ABSTRACT
Objectives
To evaluate the reporting rate of adverse events following immunization (AEFI) of the quadrivalent human papillomavirus vaccine (4vHPV) and to compare the reporting rate of AEFI following 4vHPV with those following other vaccines.
Methods
Review and describe the AEFI reported to national adverse event following immunization surveillance system (NAEFISS) in Zhejiang province from 2018 to 2020. Reporting rates of AEFI were calculated by age, city, severity of AEFI, categories of AEFI, and reaction categories. The data mining algorithm used in this study was reporting odds ratio (ROR). A value of ROR‑1.96SE >1 (standard error [SE]) was considered as positive signal.
Results
NAEFISS received 238 reports after receipt of 4vHPV and 899,282 doses of 4vHPV were administered during the study period, with a crude reporting rate of 2.7/10000 doses. Fever/redness/induration (101 reports) was the most common event reported (1.12/10000 doses). Two cases of anaphylactic shock, three cases of Guillain Barre Syndrome and two cases of acute disseminated encephalomyelitis were reported. ROR showed positive signals for seizure (ROR‑1.96SE: 2.1), syncope (ROR‑1.96SE: 1.3), allergic rash (ROR‑1.96SE: 1.6) and headache (ROR‑1.96SE: 2.1).
Conclusion
The present analysis did not identify new/unexpected safety concerns. Further epidemiological studies are required to systematically validate the data provided by NAEFISS.
Introduction
Vaccine has been considered as one of the most effective and efficient means for fighting and eradicating the infectious diseases, although it has faced the limitation to the vaccine efficacy of not 100%. The risk/benefit ratio is the highest among any other medical intervention.Citation1 It is difficult or almost impossible to identify all the possible adverse events following immunization (AEFI) of a vaccine in the pre-licensure clinical trials due to many limitations from the study design, for example, the restricted sample size, the reduced follow‑up, the appraisal of surrogate markers, and the participants with multiple comorbidities.Citation2 However, these limitations could be addressed by conducting the post-licensure monitoring on vaccine safety.Citation3
Human papillomaviruses (HPVs) are an enormous and diverse group of viruses, which are classified into low‑ and high‑risk types based on their ability to promote malignant transformation.Citation4 Two HPV types, i.e. HPV‑16 and HPV‑18, cause 70% of the cervical cancers and the precancerous cervical lesions. HPV infections and HPV‑associated cancers can effectively be prevented by the HPV vaccine. The quadrivalent (types 6, 11, 16, 18) HPV vaccine (4vHPV) was first licensed by China Drug Administration in May 2017. The 4vHPV was approved for using in the females aged 20–45 years when it was first licensed and the applicable age range was extended to 9–45 years in Nov 2020. 4vHPV can prevent the vaccine type-associated cervical, other anogenital cancers, head-and-neck cancer, neoplasias, and warts. It is recommended as a three-dose series for 4vHPV over a 6-month period (0-2-6 months).Citation5 The most common AEFIs observed in the pre-licensure clinical trials included:Citation5–7 local reactions (pain, redness, swelling, and pruritus at injection site), and systemic reactions (pyrexia, nausea, dizziness, diarrhea, vomiting, fatigue, upper respiratory tract infections, oropharyngeal pain, myalgia, and headache). Of these reactions, the injection site pain and mild systemic reaction occurred most commonly.
