ABSTRACT
The coronavirus disease 2019 (COVID-19) pandemic highlights the urgent clinical need for efficient virus therapies and vaccines. Although the functional importance of antibodies is indisputable in viral infections, there are still significant unmet needs that require vast improvements in antibody-based therapeutics. The IgG Fc domain can be engineered to produce antibodies with tailored and potent responses that will meet these clinical demands. Engaging Fc receptors (FcRs) to perform effector functions as cytotoxicity, phagocytosis, complement activation, intracellular neutralization and controlling antibody persistence. Furthermore, it produces vaccine-like effects by activating signals to stimulate T-cell responses, have proven to be required for protection, as neutralization alone does not off the full protection capacity of antibodies. This review highlights antiviral Fc functions and FcRs’ contributions in linking innate and adaptive immunity against viral threats. Moreover, it provides the latest Fc engineering strategies to improve the safety and efficacy of human antiviral antibodies and vaccines.
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has caused significant morbidity and mortality worldwide. The urgent need to develop effective therapies and vaccines during COVID-19 pandemic has led to intense research efforts to overcome the limitations of current clinical treatment options. Although past and some of the current therapies have failed to show promising results, antibody-based treatments offer a significant benefit for SARS-COV-2 patients with an 80% reduction in hospitalization and disease progression rates.Citation1–3
After a viral infection, the human immune response generates antiviral antibodies to control viral spread in addition to preventing and providing long-term protection against reinfection.Citation4 IgG antibodies are the most abundant immunoglobulin present in plasma and have been widely studied and applied in various clinical settings. Their characteristic features, such as their long half-life that is produced by their interactions with the neonatal Fc receptor (FcRn), neutralization potency and mediating Fc effector functions that result from their interactions with FcγRs and the complement system, have made them of particular interest.Citation5
The four isotypes of human IgG are IgG1, IgG2, IgG3 and IgG4. These isotypes differ in their structural and functional properties, such as their binding affinities and interactions with immune effector cells, which affect their functional outcomes. It has been suggested that during natural infection, IgG isotypes have specific roles and differ in their responses to particular pathogens, antigens and epitopes.Citation5
In viral infections, IgG1 and IgG3 are the most commonly induced isotypes, and they have the highest binding affinity for FcγRs, complement activation and potent effector functions, such as cellular cytotoxicity and the phagocytosis response.Citation6,Citation7 Due to the short half-life of IgG3, the most preferred isotype for therapeutic activity is IgG1.Citation8 In addition, this isotype is the most frequently applied to human antiviral antibodies.Citation9
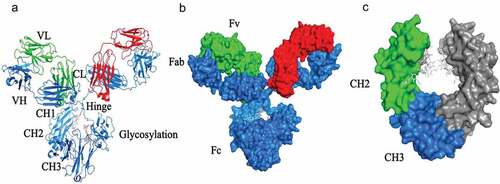
As shown in , human antibodies are characterized by a Y-shaped structure. They consist of two fragment antigen-binding domains (Fabs) and a fragment crystallizable domain (Fc). The two domains are connected at their hinge regions, and both have important functions.Citation10 When fighting viruses, the Fab domains mainly mediate virus neutralization, while the Fc domain activates a wide range of FcRs, which are expressed on vital effector immune cells and produce potent effects.Citation11 This activation triggers the elimination of viruses and the killing of infected cells by Fc effector functions, which include antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP) and complement-dependent cytotoxicity (CDC).Citation12
Figure 1. The structural insights of the complete structure of IgG antibody. (A) Heavy chains are in blue, while light chains are in green and red. Glycosylation location is in white and light orange. PDB entry 1IGY. (B) surface IgG structure by domains: variable fragment (Fv), antigen-binding fragment (Fab) and fragment crystallizable region (Fc). PDB entry 1IGY. (C) IgG Fc domains: CH2 in green and CH3 in blue. Glycosylation site in light gray. PDB entry 1FC1.
Interestingly, antibodies can facilitate immune signals that stimulate T-cell responses by interacting with major immune antigen-presenting cells (APCs) to produce vaccine-like effects.Citation13–15 However, the use of antibodies and the number of approved antiviral monoclonal antibodies (mAbs) to combat viral threats are still limited.Citation16 There are multiple reasons for this limited use, including a poor understanding of the actual mechanism of antibodies’ modes of action once injected into patients, the effect of different binding affinities among Fc and FcRs and the effect of antibodies’ serum concentrations required to reach the optimal level.Citation17
The role of Fc-mediated activities in viral infections has been well studied and investigated,Citation18,Citation19 but its clinical application remains a challenge. In the absence of human-augmented natural antibody responses,Citation20 several obstacles remain, including the specific contribution of different FcRs in viral infection and recovery, the involved signaling pathways and key receptors, the cell types that impact antiviral activity, the diversity of FcγRs across different species and the structural complexity of the different receptors that interact with the Fc domain.Citation21–23 Moreover, a better understanding of the selective engagement of antibody effector functions that mediate a protective response is required before using the antibody Fc domain to provide protection by inducing a T-cell response.Citation9,Citation24,Citation25
Antibody engineering is used to minimize and overcome some limitations and improve therapeutic outcomes.Citation20 Antibody Fc domain engineering has been extensively used to address the limitations that restrain antibody functionality for human therapy. This type of engineering can modulate the effector functions and circulation half-life and also optimizes the biophysical and biochemical properties of antibodies.Citation26–28 The new generation of therapeutic antibodies is heavily engineered with a main focus on the Fc region.Citation29
This review article aims to summarize advances in IgG Fc domain engineering to present the most effective antiviral responses that can lead to long-term viral protection. It also intends to highlight the mechanism of actions and signaling pathways of IgG Fc and its different receptors (FcRs) that trigger effector functions. Furthermore, the article aims to enhance Fc domain performance by providing the latest strategies and technologies being used to improve the overall response of human antiviral antibodies. The reader will gain knowledge about how to tailor the antibody response by altering its Fc domain and how selective activation or inhibition could result in protective activity.
