ABSTRACT
Antibodies can provide antiviral protection through neutralization and recruitment of innate effector functions through the Fc domain. While neutralization has long been appreciated for its role in antibody-mediated protection, a growing body of work indicates that the antibody Fc domain also significantly contributes to antiviral protection. Recruitment of innate immune cells such as natural killer cells, neutrophils, monocytes, macrophages, dendritic cells and the complement system by antibodies can lead to direct restriction of viral infection as well as promoting long-term antiviral immunity. Monoclonal antibody therapeutics against viruses are increasingly incorporating Fc-enhancing features to take advantage of the Fc domain, uncovering a surprising breadth of mechanisms through which antibodies can control viral infection. Here, we review the recent advances in our understanding of antibody-mediated innate immune effector functions in protection from viral infection and review the current approaches and challenges to effectively leverage innate immune cells via antibodies.
Introduction
Antibodies are the effector molecules of the humoral immune system that circulate throughout the body, providing both short-term and long-term immunity against pathogens. The antibody molecule at its most general can be divided into the antigen binding fragment domain (Fab) and the antibody constant domain (Fc). The Fab domain confers the specificity of the antibody and the Fc domain interacts with Fc-receptor bearing innate and adaptive immune cells and effector molecules.
There are many roles that antibodies play in antiviral protection: blocking initial viral infection into host cells via neutralization, limiting dissemination of infection throughout host tissues, killing/clearing infected cells, initiation of inflammation, enhancing antigen presentation, enhancing development of T cell responses, and dampening of inflammation. Thus, while the induction of antibodies specific for a given pathogen is often used as a measure of immunity, the mechanisms through which antibodies can provide protection may differ significantly across pathogens. Simply the induction of an antibody response may be insufficient to protect if those antibodies do not have the specific features needed to provide protection against that pathogen; thus, the ability to provide protection is related to the quantity and quality of pathogen-specific antibodies.
As antibodies can limit viral infection through multiple mechanisms and cells, antibody-based therapies are increasingly becoming an attractive approach to treat viral infection, especially for viruses with high rates of mortality or cause severe disease. Most of the current approaches to identify and develop new antibodies focus on neutralization as the sole mediator of protection, yet the role of Fc domain is becoming increasingly appreciated as a critical feature of antiviral immunity, and thus, there is a growing interest in identifying approaches to harness and leverage the Fc domain. In this review, we will discuss the role of antibody-mediated induction of innate immune effector functions in protection from viral infection and review the current approaches that are under investigation to effectively leverage innate immune cells via antibodies.
Antibody isotypes and Fc-receptors
Humans have several different antibody isotypes, each serving unique roles in humoral immunity. Immunoglobulin G (IgG) is the most abundant antibody isotype in serum and is further classified into IgG subclasses (IgG1, IgG2, IgG3, and IgG4). The IgG subclasses differ in abundance with IgG1 representing approximately 60% of circulating IgG, IgG2 represent 32%, and IgG3 and IgG4 accounting for the rest. Immunoglobulin A (IgA) is the second most abundant antibody isotype in serum, although when accounting for levels within tissues and in mucosal secretions and saliva, IgA is the most abundant antibody in the body. IgA can be further classified into IgA1 and IgA2 subclasses, which differ mainly in hinge length, and can be produced either as monomeric in the serum or dimeric (two monomers connected by a secretory J-chain) in the mucosa. Immunoglobulin M (IgM) is the largest of the antibody isotypes, present as pentamers or hexamers, and is the first antibody to be generated in a humoral immune response. IgE and IgD are two additional isotypes with roles in allergic reactions and B cell maturation, respectively.
Antibodies can activate innate immune cells via engagement of type I and type II Fc receptors (FcRs). Human type I FcRs include FcγRs, and FcαR, and type II FcRs include CD23/FcεRII and DC-SIGNCitation1. The type I FcRs that engage IgG are the activating receptors FcγR1/CD64, FcγR2A/CD32A, FcγR3A/CD16A, FcγR3B/CD16B and the inhibitory receptor FcγR2B/CD32B; the FcR that binds IgA is FcαR/CD89; and IgE binds to FcεR/CD23. All innate immune cells express FcRs (monocytes, macrophage, neutrophils, dendritic cells, eosinophils, basophils, NK cells) in varying combinations and levels.Citation2 Moreover, the expression of FcRs is not limited to the classic set of innate immune cells – B cells, platelets,Citation3 a subset of γδ T cells,Citation4 and a small subset of resting and activated CD4+ T cellsCitation5,Citation6 also express FcγRs – expanding the repertoire of cells that can respond to antibodies. Importantly, as different cell types express different levels and combinations of FcγRs/FcαRs, the combination of FcR expression, the balance of activating and inhibitory FcγR binding, cell type, and location together confer an incredibly diverse set of innate responses that can collectively shape antiviral immunity and disease ().
Figure 1. Protection from both ends of the antibody.
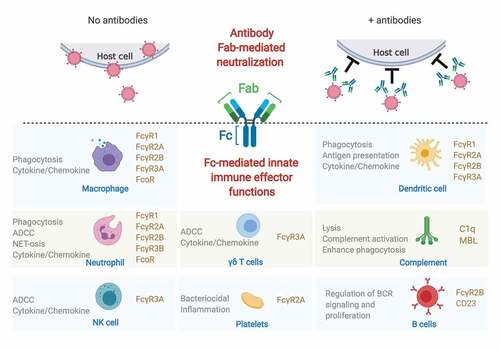
The functional diversity is further amplified by antibody features that tune the interaction with FcRs: antibody isotype and IgG subclass, antibody glycosylation, and the Fab-epitope. The four human IgG subclasses differ in affinity for FcγRs with IgG3> IgG1> IgG4> IgG2,Citation7 thus the composition of a polyclonal IgG response can set different thresholds for induction of innate immune effector functions. Antibody glycosylation plays a notable role in further modulating and tuning IgG affinity for Fcγ-receptors in both directions, where fucose-free (afucosylated) glycans enhance affinity for the activating FcγR3A and FcγR3B,Citation8 and sialyation has been proposed as a mechanism to skew antibody affinity from type I FcRs toward type II FcRs.Citation9 The role of the Fab in further shaping Fc-FcR interactions is only beginning to be appreciated, whereby affinity of Fab-antigen interactions, distance from target cell membrane, valency of antibody-antigen binding and immune complex size can all impact induction of effector function.Citation10–13
Activation of immune cells through type I FcγRs occurs via direct interaction between the FcγR and the Cγ2 domain of the human IgG that have been demonstrated through a number of structural studies (reviewed elsewhere inCitation14). Antibody-mediated cross-linking of the activating FcγR induces signaling through a cytoplasmic immunoreceptor tyrosine-based activation motif (ITAM) domain, ultimately leading to the induction of antibody-dependent effector functions, such as ADCC and ADCP, described in more detail below. Conversely, antibody-mediated cross-linking of the inhibitory FcγR2B acts to inhibit key activating molecules though through the cytoplasmic immunoreceptor tyrosine-based inhibitory motif (ITIM) domain, providing a balance to activating FcγRs in innate immune cells. FcγR2B is the only FcγR expressed on B cells and serves to limit the B cell receptor (BCR) signaling and B cell proliferation in the absence of BCR engagement, a process critical to the generation of high affinity antibodies, B cell selection, and tolerance.Citation15,Citation16
Engagement of the type II FcRs CD23 and DC-SIGN have, to date, been primarily associated with negative regulation of immune responses, as demonstrated by the anti-inflammatory properties of intravenous immunoglobulin (IVIG).Citation17,Citation18 An elegant model of IVIG-mediated amelioration of inflammation indicated that sialylated IgG-induced signaling through DC-SIGN in dendritic cells induces a cascade of events that ultimately leads to upregulation of the inhibitory type I FcγR2B on effector cells to limit inflammatory responses.Citation17,Citation19 Sialylated antibodies have also been proposed to activate CD23-mediated regulation of B cell responses through upregulation of FcγR2B.Citation20 The mechanisms through which IgG activates type II FcRs is still unclear, as whether IgG interacts directly with CD23 and DC-SIGN has been the subject of debate,Citation21–23 yet these studies highlight the interplay between type I and type II FcRs in shaping the immune response.
Direct and indirect Fc-mediated innate immune effector functions limit viral infection and dissemination
Direct Fc-mediated innate immune effector functions
ADCC
One of the primary effector functions that can directly limit viral infection is antibody-dependent cellular cytotoxicity (ADCC), which refers to the killing of target cells by innate immune cells. In humans, NK cells and neutrophils are the primary mediators of ADCC and can rapidly kill opsonized infected cells, and several studies demonstrate that FcγR3A-expressing γδ T cells are also mediators of ADCC.Citation4,Citation24 NK cells in most individuals express a single Fc-receptor, FcγR3A, and thus FcγR3A is often considered as the “ADCC receptor.” Measuring ADCC requires target cells that are expressing the pathogen antigen of interest and effector cells, such as primary NK cells or a modified NK cell line, such as NK92 cells expressing FcγR3A/CD16. Ideally, the target cell would be an infected cell or pathogen, yet the use of such target cells has traditionally been a challenge due to high containment requirements of many pathogens, variability between experiments, and are often low-throughput. As an alternative, many laboratories use reporter cell lines that express FcγR3A as an ADCC surrogate, although it should be noted that these assays do not measure cellular cytotoxicity, and thus may not accurately reflect in vivo ADCC activity. Similarly, even “true ADCC” assays that measure cellular cytotoxicity in vitro may not recapitulate in vivo ADCC activity, as seen in the context of HIV, where specific epitopes on the HIV protein gp120 are exposed when using recombinant antigen but are hidden or not exposed in the context of replicating virus.Citation25,Citation26
In mice, ADCC is predominantly mediated by macrophage and neutrophils rather than NK cells via the murine-specific FcγRIV,Citation27 highlighting conserved effector mechanisms but divergence in cell- and FcR-mediators between species. Following activation of immune cells via FcR ligation, the activated cell secretes cytotoxic molecules such as perforin and granzyme toward the target cell, leading to cell death. High-throughput assays using labeled target cells and primary human NK cells to measure ADCC have been described,Citation28 and NK cell activation but not cellular cytotoxicity can also be measured in the absence of target cells using antigen-coated immunoassay plates.Citation29,Citation30
ADCP
Antibody-dependent cellular phagocytosis (ADCP) by phagocytic cells such as monocytes, macrophage, dendritic cells, and neutrophils contribute to clearance of pathogens and infected cells. Following engagement of FcRs on phagocytic cells, the opsonized pathogen/target cell is engulfed into a phagosomal compartment, which then matures, acidifies, and fuses with the lysosome to ultimately kill the pathogen/target cell. Phagocytosis of antigen-coated target cells or beads can be experimentally measured in a number of different cell types,Citation31,Citation32 and advances in multiplexing analysis allow for simultaneous measurement of phagocytosis of different immune complexes.Citation33 Similar to the challenges with measuring ADCC, the phagocytosis of labeled target cells or recombinant antigen-coated beads rather than infected cells or pathogens may not accurately reflect in vivo activity due to different antigenic targets that may be exposed in vivo as well as a myriad of other cellular signals on infected cells that may augment or inhibit phagocytic activity.
Monocytes, macrophages, and dendritic cells express high levels of FcγR2A, and as functional blocking of FcγR2A reduces ADCP in monocytes,Citation31,Citation34 signaling via FcγR2A is often used in reporter cell lines as an ADCP surrogate, although it should be noted that these assays do not actually measure cellular phagocytosis. Moreover, different cell types may use different FcRs to induce phagocytosis. For example, neutrophil phagocytic activity is also mediated by FcαR and FcγRI upon activation.Citation35 Regardless of which FcR induces phagocytosis, as phagocytic cells are often the first cells at a site of infection, ADCP can be a potent mechanism to rapidly and specifically target opsonized pathogens and infected cells.
ADCD/CDC
Antibody-dependent complement activation and deposition (ADCD) or complement-dependent cytotoxicity (CDC) can enhance neutralization through lysis of infected cells and pathogens via formation of the membrane attack complex. ADCD can also augment phagocytosis through complement receptor CR3/CD11b/Mac-1 on phagocytic cells.Citation36 Antibodies predominantly activate the complement cascade via C1q and the classical pathway, with IgM and IgG3 having the highest complement-activating activity, followed by IgG1 and IgG2. IgG4 does not activate the complement cascade. ADCD can be measured through deposition of the main central complement component, C3, onto opsonized targets,Citation37 or through lysis of target cells in the presence of complement.Citation38
Together, ADCC, ADCP, and ADCD have been the most commonly studied Fc-dependent mechanisms of viral control, yet a growing body of literature has uncovered the additional ways that antibodies can activate immune cells and induce effector mechanisms. For example, antibody-dependent cellular trogocytosis (ADCT) has emerged as a mechanism leading to infected target cell death whereby the Fc-receptor expressing effector cell, such as neutrophils and monocytes, “nibbles” on an antibody-opsonized target cell.Citation39,Citation40 The “nibbled” part of the target cell may then be expressed on the surface of the effector cell, distinguishing trogocytosis from phagocytosis,Citation41 and during this process of “nibbling” and transferring there is significant mechanical disruption to the target cell membrane that can induce necrotic and highly inflammatory cell death, recently termed as trogoptosis.Citation40 Trogocytosis has been shown to be induced by HIV-specific antibodiesCitation39,Citation41 and SARS-CoV-2 spike-specific antibodies,Citation42 and plays a critical role in antibody-mediated killing of cancer cells by neutrophils.Citation40 The specific role of ADCT controlling viral infection is still unclear, yet these studies have expanded our understanding of the breadth of mechanisms through which antibodies can induce killing of target cells.