The World Health Organization (WHO) has recommended 4vHPV to all eligible women worldwide,Citation8 but the public concern regarding adverse reactions had threatened the successful introduction in some countries. For example, Japan had withdrawn its recommendations for HPV vaccine for the fears of the adverse reactions. After several years of 4vHPV use, concern about possible associations with autoimmune disorders and unusual and rare AEFIs, including postural orthostatic tachycardia syndrome and complex regional pain syndrome began to emerge, despite a lack of epidemiologic evidence or defined biologically plausible mechanisms.Citation4 There were few post-licensure studies conducted previously to assess the AEFI of 4vHPV globally and those reports were limited to a specific adverse events or a constrained subpopulation. Up to date, there were few reports done to evaluate the post-licensure safety profile on 4vHPV.Citation4,Citation9,Citation10 The evidence for the association between 4vHPV and the neurological disorders was inconsistent. For example, some studies revealed the higher than expected reporting rates for venous thromboembolism and syncope.Citation9–11 However, a subsequent study in Europe did not detect an increased risk of venous thromboembolism following 4vHPV.Citation12 A study from France found an increased risk of Guillain-Barré syndrome (GBS) following 4vHPV while another study from the U.S. vaccine safety datalink found no evidence of an increased risk of GBS following 4vHPV.Citation13
These aforementioned studies covered reports only from the initial period of use after the licensure of 4vHPV and many new categories of AEFIs would have unfolded due to the extended use. The limited number of safety surveillance studies could result in an ambiguous situation on the safety profile of 4vHPV. This study aimed to evaluate the reporting rate of AEFI following 4Vhpv by demographic variables on the basis of the national adverse event following immunization surveillance system (NAEFISS)Citation14 database from 2018 to 2020, and the reporting rate of AEFI following 4vHPV was also compared with those following other vaccines.
Methods
Study area
Zhejiang province is located at the east coastline of China, with a dense population of 70 million. The expanded program on immunization (EPI) was initiated in 1978, and it continued with 11 vaccines up to date and over 25 million vaccination doses were administered in every year. Since 2009, Zhejiang province joined in the NAEFISS, which was upgraded in 2012 for adding variables of the case reporting form and rules of data logic verification.Citation14
National adverse event following immunization surveillance system
NAEFISS is a national spontaneous reporting system for AEFI following China-licensed vaccines, which aims to detect new, unusual, or rare AEFIs, to evaluate the safety of newly licensed vaccines, to identify potential risk factors for AEFIs, to monitor increases in known AEFIs, to determine the possible reporting clusters, and to provide a reliable safety monitoring system.
NAEFISS accepts reports from patients or caregivers, healthcare providers, vaccine manufacturers, and others. The center of disease control and prevention (CDC) at county level should submit the AEFI report to the NAEFISS on the same day when it was detected. The reporting form collects the information on vaccinated individual, storage, and transportation of vaccines, vaccine administrations, and the AEFI itself. Signs and symptoms of AEFI are coded using the international classification of diseases (version 10.0, ICD-10),Citation15 a clinically validated, internationally standardized terminology. A single AEFI report may be assigned more than one term and be referred to more than one suspected vaccine.
All AEFI records are divided into five categories:Citation14 (1) vaccine product-related reaction (non-serious reaction and serious reaction); (2) vaccination error; (3) vaccine quality defect-related reaction; (4) coincidental event; (5) anxiety reaction. All AEFI records are assessed as non-serious or serious:Citation14 (1) non-serious, with no intervention necessary or with physician visit or event interfering with daily activities or loss of working hours; (2) serious, with any untoward medical occurrence that results in death, hospitalization, prolongation of hospitalization, persistent or significant disability/incapacity, life threatening or birth defect.
Data resource
The AEFI reports following 4vHPV from 2018 to 2020 were extracted from the NAEFISS on Apr 1, 2021, to account for data lags due to the correction and cleaning. The number of doses of 4vHPV administered during the same period was obtained from Zhejiang provincial immunization information system (ZJIIS), which was established in 2005 and its functions could be found elsewhere.Citation16
Study design
The descriptive analyses of NAEFISS data were conducted to calculate the crude 4vHPV reporting. We also performed clinical review of reports for selected specified conditions of interest, and identified the reporting rate of AEFI following 4vHPV whether higher than those of the other licensed vaccines.
Category of AEFI
All AEFI reports were divided into five categories according to the guidelines issued by health commission of China:Citation17 (1) vaccine product-related reaction (minor reaction and severe reaction); (2) vaccination error; (3) vaccine quality defect-related reaction; (4) coincidental event; (5) anxiety reaction. Severity of AEFI was assessed as non-serious or serious based on the guidelines:Citation17 (1) non-serious, with no intervention necessary or with physician visit or event interfering with daily activities or loss of working hours; (2) serious, with any untoward medical occurrence that results in death, hospitalization, prolongation of hospitalization, persistent or significant disability/incapacity, life threatening or birth defect.
