?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Given the urgent global need for vaccinating individuals of all ages against the COVID-19 pandemic, understanding the extent and reasons for parents’ willingness to get their children vaccinated is important. This study used an internet-based questionnaire survey to determine parents’ willingness to get their children (0 to 15 years) vaccinated in Japan and was conducted in April 2021 before COVID-19 vaccination for children began. Socio-demographic information, information about parents’ willingness to get children vaccinated, reasons for their responses, and parents’ willingness to get themselves vaccinated were obtained. Descriptive statistics were used to evaluate parents’ willingness to get children vaccinated based on the other variables. Logistic regression analyses were conducted to identify the characteristics of parents who indicated willingness to get their children vaccinated and to identify the reasons for vaccine willingness. Of the 1100 respondents, 472 were willing to get children vaccinated, 470 were not sure, and 158 did not want to get children vaccinated. Considerable differences were found in the willingness to get children vaccinated across gender, age groups, occupation, annual income, respondent’s academic background, and respondent’s willingness to get COVID-19 vaccination for themselves. Reasons for being unsure about the vaccination included the vaccine’s potential side effects (84.9%), uncertainty about vaccine safety (54.7%), and not trusting vaccine efficiency (25.7%). Parents’ willingness to get the COVID-19 vaccine for themselves was a significant factor for their willingness to get children vaccinated after adjusting all other factors. The study recommends disseminating more and correct information to the public to increase vaccine willingness.
Introduction
The number of cases of vaccine-preventable diseases (VPDs) should ideally reduce over time and not be a public health concern. However, the number of cases for only two VPDs, smallpox and polio, have significantly reduced,Citation1 and many other VPDs continue to become recurring epidemics worldwide. One of the reasons for the lack of significant decline in VPDs is “vaccine hesitancy.” Opposition to vaccination dates back to the 1800s – to the first vaccine ever created by Edward Jenner. Despite the public’s growing scientific sophistication, the hesitancy has never completely been eliminated. A variety of factors contribute to modern vaccine hesitancy, including the layperson’s heuristic thinking about balancing risks and benefits as well as a number of other features of vaccination, including falling victim to its own success.Citation2
Despite the high rate of childhood vaccination coverage in most developed countries, some studies have shown that not a small number of parents worry about their children’s vaccination.Citation3–5 Vaccine hesitation is one of the most common public health threats in the world.
Vaccine hesitancy is apparent in the context of the COVID-19 vaccine as well: a June 2020 survey on willingness to vaccinate in Europe, which was conducted when the COVID-19 vaccine was still in its development stage, found that 15–35% of the respondents expressed vaccine hesitancy in each surveyed country.Citation6 In Japan, a September 2020 survey, which was conducted when the COVID-19 vaccine was still in its developmental stage, showed that 22% of the population was unsure about vaccination.Citation7
Although the mRNA-based COVID-19 vaccine was licensed in the U.S. at the end of 2020 and vaccination gradually expanded, the vaccination rate began to show sluggish growth approximately six months after vaccination began. In Japan, the first COVID-19 vaccinations were administered to healthcare workers in February 2021, and by the end of June 2021, large-scale mass vaccination of older people as well as of the employees of some large corporations and university students had also begun.Citation8
After sharing with regulatory bodies Phase-3 trial data indicating that Pfizer-BioNTech’s mRNA BNT162b2 vaccine was efficacious, immunogenic, and safe for children aged 12–15 years, the United States authorized the use of the vaccine for this age group.Citation9 Pfizer-BioNTech is thought to be considering the possibility of gradually lowering the age range for the vaccination and eventually making the vaccine available to all age groups, thereby treating COVID-19 as a VPD. Some pediatricians have advocated the idea that vaccinating children against COVID-19 could also help protect adults who come in contact with them, and when combined with vaccinating young adults, the elderly would be protected from infection.Citation10 A safe and effective vaccine would thus offer significant benefits to minors, their families, and society in general.Citation11 The key to widespread vaccination for children in the future will be the presence of parents who have strong opinions about the importance of vaccinating their children. Given that the pediatric COVID-19 vaccine has not yet been commercialized, to promote vaccine use in the future, it is extremely important to understand what percentage of parents are hesitant as well as the reasons for their hesitation. Therefore, we conducted an internet-based questionnaire survey to determine the attitudes of parents in Japan in April 2021, when the COVID-19 vaccination of children had not yet begun. We believe that the survey findings will clarify what parents in Japan think about the COVID-19 vaccine and enable us to suggest appropriate measures for encouraging children’s vaccination in the future.