Analysis of the role of antibody-mediated activation of FcR-bearing γδ T cells or platelets in infection has been limited to date yet may contribute to anti-pathogen control and disease outcome. For example, the expansion of FcγR3A expressing γδ T cells has been observed in the context of human cytomegalovirus (HCMV) infectionCitation43 and in chronic malaria exposure,Citation44 and is associated with antibody-mediated restriction of viral replication in HCMV-infected cells. Bactericidal activity of antibody-activated platelets has been observed,Citation45,Citation46 yet the role of platelets in viral infection has been more commonly associated with pathology. Most recently, hyperactivation of platelets via FcγR2A was observed in severe COVID-19 patients and was associated with cardiac dysfunction markers.Citation47 Future studies will likely uncover novel mechanisms by which these cells contribute to control or disease.
Indirect Fc-mediated innate immune effector functions
In addition to directly helping to control infection through targeting the pathogen and/or the infected cell, antibodies can also mitigate infection indirectly by inducing cytokine and chemokine secretion from innate immune cells that serves to activate antiviral pathways in uninfected target cells. For example, production of interferon gamma from antibody-activated NK cells and CD16+ γδ T cells markedly reduced HCMV replication,Citation43 and stimulates type II interferon-mediated gene expression in neighboring cells, potentially limiting interferon-sensitive viruses. Activation of the complement cascade via antibodies induces not only lysis of infected cells but also the generation of the chemo-attractants and immunomodulators C3a and C5a resulting in influx of monocytes, macrophage and NK cells.
Sialylated antibodies have been proposed to help develop better humoral immune responses through increased delivery of antigen to antigen-presenting cells and setting higher thresholds for B cell activation, leading to the production of higher affinity antibodies.Citation20,Citation48 In the context of B cells, the skewing of sialylated antibodies to bind to the type II FcR CD23 on B cells drives increased expression of the FcγR2B, increasing the threshold for B cell proliferation within the germinal center, leading to preferential expansion of high affinity antibodies.Citation20 Increased delivery of viral antigen to follicular dendritic cells can be enhanced via sialylated antibody-mediated activation of complement,Citation48 and the formation of immune complexes resulting from ADCC help generate long-term CD8+ T cell immunity via increased presentation by dendritic cells.Citation49
As antibodies can limit viral infection through multiple mechanisms and cells, antibody-based therapies are increasingly becoming an attractive approach to treat viral infection. However, given the vast mechanisms that antibodies can engage immune cells, comprehensive approaches to define drivers of antiviral immunity and modulation of disease are critical to effectively and safely use monoclonal antibodies for infectious diseases. Along those lines, profiling approaches such as systems serology that measure qualitative and quantitative features of monoclonal and polyclonal antibodies have helped identify functional correlates of protection against a number of viral infections.Citation50–53 These correlates may be tested to determine if they are mechanistic correlates through antibody engineering aimed at enhancing/ablating specific Fc effector functions. Thus together, these approaches may help define how specific antibody functions contribute to antiviral immunity.
Passive immunity: functional features of effective antiviral immunotherapeutics
High-throughput isolation and identification platforms have accelerated antibody discovery for infectious diseases
There has been an explosive interest in the development of antibody-based therapeutics to treat a range of infectious diseases over the past 10 years, culminating in the recent FDA approval of monoclonal antibody cocktails for Ebola virus and emergency use authorization for SARS-CoV-2 infection.Citation54,Citation55 Platforms to rapidly identify, clone, and characterize antibodies from B cells isolated from convalescent patients of various diseases and immunization of genetically engineered mice that express human immunoglobulin genes have accelerated the generation of antibody-based therapeutics.Citation56–58 Coupled with recombinant monoclonal antibody production, these new platforms can rapidly identify hundreds of virus-specific antibodies, sometimes even from a single convalescent donor,Citation59 that can be screened for neutralization, cross-neutralization, or binding to a specific viral epitope.Citation60
These platforms provide the ability to rapidly produce effective therapeutics for use in an outbreak and were put to the test in the SARS-CoV-2 pandemic. Fortunately, these efforts were successful and resulted in the availability of several monoclonal antibodies in clinical evaluation and use within a matter of months. The success of these platforms has prompted efforts to develop panels of monoclonal antibodies against other highly pathogenic viruses with emergent pandemic potential, such as vector-borne viruses including bunyaviruses and alphaviruses.Citation61–65 Incidence of disease caused by vector-borne viruses threaten human health as the range of the host vector increases and spreads,Citation66–68 and coupled with high morbidity and mortality, the development of such therapeutics will be critical for the treatment of patients and add to the antiviral toolkit that we have to combat viruses.
While the Fab domains clearly need to be selected based on virus-specificity and neutralization potency, a common strategy to rapidly identify the necessary Fc features to enhance antibody efficacy may further accelerate development of antibody therapeutics. Single B cell sequencing and serum proteomic analysis approaches that are used in the high-throughput antibody discovery platforms enables the identification of the native Fab variable domain and IgG subclass/antibody isotype pairing, providing some insight into the natural function of isolated antibodies.Citation69 However, whether the same antibody features/effector mechanisms are required to control different viruses is not clear, and more importantly, it will be critical to determine if enhancement of a specific function may contribute to immunopathology, disease or enhanced infection. The latter has already been demonstrated in the context of respiratory syncytial virus and dengue virus where activation of a subset of innate immune cells via specific FcRs contributes to more severe disease,Citation70–72 thus highlighting the critical importance of balancing protective and pathologic responses.
Lessons from monoclonal antibody therapeutics against viruses
Whether effector function is required for antibody-mediated protection is still being defined for many viruses, and thus defining and selectively enhancing specific functions required for protection against a given pathogen remains the next frontier for monoclonal antibody therapeutics. However, there are still many lessons to be gleaned from the current body of literature regarding the functional antibody features that contribute to protection (). One of the most comprehensive analyses of the role of antibody effector functions has come from the study of monoclonal antibodies against Ebola virus, due in part to the sheer number of antibodies that have been cloned and characterized against this highly pathogenic virus and the collaborative work of the filovirus scientific community.
Figure 2. Lessons from monoclonal antibody therapeutics against viruses.
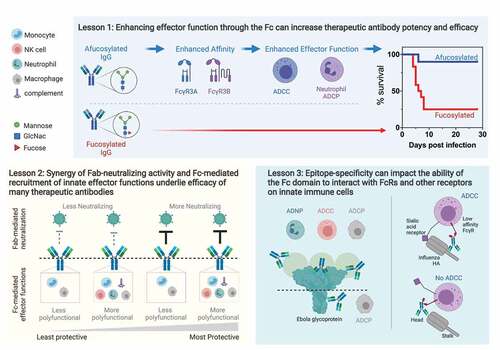
Lesson 1: enhancing effector function through the Fc can increase therapeutic antibody potency and efficacy
The development of monoclonal antibody therapeutics for Ebola virus stemmed in part from the need to have effective countermeasures for highly pathogenic viruses with high rates of mortality that could be used in the event of a known exposure. The first generation of antibodies that were evaluated for post-exposure prophylaxis included the neutralizing antibody KZ52, isolated from an Ebola virus survivor,Citation73 along with a handful of additional antibodies isolated from vaccinated mice that were subsequently chimerized to a human IgG1.Citation74 These antibodies targeted different regions of the Ebola virus glycoprotein, and importantly, cocktails of antibodies that consisted of either neutralizing and non-neutralizing antibodies together or non-neutralizing antibodies alone were able to provide post-exposure protection in non-human primate modelsCitation75,Citation76 pointing to a role for Fc-effector function in antibody-mediated protection.
The rapid and widespread emergence of an Ebola virus outbreak in West Africa in 2013 highlighted the need for effective vaccines and therapeutics for treatment of human disease. One of the most attractive therapeutics to treat Ebola infection was monoclonal antibody therapy, due in part from the existing body of work showing post-exposure efficacy in animal models and the established clinical use of monoclonal antibodies for infectious diseases. Efforts by the scientific community to further the development of monoclonal antibody-based therapies led to the identification of new antibodies that displayed potent neutralizing activity and enhanced effector functionality,Citation59,Citation77–82 ultimately leading to the approval of an antibody cocktail, Inmazeb, by the FDA for use in humans in October 2020 following clinical efficacy trials carried out in west Africa and in the DRC.Citation83,Citation84 The sudden abundance of new monoclonal antibodies against Ebola offered an opportunity to systematically analyze and define the Fab and Fc features of protective antibodies against a lethal Ebola virus infection and to date, dozens of antibody panels have been evaluated,Citation51,Citation59,Citation81,Citation85–88 leading to general conclusions regarding broadly neutralizing epitopes and the role of effector function in protection. Broadly speaking, polyfunctional antibodies are more likely to be protective in animal models with the importance of effector function increasing as neutralization activity decreases.Citation52 Specifically, the ability to induce multiple innate immune effector functions correlated with protective efficacy for monoclonal antibodies with moderate neutralizing activity, but not for antibodies with potent neutralizing activity,Citation52 highlighting the synergistic and diverse ways that antibodies can provide protection.
One of the current strategies to enhance effector functionality of monoclonal antibodies is through the production of afucosylated recombinant neutralizing antibodies in glyco-engineered cell lines. Afucosylated antibodies demonstrate increased affinity for FcγR3A,Citation8 thus enhancing FcγR3A-driven effector functions such as ADCC. Production of afucosylated versions of both neutralizing and non-neutralizing Ebola antibodies boosted protective efficacy and antibody potency coincident with enhanced ADCC activity or binding to murine FcRs.Citation89,Citation90 Beyond viruses, afucosylated cancer antibodies also show increased efficacy against cancer targets,Citation91,Citation92 highlighting the broader utility of leveraging the Fc domain for therapeutic antibodies. Similarly, several recombinant monoclonal antibodies that encode point mutations within the Fc domain that increase affinity for multiple FcγRs display increased protective efficacy against HIV and influenza, leading to increased survival of challenged animals and accelerated viral clearance from infected tissues.Citation93,Citation94 However, it must be noted that increasing FcγR3A does not always translate to increased protective efficacy in vivo and is monoclonal antibody, and disease/animal model specific. For example, afucosylation of the HIV broadly neutralizing monoclonal antibody b12 did not enhance pre-exposure protection against a mucosal challenge in a rhesus macaque model, despite in vitro enhancement of ADCC activity,Citation95 yet post-exposure therapeutic efficacy against an intraperitoneal challenge was enhanced via increased ADCC/ADCP accompanied by lower viral loads for a highly potent broadly neutralizing HIV antibody, 3BNC117, in a humanized mouse model.Citation94 Importantly, given recent associations between afucosylated antibodies and disease severity in the context of Dengue virus and SARS-CoV-2,Citation70,Citation96–98 reducing interaction with FcγR3A may be critical to avoid monoclonal antibody-mediated immunopathology for some viral infections.
Lesson 2: synergy of Fab-neutralizing activity and Fc-mediated recruitment of innate effector functions underlie efficacy of many therapeutic antibodies
Monoclonal antibodies are often categorized into “neutralizing” and “non-neutralizing,” with the implication being that the mechanism of protection is either neutralization or induction of effector function. Yet, there is an increasing appreciation for the role of collaboration between Fab and Fc-activities in protection. Ablation of FcγR interactions in HIV-specific broadly neutralizing antibodies resulted in reduced efficacy in non-human primates,Citation99 and the use of Fc-enhanced variants of broadly neutralizing antibodies displays enhanced viral clearance in humanized mouse models.Citation94,Citation100 Importantly, it is becoming increasingly clear that the synergy of a neutralizing Fab and functional Fc domain likely underlies the protective efficacy of highly potent antibodies in the context of many different viruses, including Ebola virus,Citation52,Citation77,Citation87 influenza,Citation93,Citation101 SARS-CoV-2,Citation102–106 and alphaviruses.Citation107,Citation108 Of note, a recent study that was designed to quantify the contribution of effector function to antiviral efficacy of HIV-specific neutralizing monoclonal antibodies found that effector function accounted for up to 39% of antiviral activity across several different animal models.Citation109 Next generation antibodies for many viruses, including HIV and SARS-CoV-2, are increasingly being selected for potent neutralizing activity and some of these antibodies are able to provide complete prophylactic protection in the absence of Fc-effector functions.Citation103,Citation110 However, it is important to note that the role of a functional Fc domain for neutralizing antibodies is likely most important in the context of post-exposure therapeutic settings, where infection has already been established, and thus clearing infected cells via Fc-dependent mechanisms in addition to preventing infection of new cells via neutralization is critical for protection. The latter situation was recently demonstrated in the context of SARS-CoV-2 monoclonal antibodies,Citation111 and highlights that different antiviral mechanisms that antibodies need to harness when administered before or after infection.
In addition to the production of antibodies in glyco-engineered cells lines, recent advances in antibody engineering platforms have allowed for the pairing neutralizing Fab domains with highly functional Fc domains and provide an avenue to rapidly develop recombinant monoclonal antibodies with increased potency and directed effector functionality.Citation112 Thus, these production and engineering strategies, described in more detail below, allow for next-generation monoclonal antibodies that take advantage of both ends of the antibody.
Lesson 3: epitope-specificity can impact the ability of the Fc domain to interact with FcRs and other receptors on innate immune cells
With respect to IgG, there are two well-known features of the Fc that modulate effector function: IgG subclass and glycosylation. A majority of monoclonal antibodies are produced as a human IgG1 and are increasingly being analyzed for glycosylation or produced in cell lines to generate antibodies with homogenous glycosylation. Yet, differences in functional activity have still been noted between antibodies despite identical subclass and glycosylation,Citation52 and these differences may be attributed to the antigenic epitope targeted by the antibody Fab domain. The epitope-skewing of effector function has been observed in the context of Ebola virus,Citation11,Citation113 influenza,Citation12,Citation114 and HIV,Citation115,Citation116 adding another feature that can modulate the effector potential of antibodies.