Outcome and data analysis
A database was organized as an Excel file (Microsoft Office Excel 2020). The basic characteristics of reports were summarized by causal category, type of reporter, severity, city, patient age, and interval of AEFI onset. The most common ICD-10 preferred terms from 4vHPV reports were evaluated. The reporting rates of the specified conditions, which were chosen based on the information from previous studies or surveillance work or public concern about specific AEFIs, were calculated. The AEFI reporting rates of 4vHPV for all reports and serious reports were calculated. We graphically depicted monthly reporting rates during the study period to display its trends. Onset interval was defined as the number of days from the time of vaccination to the onset of earliest reported symptoms. In this study, we presented the proportions of AFEI with the different onset intervals.
Disproportionality analysis was applied and the data mining algorithm used in this study was the reporting odds ratio (ROR).Citation18,Citation19 The ROR is defined as the ratio of the odds of reporting of one specific AEFI versus all other AEFIs for a given vaccine compared to the reporting odds for all other vaccines present in the database. Generally, a value of ROR‑1.96SE >1 (standard error [SE]) is considered as a cutoff value. The ROR is considered as a positive signal if it is above the threshold value. The higher the value, the stronger the disproportion appears to be.
Results
From 2018 to 2020, NAEFISS received 238 AEFI reports following 4vHPV without any duplicated reports and death reports. Overall, 50 reports (21.01%) were serious and most reports (58.82%) were in women aged 30–39 years. The majority reports came from healthcare providers (78.99%), followed by patients (16.39%). Of the AEFI reports, 183 (76.89%) were categorized as the vaccine product-related reactions (minor reaction) and 38 (15.97%) were categorized as the serious reactions. The majority reports indicated that the time interval from receipt of 4vHPV to start of symptoms was within 48 hours (82.35% for the total AEFI reports and 7.12% for the serious AEFI reports). In the 31 AEFI reports following 4vHPV, inactivated influenza vaccine was administered concomitantly ().
Table 1. Characteristics of AEFI reports following human papillomavirus quadrivalent vaccine (4vHPV) from 2018–2020 (N = 238)
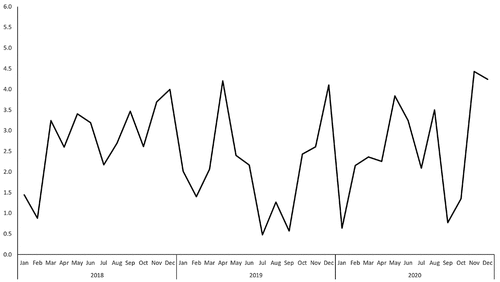
During the study period, 889,282 doses of 4vHPV were administered, with a crude reporting rate of 2.68/10000 doses. The reporting rate peaked in Nov 2020 at around 4.43/10000 doses (). The AEFI reporting rate was highest in Ningbo city (4.26/10000 doses) and lowest in Shaoxing city (1.48/10000 doses). The AEFI reporting rate for serious AEFI was highest in Ningbo city (1.09/10000 doses), while no serious AEFI report was observed in Zhoushan city ().
Table 2. Serious AEFI and non-serious AEFI reports following human papillomavirus quadrivalent vaccine (4vHPV) from 2018–2020, by city
Figure 1. Reporting rate of adverse event following immunization of 4vHPV from 2018 to 2020, Zhejiang province (/10000 doses).
After a thorough review of the clinical diagnoses of the 238 reports, 38 were severe vaccine product-related reactions. Of them, two cases of anaphylactic shock (0.02/10000 doses), three cases of Guillain Barre Syndrome (0.03/10000 doses) and two cases of acute disseminated encephalomyelitis (0.02/10000 doses) were observed. Among the 183 minor vaccine product-related reactions, fever/redness/induration (101 reports) was the most common AEFI (1.14/10000 doses), followed by headache (0.54/10000 doses) and allergic rash (0.53/10000 doses). The positive signals were obtained for seizure (ROR‑1.96SE: 2.09), syncope (ROR‑1.96SE: 1.33), allergic rash (ROR‑1.96SE: 1.58), and headache (ROR‑1.96SE: 2.08) ().