Materials and methods
This study was conducted in April 2021 in Japan. We used internet research panel data from Freeasy, operated by iBRIDGE Inc., Tokyo, Japan. More than five million people are registered on this research panel. Participants were selected from the approximately 5 million people who had originally registered with the company and responded to an application via e-mail notification. The sample size was calculated using a margin of error of 3%, a confidence level of 95%, a response distribution of 50%, and a target population of 110 million. According to the Hamed et al, the formula of sample size was below;
While n is the required sample size, p is the percentage occurrence of a state, E is the percentage maximum error required, z is the value corresponding to level of confidence required.Citation12 We followed this formula and apply our numbers, we get the following equation. n = 0.5(1–0.5)1.962/0.032. So the minimum sample size was calculated to be 1067.Citation12,Citation13 Therefore, the sample consisted of 1100 respondents. This survey was administered to parents aged between 18 and 60 years with children aged between 0 and 15 years in Japan. The questionnaire sought the following information: (1) respondent’s sex; (2) respondent’s age; (3) place of residence; (4) annual income (5) respondent’s academic background; (6) respondent’s occupation; (7) number of children and their age; (8) vaccine willingness for pediatric COVID-19 vaccine (Would you be willing to get your children vaccinated when the pediatric COVID-19 vaccine is developed? “Yes,” “Unsure,” “No”); (9) reasons for previous answer (multiple answers); (10) respondent’s vaccine willingness (or already vaccinated) for COVID-19 vaccine.
Descriptive statistics were used to evaluate parents’ willingness to get their children vaccinated by gender, age group, place of residence, annual income, academic background, occupation, number of children, and respondent’s vaccine willingness. While indicating place of residence, prefectural levels were originally used; however, because it proved to be too detailed to understand the tendency, we dichotomized the areas as “Central area” (Kanto area: around Tokyo metropolis and Kansai area: around Osaka metropolis) and “Others.” The reasons for wanting, not wanting, and hesitating to be vaccinated were evaluated by gender. For each statistical analysis, a chi-squared test was used to evaluate categorical variables. We also analyzed the characteristics of the main reason for parents’ willingness to get their children vaccinated using logistic regression analysis, with gender, age, place of residence, annual income, academic background, and respondent’s vaccine willingness as independent variables. The significance level was set at p < .05. JMP Pro 14.1.0 (SAS Institute Inc. Cary, NC, USA) was used for all the analyses.
The study was approved by the ethical committee of Kawasaki Medical School (Approval number: 5187–00). Implied consent was used rather than formal written consent to assure the anonymity of participants. The participants clicked the “I agree” button before starting the survey to indicate their consent.
Results
Of the 1100 participants, 468 were male (42.5%), and the average age was 40.2 years. The average number of children was 1.6, and average age of the children was 7.4 years. Almost all the respondents were married (94.9%). A total of 632 (57.5%) were living in the central area. The results of the other variables are shown in .
Table 1. Characteristics of respondents
Overall, 472 (42.9%) of 1100 participants stated that they were willing to get their children vaccinated against COVID-19 if a pediatric vaccine was available. Of the total participants, 470 (42.7%) stated that they were not sure, and 158 (14.4%) stated that they did not want their children to get vaccinated. As shown in , we found considerable differences in the willingness to get vaccinated across genders, age groups, occupation, annual income, respondent’s academic background, and respondent’s willingness to get COVID-19 vaccination for themselves.
Table 2. Parents’ willingness to get their children vaccinated against COVID-19
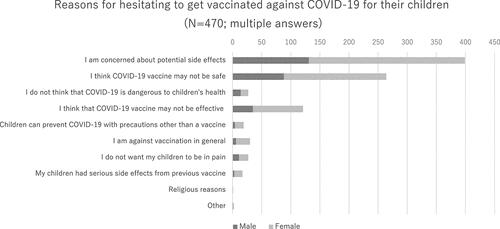
We asked participants who were unsure about getting their children vaccinated about their main reasons (). A total of 399 out of 470 people (84.9%) said that they were concerned about the potential side effects of the vaccine, although this concern was more common among women than men. A total of 264 out of 470 people (54.7%) said that they suspected the safety of the vaccination itself. Nearly one-fourth (25.7%) of the participants did not trust the vaccine efficiency.