An overview of IgG FcRs
FcRs are expressed in leukocytes, such as neutrophils, eosinophils, monocytes, macrophages, dendritic cells, natural killer (NK) cells, B-cells and T-cells; FcγRIIB on multipotent CD8 +Citation30 and FcγRIIIA are expressed in subpopulations of activated CD4 + T-cells.Citation31 There are two main types of FcRs in humans: classical and non-classical FcRs. Classical type I FcγRs (FcγRI) are broadly classified as activation and inhibition receptors based on the presence of immunoreceptor tyrosine-based activation motif or immunoreceptor tyrosine-based inhibitory motif intracellular domains. Activating FcγRs include FcγRI, FcγRIIA, FcγRIIC, FcγRIIIA and FcγIIIB. FcγRIIB is the inhibitory receptor for FcγRs.Citation25 Most of the effector leukocytes co-express both activitory and inhibitory FcγR; this cellular feature provides a sufficient balance and also allows regulation between receptors.Citation32
Although all the signaling events that activate FcγRs are similar, the specific biological outcomes for the effector functions are quite diverse.Citation25 The non-classical type of human FcRs involves C-type lectin receptors, such as DC-SIGN and FcεRII (CD23). Other non-classical FcRs include FcRn and tripartite motif-containing-21 (TRIM-21).Citation33 These receptors have distinctions in their structures, binding interfaces, ligand specificity and functions compared to type I FcγRs.Citation34 The final outcomes of these receptors’ interactions are influenced by several factors, such as the antibody isotype, the FcR type, the glycosylation state, the conformational state of the Ig Fc domain and the involvement of other signaling molecules.Citation35
Fc: FcγR’s effector functions
Antibody-dependent cellular phagocytosis (ADCP)
ADCP is a significant Fc effector function for clearing IgG-opsonized pathogens and also plays a role in antigen presentation.Citation36 This process is mediated by phagocytic cells (such as neutrophils and macrophages) and is initiated by interacting with FcγR’s receptors.Citation37 FcγRIIA is the prime mediator involved in ADCP to combat different viruses or virus-infected cells.Citation38 However, the signaling pathway for FcγR cross-linking in phagocytes is activated by the phosphorylation of Src family tyrosine kinases, which triggers the activation of other kinases (such as Akt, p70S6 and inositol 3-kinases).Citation39,Citation40
Next, antibody-opsonized viruses are guided to lysosomes; thus, antigens are being processed into peptides .Citation41 The cell type is a crucial factor that determines the outcome of phagocytosis. To elaborate, when macrophages drive the antibody phagocytosis process, it results in improved pathogen destruction and enhanced antigen presentation.Citation36 Conversely, when plasmacytoid dendritic cells drive the antibody phagocytosis process, they secrete IFN-α,Citation42 which is considered a major antimicrobial defense mechanism.Citation43,Citation44
Antibody-dependent cellular cytotoxicity (ADCC)
ADCC is one of the major IgG Fc-dependent effector functions. It plays an important role in antiviral immunity and the clearance of viral infections and is usually mediated by NK cells; recent studies have also implicated neutrophils as mediators.Citation45 To induce an efficient ADCC response, an antibody’s Fc domain interacts with NK cells (Fc receptor-bearing cells) that express FcγRIIIA, which results in the production of cytotoxic granules, perforins and granzymes.Citation46 In viral infections, perforins create pores in the virus-infected cell’s surface, facilitating the entry of granzymes to cleave death substrates and drive infected cells into apoptosis.Citation47
Complement-dependent cytotoxicity (CDC)
Complement activation, which leads to virolysis and direct cell lysis, is considered one of the key effector functions for fighting viral infections. Antibodies are capable of triggering complement activation. This mechanism contributes to pathogen elimination in two ways: either directly via CDC or indirectly via phagocytic clearance.Citation12 The classical complement activation pathway is initiated when C1q binds to the Fc CH2 domain of antibodies bound to virus-infected cells.Citation48,Citation49 Upon binding, the proteolytic cascade is activated, which leads to the release of anaphylatoxins (such as C3a and C5a) and the formation of the membrane attack complex (MAC), which forms pores that cause the lysis of target cells.Citation50
Triggering Fc effector functions to induce antiviral responses and vaccine-like effects by linking innate and adaptive immunity
Antigen presenting cells (DC): FcγRs
To activate a T-cell response, three signals are required: Signal 1 is the interaction between T-cell receptors (TCRs) and MHC I or MHC II, which present the antigen peptide. Signal 2 involves the expression of co-stimulatory molecules, and signal 3 is characterized by the release of anti-inflammatory cytokines.Citation13 By engaging FcγR activation in APCs as DC, antibodies can induce T-cell responses by binding to immune complexes and triggering antigen processing for their presentation to CD4+ and CD8 + T-cells.Citation15,Citation51 A unique feature of DCs is that they express both the activitory FcγRIIA and the inhibitory FcγRIIB receptor to maintain a balanced regulation of DC maturation.Citation52
The selective engagement of FcγRIIA in DC stimulates protective CD8 + T-cell responses and promotes long-term protection against viral infections.Citation22 Upon the cross-linking of FcγRs with DC via FcγRIIA receptors, several biological effects take place that trigger all components required to activate T-cell responses for antigen processing and presentation.Citation53 Moreover, there is also an increase in the expression of co-stimulatory molecules,Citation13,Citation54 such as CD80, CD86Citation55 and CD40.Citation55,Citation56
Cytokine production due to the activation of FcγRs stimulates host antiviral immunity,Citation57 leading to the delivery of monocytes and DCs to the infection site to clear viral infections.Citation58 Examples of pro-inflammatory cytokines are tumor necrosis factor alphaCitation59 and interleukin-6.Citation60 Further evidence to support vaccine-like T-cell responses mediated by FcγR signaling in DC was offered by DiLillo and Ravetch,Citation15 who investigated the mechanisms of inducing anti-tumor vaccinal effects in lymphoma patients treated with Rituximab. Their findings showed improved therapeutic outcomes via specific anti-idiotype T-cell responses that developed after mAb treatment. DiLillo and RavetchCitation15 utilized FcγR-humanized mice and observed that the selective engagement of hFcγRIIA with DCs stimulated long-term immunity and generated a potent vaccine-like effect. However, further research and studies are needed to understand the role of FcγRs in producing potent and directed T-cell responses in viral infections and to determine how the modulation of cytokines can shape the properties of FcγRs during viral infections.Citation57