In the context of Ebola virus, antibodies targeting different regions of the glycoprotein differ in the ability to recruit specific innate effector functions. For example, antibodies that bind to the top tier of the glycoprotein and/or the secreted glycoprotein (sGP) on average induce multiple effector functions, including high levels of ADCC and ADCP,Citation11 whereas those targeting the base of the glycoprotein were more limited in functional activity, recruiting phagocytic functions but not ADCC, despite identical IgG subclass and glycosylation.Citation52 One possible reason for this difference may be that binding to epitopes that are closer to the viral/cellular membrane constrain the Fc domain from engaging with FcγRs. In addition, another hypothesis is that the differences in FcR/cytoskeletal architecture and interactions between NK cells and monocytes/macrophages may further limit interactions between constrained Fc domains with FcγRs and may explain why potentially constrained Fcs can still induce ADCP but not ADCC.Citation10 As different epitopes are linked with differential induction of effector functions, the inclusion of antibodies that target different regions of the Ebola glycoprotein appears to be critical to the success and efficacy of monoclonal antibody cocktails. Specifically, the inclusion of polyfunctional antibodies targeting the glycan cap/sGP in addition to neutralizing antibodies are important in both the two-antibody version of the ZMapp cocktail,Citation117 and in the three antibody Inmazeb cocktail,Citation80 indicating the complementary and synergistic combinations of antibodies.
Epitope-directed effector function has also been noted in the context of influenza, where antibodies that bind to the stalk domain of hemagglutinin (HA) are able to induce ADCC activity whereas neutralizing antibodies that bind to the head domain do not.Citation12,Citation118 The mechanism underlying this epitope specificity is that the induction of effector function against influenza requires not only engagement of the FcR but also interaction between a sialic acid-bearing receptor on the effector cell and the head domain of the HA, which may be occluded by neutralizing head-binding antibodies.Citation114 Interestingly, this two-contact requirement was restricted to low-affinity FcγRs, such as FcγR3A and FcγR2A, as HA head-binding antibodies can still induce activation via the high affinity FcγRI.Citation119 Thus, the anti-HA head antibodies likely selectively recruit effector function from cells expressing FcγRI, which include activated phagocytic cells (neutrophils, dendritic cells, monocytes/macrophages), but not NK cells.
FcR polymorphisms and FcR and innate immune cell dynamics during infection may impact therapeutic antibody efficacy and mechanisms of protection
Polymorphisms in the low affinity FcγRs, FcγR2A and FcγR3A, have been shown to alter IgG affinity for the respective FcγR. For example, a histidine at amino acid position 131 (H131) in FcγR2A notably increases human IgG2 binding over arginine (R131),Citation7 and valine at position 176 (V176) in FcγR3A increasing human IgG1 affinity over phenylalanine (F176),Citation7 leading to elevated levels of ADCP and ADCC, respectively.Citation120 These and other known FcR genetic polymorphisms can impact the efficacy of monoclonal antibodies in the context of cancer therapeutics.Citation121 Moreover, recent evidence suggests that polymorphism within FcγR2C, which is only expressed in approximately 20% of individuals,Citation122,Citation123 was associated with decreased risk of HIV infection in the moderately protective RV144 vaccine trial,Citation124 yet was associated with infection risk following the non-protective Adenovirus 5-vectored HIV clinical vaccine trial.Citation125 Together these studies highlight that FcR variation between individuals is an additional factor that should be taken into account when considering antibody-based therapeutics.
While assays for in vitro characterization of antibody effector function have been established and have predictive value for in vivo protection, there remains the question of how infection impacts FcR expression and innate immune cell function that are critical to consider particularly in the context of post-exposure therapeutic antibodies. The effector function potential of antibodies is typically evaluated in vitro one function at a time using resting innate cells from healthy human donors or in cell culture, but infection itself may modulate abundance of FcR-expressing cells, FcR expression, and activation of innate cells, thereby altering the repertoire of cells that antibodies can leverage during infection. For example, activation of neutrophils leads to upregulation of the high affinity FcγR, FcγRI, which enables enhanced phagocytosis and ADCC activity mediated by monomeric antibodies.Citation126,Citation127 In addition, in vitro polarized macrophages show distinct impacts on phagocytosis and reactive oxygen species (ROS) depending on the mechanism of polarization. Specifically, macrophages polarized by IFNγ (typically considered an M1-polarizing cytokine) displayed induction of higher levels of ROS despite lower phagocytic activity following antibody-mediated phagocytosis compared with macrophages polarized by IL-10 (typically considered an M2-polarizing cytokine),Citation128 indicating that the downstream indirect antibody-effector functions can be modulated by different cytokine milieus.
Developing a fever is the most common symptom of infection by any pathogen and is thought to aid the immune system in combating infection.Citation129 Beyond impacting the stability and infectious potential of the pathogen itself, fever and thermal stress have been shown to increase recruitment of both innate and adaptive immune cells to sites of infectionCitation130,Citation131 and enhance a broad range of anti-pathogen activities in innate immune cells, including respiratory burst, antigen processing, and phagocytosis.Citation132–134 Moreover, viral envelope structural dynamics are impacted by temperature, allowing for antibody access to temporarily exposed epitopes during viral breathing,Citation135 which may be further enhanced during fever. Thus, the efficacy of antibodies to mediate both neutralization and induce innate immune effector functions may be altered in the setting of fever.
Finally, as innate immune cells are often targets of viral infection, the loss of FcR-bearing cells impacts the efficacy of therapeutic antibodies. Ebola virus infection, for example, results in loss of non-classical FcγR+ monocytes early during infection which ultimately recovers,Citation136 and tracks with survival.Citation137 Thus, use of therapeutic antibodies that can harness multiple different innate immune cells may be beneficial depending on the stage of infection the antibody is administered.
Convalescent plasma: polyclonal therapeutics
Passive transfer of polyclonal antibodies from convalescent individuals into patients with acute infection has long been an attractive possible alternative therapy to monoclonal antibodies. However, the utility of convalescent plasma in treatment of viral infection remains unclear. Early studies in the context of Ebola virus showed promise,Citation138 yet follow-up studies have yielded variable results,Citation139–142 but ultimately indicate that the efficacy of Ebola virus disease (EVD) convalescent plasma is correlated to antibody titers and high levels of neutralizing antibodies.Citation143 A majority of human survivors of EVD develop neutralizing antibodies, yet EVD survivors differ in the ability to recruit innate immune effector functions,Citation144 raising the possibility that some convalescent donor plasma may lack effector functionality, despite having neutralizing activity. Thus, a strategy aimed at screening donor plasma for at least one effector function in addition to neutralization may increase the utility and efficacy of convalescent plasma in the treatment of patients.
Passive transfer of convalescent plasma is being explored as a therapy for SARS-CoV-2,Citation145 yet to date, the correlates of convalescent plasma efficacy have yet to be defined. Interestingly, analysis of COVID convalescent donors demonstrated that only a small fraction of donors had polyfunctional antiviral activities,Citation146 further highlighting the need for comprehensive screening of donors prior to infusion.
As an alternative to donor-based convalescent plasma, production of human polyclonal antibodies in trans-chromosomal (Tc) cattle may offer a controlled source of polyclonal neutralizing and functional antibodies for use in patients with a number of different viral infections.Citation147–149 These genetically modified cattle express human immunoglobulin genes to produce human virus-specific antibodies following immunization with a viral antigen, and passive transfer of the Tc-derived antibodies into non-human primates were protective against Ebola virus, coincident with high levels of neutralizing activity and recruitment of effector function.Citation150
Functional features of vaccine-mediated immunity
The development of vaccines against infectious diseases remains the most effective defense against localized and widespread incidence of disease. The induction of a polyclonal antibody response against a given pathogen via vaccination can target multiple protective epitopes that can limit viruses at multiple steps of infection and dissemination. Correlates analysis of vaccines against both bacterial and viral pathogens indicate that the ability to recruit innate immune effector function may be a critical component of vaccine-mediated protection,Citation151–153 and the functional humoral response can be shaped by vaccine vectors, adjuvants, and vaccine regimens.Citation154
In the context of HIV vaccines, the induction of broadly neutralizing antibodies via vaccination has long been elusive. However, the modestly successful HIV trial RV144 induced ADCC-recruiting antibodies that were identified as correlates of protection.Citation152 In natural HIV infection, elevated levels of effector function-inducing antibodies have been found in elite and viremic controller individuals, suggesting that functional antibodies may play a role in natural control of HIV.Citation155,Citation156 Thus, identifying strategies to boost the induction of functional antibodies has been of great interest to the HIV vaccine community.
Different prime-boosting strategies have been shown to impact IgG subclass selection which in turn affects the ability of antibodies to recruit effector functions. Specifically, repeated protein boosting regimens, such as those used in the non-protective VAX-003 trial, forced class-switching to IgG2 and IgG4, leading to less functional antibodies,Citation89,Citation157–159 in contrast to the elevated IgG3 levels observed in RV144 linked to increased effector functions induced by a canarypox-vectored prime and two protein boosts.Citation157 However, subsequent boosts of uninfected RV144 trial participants with either vector or the protein alone failed to recall IgG3 responses, although functional IgG1 responses were recalled by the protein boost alone,Citation160 highlighting differences between the composition of the primary immune responses and long-term boosts.
Different viral vectors have been evaluated for the ability to skew induction of functional antibodies. Adenoviral vectors have shown promise across several different pathogens, and antibodies induced by the Adenovirus serotype 26 (Ad26) vector are polyfunctional in the context of both HIV and SARS-CoV-2.Citation153,Citation161,Citation162 The protective efficacy of the vesicular stomatitis virus (VSV)-vectored Ebola vaccine, Ervebo, is linked to the induction of antibodies,Citation163 and the functional activity of vaccine-induced antibodies is skewed toward ADCC rather than ADCP.Citation164 Interesting, while the vaccine is highly effective in non-human primates and in humans,Citation165,Citation166 the functional activity of vaccine-induced antibodies is reduced when compared to natural survivors of EVD,Citation164 suggesting that the activity of vaccine-induced antibodies is sufficient for protection. Of note, vaccine-induced IgM is posited to play a critical role in neutralizing Ebola,Citation167 and thus, ADCD activation by IgM may further contribute to vaccine-mediated protection, yet to date, has not been reported.
Finally, vaccine adjuvants are used to boost magnitude, durability, and quality of the immune response, often acting through stimulation of innate immunity and antigen uptake by antigen presenting cells, and increasing evidence suggests that different adjuvants may qualitatively impact antibody function. For example, MF59 induced more ADCC-activating antibodies compared with alum in the context of influenza vaccination;Citation168 a toll-like receptor (TLR)-7 stimulating adjuvant induced production of antibodies with high ADCD activity; and a TLR-3 stimulating adjuvant induced polyfunctional antibodies.Citation168,Citation169
Approaches to modify effector function in monoclonal antibodies
Isotypes and IgG subclasses
Human IgG3 binds with the highest affinity to FcγRs, followed by IgG1, IgG4, and then IgG2.Citation7 Despite the higher affinity to FcγRs conferred by IgG3 and association with increased pathogen control in natural and vaccine-mediated protection, virtually all monoclonal antibody therapeutics have been generated as human IgG1. Concerns regarding potential immunogenicity of different human IgG3 allotypes, short half-life, stability and purification methods have long hampered the investment in IgG3-based therapeutics.Citation170 However, advances in engineering have increased the stability and manufacturability of recombinant IgG3,Citation171,Citation172 which may open the door to the development of IgG3-therapeutic antibodies with high levels of effector function for a range of infectious diseases.
There has been increased interest in the development of IgA-based prophylactics and therapeutics due to the enrichment of IgA at mucosal sites, which are common sites of entry for many pathogens.Citation173 Recent reports that IgA can neutralize SARS-CoV-2 with elevated potency compared to IgG1Citation174,Citation175 have added to the growing interest in utilizing IgA-based therapeutics against viral infection. FcαR (CD89), and IgA-mediated activation of neutrophils results in phagocytosis, ADCC, and production of neutrophil extracellular traps against viruses such as Ebola virus and HIV.Citation176 Additional unique properties of IgA may further contribute to antiviral efficacy, especially for influenza virus. The conserved cytoplasmic tail of IgA that allows for the dimerization of two IgA molecules has blocks influenza virus entry via competition between binding to the sialic acid receptors that influenza uses as an entry receptor via sialylated glycans on the IgA tail.Citation177
Glyco-engineering
IgG are glycosylated with biantennary glycan structures at a single site on each heavy chain of the Fc domain, and afucosylated glycans increase IgG affinity for FcγR3A by 100-fold,Citation8 resulting in elevated ADCC activity, but does not enhance binding to other FcγRs receptors. As a result, no major effect on monocyte/macrophage FcγR2A-mediated phagocytosis has been observed. Production of afucosylated recombinant antibodies in mammalian cell lines that lack the fucosyltransferase FUT8 or in Nicotiana benthamiana plants has shown to increase potency and efficacy of Ebola monoclonal antibodies.Citation76,Citation89,Citation90,Citation178 Novel approaches such as adenovirus-associated virus delivery of short-hairpin RNAs to transiently knock down FUT8 with simultaneous expression of broadly neutralizing HIV antibodies offers an exciting new opportunity to deliver and sustain high levels of afucosylated antibodies in vivo.Citation179
Agalactosylated antibodies, a well-established biomarker of rheumatoid arthritisCitation180 and HIV,Citation181 have been linked to increased phagocytic activity induced by HIV-specific antibodiesCitation182 yet has also been shown to reduce complement-mediated inflammation via FcγRIIb.Citation183 As the role of antibody galactose in effector function has yet to be fully resolved, to date there has been little movement in the development of galactosylated antibody therapeutics but could represent a new avenue to modify phagocytosis and complement.