Furthermore, logistic regression analyses were conducted to identify the characteristics of parents who indicated willingness to get their children vaccinated against COVID-19. To identify the reasons for vaccine willingness, the dependent variable was dichotomized into those who responded that they wanted their children to get the COVID-19 vaccine and those who provided other responses (“unsure” and “no”). We created three logistic regression models: the first with parents’ willingness to get themselves vaccinated as an independent variable, the second with the factors showing significant differences in as independent variables, and the third with all the factors as independent variables. The results of the logistic regression analyses revealed that parents’ willingness to get themselves vaccinated was a significant factor in determining their willingness to get their children vaccinated, after adjusting all other considerable factors (OR: 54.28 compared with parents who were unsure about their COVID-19 vaccine, 95% CI: 32.99–89.29) ().
Table 3. Logistic regression analysis with parents’ willingness to get their children vaccinated against COVID-19 as the dependent variable
Discussion
Our results suggested that the number of parents (approximately 42%) who wanted to get their children vaccinated against COVID-19 and those who were hesitant was almost equal. The results were almost same as former England survey.Citation14 In the England survey, 48.2% of parents wanted their children to be vaccinated against COVID-19, while 40.9% were hesitant. The main reason for vaccine hesitation against COVID-19 was worry about potential side effects. Almost all parents said that they were concerned about the potential side effects of the COVID-19 vaccine. All vaccines are known to cause adverse reactions to some extent. Even with the COVID-19 vaccine, there have been many reports of adverse reactions in adults.Citation15–17 Recent data suggest the incidence of anaphylaxis episodes attributable to the Pfizer/BioNTech vaccine in roughly 1:200,000 individuals.Citation15,Citation18 This is in sharp contrast to the rate of less than 1 per million doses for most vaccines. The causative antigen and associated mechanisms continue to be under investigation, although polyethylene glycol has been proposed as the potential culprit.Citation19 The FDA EUA guidance for both vaccines is to not administer the vaccine to individuals with a known history of anaphylaxis to any component of the COVID-19 vaccine. The Centers for Disease Control and Prevention additionally advises that individuals with a history of an immediate allergic reaction to a vaccine or injectable or any history of anaphylaxis be observed for 30 minutes after the COVID-19 vaccination. All other individuals should be observed for 15 minutes after the COVID-19 vaccination.Citation20 Staff at vaccine clinics must be able to identify and manage anaphylaxis,Citation21 although these serious adverse reactions are rare and not as frequent for other vaccines.Citation22
Currently, accurate prediction of adverse reactions is difficult because the COVID-19 vaccination has not yet been commercialized for children. However, some vaccines have already been administered to children older than 12 years, and reports of significantly more cases of myocarditis and pericarditis among these children have emerged, particularly in young males.Citation23,Citation24 Nevertheless, the number of reports is very small considering that millions of people have been vaccinated, and patients are recovering quickly after being treated with NSAIDs (some require steroids).Citation25 Such information should be made available to the public so that they can make better and more informed decisions about vaccination.
Meanwhile, the results in show that the older the age group of the parents and the higher their annual income and education, the more willing they are to vaccinate their children. The results also suggest that parents who want to vaccinate themselves or have already done so tend to be more willing to vaccinate their children. We also found that the common characteristic of those who expressed willingness to get their children vaccinated was that they had the highest odds ratio of being willing to receive the COVID-19 vaccine themselves (or having already been vaccinated) from results of logistic regression analysis. The results of logistic regression analysis also suggested that parental age of 40 years or older and annual income (6,000,000–9,000,000 JPY) were also a factor that influences the willingness to be vaccinated. Previous studies in other countries and Japan have also shown that older age groups tend to be more willing to be vaccinated,Citation7,Citation26 yet the influence of annual income was unknown.Citation26,Citation27 Although there are a few opinions that providing the COVID-19 vaccination to children is not necessaryCitation28,Citation29 or should not be recommended,Citation30 many pediatric societies and experts say that it is worthwhile to actively provide COVID-19 vaccine to children.Citation10,Citation11,Citation31,Citation32
Our study has some potential limitations. First, as our study was a cross-sectional study, we could not derive any causality from our results. Secondly, we did not enquire about the willingness to pay for the COVID-19 vaccine because at the time of the study, vaccination was available for free. However, the willingness to pay may also play an important role.Citation33 Thirdly, we could not completely avoid the selectivity bias because we use the internet-based research. We adjusted the number of participants after calculating the sample size in advance, so we believe that we have ensured the representativeness of the general population, but we could not eliminate the bias in the initial selection.
According to a previous study, the determinants of vaccine hesitancy can be classified into three categories: (1) contextual influences: influences arising due to historic, socio-cultural, environmental, health system/institutional, economic or political factors; (2) individual and group influences: influences arising from personal perception of the vaccine or from the social/peer environment; and (3) vaccine-/vaccination-specific issues: directly related to the vaccine or vaccination.Citation34 We consider that these determinants are largely influenced by the release of correct information. With the COVID-19 pandemic still showing no signs of abating globally, vaccination plays an important role in protecting the health of children. Although vaccination should certainly not be made compulsory, it is important that parents make the right decision by allowing children to be vaccinated without hesitation.