Table 3. Clinical diagnosis of AEFI reports following human papillomavirus quadrivalent vaccine (4vHPV) from 2018–2020
Discussion
Although NAEFISS shares the immanent limitations of all passive surveillance systems, it has a horizon of scope and could provide the important signals that may need further evaluation.Citation20 Such post-licensure monitoring allows for the potential detection of rare AEFIs as more people are vaccinated. As expected with the increased numbers of vaccination in the post-licensure settings, rare AEFIs can be observed more frequently than in the pre-licensure trails.
Our analysis of a 3-year period (2018 through 2020) of the post-licensure safety data on 4vHPV in the NAEFISS did not detect any unusual or unexpected patterns of AEFI reports, which could suggest the new safety concerns. With a review of 899,282 doses administered, the reporting rate of AEFI in this study was higher than that from U.S. in both female and male (0.47/10000 doses). Besides, the reporting rate of serious AEFI was also higher than that from the same study (0.18/10000 doses). It could be due to the difference in the sensitivity of AEFI surveillance system or the definitions of AEFI or serious AEFI, which could reappear in our previous studies.Citation21,Citation22 The percentages of non-serious and serious AEFI were similar to those reported in the previous publication. The injection site reactions, headache were commonly reported AEFIs following 4vHPV.Citation4,Citation10 In general, the safety profile of 4vHPV developed through our study was consistent with data from pre-licensure clinical trials and other post-licensure surveillance and epidemiologic reports.
Compared with a previous post-licensure report, most of the AEFI reports in our study were submitted by healthcare provider or patients/caregivers.Citation4 It could address the limitation that the reports submitted by manufacturers did not include the sufficient identifying information to allow the following medical review, which would induce the category of AEFI not be confirmed. Temporal analysis revealed that there was no reporting peak of AEFI following 4vHPV during the study period. It indicated that the reporting rate of AEFI showed a steady trend over the whole study period. As expected, the age group of 20–40 years accounted for 86% of the total reports. The main reason was that this group was routinely recommended for receiving the 4Vhpv during the most of the study period. Only one individual report under 20 years old was observed, which was demonstrated as a vaccination error. A study conducted by Hibbs et al. showed that HPV and rotavirus vaccine were the most frequently involved in vaccination error reports.Citation23 Most of AEFI occurred on the day of vaccination, which was similar to the findings from our previous report on the surveillance on AEFI for all vaccines used in Zhejiang province.Citation17 As we known, the minor adverse reactions like fever and rash were the most common either 4vHPV or other vaccines, while the onset interval for these common adverse reactions were very short.
In this study, we found the reporting rates of the total AEFI and the serious AEFI were different between cities. It might be due to the sensitivity of the AEFI surveillance system in the individual city was different and some cities had a lower sensitivity. For example, some cities had a high reporting rate of serious AEFI but with a low reporting rate of non-serious AEFI concurrently, which would indicate the non-serious AEFI would be under reported. This pattern suggested that it was likely to be related, to some extent, to know the disparities in the notification or investigation procedures of AEFI. Further studies to evaluate and compare AEFI surveillance sensitivity across cities would help to elucidate this problem.
The autoimmune disorders such as postural orthostatic tachycardia syndrome, complex regional pain syndrome were not reported in our study, which was inconsistent with the previous publications.Citation24,Citation25 GBS is a nervous system disorder which is the most frequent cause of acute flaccid paralysis worldwide. Ojha et al. conducted a study to analyze the post-licensure surveillance data following 4vHPV vaccination, which indicated that GBS was not a frequently reported AEFI.Citation25 In this study, the data mining analysis also did not identify any disproportionate reporting signal for GBS following 4vHPV. Our finding was inconsistent with the previous reports from other settings,Citation24,Citation25 which considered the GBS as one of the most common concerns for HPV vaccine safety. We assumed that the complex clinical diagnosis standard and the difficulty in determining the association between vaccination and GBS would induce the under-reporting of GBS.