Point mutations
Mutational screens and co-crystal structure analysis of antibodies in complex with FcRs have identified critical amino acids in both the hinge and constant domains of the antibody Fc domain that alter binding affinity to FcγRs,Citation34,Citation184–192 complement,Citation184,Citation188,Citation193–195 and/or the neonatal Fc-receptor (FcRn) to increase antibody half-life.Citation196–200 Engineering these mutations into monoclonal antibodies has allowed for the analysis of the role of FcR-mediated activation in infection, dissection of mechanisms, and highlighted potential opportunities to enhance efficacy of monoclonal therapeutics.
Several approaches have been developed to take advantage of these mutations, and the most recent platform, Rationally Engineered and Functionally Optimized Monoclonal antibodies (REFORM), utilizes a high-throughput cloning approach to rapidly generate Fc-variants, and a systems serology approach to profile effector functions across the panel of antibodies that then can be tested in vivo to determine the impact of specific effector profiles on antibody efficacy.Citation112 Using this approach in the context of Ebola virus revealed the role of antibody-dependent complement activation and moderate not maximal ADCC activity in combination with neutralizing activity in complete protection against disease and death in an animal model, highlighting that combinations and levels of induction of effector functions may be key to more precise and highly effective antibodies.Citation112 A similar approach based on the SARS-CoV-2 specific non-neutralizing antibody CR3022 demonstrated that while enhancing Fc effector function led to reduced viral loads, increased lung pathology and weight loss were observed,Citation201 further highlighting the critical need to balance protective and pathologic functions of antibodies and warranting the need to carefully select induction of specific effector functions.
Conversely, some viral diseases can be enhanced by Fc-dependent mechanisms, and thus strategies to ablate Fc-FcR interactions may be needed. For example, increased antibody function is associated with more severe disease in the context of Dengue virus infection,Citation70 and cross-reactive Dengue virus antibodies have been demonstrated to enhance Zika virus, a related flavivirus, infection.Citation202,Citation203 Engineering of mutations to ablate effector functions in neutralizing antibodies such as L234A/L235A (LALA) or the glycosylation site N297, which disrupts Fc-FcR interactions, have been shown to block infection without increasing the risk of antibody-dependent enhancement of infection and disease.Citation202,Citation204
Translating the role of antibody effector function between animal models
It is worthwhile briefly discussing the challenges of translating the role of effector functions in controlling viral infection between different animal models and humans. There are significant differences in expression of FcRs across different species, often complicating the analysis of mechanism of action of therapeutic antibodies. Some innate functions, such as antibody-dependent complement activation, can be readily translated across species as the complement pathway is highly conserved,Citation205 but other functions are more difficult to translate. Mice express a unique FcγR, FcγRIV, which mediates ADCC in mice and is expressed on macrophage and neutrophils but not NK cells,Citation27 and non-human primates have multiple FcR polymorphisms that can modulate induction of effector functions.Citation206,Citation207 Chimerized antibodies composed of human Fab domains with species-specific subclasses is one approach to determining if effector function enhances protective efficacy,Citation208 but still does not provide direct translation to efficacy in humans. Transgenic expression of human FcRs in mice is another alternative to evaluate human IgG in mouse models,Citation187 yet is still in the setting of a mouse innate immune system and mechanisms of antibody-mediated protection may not directly translate to humans. However, these approaches have been fundamental in demonstrating the vast repertoire of functions that antibodies can mediate beyond neutralization.
Moving forward, development of bridging analyses to help translate findings between the most commonly used animal models for viral infection (mice, non-human primates, and guinea pigs) and humans are needed. Comparative analysis of human antibody binding to recombinant FcRs from other species has provided a preliminary framework for these bridging analyses,Citation112,Citation209 but additional comparative analyses using innate immune cells from different animal model species are needed to fully develop cross-species maps to define mechanistic translatability across model systems.
Concluding remarks
Our understanding of the repertoire of antiviral effector functions that can be deployed by antibodies is constantly expanding and offers the opportunity to harness the antibody Fc domain to enhance efficacy and potency of monoclonal antibody therapeutics against viral infection. Despite the advances in experimental assays, production techniques, and antibody engineering technology that allow for modulation of antibody effector functions, there are still many unknowns that are critical to examine in order to safely and effectively direct innate functions. Specifically, defining the role of specific effector functions in antiviral control/modulation of infection and the impact of infection on expression of FcRs on innate cells will be critical next steps. Advances in antibody engineering technology together with a deeper understanding of the antibody and innate features that regulate antibody Fc-effector function will undoubtedly lead to the development of highly effective next generation antibody therapeutics and vaccines against viral infection in the future.
Disclosure of potential conflicts of interest
The authors have declared that there are no conflicts of interest.
Acknowledgments
We would like to thank members of the Gunn Lab for helpful discussions. We would like to acknowledge funding support from the National Institutes of Allergy and Infectious Diseases U19AI142777, the National Cancer Institute U54CA260581, and the Washington State University College of Veterinary Medicine Intramural Research Grant Fund. Figures were generated with an academic publishing license in Biorender.
References
- Pincetic A, Bournazos S, DiLillo DJ, Maamary J, Wang TT, Dahan R, Fiebiger B-M, Ravetch JV. Type I and type II Fc receptors regulate innate and adaptive immunity. Nat Immunol. 2014;15(8):707–16. doi:10.1038/ni.2939.
- Bruhns P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood. 2012;119:5640–49. doi:10.1182/blood-2012-01-380121.
- Rosenfeld SI, Looney RJ, Leddy JP, Phipps DC, Abraham GN, Anderson CL. Human platelet Fc receptor for immunoglobulin G. Identification as a 40,000-molecular-weight membrane protein shared by monocytes. J Clin Invest. 1985;76:2317–22. doi:10.1172/JCI112242.
- Lanier LL, Kipps TJ, Phillips JH. Functional properties of a unique subset of cytotoxic CD3+ T lymphocytes that express Fc receptors for IgG (CD16/Leu-11 antigen). J Exp Med. 1985;162:2089–106. doi:10.1084/jem.162.6.2089.
- Bertagnolli LN, White JA, Simonetti FR, Beg SA, Lai J, Tomescu C, Murray AJ, Antar AAR, Zhang H, Margolick JB, et al. The role of CD32 during HIV-1 infection. Nature. 2018;561:E17–E19. doi:10.1038/s41586-018-0494-3.
- Badia R, Ballana E, Castellví M, García-Vidal E, Pujantell M, Clotet B, Prado JG, Puig J, Martínez MA, Riveira-Muñoz E, et al. CD32 expression is associated to T-cell activation and is not a marker of the HIV-1 reservoir. Nat Commun. 2018;9:2739. doi:10.1038/s41467-018-05157-w.
- Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, Daëron M. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113:3716–25. doi:10.1182/blood-2008-09-179754.
- Ferrara C, Grau S, Jäger C, Sondermann P, Brünker P, Waldhauer I, Hennig M, Ruf A, Rufer AC, Stihle M, et al. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc Natl Acad Sci U S A. 2011;108:12669–74. doi:10.1073/pnas.1108455108.
- Sondermann P, Pincetic A, Maamary J, Lammens K, Ravetch JV. General mechanism for modulating immunoglobulin effector function. Proc Natl Acad Sci U S A. 2013;110:9868–72. doi:10.1073/pnas.1307864110.
- Patel KR, Roberts JT, Barb AW. Multiple variables at the leukocyte cell surface impact Fc gamma receptor-dependent mechanisms. Front Immunol. 2019;10:223. doi:10.3389/fimmu.2019.00223.
- Saphire EO, Schendel SL, Gunn BM, Milligan JC, Alter G. Antibody-mediated protection against Ebola virus. Nat Immunol. 2018;19:1169–78. doi:10.1038/s41590-018-0233-9.
- He W, Tan GS, Mullarkey CE, Lee AJ, Lam MMW, Krammer F, Henry C, Wilson PC, Ashkar AA, Palese P, et al. Epitope specificity plays a critical role in regulating antibody-dependent cell-mediated cytotoxicity against influenza A virus. Proc Natl Acad Sci U S A. 2016;113(42):11931–36. doi:10.1073/pnas.1609316113.
- Bakalar MH, Joffe AM, Schmid EM, Son S, Podolski M, Fletcher DA. Size-dependent segregation controls macrophage phagocytosis of antibody-opsonized targets. Cell. 2018;174(1):131–142 e113. doi:10.1016/j.cell.2018.05.059.
- Hanson QM, Barb AW. A perspective on the structure and receptor binding properties of immunoglobulin G Fc. Biochemistry. 2015;54:2931–42. doi:10.1021/acs.biochem.5b00299.
- Pearse RN, Kawabe T, Bolland S, Guinamard R, Kurosaki T, Ravetch JV. SHIP recruitment attenuates Fc gamma RIIB-induced B cell apoptosis. Immunity. 1999;10:753–60. doi:10.1016/s1074-7613(00)80074-6.
- Bournazos S, Wang TT, Dahan R, Maamary J, Ravetch JV. Signaling by antibodies: recent progress. Annu Rev Immunol. 2017;35:285–311. doi:10.1146/annurev-immunol-051116-052433.
- Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 2011;475:110–13. doi:10.1038/nature10134.
- Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008;320(5874):373–76. doi:10.1126/science.1154315.
- Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci U S A. 2008;105:19571–78. doi:10.1073/pnas.0810163105.
- Wang TT, Maamary J, Tan G, Bournazos S, Davis C, Krammer F, Schlesinger S, Palese P, Ahmed R, Ravetch J, et al. Anti-HA glycoforms drive B cell affinity selection and determine influenza vaccine efficacy. Cell. 2015;162:160–69. doi:10.1016/j.cell.2015.06.026.
- Temming AR, Dekkers G, van de Bovenkamp FS, Plomp HR, Bentlage AEH, Szittner Z, Derksen NIL, Wuhrer M, Rispens T, Vidarsson G, et al. Author correction: human DC-SIGN and CD23 do not interact with human IgG. Sci Rep. 2020;10:12560. doi:10.1038/s41598-020-68760-2.
- Yu X, Vasiljevic S, Mitchell DA, Crispin M, Scanlan CN. Dissecting the molecular mechanism of IVIg therapy: the interaction between serum IgG and DC-SIGN is independent of antibody glycoform or Fc domain. J Mol Biol. 2013;425:1253–58. doi:10.1016/j.jmb.2013.02.006.
- Tjon AS, Van Gent R, Geijtenbeek TB, Kwekkeboom J. Differences in anti-inflammatory actions of intravenous immunoglobulin between mice and men: more than meets the eye. Front Immunol. 2015;6:197. doi:10.3389/fimmu.2015.00197.
- Braakman E, van de Winkel JG, van Krimpen BA, Jansze M, Bolhuis RL. CD16 on human gamma delta T lymphocytes: expression, function, and specificity for mouse IgG isotypes. Cell Immunol. 1992;143:97–107. doi:10.1016/0008-8749(92)90008-d.
- Richard J, Prévost J, Baxter AE, von Bredow B, Ding S, Medjahed H, Delgado GG, Brassard N, Stürzel CM, Kirchhoff F, et al. Uninfected bystander cells impact the measurement of HIV-specific antibody-dependent cellular cytotoxicity responses. mBio. 2018;9. doi:10.1128/mBio.00358-18.
- Prevost J, Richard J, Medjahed H, Alexander A, Jones J, Kappes JC, Ochsenbauer C, Finzi A. Incomplete downregulation of CD4 expression affects HIV-1 Env conformation and antibody-dependent cellular cytotoxicity responses. J Virol. 2018:92. doi:10.1128/JVI.00484-18.
- Nimmerjahn F, Bruhns P, Horiuchi K, Ravetch JV. FcgammaRIV: a novel FcR with distinct IgG subclass specificity. Immunity. 2005;23:41–51. doi:10.1016/j.immuni.2005.05.010.
- Gomez-Roman VR, Florese RH, Patterson LJ, Peng B, Venzon D, Aldrich K, Robert-Guroff M. A simplified method for the rapid fluorometric assessment of antibody-dependent cell-mediated cytotoxicity. J Immunol Methods. 2006;308:53–67. doi:10.1016/j.jim.2005.09.018.
- Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22. doi:10.1016/j.jim.2004.08.008.
- Chung AW, Rollman E, Center RJ, Kent SJ, Stratov I. Rapid degranulation of NK cells following activation by HIV-specific antibodies. J Immunol. 2009;182:1202–10. doi:10.4049/jimmunol.182.2.1202.
- Ackerman ME, Moldt B, Wyatt RT, Dugast A-S, McAndrew E, Tsoukas S, Jost S, Berger CT, Sciaranghella G, Liu Q, et al. A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples. J Immunol Methods. 2011;366(1–2):8–19. doi:10.1016/j.jim.2010.12.016.
- Karsten CB, Mehta N, Shin SA, Diefenbach TJ, Slein MD, Karpinski W, Irvine EB, Broge T, Suscovich TJ, Alter G, et al. A versatile high-throughput assay to characterize antibody-mediated neutrophil phagocytosis. J Immunol Methods. 2019;471:46–56. doi:10.1016/j.jim.2019.05.006.
- Butler AL, Fallon JK, Alter G. A sample-sparing multiplexed ADCP assay. Front Immunol. 2019;10:1851. doi:10.3389/fimmu.2019.01851.
- Richards JO, Karki S, Lazar GA, Chen H, Dang W, Desjarlais JR. Optimization of antibody binding to FcgammaRIIa enhances macrophage phagocytosis of tumor cells. Mol Cancer Ther. 2008;7:2517–27. doi:10.1158/1535-7163.MCT-08-0201.
- Bakema JE, van Egmond M. The human immunoglobulin A Fc receptor FcalphaRI: a multifaceted regulator of mucosal immunity. Mucosal Immunol. 2011;4:612–24. doi:10.1038/mi.2011.36.