Conclusion
The study found that 42.9% of Japanese parents would like their children to be vaccinated with COVID-19. About the same number of parents said they would be hesitant about the COVID-19 vaccine. Parents who wanted their children to receive the COVID-19 vaccine were more likely than other parents to be in the older age group (40 years or more) and to want to receive the vaccine themselves (or to have already received the vaccine). The most common reason given for hesitation in giving the COVID-19 vaccine to children was “fear of potential side effects” (Approximately 85%). Early development and commercialization of the vaccine is key to preventing COVID-19 infections in children, and the sharing and dissemination of correct information is a major factor in improving vaccination rates.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, [TY]. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
Additional information
Funding
References
- Centers for Disease Control and Prevention (CDC).Ten great public health achievements–United States, 1900-1999. MMWR Morb Mortal Wkly Rep. 1999;48:241–43.
- Jacobson RM, St Sauver JL, Finney Rutten LJ. Vaccine hesitancy. Mayo Clin Proc. 2015;90:1562–68. doi:10.1016/j.mayocp.2015.09.006.
- Kennedy A, Lavail K, Nowak G, Basket M, Landry S. Confidence about vaccines in the United States: understanding parents’ perceptions. Health Aff (Millwood). 2011;30:1151–59. doi:10.1377/hlthaff.2011.0396.
- Wu AC, Wisler-Sher DJ, Griswold K, Colson E, Shapiro ED, Holmboe ES, Benin AL. Postpartum mothers’ attitudes, knowledge, and trust regarding vaccination. Matern Child Health J. 2008;12:766–73. doi:10.1007/s10995-007-0302-4.
- Casiday R, Cresswell T, Wilson D, Panter-Brick C. A survey of UK parental attitudes to the MMR vaccine and trust in medical authority. Vaccine. 2006;24:177–84. doi:10.1016/j.vaccine.2005.07.063.
- Neumann-Böhme S, Varghese NE, Sabat I, Barros PP, Brouwer W, van Exel J, Schreyögg J, Stargardt T. Once we have it, will we use it? A European survey on willingness to be vaccinated against COVID-19. Eur J Health Econ. 2020;21:977–82. doi:10.1007/s10198-020-01208-6.
- Yoda T, Katsuyama H. Willingness to receive COVID-19 vaccination in Japan. Vaccines. 2021;9:48. doi:10.3390/vaccines9010048.
- Takahashi R, Sugiyama S, Osaki T. Workplace and university vaccinations ramp up in Japan. Tokyo (Japan): Japan Times; 2021 June 21 [accessed 2021 July 1]. https://www.japantimes.co.jp/news/2021/06/21/national/vaccination-drive-pace/.
- Wallace M, Woodworth KR, Gargano JW, Scobie HM, Blain AE, Moulia D, Chamberland M, Reisman N, Hadler SC, MacNeil JR, et al. The advisory committee on immunization practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine in adolescents aged 12-15 years - United States, May 2021. MMWR Morb Mortal Wkly Rep. 2021;70:749–52. doi:10.15585/mmwr.mm7020e1.
- Plotkin SA, Levy O. Considering mandatory vaccination of children for COVID-19. Pediatrics. 2021;147(6):e2021050531. doi:10.1542/peds.2021-050531.
- Mintz K, Jardas E, Shah S, Grady C, Danis M, Wendler D. Enrolling minors in COVID-19 vaccine trials. Pediatrics. 2021;147(3):e2020040717. doi:10.1542/peds.2020-040717.
- Hamed T. Determining sample size; how to calculate survey sample size. Int J Econ Manage Syst. 2017;2:237–39.
- Bartlett JE, Kotrlik J, Higgins C. Organizational research: determining appropriate sample size in survey research. Inform Technol Learn Perform J. 2001;19:43–50.
- Bell S, Clarke R, Mounier-Jack S, Walker JL, Paterson P. Parents’ and guardians’ views on the acceptability of a future COVID-19 vaccine: a multi-methods study in England. Vaccine. 2020;38:7789–98. doi:10.1016/j.vaccine.2020.10.027.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–15. doi:10.1056/NEJMoa2034577.
- Walsh EE, Frenck RW Jr., Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R, et al. Safety and immunogenicity of two RNA-Based Covid-19 vaccine candidates. N Engl J Med. 2020;383:2439–50. doi:10.1056/NEJMoa2027906.