TRIM-21: Fc
In viral infections, antibodies can be carried inside a cell’s cytosol, where they bind to TRIM-21.Citation61 The intracellular Fc receptor TRIM-21 is activated when it binds to the Fc region of virus-associated antibodies and drives the formed immune complex into rapid proteasomal degradation. This mechanism is known as antibody-dependent intracellular neutralization (ADIN).Citation62 ADIN that is mediated by TRIM-21 performs a dual effector mechanism inside cells; it eliminates viruses and also prevents their replication through proteasomal degradation.Citation62 In addition, ADIN triggers innate signaling pathways to produce proinflammatory cytokines, such as NF-kB, AP-1, IRF3, IRF5 and IRF7, thus generating an antiviral state.Citation63 ADIN stimulation against incoming virions results in infection blocking,Citation61 while the targeting of specific proteins leads to selective depletion, which is also called TRIM-Away.Citation64
Furthermore, activating the TRIM-21 Fc receptor mediates immune protection by inducing a cytotoxic T-cell killing response against viral infections. Caddy, VaysburdCitation65 used the lymphocytic choriomeningitis virus (LCMV) as a model system to analyze the role of non-neutralizing anti-nucleoprotein (N) antibodies in providing protection by stimulating TRIM-21 interaction. After forming the phagocytosed antibody-N-protein immune complex, it was imported into the cytosol where it was recognized and detected by TRIM-21, which triggers ubiquitination and proteasomal degradation of the immune complex.Citation65 The results showed that TRIM-21 drives cross-presentation to display the proteasome-derived peptides for MHC class I and activates CD8 + T-cells, which may have future implications for designing vaccines.Citation65
Viruses continue to develop complicated pathways to evade antibody responses, such as mutating to escape from neutralizing antibodies. For example, the human immunodeficiency virus (HIV) mutates its viral neutralizing determinants to evade the responses of neutralizing antibodies during infection.Citation66 Such issues highlight the importance of the role of antibody cellular neutralization and other Fc effector functions in enhancing the protective efficacy of neutralizing and of N antibodies.
FcRn: Fc
FcRn plays a vital role in controlling antibodies’ persistence in the circulation;Citation67 it mediates virus neutralizationCitation68 and facilitates antigen presentation to drive T-cell responses.Citation69 As a pH-dependent receptor, FcRn protects antibodies from lysosomal degradation by engulfing the antibody in acidic media (pH: 6–6.5) and then recycling it back into circulation, leading to a long half-life for antibodies.Citation70
Bai, YeCitation68 studied the intracellular neutralizing activity of an influenza hemagglutinin-specific monoclonal antibody (Y8-mAb), which is characterized by mediating viral neutralization at acidic pHs only. The results of a mice model indicated a significant decrease in viral replication, pulmonary virus titers and viral inflammation in the lungs.Citation68 The mechanisms of Y8-mAb, which caused a reduction in viral replication, may be due to the interruption in viral fusion that occurs at an acidic endosomal pH, which blocks the interaction, while FcRn facilities the ordination of this interaction by organizing IgG in the endosome and also may boost the IgG endosomal concentration to block viral binding.Citation68
Moreover, Qiao, KobayashiCitation71 proposed that a further function of FcRn may be antibody-mediated antigen presentation in dendritic cells. Baker, RathCitation72 showed that FcRn enhances CD8 + T-cell responses and plays a significant role in the mucosal immune system. Due to these characteristic protection features, FcRn has become an attractive target for enhancing protective humoral immunity, pharmacological interventions and vaccination strategies.Citation73
Vaccines induce Fc effector functions
There is a significant body of evidence that describes the importance of Fc effector functions in infectious disease control and protection; therefore, designing vaccines to induce optimum Fc effector functions has become a promising strategy for vaccine development.Citation4,Citation74
Vaccines are currently being evaluated to induce neutralizing antibodies with Fc effector functions, as with the ChAdOx1 nCov-19 vaccineCitation75 and the RTS, S/AS01 malaria vaccine.Citation76,Citation77 Designing vaccine adjuvants to stimulate Fc effectors is a key strategy. Coler, DayCitation78 designed the ID93 Tuberculosis (TB) vaccine and used ID93 with GLA-SE, which is a fusion protein TB vaccine candidate combined with a toll-like receptor- 4 (TLR-4) agonist adjuvant. The results from the first human trial revealed the beneficial antibody Fc effector functions of ADCC and ADCP along with supported safety and immunogenicity profiles.Citation78 In addition, it was now possible to design immunogens to produce both a neutralizing and Fc effector response. A key factor to consider here is the steric hindrance effect caused by antibodies that target particular epitopes, as they may cause structural constraints that affect FcγR binding.Citation74
To elaborate further, Bournazos, DiLilloCitation79 characterized anti-Ebola virus (EBOV) antibodies that target glycoprotein epitopes based on their FcγR dependence. They found that epitopes located on glycoproteins induced optimal in vivo antiviral activity by mediating Fc effector activity. In contrast, epitopes that target membrane-mediated Fab activities and neutralization without depending on Fc–FcγR interactions to confer activity have also been described.Citation79
This particular area of research has recently gained attention with the publication of actual clinical applications, such as the second dose of the ChAdOx1 nCov-19 vaccine, which was able to induce effector functions via antibody-dependent neutrophil/monocyte phagocytosis, complement activation and NK cells activation.Citation75
Several factors may impact the quality of vaccines to induce Fc effector functions, including the route of immunization and the required number of immunizations, along with the minimum and maximum therapeutic dose.Citation74,Citation80 Additional vaccines that have induced Fc effector functions are listed in .