Our study identified a positive signal and a total of four syncope cases, which was in line with the previous report.Citation26 To our knowledge, syncope is an anxiety reaction and most of the syncope documented after vaccination occurred in female or young children, resulting in hospitalization for medical evaluation because of injury. Female or young children also had a higher background rate of syncope than the general population. The predominance of females could represent the reporting bias since the 4vHPV was currently licensed for females only in China. We strongly urged that all vaccination providers must follow the Chinese immunization regulation to strongly recommend the recipients for the 30-min medical observation after vaccination to prevent syncope‑related injuries.
The headache was found as a frequent minor reaction after 4vHPV vaccination and was detected as a positive signal. In the pre-licensure report, the headache was the most commonly reported systemic AEFI in both the intervention and control arms, but it accounted for 7.7% of the total AEFI reports and 1.2% for the serious AEFI reports in a post-licensure report from U.S.Citation4 Although the percentage of headache was higher in this study, they were classified into the minor reactions as no positive signs or symptoms were observed in the neurologic evaluation.
Allergic rash is a common clinical manifestation of the hypersensitivity reactions. In our analysis, we found the allergic rash as a positive signal of the AEFI associated with 4vHPV vaccination and all of them were mild. It was consistent with the pre- and post-licensure studies.Citation4,Citation27 The main reason would be due to the allergy of the recipient to the components of the vaccine, like the proteins.
Seizure is a very common adverse reaction after vaccinated with the pertussis containing vaccines, which was due to the residual. However, in the previous publications, seizure was reported very rare and had not been identified as a positive signal before.Citation26 We could not determine whether this association was actually existed or it was due to the reporting or diagnosing bias. It needed further monitoring or specialized research.
Anaphylactic shock is an acute hypersensitivity reaction with multi-organ-system involvement that can present as, or rapidly progress to, a severe life-threatening reaction. Anaphylactic shock following immunization is a serious, but rare occurrence (1–10/million doses).Citation28 Anaphylactic shock had rarely been reported by the previous studies; however, two anaphylactic shock were reported in our study. On one hand, we assumed that the component of vaccine could induce the anaphylaxis reactions theoretically since the anaphylaxis might occur following exposure to allergens from a variety of sources including food, aeroallergens, insect venom, drugs, and immunization. On the other hand, anaphylactic shock could be misdiagnosed without enough diagnostic basis as vaccination providers must give the first aid (like epinephrine injection) when the atypical early symptoms appeared.
There were still several limitations regarding this study. First, signals detected through the analysis of passive surveillance data on AEFI required confirmation through a controlled study. It should be used simultaneously with the medical judgment since these results did not mean a causal relationship between the AEFI and the vaccination existed. Extensive studies and pharmacological evidence were needed to prove it. Second, other drawbacks included underreporting (vaccination of an illiterate individual who is unable to report properly), the absence of control group, lack of verification of reported diagnosis, insufficient clinical information, and mix-up with a non-HPV vaccination could affect the accuracy of the results and this limitation should be addressed in the future active surveillance studies.
Conclusion
Vaccination with 4vHPV has a great potential to decrease the global morbidity and mortality of HPV‑associated disorders, including cervical cancer. Our analysis did not identify new/unexpected safety concern in the review of AEFI reports following 4vHPV from 2018 to 2020, which was broadly consistent with the safety data from the previous pre- or post-licensure trials. Vaccination providers should be cautious about the plausibility to encounter the AEFI associated with 4vHPV observed in post-licensure surveillance. Further epidemiological researches are recommended to systematically validate the data from NAEFISS. Our findings would serve as a reference for discussing the benefits and risks of 4vHPV vaccination. Since the NAEFISS data must be interpreted cautiously and could not generally be used to infer causal associations between vaccine and AEFI, the identified signals should be validated by using epidemiologic observational studies.
Author contributions
Y.H., HK.L. and XJ. P conceived and designed the experiments; H.L. and Y.C. performed the experiments; H. L. and Y.W. analyzed the data; LZ.S. and FX. C. contributed reagents/materials/analysis tools; XJ. P and Y.H. wrote the paper.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the author(s).