- Meo SA, Bukhari IA, Akram J, Meo AS, Klonoff DC. COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and moderna vaccines. Eur Rev Med Pharmacol Sci. 2021;25:1663–69.
- Shimabukuro TT, Cole M, Su JR. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US-December 14, 2020-January 18, 2021. Jama. 2021;325:1101–02. doi:10.1001/jama.2021.1967.
- Caminati M, Guarnieri G, Senna G. Who is really at risk for anaphylaxis due to COVID-19 vaccine? Vaccines. 2021;9:38. doi:10.3390/vaccines9010038.
- Center for Diseases Control and Prevention. Interim considerations: preparing for the potential management of anaphylaxis after COVID-19 vaccination. Washington (DC): Office of the Associate Director for Communication, Digital Media Branch, Division of Public Affairs; 2021 Feb 26 [accessed 2021 July 1]. https://www.cdc.gov/vaccines/covid-19/downloads/IntermConsid-Anaphylaxis-covid19-vaccine-sites.pdf.
- Banerji A, Wickner PG, Saff R, Stone CA Jr., Robinson LB, Long AA, Wolfson AR, Williams P, Khan DA, Phillips E, et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2021;9:1423–37. doi:10.1016/j.jaip.2020.12.047.
- Centers for Disease Control and Prevention (CDC) Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine - United States, December 14- 23,2020. MMWR Morb Mortal Wkly Rep. 2021;70:46–51. doi:10.15585/mmwr.mm7002e1.
- Abu Mouch S, Roguin A, Hellou E, Ishai A, Shoshan U, Mahamid L, Zoabi M, Aisman M, Goldschmid N, Berar Yanay N, et al. Myocarditis following COVID-19 mRNA vaccination. Vaccine. 2021;39:3790–93. doi:10.1016/j.vaccine.2021.05.087.
- Kim HW, Jenista ER, Wendell DC, Azevedo CF, Campbell MJ, Darty SN, Parker MA, Kim RJ. Patients with acute myocarditis following mRNA COVID-19 vaccination. JAMA Cardiol. 2021. doi:10.1001/jamacardio.2021.2828.
- Wise J. Covid-19: should we be worried about reports of myocarditis and pericarditis after mRNA vaccines? BMJ. 2021;373:n1635. doi:10.1136/bmj.n1635.
- Freeman D, Loe BS, Chadwick A, Vaccari C, Waite F, Rosebrock L, Jenner L, Petit A, Lewandowsky S, Vanderslott S, et al. COVID-19 vaccine hesitancy in the UK: the Oxford coronavirus explanations, attitudes, and narratives survey (Oceans) II. Psychol Med. 2020;1–15. doi:10.1017/S0033291720005188.
- Wong LP, Alias H, Wong PF, Lee HY, AbuBakar S. The use of the health belief model to assess predictors of intent to receive the COVID-19 vaccine and willingness to pay. Hum Vaccin Immunother. 2020;16: 2204–14. doi:10.1080/21645515.2020.1790279.
- The Lancet Infectious D. Should we vaccinate children against SARS-CoV-2? Lancet Infect Dis. 2021;21:889. doi:10.1016/S1473-3099(21)00339-X.
- Obaro S. COVID-19 herd immunity by immunisation: are children in the herd? Lancet Infect Dis. 2021;21:758–59. doi:10.1016/S1473-3099(21)00212-7.
- Opel DJ, Diekema DS, Ross LF. Should we mandate a COVID-19 vaccine for children? JAMA Pediatr. 2021;175:125–26. doi:10.1001/jamapediatrics.2020.3019.
- Anderson EJ, Campbell JD, Creech CB, Frenck R, Kamidani S, Munoz FM, Nachman S, Spearman P. Warp Speed for Coronavirus Disease 2019 (COVID-19) Vaccines: Why Are Children Stuck in Neutral? Clin Infect Dis. 2021;73:336-40. doi:10.1093/cid/ciaa1425. .
- Kamidani S, Rostad CA, Anderson EJ. COVID-19 vaccine development: a pediatric perspective. Curr Opin Pediatr. 2021;33:144–51. doi:10.1097/MOP.0000000000000978.
- Catma S, Reindl D. Parents' willingness to pay for a COVID-19 vaccine for themselves and their children in the United States. Hum Vaccin Immunother. 2021;17:2919–25. doi: 10.1080/21645515.2021.1919453. .
- MacDonald NE. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33:4161–64. doi:10.1016/j.vaccine.2015.04.036.