- Huang ZY, Hunter S, Chien P, Kim M-K, Han-Kim T-H, Indik ZK, Schreiber AD. Interaction of two phagocytic host defense systems: fcgamma receptors and complement receptor 3. J Biol Chem. 2011;286:160–68. doi:10.1074/jbc.M110.163030.
- Fischinger S, Fallon JK, Michell AR, Broge T, Suscovich TJ, Streeck H, Alter G. A high-throughput, bead-based, antigen-specific assay to assess the ability of antibodies to induce complement activation. J Immunol Methods. 2019;473:112630. doi:10.1016/j.jim.2019.07.002.
- Duensing TD, Watson SR. Complement-dependent cytotoxicity assay. Cold Spring Harb Protoc. 2018:2018. doi:10.1101/pdb.prot093799.
- Richardson SI, Chung AW, Natarajan H, Mabvakure B, Mkhize NN, Garrett N, Abdool Karim S, Moore PL, Ackerman ME, Alter G, et al. HIV-specific Fc effector function early in infection predicts the development of broadly neutralizing antibodies. PLoS Pathog. 2018;14(4):e1006987. doi:10.1371/journal.ppat.1006987.
- Matlung HL, Babes L, Zhao XW, van Houdt M, Treffers LW, van Rees DJ, Franke K, Schornagel K, Verkuijlen P, Janssen H, et al. Neutrophils kill antibody-opsonized cancer cells by trogoptosis. Cell Rep. 2018;23:3946–3959 e3946. doi:10.1016/j.celrep.2018.05.082.
- Richardson SI, Crowther C, Mkhize NN, Morris L. Measuring the ability of HIV-specific antibodies to mediate trogocytosis. J Immunol Methods. 2018;463:71–83. doi:10.1016/j.jim.2018.09.009.
- Lee WS, Selva KJ, Davis SK, Wines BD, Reynaldi A, Esterbauer R, Kelly HG, Haycroft ER, Tan H-X, Juno JA, et al. Decay of Fc-dependent antibody functions after mild to moderate COVID-19. Cell Rep Med. 2021;2(6):100296. doi:10.1016/j.xcrm.2021.100296.
- Couzi L, Pitard V, Sicard X, Garrigue I, Hawchar O, Merville P, Moreau J-F, Déchanet-Merville J. Antibody-dependent anti-cytomegalovirus activity of human gammadelta T cells expressing CD16 (FcgammaRIIIa). Blood. 2012;119:1418–27. doi:10.1182/blood-2011-06-363655.
- Farrington LA, Callaway PC, Vance HM, Baskevitch K, Lutz E, Warrier L, McIntyre TI, Budker R, Jagannathan P, Nankya F, et al. Opsonized antigen activates Vdelta2+ T cells via CD16/FCgammaRIIIa in individuals with chronic malaria exposure. PLoS Pathog. 2020;16:e1008997. doi:10.1371/journal.ppat.1008997.
- Palankar R, Kohler TP, Krauel K, Wesche J, Hammerschmidt S, Greinacher A. Platelets kill bacteria by bridging innate and adaptive immunity via platelet factor 4 and Fcγ RIIA. J Thromb Haemost. 2018;16:1187–97. doi:10.1111/jth.13955.
- Riaz AH, Tasma BE, Woodman ME, Wooten RM, Worth RG. Human platelets efficiently kill IgG-opsonized E. coli. FEMS Immunol Med Microbiol. 2012;65:78–83. doi:10.1111/j.1574-695X.2012.00945.x.
- Apostolidis SA, Sarkar A, Giannini HM, Goel RR, Mathew D, Suzuki A, Baxter AE, Greenplate AR, Alanio C, Abdel-Hakeem M, et al. Signaling through FcgammaRIIA and the C5a-C5aR pathway mediates platelet hyperactivation in COVID-19. bioRxiv. 2021. doi:10.1101/2021.05.01.442279.
- Lofano G, Gorman MJ, Yousif AS, Yu W-H, Fox JM, Dugast A-S, Ackerman ME, Suscovich TJ, Weiner J, Barouch D, et al. Antigen-specific antibody Fc glycosylation enhances humoral immunity via the recruitment of complement. Sci Immunol. 2018;3. doi:10.1126/sciimmunol.aat7796.
- DiLillo DJ, Ravetch JV. Differential Fc-receptor engagement drives an anti-tumor vaccinal effect. Cell. 2015;161:1035–45. doi:10.1016/j.cell.2015.04.016.
- Chung AW, Kumar M, Arnold K, Yu W, Schoen M, Dunphy L, Suscovich T, Frahm N, Linde C, Mahan A, et al. Dissecting polyclonal vaccine-induced humoral immunity against HIV using systems serology. Cell. 2015;163(4):988–98. doi:10.1016/j.cell.2015.10.027.
- Saphire EO, Schendel SL, Fusco ML, Gangavarapu K, Gunn BM, Wec AZ, Halfmann PJ, Brannan JM, Herbert AS, Qiu X, et al. Systematic analysis of monoclonal antibodies against ebola virus GP defines features that contribute to protection. Cell. 2018;174(4):938–952 e913. doi:10.1016/j.cell.2018.07.033.
- Gunn BM, Yu W-H, Karim MM, Brannan JM, Herbert AS, Wec AZ, Halfmann PJ, Fusco ML, Schendel SL, Gangavarapu K, et al. A role for Fc function in therapeutic monoclonal antibody-mediated protection against ebola virus. Cell Host Microbe. 2018;24(2):221–233 e225. doi:10.1016/j.chom.2018.07.009.
- Atyeo C, Fischinger S, Zohar T, Slein MD, Burke J, Loos C, McCulloch DJ, Newman KL, Wolf C, Yu J, et al. Distinct early serological signatures track with SARS-CoV-2 survival. Immunity. 2020;53(3):524–532 e524. doi:10.1016/j.immuni.2020.07.020.
- FDA. FDA approves first treatment for ebola virus. Press Release; 2020.
- FDA. Coronavirus (COVID-19) update: FDA authorizes monoclonal antibodies for treatment of COVID-19. Press Release; 2020.
- Xu Y, Roach W, Sun T, Jain T, Prinz B, Yu T-Y, Torrey J, Thomas J, Bobrowicz P, Vasquez M, et al. Addressing polyspecificity of antibodies selected from an in vitro yeast presentation system: a FACS-based, high-throughput selection and analytical tool. Protein Eng Des Sel. 2013;26(10):663–70. doi:10.1093/protein/gzt047.
- Smith SA, Crowe JE Jr. Use of human hybridoma technology to isolate human monoclonal antibodies. Microbiol Spectr. 2015;3:AID-0027-2014. doi:10.1128/microbiolspec.AID-0027-2014.
- Corti D, Lanzavecchia A. Efficient methods to isolate human monoclonal antibodies from memory B cells and plasma cells. Microbiol Spectr. 2014:2. doi:10.1128/microbiolspec.AID-0018-2014.
- Bornholdt ZA, Turner HL, Murin CD, Li W, Sok D, Souders CA, Piper AE, Goff A, Shamblin JD, Wollen SE, et al. Isolation of potent neutralizing antibodies from a survivor of the 2014 Ebola virus outbreak. Science. 2016;351(6277):1078–83. doi:10.1126/science.aad5788.
- Flyak AI, Kuzmina N, Murin CD, Bryan C, Davidson E, Gilchuk P, Gulka CP, Ilinykh PA, Shen X, Huang K, et al. Broadly neutralizing antibodies from human survivors target a conserved site in the Ebola virus glycoprotein HR2-MPER region. Nat Microbiol. 2018;3:670–77. doi:10.1038/s41564-018-0157-z.
- Duehr J, McMahon M, Williamson B, Amanat F, Durbin A, Hawman DW, Noack D, Uhl S, Tan GS, Feldmann H, et al. Neutralizing monoclonal antibodies against the Gn and the Gc of the Andes virus glycoprotein spike complex protect from virus challenge in a preclinical hamster model. mBio. 2020;11(2). doi:10.1128/mBio.00028-20.
- Garrido JL, Prescott J, Calvo M, Bravo F, Alvarez R, Salas A, Riquelme R, Rioseco ML, Williamson BN, Haddock E, et al. Two recombinant human monoclonal antibodies that protect against lethal Andes hantavirus infection in vivo. Sci Transl Med. 2018;10(468):eaat6420. doi:10.1126/scitranslmed.aat6420.
- Chapman NS, Zhao H, Kose N, Westover JB, Kalveram B, Bombardi R, Rodriguez J, Sutton R, Genualdi J, LaBeaud AD, et al. Potent neutralization of rift valley fever virus by human monoclonal antibodies through fusion inhibition. Proc Natl Acad Sci U S A. 2021;118. doi:10.1073/pnas.2025642118.
- Allen ER, Krumm SA, Raghwani J, Halldorsson S, Elliott A, Graham VA, Koudriakova E, Harlos K, Wright D, Warimwe GM, et al. A protective monoclonal antibody targets a site of vulnerability on the surface of rift valley fever virus. Cell Rep. 2018;25(13):3750–3758 e3754. doi:10.1016/j.celrep.2018.12.001.
- Pal P, Dowd KA, Brien JD, Edeling MA, Gorlatov S, Johnson S, Lee I, Akahata W, Nabel GJ, Richter MK, et al. Development of a highly protective combination monoclonal antibody therapy against Chikungunya virus. PLoS Pathog. 2013;9:e1003312. doi:10.1371/journal.ppat.1003312.
- Kuehnert PA, Stefan CP, Badger CV, Ricks KM. Crimean-Congo hemorrhagic fever virus (CCHFV): a Silent but widespread threat. Curr Trop Med Rep. 2021:1–7. doi:10.1007/s40475-021-00235-4.
- Kruger DH, Figueiredo LT, Song JW, Klempa B. Hantaviruses–globally emerging pathogens. J Clin Virol. 2015;64:128–36. doi:10.1016/j.jcv.2014.08.033.
- Swei A, Couper LI, Coffey LL, Kapan D, Bennett S. Patterns, drivers, and challenges of vector-borne disease emergence. Vector Borne Zoonotic Dis. 2020;20:159–70. doi:10.1089/vbz.2018.2432.
- Wine Y, Horton AP, Ippolito GC, Georgiou G. Serology in the 21st century: the molecular-level analysis of the serum antibody repertoire. Curr Opin Immunol. 2015;35:89–97. doi:10.1016/j.coi.2015.06.009.
- Wang TT, Sewatanon J, Memoli MJ, Wrammert J, Bournazos S, Bhaumik SK, Pinsky BA, Chokephaibulkit K, Onlamoon N, Pattanapanyasat K, et al. IgG antibodies to dengue enhanced for FcgammaRIIIA binding determine disease severity. Science. 2017;355:395–98. doi:10.1126/science.aai8128.
- Gomez RS, Ramirez BA, Céspedes PF, Cautivo KM, Riquelme SA, Prado CE, González PA, Kalergis AM. Contribution of Fcγ receptors to human respiratory syncytial virus pathogenesis and the impairment of T-cell activation by dendritic cells. Immunology. 2016;147:55–72. doi:10.1111/imm.12541.
- Kuzmina NA, Younan P, Gilchuk P, Santos RI, Flyak AI, Ilinykh PA, Huang K, Lubaki NM, Ramanathan P, Crowe JE, et al. Antibody-dependent enhancement of ebola virus infection by human antibodies isolated from survivors. Cell Rep. 2018;24(7):1802–1815 e1805. doi:10.1016/j.celrep.2018.07.035.
- Maruyama T, Rodriguez LL, Jahrling PB, Sanchez A, Khan AS, Nichol ST, Peters CJ, Parren PW, Burton DR. Ebola virus can be effectively neutralized by antibody produced in natural human infection. J Virol. 1999;73:6024–30. doi:10.1128/JVI.73.7.6024-6030.1999.
- Wilson JA, Hevey M, Bakken R, Guest S, Bray M, Schmaljohn AL, Hart MK. Epitopes involved in antibody-mediated protection from Ebola virus. Science. 2000;287:1664–66. doi:10.1126/science.287.5458.1664.
- Olinger GG Jr., Pettitt J, Kim D, Working C, Bohorov O, Bratcher B, Hiatt E, Hume SD, Johnson AK, Morton J, et al. Delayed treatment of Ebola virus infection with plant-derived monoclonal antibodies provides protection in rhesus macaques. Proc Natl Acad Sci U S A. 2012;109:18030–35. doi:10.1073/pnas.1213709109.
- Qiu X, Wong G, Audet J, Bello A, Fernando L, Alimonti JB, Fausther-Bovendo H, Wei H, Aviles J, Hiatt E, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014;514(7520):47–53. doi:10.1038/nature13777.
- Misasi J, Gilman MSA, Kanekiyo M, Gui M, Cagigi A, Mulangu S, Corti D, Ledgerwood JE, Lanzavecchia A, Cunningham J, et al. Structural and molecular basis for Ebola virus neutralization by protective human antibodies. Science. 2016;351(6279):1343–46. doi:10.1126/science.aad6117.
- Wec AZ, Bornholdt ZA, He S, Herbert AS, Goodwin E, Wirchnianski AS, Gunn BM, Zhang Z, Zhu W, Liu G, et al. Development of a human antibody cocktail that deploys multiple functions to confer pan-ebolavirus protection. Cell Host Microbe. 2019;25:39–48 e35. doi:10.1016/j.chom.2018.12.004.
- Flyak AI, Shen X, Murin C, Turner H, David J, Fusco M, Lampley R, Kose N, Ilinykh P, Kuzmina N, et al. Cross-reactive and potent neutralizing antibody responses in human survivors of natural ebolavirus infection. Cell. 2016. doi:10.1016/j.cell.2015.12.022.