Table 1. Human vaccines that induced Fc effector functions and produced protection or reduced the infection risk. Abbreviations: Human cytomegalovirus (HCMV)
However, further work is needed to address the obstacles and the limitations in this area, such as assay standardization, understanding the molecular mechanism that drives these Fc effector functions in vaccination and how to translate the promising findings of non-human models to humans.Citation74 Fc engineering strategies are described below with recent examples to improve the link between innate and adaptive immunity.
Fc antibody engineering to produce potent antiviral responses and vaccine-like effects
Fc antibody engineering for improved IgG effector functions
Optimizing the binding affinity of human FcγRs is a critical feature of therapeutic antibody functions. Increasing the binding affinity of Fc to activate selected receptors of FcγRs is a well-established engineering approach. This approach is important, as most FcγRs have a low affinity for their ligand (>1 μM), except for FcγRI.Citation87
Fc engineering for improved ADCC
NK-mediated ADCC is influential in tailoring the efficacy and potency of therapeutic antibodies. ADCC can by modified in two ways: amino acid mutations and glyco-engineering. FcγRIIIA is the key receptor involved in mediating this interaction, and it has been targeted in many studies that aimed to enhance the binding affinity of FcγRIIIA to develop antibodies with a potent ADCC response .Citation88
Stavenhagen, GorlatovCitation89 employed yeast surface display techniques to identify five mutations in a novel Fc, F243L/R292P/Y300L/V305I/P396L (LPLIL), which improve FcγRIIIA’s binding affinity. Introducing these mutations into the IgG1 Fc domain has dramatically enhanced FcγRIII binding, and ADCC activity was boosted by up to 100 times.Citation89 Clinically, LPLIL was utilized in Margetuximab (Fc-optimized anti-HER2 mAb).Citation90 It showed decreased FcγRIIB binding, stimulated macrophages and activated HER2 antigen presentation.Citation91,Citation92 The Fc-optimized Margetuximab has reached phase-III clinical trials, and promising results have been presented by Rugo, Im.Citation93
Shields, NamenukCitation94 used alanine scanning mutagenesis to identify three Fc variants (S298A, E333A and K334A), which are known as AAA. AAA mutations increased IgG1’s binding affinity for FcγRIIIA while at the same time decreasing its affinity for binding to FcγRIIB. This alteration resulted in potent ADCC activity mediated by human NK cells. The same research team also identified selected Fc mutations E333A, K334A and A339T, which selectively enhance the binding to FcγRIIIA.Citation94 When AAA Fc mutations were inserted in Trastuzumab (used for breast cancer treatment), a 50–100-time enhanced killing of Her2+ cells was demonstrated as a result of the improved FcγRIIIA binding.Citation94
Fc glycoengineering to improve ADCC
All human IgG molecules have one conserved glycosylation site at N297.Citation95 Consequently, the attached glycans at N297 in Fc are significant for effective ADCC responses.Citation96,Citation97 Since human sera contain fucosylated IgGs,Citation98 engineered afucosylated antibodies created by removing the Fc N-glycan fucose residues can increase the ADCC response by improving the binding affinity of FcγRIIIA.Citation99,Citation100 Shields, LaiCitation101 found that antibodies deficient in fucose had a 50-fold increased binding affinity for Fc FcγRIIIA, which resulted in an improved ADCC response. In addition, a synergistic effect was observed when combining the afucosylated antibodies with the enhanced ADCC IgG1 Fc variant.Citation101 Mogamulizumab (afucosylated mAb for mycosis fungoides [MF]) is an example of an approved afucosylated antibody with a potent ADCC response.Citation102,Citation103
Two technologies have been applied to engineer antibodies with reduced fucosylation: GlycoMab® technologyCitation104 and Potelligent® technology.Citation105 However, antibody afucosylation remains a widely accepted glycoengineering strategy for improving ADCC activityCitation105 and producing enhanced antiviral activity.Citation106
Fc engineering for improved ADCP
Macrophage-mediated ADCP is crucial for designing antiviral antibodies with an effective antiviral response. FcγRIIA is commonly used by macrophages in ADCP and has been the focus of previous antibody research.Citation107–109
Richards, KarkiCitation110 screened up to 900 Fc variants to study the effects of different binding affinities of FcγR and the effect of inserting enhancement mutations. That study concluded that the G236A mutation (GA) alone improved the binding affinity of selective FcγRIIA by six to seven times in both alleles, which led to a strong ADCP response (FcγRIIA-dependent phagocytosis). This Fc mutation increased the binding affinity of FcγRIIA without affecting FcγRIIB but also decreased the IgG1 binding affinity. This issue was resolved by inserting the following combination of three mutations: G236A, S239D and I332E. This solution improved the phagocytosis of macrophages by up to 70 times and enhanced the FcγRIIIA-mediated ADCC of antibody-coated target cells by up to 31 times.Citation110 Consequently, the GA mutation was inserted into the humanized IgG1 anti-EpCAM antibody, and elevated ADCP activity was indicated compared to the wild-type IgG.Citation110
Lazar, DangCitation111 used both computational design algorithms and high-throughput screening methods to design optimized Fc mutations. They identified three Fc mutants (S239D, A330L, I332E [DLE]) with an enhanced binding affinity for FcγRIIIA. As expected, the ADCC response increased when DLE was inserted into MEDI-522 (anti-integrin antibody). In addition, inserting DLE into Rituximab resulted in an enhanced ADCP response.Citation111
Smith, DiLilloCitation112 used FcγR humanized mice to selectively enhance the binding affinities of FcγRIIA and FcγRIIIA. They generated Fc mutations that consisted of G236A/S239D/A330L/I332E (GASDALIE).Citation113 Accordingly, FcγRIIA was improved by 25 times, and FcγRIIIA was enhanced by 30 times.Citation112 Although these mutations increased the binding affinity of FcγRIIA, the effects of ADCP have yet to be determined in humans. Only a limited number of studies have attempted to engineer FcγRIIA for antiviral therapies, and several molecular mechanisms have yet to be identified.