Ethics approval and consent to participate
This study was approved by the ethical review board of Zhejiang provincial CDC. All the data were anonymous when we exported them from ZJIIS and kept confidential without individual identifiers.
Acknowledgments
We are grateful to all physicians in all health centers for providing AEFI reports in Zhejiang province. We thank our colleagues of CDCs at municipal and county levels for collecting and collating data.
Additional information
Funding
References
- Grammatikos AP, Mantadakis E, Falagas ME. Meta-analyses on pediatric infections and vaccines. Infect Dis Clin North Am. 2009;23:431–57. doi:10.1016/j.idc.2009.01.008.
- Ahmad SR. Adverse drug event monitoring at the food and drug administration. J Gen Intern Med. 2003;18:57–60. doi:10.1046/j.1525-1497.2003.20130.x.
- De St Maurice A, Edwards KM. Post-licensure monitoring to evaluate vaccine safety. J Pediatr. 2015;166:513–15. doi:10.1016/j.jpeds.2014.12.031.
- Slade BA, Leidel L, Vellozzi C, Woo EJ, Hua W, Sutherland A, Izurieta HS, Ball R, Miller N, Braun MM, et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA. 2009;302:750–57. doi:10.1001/jama.2009.1201.
- Villa LL, Costa RL, Petta CA, Andrade RP, Paavonen J, Iversen OE, Olsson S-E, Høye J, Steinwall M, Riis-Johannessen G, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer. 2006;95:1459–66. doi:10.1038/sj.bjc.6603469.
- Siddiqui MA, Perry CM. Human papillomavirus quadrivalent (types 6, 11, 16, 18) recombinant vaccine (Gardasil): profile report. BioDrugs. 2006;20:313–16. doi:10.2165/00063030-200620050-00006.
- Vaccine package insert for human papillomavirus quadrivalent (types 6, 11, 16, 18) recombinant vaccine (Gardasil). [accessed 2021 June 15]. https://www.merck.com/product/usa/pi_circulars/g/gardasil/gardasil_pi.pdf.
- Meeting of the WHO human papillomavirus vaccine advisory committee, April 2010. Releve epidemiologique hebdomadaire / Section d’hygiene du Secretariat de la Societe des Nations = Weekly epidemiological record / Health Section of the Secretariat of the League of Nations. 2011;86:227–31.
- Arana JE, Harrington T, Cano M, Lewis P, Mba-Jonas A, Rongxia L, Stewart B, Markowitz LE, Shimabukuro TT. Post-licensure safety monitoring of quadrivalent human papillomavirus vaccine in the vaccine adverse event reporting system (VAERS), 2009–2015. Vaccine. 2018;36:1781–88. doi:10.1016/j.vaccine.2018.02.034.
- Sy LS, Meyer KI, Klein NP, Chao C, Velicer C, Cheetham TC, Ackerson BK, Slezak JM, Takhar HS, Hansen J, et al. Postlicensure safety surveillance of congenital anomaly and miscarriage among pregnancies exposed to quadrivalent human papillomavirus vaccine. Hum Vaccin Immunother. 2018;14:412–19. doi:10.1080/21645515.2017.1403702.
- Gee J, Naleway A, Shui I, Baggs J, Yin R, Li R, Kulldorff M, Lewis E, Fireman B, Daley MF, et al. Monitoring the safety of quadrivalent human papillomavirus vaccine: findings from the vaccine safety datalink. Vaccine. 2011;29(46):8279–84. doi:10.1016/j.vaccine.2011.08.106.
- Ward D, Thorsen NM, Frisch M, Valentiner-Branth P, Molbak K, Hviid A. A cluster analysis of serious adverse event reports after human papillomavirus (HPV) vaccination in Danish girls and young women, September 2009 to August 2017. Euro Surveillance. 2019;24:1800380.
- Hviid A, Svanstrom H, Scheller NM, Gronlund O, Pasternak B, Arnheim-Dahlstrom L. Human papillomavirus vaccination of adult women and risk of autoimmune and neurological diseases. J Intern Med. 2018;283:154–65. doi:10.1111/joim.12694.