- Pascal KE, Dudgeon D, Trefry JC, Anantpadma M, Sakurai Y, Murin CD, Turner HL, Fairhurst J, Torres M, Rafique A, et al. Development of clinical-stage human monoclonal antibodies that treat advanced ebola virus disease in nonhuman primates. J Infect Dis. 2018;218(suppl_5):S612–S626. doi:10.1093/infdis/jiy285.
- Zhao X, Howell KA, He S, Brannan JM, Wec AZ, Davidson E, Turner HL, Chiang C-I, Lei L, Fels JM, et al. Immunization-elicited broadly protective antibody reveals ebolavirus fusion loop as a site of vulnerability. Cell. 2017;169(5):891–904 e815. doi:10.1016/j.cell.2017.04.038.
- Holtsberg FW, Shulenin S, Vu H, Howell KA, Patel SJ, Gunn B, Karim M, Lai JR, Frei JC, Nyakatura EK, et al. Pan-ebolavirus and pan-filovirus mouse monoclonal antibodies: protection against ebola and Sudan viruses. J Virol. 2016;90(1):266–78. doi:10.1128/JVI.02171-15.
- Mulangu S, Dodd LE, Davey RT, Tshiani Mbaya O, Proschan M, Mukadi D, Lusakibanza Manzo M, Nzolo D, Tshomba Oloma A, Ibanda A, et al. A randomized, controlled trial of ebola virus disease therapeutics. N Engl J Med. 2019;381(24):2293–303. doi:10.1056/NEJMoa1910993.
- PREVAIL II Writing Group, et al. A randomized, controlled trial of ZMapp for Ebola virus infection. N Engl J Med. 2016;375:1448–56. doi:10.1056/NEJMoa1604330.
- Wec AZ, Herbert AS, Murin CD, Nyakatura EK, Abelson DM, Fels JM, He S, James RM, De La Vega M-A, Zhu W, et al. Antibodies from a human survivor define sites of vulnerability for broad protection against ebolaviruses. Cell. 2017;169:878–890 e815. doi:10.1016/j.cell.2017.04.037.
- Howell KA, Qiu X, Brannan JM, Bryan C, Davidson E, Holtsberg FW, Wec AZ, Shulenin S, Biggins JE, Douglas R, et al. Antibody treatment of Ebola and Sudan virus infection via a uniquely exposed epitope within the glycoprotein receptor-binding site. Cell Rep. 2016;15(7):1514–26. doi:10.1016/j.celrep.2016.04.026.
- Gilchuk P, Kuzmina N, Ilinykh PA, Huang K, Gunn BM, Bryan A, Davidson E, Doranz BJ, Turner HL, Fusco ML, et al. Multifunctional pan-ebolavirus antibody recognizes a site of broad vulnerability on the ebolavirus glycoprotein. Immunity. 2018;49(2):363–374 e310. doi:10.1016/j.immuni.2018.06.018.
- Ilinykh PA, Huang K, Santos RI, Gilchuk P, Gunn BM, Karim MM, Liang J, Fouch ME, Davidson E, Parekh DV, et al. Non-neutralizing antibodies from a Marburg infection survivor mediate protection by Fc-Effector functions and by enhancing efficacy of other antibodies. Cell Host Microbe. 2020;27:976–991 e911. doi:10.1016/j.chom.2020.03.025.
- Zeitlin L, Pettitt J, Scully C, Bohorova N, Kim D, Pauly M, Hiatt A, Ngo L, Steinkellner H, Whaley KJ, et al. Enhanced potency of a fucose-free monoclonal antibody being developed as an Ebola virus immunoprotectant. Proc Natl Acad Sci U S A. 2011;108:20690–94. doi:10.1073/pnas.1108360108.
- Bornholdt ZA, Herbert AS, Mire CE, He S, Cross RW, Wec AZ, Abelson DM, Geisbert JB, James RM, Rahim MN, et al. A two-antibody pan-ebolavirus cocktail confers broad therapeutic protection in ferrets and nonhuman primates. Cell Host Microbe. 2019;25:49–58 e45. doi:10.1016/j.chom.2018.12.005.
- Golay J, Da Roit F, Bologna L, Ferrara C, Leusen JH, Rambaldi A, Klein C, Introna M. Glycoengineered CD20 antibody obinutuzumab activates neutrophils and mediates phagocytosis through CD16B more efficiently than rituximab. Blood. 2013;122:3482–91. doi:10.1182/blood-2013-05-504043.
- Reddy V, Klein C, Isenberg DA, Glennie MJ, Cambridge G, Cragg MS, Leandro MJ. Obinutuzumab induces superior B-cell cytotoxicity to rituximab in rheumatoid arthritis and systemic lupus erythematosus patient samples. Rheumatology (Oxford). 2017;56(7):1227–37. doi:10.1093/rheumatology/kex067.
- DiLillo DJ, Palese P, Wilson PC, Ravetch JV. Broadly neutralizing anti-influenza antibodies require Fc receptor engagement for in vivo protection. J Clin Invest. 2016;126:605–10. doi:10.1172/JCI84428.
- Bournazos S, Klein F, Pietzsch J, Seaman M, Nussenzweig M, Ravetch J. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell. 2014;158:1243–53. doi:10.1016/j.cell.2014.08.023.
- Moldt B, Shibata-Koyama M, Rakasz EG, Schultz N, Kanda Y, Dunlop DC, Finstad SL, Jin C, Landucci G, Alpert MD, et al. A nonfucosylated variant of the anti-HIV-1 monoclonal antibody b12 has enhanced FcgammaRIIIa-mediated antiviral activity in vitro but does not improve protection against mucosal SHIV challenge in macaques. J Virol. 2012;86:6189–96. doi:10.1128/JVI.00491-12.
- Chakraborty S, Gonzalez J, Edwards K, Mallajosyula V, Buzzanco AS, Sherwood R, Buffone C, Kathale N, Providenza S, Xie MM, et al. Proinflammatory IgG Fc structures in patients with severe COVID-19. Nat Immunol. 2021;22(1):67–73. doi:10.1038/s41590-020-00828-7.
- Bournazos S, Vo HTM, Duong V, Auerswald H, Ly S, Sakuntabhai A, Dussart P, Cantaert T, Ravetch JV. Antibody fucosylation predicts disease severity in secondary dengue infection. Science. 2021;372(6546):1102–05. doi:10.1126/science.abc7303.
- Larsen MD, de Graaf EL, Sonneveld ME, Plomp HR, Nouta J, Hoepel W, Chen H-J, Linty F, Visser R, Brinkhaus M, et al. Afucosylated IgG characterizes enveloped viral responses and correlates with COVID-19 severity. Science. 2021;371(6532):eabc8378. doi:10.1126/science.abc8378.
- Hessell AJ, Hangartner L, Hunter M, Havenith CEG, Beurskens FJ, Bakker JM, Lanigan CMS, Landucci G, Forthal DN, Parren PWHI, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449(7158):101–04. doi:10.1038/nature06106.
- Halper-Stromberg A, Lu C-L, Klein F, Horwitz J, Bournazos S, Nogueira L, Eisenreich T, Liu C, Gazumyan A, Schaefer U, et al. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell. 2014;158(5):989–99. doi:10.1016/j.cell.2014.07.043.
- DiLillo DJ, Tan GS, Palese P, Ravetch JV. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcgammaR interactions for protection against influenza virus in vivo. Nat Med. 2014;20:143–51. doi:10.1038/nm.3443.
- Rappazzo CG, Tse LV, Kaku CI, Wrapp D, Sakharkar M, Huang D, Deveau LM, Yockachonis TJ, Herbert AS, Battles MB, et al. Broad and potent activity against SARS-like viruses by an engineered human monoclonal antibody. Science. 2021;371(6531):823–29. doi:10.1126/science.abf4830.
- Schafer A, Muecksch F, Lorenzi JCC, Leist SR, Cipolla M, Bournazos S, Schmidt F, Maison RM, Gazumyan A, Martinez DR, et al. Antibody potency, effector function, and combinations in protection and therapy for SARS-CoV-2 infection in vivo. J Exp Med. 2021;218(3). doi:10.1084/jem.20201993.
- Winkler ES, Gilchuk P, Yu J, Bailey AL, Chen RE, Zost SJ, Jang H, Huang Y, Allen JD, Case JB, et al. Human neutralizing antibodies against SARS-CoV-2 require intact Fc effector functions and monocytes for optimal therapeutic protection. bioRxiv. 2020. doi:10.1101/2020.12.28.424554.
- Pinto D, Park Y-J, Beltramello M, Walls AC, Tortorici MA, Bianchi S, Jaconi S, Culap K, Zatta F, De Marco A, et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583(7815):290–95. doi:10.1038/s41586-020-2349-y.
- Hansen J, Baum A, Pascal KE, Russo V, Giordano S, Wloga E, Fulton BO, Yan Y, Koon K, Patel K, et al. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science. 2020;369(6506):1010–14. doi:10.1126/science.abd0827.
- Fox JM, Roy V, Gunn BM, Huang L, Edeling MA, Mack M, Fremont DH, Doranz BJ, Johnson S, Alter G, et al. Optimal therapeutic activity of monoclonal antibodies against chikungunya virus requires Fc-FcgammaR interaction on monocytes. Sci Immunol. 2019;4:eaav5062. doi:10.1126/sciimmunol.aav5062.
- Earnest JT, Basore K, Roy V, Bailey AL, Wang D, Alter G, Fremont DH, Diamond MS. Neutralizing antibodies against Mayaro virus require Fc effector functions for protective activity. J Exp Med. 2019;216(10):2282–301. doi:10.1084/jem.20190736.
- Wang P, Gajjar MR, Yu J, Padte NN, Gettie A, Blanchard JL, Russell-Lodrigue K, Liao LE, Perelson AS, Huang Y, et al. Quantifying the contribution of Fc-mediated effector functions to the antiviral activity of anti-HIV-1 IgG1 antibodies in vivo. Proc Natl Acad Sci U S A. 2020;117:18002–09. doi:10.1073/pnas.2008190117.
- Parsons MS, Lee WS, Kristensen AB, Amarasena T, Khoury G, Wheatley AK, Reynaldi A, Wines BD, Hogarth PM, Davenport MP, et al. Fc-dependent functions are redundant to efficacy of anti-HIV antibody PGT121 in macaques. J Clin Invest. 2019;129(1):182–91. doi:10.1172/JCI122466.
- Winkler ES, Gilchuk P, Yu J, Bailey AL, Chen RE, Chong Z, Zost SJ, Jang H, Huang Y, Allen JD, et al. Human neutralizing antibodies against SARS-CoV-2 require intact Fc effector functions for optimal therapeutic protection. Cell. 2021;184(7):1804–1820.e16. doi:10.1016/j.cell.2021.02.026.
- Gunn BM, Lu R, Slein MD, Ilinykh PA, Huang K, Atyeo C, Schendel SL, Kim J, Cain C, Roy V, et al. A Fc engineering approach to define functional humoral correlates of immunity against Ebola virus. Immunity. 2021;54(4):815–828 e815. doi:10.1016/j.immuni.2021.03.009.
- Bournazos S, DiLillo DJ, Goff AJ, Glass PJ, Ravetch JV. Differential requirements for FcgammaR engagement by protective antibodies against Ebola virus. Proc Natl Acad Sci U S A. 2019;116:20054–62. doi:10.1073/pnas.1911842116.
- Leon PE, He W, Mullarkey CE, Bailey MJ, Miller MS, Krammer F, Palese P, Tan GS. Optimal activation of Fc-mediated effector functions by influenza virus hemagglutinin antibodies requires two points of contact. Proc Natl Acad Sci U S A. 2016;113(40):E5944–E5951. doi:10.1073/pnas.1613225113.
- Tolbert WD, Sherburn R, Gohain N, Ding S, Flinko R, Orlandi C, Ray K, Finzi A, Lewis GK, Pazgier M, et al. Defining rules governing recognition and Fc-mediated effector functions to the HIV-1 co-receptor binding site. BMC Biol. 2020;18(1):91. doi:10.1186/s12915-020-00819-y.
- Guan Y, Pazgier M, Sajadi MM, Kamin-Lewis R, Al-Darmarki S, Flinko R, Lovo E, Wu X, Robinson JE, Seaman MS, et al. Diverse specificity and effector function among human antibodies to HIV-1 envelope glycoprotein epitopes exposed by CD4 binding. Proc Natl Acad Sci U S A. 2013;110(1):E69–78. doi:10.1073/pnas.1217609110.
- Qiu X, Audet J, Lv M, He S, Wong G, Wei H, Luo L, Fernando L, Kroeker A, Bovendo HF, et al. Two-mAb cocktail protects macaques against the Makona variant of Ebola virus. Sci Transl Med. 2016;8(329):329ra333. doi:10.1126/scitranslmed.aad9875.
- Mullarkey CE, Bailey MJ, Golubeva DA, Tan GS, Nachbagauer R, He W, Novakowski KE, Bowdish DM, Miller MS, Palese P, et al. Broadly neutralizing hemagglutinin stalk-specific antibodies induce potent phagocytosis of immune complexes by neutrophils in an Fc-dependent manner. mBio. 2016;7(5). doi:10.1128/mBio.01624-16.
- Freyn AW, Han J, Guthmiller JJ, Bailey MJ, Neu K, Turner HL, Rosado VC, Chromikova V, Huang M, Strohmeier S, et al. Influenza hemagglutinin-specific IgA Fc-effector functionality is restricted to stalk epitopes. Proc Natl Acad Sci U S A. 2021;118. doi:10.1073/pnas.2018102118.
- Temming AR, De Taeye SW, de Graaf EL, de Neef LA, Dekkers G, Bruggeman CW, Koers J, Ligthart P, Nagelkerke SQ, Zimring JC, et al. Functional attributes of antibodies, effector cells, and target cells affecting NK cell-mediated antibody-dependent cellular cytotoxicity. J Immunol. 2019;203:3126–35. doi:10.4049/jimmunol.1900985.
- Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–58. doi:10.1182/blood.v99.3.754.
- Breunis WB, van Mirre E, Bruin M, Geissler J, De Boer M, Peters M, Roos D, De Haas M, Koene HR, Kuijpers TW, et al. Copy number variation of the activating FCGR2C gene predisposes to idiopathic thrombocytopenic purpura. Blood. 2008;111(3):1029–38. doi:10.1182/blood-2007-03-079913.
- Ernst LK, Metes D, Herberman RB, Morel PA. Allelic polymorphisms in the FcgammaRIIC gene can influence its function on normal human natural killer cells. J Mol Med (Berl). 2002;80:248–57. doi:10.1007/s00109-001-0294-2.
- Li SS, Gilbert PB, Tomaras GD, Kijak G, Ferrari G, Thomas R, Pyo C-W, Zolla-Pazner S, Montefiori D, Liao H-X, et al. FCGR2C polymorphisms associate with HIV-1 vaccine protection in RV144 trial. J Clin Invest. 2014;124(9):3879–90. doi:10.1172/JCI75539.
- Li SS, Gilbert PB, Carpp LN, Pyo C-W, Janes H, Fong Y, Shen X, Neidich SD, Goodman D, deCamp A, et al. Fc gamma receptor polymorphisms modulated the vaccine effect on HIV-1 Risk in the HVTN 505 HIV vaccine trial. J Virol. 2019;93(21). doi:10.1128/JVI.02041-18.
- Repp R, Valerius T, Sendler A, Gramatzki M, Iro H, Kalden JR, Platzer E. Neutrophils express the high affinity receptor for IgG (Fc gamma RI, CD64) after in vivo application of recombinant human granulocyte colony-stimulating factor. Blood. 1991;78:885–89. doi:10.1182/blood.V78.4.885.885.
- Wang Y, Jonsson F. Expression, role, and regulation of neutrophil Fcgamma receptors. Front Immunol. 2019;10:1958. doi:10.3389/fimmu.2019.01958.
- Mendoza-Coronel E, Ortega E. Macrophage polarization modulates FcgammaR- and CD13-mediated phagocytosis and reactive oxygen species production, independently of receptor membrane expression. Front Immunol. 2017;8:303. doi:10.3389/fimmu.2017.00303.
- Evans SS, Repasky EA, Fisher DT. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat Rev Immunol. 2015;15:335–49. doi:10.1038/nri3843.
- Rice P, Martin E, He J-R, Frank M, DeTolla L, Hester L, O’Neill T, Manka C, Benjamin I, Nagarsekar A, et al. Febrile-range hyperthermia augments neutrophil accumulation and enhances lung injury in experimental gram-negative bacterial pneumonia. J Immunol. 2005;174:3676–85. doi:10.4049/jimmunol.174.6.3676.
- Lin C, Zhang Y, Zhang K, Zheng Y, Lu L, Chang H, Yang H, Yang Y, Wan Y, Wang S, et al. Fever promotes T lymphocyte trafficking via a thermal sensory pathway involving heat shock protein 90 and alpha4 integrins. Immunity. 2019;50:137–151 e136. doi:10.1016/j.immuni.2018.11.013.
- Hasday JD, Garrison A, Singh IS, Standiford T, Ellis GS, Rao S, He J-R, Rice P, Frank M, Goldblum SE, et al. Febrile-range hyperthermia augments pulmonary neutrophil recruitment and amplifies pulmonary oxygen toxicity. Am J Pathol. 2003;162(6):2005–17. doi:10.1016/S0002-9440(10)64333-7.
- Zanker KS, Lange J. Whole body hyperthermia and natural killer cell activity. Lancet. 1982;1:1079–80. doi:10.1016/s0140-6736(82)92142-0.
- Hatzfeld-Charbonnier AS, Lasek A, Castera L, Gosset P, Velu T, Formstecher P, Mortier L, Marchetti P. Influence of heat stress on human monocyte-derived dendritic cell functions with immunotherapeutic potential for antitumor vaccines. J Leukoc Biol. 2007;81(5):1179–87. doi:10.1189/jlb.0506347.
- Dowd KA, Pierson TC. The many faces of a dynamic virion: implications of viral breathing on flavivirus biology and immunogenicity. Annu Rev Virol. 2018;5:185–207. doi:10.1146/annurev-virology-092917-043300.
- McElroy AK, Akondy RS, Mcllwain DR, Chen H, Bjornson-Hooper Z, Mukherjee N, Mehta AK, Nolan G, Nichol ST, Spiropoulou CF, et al. Immunologic timeline of Ebola virus disease and recovery in humans. JCI Insight. 2020;5(10). doi:10.1172/jci.insight.137260.
- Ludtke A, Ruibal P, Becker-Ziaja B, Rottstegge M, Wozniak DM, Cabeza-Cabrerizo M, Thorenz A, Weller R, Kerber R, Idoyaga J, et al. Ebola virus disease is characterized by poor activation and reduced levels of circulating CD16+monocytes. J Infect Dis. 2016;214(suppl 3):S275–S280. doi:10.1093/infdis/jiw260.
- Mupapa K, Massamba M, Kibadi K, Kuvula K, Bwaka A, Kipasa M, Colebunders R, Muyembe‐Tamfum J. Treatment of Ebola hemorrhagic fever with blood transfusions from convalescent patients. International scientific and technical committee. J Infect Dis. 1999;179(Suppl 1):S18–23. doi:10.1086/514298.
- Dye JM, Herbert AS, Kuehne AI, Barth JF, Muhammad MA, Zak SE, Ortiz RA, Prugar LI, Pratt WD. Postexposure antibody prophylaxis protects nonhuman primates from filovirus disease. Proc Natl Acad Sci U S A. 2012;109:5034–39. doi:10.1073/pnas.1200409109.
- Mire CE, Geisbert JB, Agans KN, Thi EP, Lee ACH, Fenton KA, Geisbert TW. Passive immunotherapy: assessment of convalescent serum against ebola virus makona infection in nonhuman primates. J Infect Dis. 2016;214:S367–S374. doi:10.1093/infdis/jiw333.
- Van Griensven J, Edwards T, De Lamballerie X, Semple MG, Gallian P, Baize S, Horby PW, Raoul H, Magassouba N, Antierens A, et al. Evaluation of convalescent plasma for ebola virus disease in Guinea. N Engl J Med. 2016;374(1):33–42. doi:10.1056/NEJMoa1511812.
- Van Griensven J, Edwards T, Baize S, Ebola-Tx C. Efficacy of convalescent plasma in relation to dose of ebola virus antibodies. N Engl J Med. 2016;375:2307–09. doi:10.1056/NEJMc1609116.
- Brown JF, Dye JM, Tozay S, Jeh-Mulbah G, Wohl DA, Fischer WA, Cunningham CK, Rowe K, Zacharias P, van Hasselt J, et al. Anti-ebola virus antibody levels in convalescent plasma and viral load after plasma infusion in patients with ebola virus disease. J Infect Dis. 2018;218:555–62. doi:10.1093/infdis/jiy199.
- Gunn BM, Roy V, Karim MM, Hartnett JN, Suscovich TJ, Goba A, Momoh M, Sandi JD, Kanneh L, Andersen KG, et al. Survivors of ebola virus disease develop polyfunctional antibody responses. J Infect Dis. 2020;221(1):156–61. doi:10.1093/infdis/jiz364.
- Joyner MJ, Carter RE, Senefeld JW, Klassen SA, Mills JR, Johnson PW, Theel ES, Wiggins CC, Bruno KA, Klompas AM, et al. Convalescent plasma antibody levels and the risk of death from Covid-19. N Engl J Med. 2021;384(11):1015–27. doi:10.1056/NEJMoa2031893.
- Natarajan H, Crowley AR, Butler SE, Xu S, Weiner JA, Bloch EM, Littlefield K, Wieland-Alter W, Connor RI, Wright PF, et al. Markers of polyfunctional SARS-CoV-2 antibodies in convalescent plasma. mBio. 2021;12(2). doi:10.1128/mBio.00765-21.
- Kuroiwa Y, Kasinathan P, Sathiyaseelan T, Jiao J-A, Matsushita H, Sathiyaseelan J, Wu H, Mellquist J, Hammitt M, Koster J, et al. Antigen-specific human polyclonal antibodies from hyperimmunized cattle. Nat Biotechnol. 2009;27(2):173–81. doi:10.1038/nbt.1521.
- Luke T, Wu H, Zhao J, Channappanavar R, Coleman CM, Jiao J-A, Matsushita H, Liu Y, Postnikova EN, Ork BL, et al. Human polyclonal immunoglobulin G from transchromosomic bovines inhibits MERS-CoV in vivo. Sci Transl Med. 2016;8(326):326ra321. doi:10.1126/scitranslmed.aaf1061.
- Hooper JW, Brocato RL, Kwilas SA, Hammerbeck CD, Josleyn MD, Royals M, Ballantyne J, Wu H, Jiao J-A, Matsushita H, et al. DNA vaccine-derived human IgG produced in transchromosomal bovines protect in lethal models of hantavirus pulmonary syndrome. Sci Transl Med. 2014;6(264):264ra162. doi:10.1126/scitranslmed.3010082.
- Luke T, Bennett RS, Gerhardt DM, Burdette T, Postnikova E, Mazur S, Honko AN, Oberlander N, Byrum R, Ragland D, et al. Fully human immunoglobulin G from transchromosomic bovines treats nonhuman primates infected with ebola virus makona isolate. J Infect Dis. 2018;218(suppl_5):S636–S648. doi:10.1093/infdis/jiy377.
- Jin C, Hill J, Gunn BM, Yu W-H, Dahora LC, Jones E, Johnson M, Gibani MM, Spreng RL, Alam SM, et al. Vi-specific serological correlates of protection for typhoid fever. J Exp Med. 2021;218(2). doi:10.1084/jem.20201116.
- Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366(14):1275–86. doi:10.1056/NEJMoa1113425.
- Alter G, Yu W-H, Chandrashekar A, Borducchi EN, Ghneim K, Sharma A, Nedellec R, McKenney KR, Linde C, Broge T, et al. Passive transfer of vaccine-elicited antibodies protects against SIV in rhesus macaques. Cell. 2020;183(1):185–196 e114. doi:10.1016/j.cell.2020.08.033.
- Om K, Paquin-Proulx D, Montero M, Peachman K, Shen X, Wieczorek L, Beck Z, Weiner JA, Kim D, Li Y, et al. Adjuvanted HIV-1 vaccine promotes antibody-dependent phagocytic responses and protects against heterologous SHIV challenge. PLoS Pathog. 2020;16(9):e1008764. doi:10.1371/journal.ppat.1008764.
- Ackerman ME, Crispin M, Yu X, Baruah K, Boesch AW, Harvey DJ, Dugast A-S, Heizen EL, Ercan A, Choi I, et al. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J Clin Invest. 2013;123(5):2183–92. doi:10.1172/JCI65708.
- Ackerman ME, Mikhailova A, Brown EP, Dowell KG, Walker BD, Bailey-Kellogg C, Suscovich TJ, Alter G. Polyfunctional HIV-specific antibody responses are associated with spontaneous HIV control. PLoS Pathog. 2016;12(1):e1005315. doi:10.1371/journal.ppat.1005315.
- Chung AW, Ghebremichael M, Robinson H, Brown E, Choi I, Lane S, Dugast A-S, Schoen MK, Rolland M, Suscovich TJ, et al. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci Transl Med. 2014;6(228):228ra238. doi:10.1126/scitranslmed.3007736.
- Karnasuta C, Akapirat S, Madnote S, Savadsuk H, Puangkaew J, Rittiroongrad S, Rerks-Ngarm S, Nitayaphan S, Pitisuttithum P, Kaewkungwal J, et al. Comparison of antibody responses induced by RV144, VAX003, and VAX004 vaccination regimens. AIDS Res Hum Retrovir. 2017;33(5):410–23. doi:10.1089/AID.2016.0204.
- Yates NL, Liao H-X, Fong Y, deCamp A, Vandergrift NA, Williams WT, Alam SM, Ferrari G, Yang Z-Y, Seaton KE, et al. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med. 2014;6(228):228ra239. doi:10.1126/scitranslmed.3007730.
- Fischinger S, Shin S, Boudreau CM, Ackerman M, Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kim JH, Robb ML, Michael NL, et al. Protein-based, but not viral vector alone, HIV vaccine boosting drives an IgG1-biased polyfunctional humoral immune response. JCI Insight. 2020;5(12). doi:10.1172/jci.insight.135057.
- Mercado NB, Zahn R, Wegmann F, Loos C, Chandrashekar A, Yu J, Liu J, Peter L, McMahan K, Tostanoski LH, et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature. 2020;586(7830):583–88. doi:10.1038/s41586-020-2607-z.
- Barouch DH, Alter G, Broge T, Linde C, Ackerman ME, Brown EP, Borducchi EN, Smith KM, Nkolola JP, Liu J, et al. Protective efficacy of adenovirus/protein vaccines against SIV challenges in rhesus monkeys. Science. 2015;349(6245):320–24. doi:10.1126/science.aab3886.
- Marzi A, Engelmann F, Feldmann F, Haberthur K, Shupert WL, Brining D, Scott DP, Geisbert TW, Kawaoka Y, Katze MG, et al. Antibodies are necessary for rVSV/ZEBOV-GP-mediated protection against lethal Ebola virus challenge in nonhuman primates. Proc Natl Acad Sci U S A. 2013;110(5):1893–98. doi:10.1073/pnas.1209591110.
- Koch T, Rottstegge M, Ruibal P, Gomez-Medina S, Nelson EV, Escudero-Pérez B, Pillny M, Ly ML, Koundouno FR, Bore JA, et al. Ebola virus disease survivors show more efficient antibody immunity than vaccines despite similar levels of circulating immunoglobulins. Viruses. 2020;12(9):915. doi:10.3390/v12090915.