Fc engineering for improved CDC
The interaction of C1q with the Fc domain is a required step for initiating CDC pathways and leading to potent complement fixation and direct virolysis.Citation114 This was a targeted strategy for engineering antibodies to produce potent CDC responses.Citation115
Moore, ChenCitation116 designed and screened 38 Fc mutations to investigate their ability to produce effective CDC activity. They observed that the triplet mutation in S267E, H268F and S324T (EFT) had boosted C1q binding by 47-fold. As a result, the CDC activity increased 6.9-fold. However, the EFT mutation also led to low ADCP and ADCC activity as well as an unwanted increased binding affinity to the inhibitory FcγRIIB.
Adding the ADCC-enhancing mutations G236A and I332E resolved this issue. As a result, the ADCC functionality was restored to be similar to the wild-type IgG1.Citation116 Therefore, removing S267E from the EFT mutations may reduce the FcγRIIB binding affinity and lower the CDC response. Thus, this study showed the importance of balancing effector functions to reach the desired outcome. Furthermore, Idusogie, WongCitation117 utilized site-directed mutagenesis to construct Rituximab mutants to enhance the drug’s CDC activity. They found that using the double Fc mutations K326A and E333A produced an improved binding affinity of Rituximab to CIq and thus enhanced the CDC response by 50% compared to wild-type Rituximab.Citation117
Hexamerization using HexaBody technology is another strategy for enhancing C1q to produce potent CDC.Citation49 The characteristic feature of this approach is the targeting of Fc-Fc engagement to form organized antibody hexamers using E345R, E430G and S440Y (RGY) or the single mutation E345R. The formed antibody hexamers that activate the complement cascade only when the antigen has been bound to the targeted cell, which leads to more effective CDC compared to the IgG1 wild type.Citation118 Schutze, PetryCitation119 inserted the E345R HexaBody mutation into chimeric llama/human heavy-chain antibodies (hcAbs) to analyze its effect on CD38-expressing cells. The research team observed an improvement in CDC potency due to the formation of hexamers on the cell surface.Citation119 These effector functions were combined for selective activation when required via the collaborative work of many scientists ().
Table 2. The effect of Fc engineering by inserting mutations. Mutation named as S298A means Serine was mutated to Alanine. Residues numbering is based on EU numbering system
Effector-enhanced antiviral antibodies such as Elipovimab (used for HIV) are heavily Fc engineered. The FcγR binding-enhancing Fc mutations G236A, S239D, A330L, I332E, M428L and N434S were inserted to improve the Elipovimab binding affinity to FcγRIIIA, FcγRIIA and FcγRIIB.Citation32 Furthermore, VIR-3434 (mAb for chronic hepatitis B virus [HBV] infections) is Fc-engineered as well. The Fc mutations G236A, A330L, I332E, M428L and N434S were introduced to enhance VIR-3434’s binding affinity for FcγRIIIA and FcγRIIA while reducing its binding to FcγRIIB.Citation32 The clinical results of both these studies, which are currently underway, are urgently needed to determine their safety and effectiveness.Citation126
To this end, all these studies suggest that Fc engineering for optimal binding affinity and selective specificity to FcγRs stand out as a prospective and practical approach for enhancing the clinical efficacy and potency of antiviral mAbs.