- Liu D, Wu W, Li K, Xu D, Ye J, Li L, Wang H. Surveillance of adverse events following immunization in China: past, present, and future. Vaccine. 2015;33:4041–46. doi:10.1016/j.vaccine.2015.04.060.
- Rust J. Updating the international classification of diseases and related health problems, tenth revision (ICD-10). Health Inf Manag. 2010;39:40. doi:10.1177/183335831003900207.
- Li Q, Hu Y, Zhong Y, Chen Y, Tang X, Guo J, Shen L. Using the immunization information system to determine vaccination coverage rates among children aged 1–7 years: a report from Zhejiang Province, China. Int J Environ Res Public Health 2014; 11:2713–28.
- Hu Y, Li Q, Lin L, Chen E, Chen Y, Qi X. Surveillance for adverse events following immunization from 2008 to 2011 in Zhejiang Province, China. Clin Vaccine Immunol. 2013;20:211–17. doi:10.1128/CVI.00541-12.
- Kim S, Park K, Kim MS, Yang BR, Choi HJ, Park BJ. Data-mining for detecting signals of adverse drug reactions of fluoxetine using the Korea Adverse Event Reporting System (KAERS) database. Psychiatry Res. 2017;256:237–42. doi:10.1016/j.psychres.2017.06.038.
- Sakaeda T, Tamon A, Kadoyama K, Okuno Y. Data mining of the public version of the FDA adverse event reporting system. Int J Med Sci. 2013;10:796–803. doi:10.7150/ijms.6048.
- Clothier HJ, Lawrie J, Lewis G, Russell M, Crawford NW, Buttery JP. SAEFVIC: surveillance of adverse events following immunisation (AEFI) in Victoria, Australia, 2018. Commun Dis Intell. 2018;2020:44.
- Joshi J, Das MK, Polpakara D, Aneja S, Agarwal M, Arora NK. Vaccine safety and surveillance for adverse events following immunization (AEFI) in India. Indian J Pediatr. 2018;85:139–48. doi:10.1007/s12098-017-2532-9.
- Schumacher Z, Bourquin C, Heininger U. Surveillance for adverse events following immunization (AEFI) in Switzerland–1991–2001. Vaccine. 2010;28:4059–64. doi:10.1016/j.vaccine.2010.04.002.
- Hibbs BF, Moro PL, Lewis P, Miller ER, Shimabukuro TT. Vaccination errors reported to the vaccine adverse event reporting system, (VAERS) United States, 2000–2013. Vaccine. 2015;33:3171–78. doi:10.1016/j.vaccine.2015.05.006.
- Gee J, Sukumaran L, Weintraub E, Vaccine Safety Datalink T. Risk of guillain-barre syndrome following quadrivalent human papillomavirus vaccine in the vaccine safety datalink. Vaccine. 2017;35:5756–58. doi:10.1016/j.vaccine.2017.09.009.
- Ojha RP, Jackson BE, Tota JE, Offutt-Powell TN, Singh KP, Bae S. Guillain-Barre syndrome following quadrivalent human papillomavirus vaccination among vaccine-eligible individuals in the United States. Hum Vaccin Immunother. 2014;10:232–37. doi:10.4161/hv.26292.
- Neha R, Subeesh V, Beulah E, Gouri N, Maheswari E. Postlicensure surveillance of human papillomavirus vaccine using the vaccine adverse event reporting system, 2006–2017. Perspect Clin Res. 2020;11:24–30. doi:10.4103/picr.PICR_140_18.
- Munoz N, Manalastas R Jr., Pitisuttithum P, Tresukosol D, Monsonego J, Ault K, Clavel C, Luna J, Myers E, Hood S, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24–45 years: a randomised, double-blind trial. Lancet. 2009;373:1949–57. doi:10.1016/S0140-6736(09)60691-7.
- Jens U, Michael S, Jos´e MB, Michael D, Jan B, Sheila F, Glacus S, Ulrich H, Babatunde I, Ali K, et al. Anaphylaxis: Case Definition and Guidelines for Data Collection, Analysis, and Presentation of Immunization Safety Data. Vaccine. 2007;25:5675–84.