- Marzi A, Robertson SJ, Haddock E, Feldmann F, Hanley PW, Scott DP, Strong JE, Kobinger G, Best SM, Feldmann H, et al. EBOLA VACCINE. VSV-EBOV rapidly protects macaques against infection with the 2014/15 Ebola virus outbreak strain. Science. 2015;349(6249):739–42. doi:10.1126/science.aab3920.
- Henao-Restrepo AM, Camacho A, Longini IM, Watson CH, Edmunds WJ, Egger M, Carroll MW, Dean NE, Diatta I, Doumbia M, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ca Suffit!). Lancet. 2017;389:505–18. doi:10.1016/S0140-6736(16)32621-6.
- Khurana S, Fuentes S, Coyle EM, Ravichandran S, Davey RT, Beigel JH. Human antibody repertoire after VSV-Ebola vaccination identifies novel targets and virus-neutralizing IgM antibodies. Nat Med. 2016;22(12):1439–47. doi:10.1038/nm.4201.
- Boudreau CM, Yu W-H, Suscovich TJ, Talbot HK, Edwards KM, Alter G. Selective induction of antibody effector functional responses using MF59-adjuvanted vaccination. J Clin Invest. 2020;130(2):662–72. doi:10.1172/JCI129520.
- Francica JR, Zak DE, Linde C, Siena E, Johnson C, Juraska M, Yates NL, Gunn B, De Gregorio E, Flynn BJ, et al. Innate transcriptional effects by adjuvants on the magnitude, quality, and durability of HIV envelope responses in NHPs. Blood Adv. 2017;1:2329–42. doi:10.1182/bloodadvances.2017011411.
- Chu TH, Patz EF Jr., Ackerman ME. Coming together at the hinges: therapeutic prospects of IgG3. mAbs. 2021;13:1882028. doi:10.1080/19420862.2021.1882028.
- Saito S, Namisaki H, Hiraishi K, Takahashi N, Iida S. A stable engineered human IgG3 antibody with decreased aggregation during antibody expression and low pH stress. Protein Sci. 2019;28:900–09. doi:10.1002/pro.3598.
- Stapleton NM, Andersen JT, Stemerding AM, Bjarnarson SP, Verheul RC, Gerritsen J, Zhao Y, Kleijer M, Sandlie I, De Haas M, et al. Competition for FcRn-mediated transport gives rise to short half-life of human IgG3 and offers therapeutic potential. Nat Commun. 2011;2:599. doi:10.1038/ncomms1608.
- de Sousa-Pereira P, Woof JM. IgA: structure, function, and developability. Antibodies (Basel). 2019:8. doi:10.3390/antib8040057.
- Sterlin D, Mathian A, Miyara M, Mohr A, Anna F, Claër L, Quentric P, Fadlallah J, Devilliers H, Ghillani P, et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med. 2021;13(577):eabd2223. doi:10.1126/scitranslmed.abd2223.
- Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Viant C, Gaebler C, Cipolla M, Hoffmann -H-H, Oliveira TY, Oren DA, et al. Enhanced SARS-CoV-2 neutralization by dimeric IgA. Sci Transl Med. 2021;13(577):eabf1555. doi:10.1126/scitranslmed.abf1555.
- Sips M, Krykbaeva M, Diefenbach TJ, Ghebremichael M, Bowman BA, Dugast A-S, Boesch AW, Streeck H, Kwon DS, Ackerman ME, et al. Fc receptor-mediated phagocytosis in tissues as a potent mechanism for preventive and therapeutic HIV vaccine strategies. Mucosal Immunol. 2016;9(6):1584–95. doi:10.1038/mi.2016.12.
- Maurer MA, Meyer L, Bianchi M, Turner HL, Le NPL, Steck M, Wyrzucki A, Orlowski V, Ward AB, Crispin M, et al. Glycosylation of human IgA directly inhibits influenza A and other sialic-acid-binding viruses. Cell Rep. 2018;23(1):90–99. doi:10.1016/j.celrep.2018.03.027.
- Strasser R, Stadlmann J, Schähs M, Stiegler G, Quendler H, Mach L, Glössl J, Weterings K, Pabst M, Steinkellner H, et al. Generation of glyco-engineered Nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan structure. Plant Biotechnol J. 2008;6(4):392–402. doi:10.1111/j.1467-7652.2008.00330.x.
- Termini JM, Martinez-Navio JM, Gao G, Fuchs SP, Desrosiers RC. Glycoengineering of AAV-delivered monoclonal antibodies yields increased ADCC activity. Mol Ther Methods Clin Dev. 2021;20:204–17. doi:10.1016/j.omtm.2020.11.001.
- Parekh RB, Dwek RA, Sutton BJ, Fernandes DL, Leung A, Stanworth D, Rademacher TW, Mizuochi T, Taniguchi T, Matsuta K, et al. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985;316(6027):452–57. doi:10.1038/316452a0.
- Moore JS, Wu X, Kulhavy R, Tomana M, Novak J, Moldoveanu Z, Brown R, Goepfert PA, Mestecky J. Increased levels of galactose-deficient IgG in sera of HIV-1-infected individuals. Aids. 2005;19(4):381–89. doi:10.1097/01.aids.0000161767.21405.68.
- Mahan AE, Jennewein MF, Suscovich T, Dionne K, Tedesco J, Chung AW, Streeck H, Pau M, Schuitemaker H, Francis D, et al. Antigen-specific antibody glycosylation is regulated via vaccination. PLoS Pathog. 2016;12(3):e1005456. doi:10.1371/journal.ppat.1005456.
- Karsten CM, Pandey MK, Figge J, Kilchenstein R, Taylor PR, Rosas M, McDonald JU, Orr SJ, Berger M, Petzold D, et al. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcgammaRIIB and dectin-1. Nat Med. 2012;18:1401–06. doi:10.1038/nm.2862.
- Moore GL, Chen H, Karki S, Lazar GA. Engineered Fc variant antibodies with enhanced ability to recruit complement and mediate effector functions. mAbs. 2010;2:181–89. doi:10.4161/mabs.2.2.11158.
- Lazar GA, Dang W, Karki S, Vafa O, Peng JS, Hyun L, Chan C, Chung HS, Eivazi A, Yoder SC, et al. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci U S A. 2006;103:4005–10. doi:10.1073/pnas.0508123103.
- Moldt B, Schultz N, Dunlop DC, Alpert MD, Harvey JD, Evans DT, Poignard P, Hessell AJ, Burton DR. A panel of IgG1 b12 variants with selectively diminished or enhanced affinity for Fcgamma receptors to define the role of effector functions in protection against HIV. J Virol. 2011;85:10572–81. doi:10.1128/JVI.05541-11.
- Smith P, DiLillo DJ, Bournazos S, Li F, Ravetch JV. Mouse model recapitulating human Fcgamma receptor structural and functional diversity. Proc Natl Acad Sci U S A. 2012;109:6181–86. doi:10.1073/pnas.1203954109.
- Steurer W, Nickerson PW, Steele AW, Steiger J, Zheng XX, Strom TB. Ex vivo coating of islet cell allografts with murine CTLA4/Fc promotes graft tolerance. J Immunol. 1995;155:1165–74.
- Shields RL, Namenuk AK, Hong K, Meng YG, Rae J, Briggs J, Xie D, Lai J, Stadlen A, Li B, et al. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J Biol Chem. 2001;276:6591–604. doi:10.1074/jbc.M009483200.
- Stavenhagen JB, Gorlatov S, Tuaillon N, Rankin CT, Li H, Burke S, Huang L, Johnson S, Bonvini E, Koenig S, et al. Fc optimization of therapeutic antibodies enhances their ability to kill tumor cells in vitro and controls tumor expansion in vivo via low-affinity activating Fcγ receptors. Cancer Res. 2007;67:8882–90. doi:10.1158/0008-5472.CAN-07-0696.
- Xu D, Alegre M-L, Varga SS, Rothermel AL, Collins AM, Pulito VL, Hanna LS, Dolan KP, Parren PWHI, Bluestone JA, et al. In vitro characterization of five humanized OKT3 effector function variant antibodies. Cell Immunol. 2000;200(1):16–26. doi:10.1006/cimm.2000.1617.
- Chu SY, Vostiar I, Karki S, Moore GL, Lazar GA, Pong E, Joyce PF, Szymkowski DE, Desjarlais JR. Inhibition of B cell receptor-mediated activation of primary human B cells by coengagement of CD19 and FcgammaRIIb with Fc-engineered antibodies. Mol Immunol. 2008;45:3926–33. doi:10.1016/j.molimm.2008.06.027.
- Diebolder CA, Beurskens FJ, de Jong RN, Koning RI, Strumane K, Lindorfer MA, Voorhorst M, Ugurlar D, Rosati S, Heck AJR, et al. Complement is activated by IgG hexamers assembled at the cell surface. Science. 2014;343(6176):1260–63. doi:10.1126/science.1248943.
- Idusogie EE, Presta LG, Gazzano-Santoro H, Totpal K, Wong PY, Ultsch M, Meng YG, Mulkerrin MG. Mapping of the C1q binding site on rituxan, a chimeric antibody with a human IgG1 Fc. J Immunol. 2000;164:4178–84. doi:10.4049/jimmunol.164.8.4178.
- Idusogie EE, Wong PY, Presta LG, Gazzano-Santoro H, Totpal K, Ultsch M, Mulkerrin MG. Engineered antibodies with increased activity to recruit complement. J Immunol. 2001;166:2571–75. doi:10.4049/jimmunol.166.4.2571.
- Dall’Acqua WF, Woods RM, Ward ES, Palaszynski SR, Patel NK, Brewah YA, Wu H, Kiener PA, Langermann S. Increasing the affinity of a human IgG1 for the neonatal Fc receptor: biological consequences. J Immunol. 2002;169:5171–80. doi:10.4049/jimmunol.169.9.5171.
- Yeung YA, Leabman MK, Marvin JS, Qiu J, Adams CW, Lien S, Starovasnik MA, Lowman HB. Engineering human IgG1 affinity to human neonatal Fc receptor: impact of affinity improvement on pharmacokinetics in primates. J Immunol. 2009;182:7663–71. doi:10.4049/jimmunol.0804182.
- Datta-Mannan A, Witcher DR, Tang Y, Watkins J, Jiang W, Wroblewski VJ. Humanized IgG 1 variants with differential binding properties to the neonatal Fc receptor: relationship to pharmacokinetics in mice and primates. Drug Metab Dispos. 2007;35:86–94. doi:10.1124/dmd.106.011734.
- Zalevsky J, Chamberlain AK, Horton HM, Karki S, Leung IWL, Sproule TJ, Lazar GA, Roopenian DC, Desjarlais JR. Enhanced antibody half-life improves in vivo activity. Nat Biotechnol. 2010;28:157–59. doi:10.1038/nbt.1601.
- Hinton PR, Johlfs MG, Xiong JM, Hanestad K, Ong KC, Bullock C, Keller S, Tang MT, Tso JY, Vásquez M, et al. Engineered human IgG antibodies with longer serum half-lives in primates. J Biol Chem. 2004;279(8):6213–16. doi:10.1074/jbc.C300470200.
- Atyeo C, Slein MD, Fischinger S, Burke J, Schäfer A, Leist SR, Kuzmina NA, Mire C, Honko A, Johnson R, et al. Dissecting strategies to tune the therapeutic potential of SARS-CoV-2-specific monoclonal antibody CR3022. JCI Insight. 2021;6. doi:10.1172/jci.insight.143129.
- Brown JA, Singh G, Acklin JA, Lee S, Duehr JE, Chokola AN, Frere JJ, Hoffman KW, Foster GA, Krysztof D, et al. Dengue virus immunity increases zika virus-induced damage during pregnancy. Immunity. 2019;50(3):751–762 e755. doi:10.1016/j.immuni.2019.01.005.
- Zimmerman MG, Quicke KM, O’Neal JT, Arora N, Machiah D, Priyamvada L, Kauffman RC, Register E, Adekunle O, Swieboda D, et al. Cross-reactive dengue virus antibodies augment zika virus infection of human placental macrophages. Cell Host Microbe. 2018;24(5):731–742 e736. doi:10.1016/j.chom.2018.10.008.
- Balsitis SJ, Williams KL, Lachica R, Flores D, Kyle JL, Mehlhop E, Johnson S, Diamond MS, Beatty PR, Harris E, et al. Lethal antibody enhancement of dengue disease in mice is prevented by Fc modification. PLoS Pathog. 2010;6(2):e1000790. doi:10.1371/journal.ppat.1000790.
- Nonaka M, Kimura A. Genomic view of the evolution of the complement system. Immunogenetics. 2006;58:701–13. doi:10.1007/s00251-006-0142-1.
- Nguyen DC, Scinicariello F, Attanasio R. Characterization and allelic polymorphisms of rhesus macaque (Macaca mulatta) IgG Fc receptor genes. Immunogenetics. 2011;63:351–62. doi:10.1007/s00251-011-0514-z.
- Chan YN, Boesch AW, Osei-Owusu NY, Emileh A, Crowley AR, Cocklin SL, Finstad SL, Linde CH, Howell RA, Zentner I, et al. IgG binding characteristics of rhesus macaque FcgammaR. J Immunol. 2016;197:2936–47. doi:10.4049/jimmunol.1502252.
- Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310:1510–12. doi:10.1126/science.1118948.
- Dekkers G, Bentlage AEH, Stegmann TC, Howie HL, Lissenberg-Thunnissen S, Zimring J, Rispens T, Vidarsson G. Affinity of human IgG subclasses to mouse Fc gamma receptors. mAbs. 2017;9(5):767–73. doi:10.1080/19420862.2017.1323159.