The Fc engineering strategy for enhanced intracellular neutralization
The use of ADIN mediated by TRIM-21 to degrade invading viruses before they replicate has refocused interests on designing effective antibody-antiviral responses with vaccinal effects.Citation127 Ng, KaliaperumalCitation128 engineered the IgG1 Fc domain to increase its binding affinity for TRIM-21 by 100 times. Their research group used phage display technology to identify five mutations that may enhance binding affinity: H433T, N434R, Y436F, S440I and T256P. Their results showed that using engineered Fc with a high binding affinity for TRIM-21 can facilitate antigen cross-presentation, which leads to better human DC maturation and induces an antigen-specific CD8 T-cell response.Citation128 In addition to these effects, the TRIM-21 Fc receptor affects the release of IFN-γ and other proinflammatory cytokines.Citation129 This finding suggests that the TRIM-21 Fc receptor approach might be considered for use in DC-derived vaccines.Citation128 TRIM-21 also collaborates with the complement system to prevent cellular infection using different protective antiviral mechanisms, such as sensing a complement-coated virus and thus triggering viral degradation and elimination.Citation61,Citation63,Citation130,Citation131
TRIM-AWAY
TRIM-AWAY is a novel protein degradation technology used to target specific intracellular proteins tagged for degradation by an antibody.Citation132 This strategy delivers antibodies into cells and facilitates the study of protein functions without prior modifications.Citation64,Citation132 Zeng, SantosCitation133 used this technology to selectively degrade disease-causing proteins by TRIM-21-nanobody chimeras. Such studies highlight the possibility of using TRIM-AWAY technology to specifically target selective proteins for degradation, either as a research tool or for therapy.Citation133
Fc engineering to modulate antibody half-life circulation
Fc engineering to tailor the IgG half-life via modulating the interaction between FcRn and IgG has been broadly used to improve the clinical potential of antibodies.Citation67 To extend the serum persistence of IgG antibodies, the IgG-FcRn interaction has been altered by inserting Fc point mutations.Citation134
Dall’Acqua, WoodsCitation135 used rationally designed libraries and phage displays to study the effects of IgG1 Fc-FcRn binding affinities. They identified the three Fc mutations M252Y/S254T/T256E (YTE). YTE was inserted into the anti-respiratory syncytial virus (RSV) antibody (Motavizumab-YTE), which enhanced the binding affinity by 10 times. Motavizumab-YTE was safe and well tolerated by patients and demonstrated an increased serum half-life up to 70–100 days and a clearance rate that was decreased by 71–86%Citation136 (ClinicalTrials.gov registration no. NCT00578682). In addition, Zalevsky, ChamberlainCitation125 combined rational design methods with high-throughput protein screening methods to discover Fc variants with a high binding affinity for human FcRn. Their research team identified the double Fc mutations M428L/N434S (LS). IgG1 LS enhanced the binding affinity for FcRn by 11 times in vitro.
Recently, Sotrovimab, a SARS-COV-2 engineered Fc antibody, has been granted Emergency Use Authorization (EUA) by the United States Food and Drug Administration.Citation137 The Fc domain of Sotrovimab was engineered by inserting the Fc mutations M428L and N434S (LS) to extend its circulation half-life, and it influenced the disease progression in COVID-19 patients with an 85% reduction in hospitalizations.Citation138,Citation139 Other antiviral antibodies Elipovimab and VRC01LS are currently in phase-1 human clinical trials for HIV; both contain enhanced serum persistence mutations.Citation126
Fc fusion engineering for antigen binding
Using the full antibody structure of Fc has limitations due to its poor penetration into targeted tissues and weak binding affinity for certain surface molecules, such as viral glycoproteins.Citation140 Utilizing the Fc fragment instead of the full antibody structure to target viral antigen protein fusion or even the virus itselfCitation141 represents a promising approach for generating novel antigen-binding strategies.Citation142,Citation143
Immunoadhesion is a strategy used in designing antibody-like molecules using the Fc domain for protein fusion with targeted epitopes.Citation141 Consequently, this process produces a combination of two functions (dual effects) in a single well-designed molecule: a neutralizing effect and vaccine-like effects.Citation141 KruseCitation141 designed an Fc-angiotensin-converting enzyme 2 (Fc-ACE2) fusion to be used as a therapy against future coronavirus pandemics, acute respiratory distress syndrome or other viruses that use ACE2 to infect cells.
Zhang, ZhouCitation144 developed an IgG Fc fused with RSV glycoprotein F (F-Fc) to be used as a potential vaccine candidate against RSV. That study reported the induction of immunization effects and a reduction in lung injuries caused by the RSV infection.Citation144 In addition, when the MPL adjuvant was added, neutralizing antibodies were generated after immunization with the F-Fc complex.Citation144
Creating accurately oriented antibody assemblies with controlled valency is a necessary and currently unfulfilled criterion that will be required to enhance antibody performance and potency.Citation145 Divine, DangCitation145 designed nanocages that were composed of two components. The first is an antibody or Fc fusion, and the second is an Fc-binding homo-oligomer designed to direct the assembly of nanocages. The results of this approach showed improved antibody signaling to produce CD40 activation and a T-cell response.Citation145 In addition, the viral neutralization potency for a SARS-COV-2 pseudovirus was also enhanced.Citation145
Fc engineering for improved vaccine-like effects
Fc engineering can be utilized to produce strong vaccine-like effects through several methods, such as enhancing selective Fc-FcγR interactions, glycoengineering and improving FcRn interactions to regulate the antibody’s serum half-life.Citation69,Citation146–149
Pelegrin, Naranjo-GomezCitation9 reviewed the possible improvements for mAb-based antiviral therapies for producing vaccine-like effects. They were particularly interested in identifying the cellular and molecular mechanisms involved in the induction of vaccine-like effects during antiviral mAb treatment. Direct mechanisms are actively involved, such as ADCC, ADCP, CDC and antibody-dependent cell-mediated virus inhibition (ADCVI).Citation18,Citation150,Citation151 Indirect mechanisms that stimulate adaptive immunity were observed when mAb therapies were combined, which enhanced and strengthened the Fc effector functions and targeted the infected cells.Citation9 Bournazos and RavetchCitation52 explained the immunomodulatory mechanisms of the FcγRs that are involved in T-cell immunity. This process is similar to how human DCs express both FcγRIIA and FcγRIIB and maintain a balance between them to produce effective T-cell responses, such as antigen presentation and stimulating co-stimulatory molecules, which result in long-lasting protection. The three Fc mutations G236A/A330L/I332E (GAALIE)Citation121 exhibit an increased binding affinity for the selective activated human FcγRs (FcγRIIA and FcγRIIIA) but reduced binding with the inhibitory FcγRIIB receptor.Citation121
Upon further analyses, Bournazos, CortiCitation22 found that GAALIE Fc mutations induced the activation of T-cell responses for both CD8+ and CD4 + T-cells, which provide protection against infections. Additionally, when inserting LS Fc mutations, GAALIE-LS demonstrated a longer half-life and superior protection with a 5.5-fold enhanced antiviral potency in vivo.Citation22
Clinically, VIR-7832 (a neutralizing COVID-19 antibody developed by Vir Biotechnology) has been designed to include GAALIE Fc mutations.Citation152 This GAALIE-engineered antibody is currently in phase I/II clinical trials (NCT04746183).
