Reiber GE, Lipsky BA, Boyko EJ, Neuzil KM. Influenza vaccination rates of children in households with high-risk adults. Public Health Rep. 2010;125(2):192–98. PMID: 20297745. doi:10.1177/003335491012500207.
- Pavlopoulou ID, Michail KA, Samoli E, Tsiftis G, Tsoumakas K. Immunization coverage and predictive factors for complete and age-appropriate vaccination among preschoolers in Athens, Greece: a cross–sectional study. BMC Public Health. 2013;13(1):908. PMID: 24083352. doi:10.1186/1471-2458-13-908.
- Bitsori M, Ntokos M, Kontarakis N, Sianava O, Ntouros T, Galanakis E. Vaccination coverage among adolescents in certain provinces of Greece. Acta Paediatr. 2005;94(8):1122–25. PMID: 16188859. doi:10.1111/j.1651-2227.2005.tb02055.x.
- Babatsikou F, Vorou R, Vardaki Z, Galani S, Ktenas E, Koutis C. Childhood vaccination uptake and factors affecting this in Athens, Greece. Health Sci J. 2010;4:237–44.
- Sakou II, Tsitsika AK, Papaevangelou V, Tzavela EC, Greydanus DE, Tsolia MN. Vaccination coverage among adolescents and risk factors associated with incomplete immunization. Eur J Pediatr. 2011;170(11):1419–26. Epub 2011 Apr 5. PMID: 21465121. doi:10.1007/s00431-011-1456-z.
- Donadiki EM, Jiménez-García R, Hernández-Barrera V, Carrasco-Garrido P, López de Andrés A, Velonakis EG. Human papillomavirus vaccination coverage among Greek higher education female students and predictors of vaccine uptake. Vaccine. 2012;30(49):6967–70. Epub 2012 Sep 25. PMID: 23022147. doi:10.1016/j.vaccine.2012.09.028.
- Pandolfi E, Carloni E, Marino MG, Ciofi degli Atti ML, Gesualdo F, Romano M, Giannattasio A, Guarino A, Carloni R, Borgia P, et al. Immunization coverage and timeliness of vaccination in Italian children with chronic diseases. Vaccine. 2012;30(34):5172–78. Epub 2011 Mar 15. PMID: 21414380. doi:10.1016/j.vaccine.2011.02.099.
- Yank L, Nan H, Liang J, Chan YH, Chan L, Man Sum RW, Kwan YM, Zhou F, Meng H, Ping Suen LK. Influenza vaccination in older people with diabetes and their household contacts. Vaccine. 2017;35(6):889–96. Epub 2017 Jan 13. PMID: 28094076. doi:10.1016/j.vaccine.2017.01.004.
- Giannattasio A, Squeglia V, Lo Vecchio A, Russo MT, Barbarino A, Carlomagno R, Guarino A. Pneumococcal and influenza vaccination rates and their determinants in children with chronic medical conditions. Ital J Pediatr. 2010;36(1):28. PMID: 2034614. doi:10.1186/1824-7288-36-28.
- Alvarez CE, Clichici L, Patricia Guzmán-Libreros A, Navarro-Francés M, Ena J. Survey of vaccination practices in patients with diabetes: a report examining patient and provider perceptions and barriers. J Clin Transl Endocrinol. 2017;9:15–17. PMID: 29067263. doi:10.1016/j.jcte.2017.06.002.
- Alcusky MJ, Pawasauskas J. Adherence to guidelines for Hepatitis B, pneumococcal, and influenza vaccination in patients with diabetes. Clin Diabetes. 2015;33(3):116–22. PMID: 26203204. doi:10.2337/diaclin.33.3.116.
- Centers for Disease Control and Prevention. Flu vaccination coverage, United States, 2018–19 influenza season 2019 [accessed 2021 Jan 21]. https://www.cdc.gov/flu/fluvaxview/coverage-1819estimates.htm.
- Villarroel MA, Vahratian A. Vaccination coverage among adults with diagnosed diabetes: United States, 2015. NCHS Data Brief. 2016;(265):1–8. PMID: 27930282.