Recently, Ullah, PrevostCitation153 applied live bioluminescence imaging (BLI) to analyze the actual effects of antibodies in prophylaxis and therapy, by using SARS-CoV-2-nanoluciferase infected mice model (K18-hACE2). In their study, authors signified that Fc effector functions were required for ideal efficacy and protection, in agreement with other studies.Citation4,Citation80,Citation154 They engineered Fc domain by inserting GASDALIE mutations for improved FcγRs binding affinity. They observed that the GASDALIE enhanced Fc variant had provided almost complete protection, engaging FcγRs and activating immune cells as neutrophils, monocytes and NKs. Furthermore, reduced viral load, decreased virus dissemination and spreading in addition to reduction of inflammation and cytokine-storm like phenotype.Citation153
Liu, XuCitation155 used the receptor-binding domain (RBD) of the spike protein of SARS-COV-2 to design an Fc-based vaccine candidate for COVID-19 (SARS-COV-2 RBD-Fc). This recombinant subunit vaccine contains the RBD of SARS-COV-2 S1 conjugated to the human IgG Fc domain via the C-terminus of the SARS-COV-2 RBD. The results of the immunization of mice with SARS-COV-2 RBD-Fc indicated the presence of RBD-specific antibodies at high titers and also demonstrated virus neutralization. The Fc domain in this vaccine design acts as an immunopotentiator for enhancing vaccine immunogenicity by interacting with FcRs on the APC.Citation156–158 This novel vaccine candidate developed by Liu, XuCitation155 demonstrated effective neutralization for the SARS-COV-2 D614G mutation (located outside the RBD) that had raised concerns by being more infectious compared to other mutations.Citation155 These results highlight the possibility of using the Fc domain in vaccine design to induce a long-term neutralizing antibody response and broad-spectrum vaccine-like effects to prevent and protect against viruses and their mutants.Citation155
Conclusion and recommendations
Newly emerging human infectious diseases caused by viral infections pose a serious threat to public health and global economies, as seen during the COVID-19 pandemic. This ongoing event highlights the urgent need to find effective therapeutics and protective vaccines to address the global crisis. With few therapeutic options available, antibody-based therapy has proven to be an effective strategy to meet these needs. It has long been thought that antibody neutralization is the main function of Fc effectors, and recent studies have reinforced the significant roles mediated by Fc effector functions for treating and preventing viral infections.Citation4 As seen in the work of Winkler, Gilchuk,Citation159 intact Fc effector functions are required to mediate antibody protection in neutralizing human antibodies against SARS-COV-2. Vaccines that elicit Fc effector functions have become a target of vaccine development, as observed with the ChAdOx1 nCov-19 vaccineCitation75 and the RTS, S/AS01 malaria vaccine.Citation76,Citation77 In addition, antibodies obtained from infected patients are attracting further research focus. For example, SARS-COV-2 neutralizing IgG1 antibody (SC31), which was initially isolated from a convalescent COVID-19 patient, had therapeutic efficacy when administered to hamsters infected with SARS-COV-2.Citation80 SC31 has reduced the incidence of severe disease progression and also decreased the viral load and presence of lung lesions in hamsters; its effectiveness was the result of its dual functionality: neutralizing the viral infection by blocking the binding receptor and mediating Fc effector functions, and inducing an IFNγ-driven antiviral response for maximal therapeutic efficacy.Citation80 It is also noteworthy that SC31 showed no evidence of antibody-dependent enhancement (ADE); human trials will soon begin for COVID-19 patients.Citation80 SC31 and all other examples mentioned in this review are antibodies that have been discovered and developed against viral infections to confirm their real therapeutic potential. However, there is still an unmet clinical need to improve antibody therapies. Fc engineering strategies have already proven to be a successful approach to enhance the clinical effectiveness and improve the functional selectivity and safety profile efficiency of antibodies.Citation160
The newest direction of research in this area is moving toward understanding the precise mechanisms involved in activating selective FcR and signaling pathways to produce a potent, tailored and protective antiviral response, as seen by the Fc antibody engineering work of Bournazos, Corti.Citation22 In addition, it is also of interest to mimic the normal response of protective antibodies by maintaining a balance between the neutralization capacity and the required level of binding affinities to produce a sufficient level of effector functions and synergetic control interactions with adaptive immunity.Citation87 Although the basics of Fcγ receptors are well established, further work will be required to understand the specific contributions of FcRs in producing broad, long-term antiviral protection. Additionally, understanding the nature of the interaction between an antibody with a virus or the surface structural proteins of an infected cell is important to identify key factors that influence FcRs’ accessibility and the conformational changes that occur upon activation.
In regards to the vaccinal effect derived from T-cell activation, more data are needed to understand the immunological mechanisms required to stimulate DC activation via Fc-FcγR binding, FcRn antigen presentation and the intracellular neutralization mediated by TRIM-21 to address viral threats. Vaccines that induce Fc effector functions should be evaluated to optimize their efficacy and safety using well-constructed standardized assays to understand how neutralizing antibody activity can be improved by combining it with FcR engagement and also how Fc effector functions contribute to the neutralization breadth of antibodies.Citation74
Critically, as evidenced in this review, Fc engineering has shown great potential to meet clinical demands, but careful consideration must be taken when selecting the best approach to engineer the antibody Fc domain. Choosing the right approach to engineer the antibody Fc domain will depend on the desired outcomes, such as improving binding affinities and selectivity, enhancing effector function potency or even combining engineering strategies to produce tailored synergistic effects with safe developability profiles. However, in some cases, engineering Fc to have better binding properties may cause unwanted effects, such as cell destruction due to FcγR saturation level differences. Therefore, caution and further study will be required to identify the proper level of activation to understand the complex concerns required when using Fc engineering or the intact Fc domain.Citation161
Goulet and AtkinsCitation162 recently reviewed the general factors to consider in antibody design and development, but further research is still necessary to identify the factors and properties that should be avoided when designing effective antiviral antibodies with vaccine-like effects to prevent unwanted immunogenicity and other harmful side effects. Safety is at the top of these considerations, and several strategies have been proposed to enhance antibody safety and prevent ADE using Fc engineering.Citation163 One strategy used to decrease ADE activity is to replace IgG subclasses.Citation164 IgG4 was selected due to its lowered ability to recruit effector functions (i.e., its low affinity for C1q and FcγRs).Citation165
MuhammedCitation166 engineered an ACE2-IgG4-Fc fusion to avoid FcR binding while retaining viral neutralization and showed an increased binding affinity for both the SARS-COV-2 RBD and spike protein. Moreover, a stabilizing point mutation (S228P) located in the hinge was inserted to ensure the stability of the ACE2-IgG4-Fc fusion protein.Citation167 This approach has not yet been tested in humans, but the research team suggested that engineering the Fc domain to avoid FcR binding and to utilize IgG4 may have clinical applications for preventing ADE while administering therapy against viral infections.Citation166
Another proposed strategy is minimizing or abrogating the binding of FcRs by inserting key point mutations. Wang, PengCitation168 developed an Fc engineered neutralizing antibody against SARS-COV-2 called MW05. MW05 was engineered by introducing the Fc mutations L234A and L235A (LALA) to abrogate the ADE activity mediated by FcγRIIB. A prophylactic and therapeutic response was observed after a single dose of MW05/LALA. Their study also demonstrated that LALA Fc mutations were able to eliminate ADE and minimize the risk of Fc-mediated acute lung injury in vivo.Citation168
Further clinical trials with large number of patients are needed to investigate the effectiveness of published research; these results will help generate safe, potent and tailored Fc optimized antibodies with antiviral and vaccine-like responses and will also produce effective vaccines that confer long-lasting immunity. Overall, Fc antibody engineering will play a vital role in developing future next-generation antiviral antibody therapeutics and vaccines for infectious diseases in general with a particular interest against emerging viral infections.
Acknowledgments
The author would like to thank Prof Hani Faidah (Faculty of Medicine., Umm Al-Qura University), Dr Saud Almaslmani (Orthopaedic surgeon., Faculty of Medicine., Umm Al-Qura University) and Dr Faz Chowdhury (Chairman and CEO., Nemaura Medical Inc) for their critical reviews.
Disclosure statement
The author declares that she has no conflict of interest related to this study.
Additional information
Funding
References
- Chen P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J, Huhn G, Cardona J, Mocherla B, Stosor V, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384(3):229–37. doi:10.1056/NEJMoa2029849.
- Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, Musser BJ, Soo Y, Rofail D, Im J, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384(3):238–51. doi:10.1056/NEJMoa2035002.
- Gottlieb RL, Nirula A, Chen P, Boscia J, Heller B, Morris J, Huhn G, Cardona J, Mocherla B, Stosor V, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325(7):632–44. doi:10.1001/jama.2021.0202.
- Keeler SP, Fox JM. Requirement of Fc-Fc gamma receptor interaction for antibody-based protection against emerging virus infections. Viruses. 2021:13. doi:10.3390/v13061037.
- Irani V, Guy AJ, Andrew D, Beeson JG, Ramsland PA, Richards JS. Molecular properties of human IgG subclasses and their implications for designing therapeutic monoclonal antibodies against infectious diseases. Mol Immunol. 2015;67(2):171–82. doi:10.1016/j.molimm.2015.03.255.
- Ferrante A, Beard LJ, Feldman RG. IgG subclass distribution of antibodies to bacterial and viral antigens. Pediatr Infect Dis J. 1990;9(8Suppl):S16–24. doi:10.1097/00006454-199008001-00004.
- Wagner DK, Graham BS, Wright PF, Walsh EE, Kim HW, Reimer CB, Nelson DL, Chanock RM, Murphy BR. Serum immunoglobulin G antibody subclass responses to respiratory syncytial virus F and G glycoproteins after primary infection. J Clin Microbiol. 1986;24(2):304–06. doi:10.1128/jcm.24.2.304-306.1986.
- Stapleton NM, Andersen JT, Stemerding AM, Bjarnarson SP, Verheul RC, Gerritsen J, Zhao Y, Kleijer M, Sandlie I, De Haas M, et al. Competition for FcRn-mediated transport gives rise to short half-life of human IgG3 and offers therapeutic potential. Nat Commun. 2011;2(1):599. doi:10.1038/ncomms1608.
- Pelegrin M, Naranjo-Gomez M, Piechaczyk M. Antiviral monoclonal antibodies: can they be more than simple neutralizing agents? Trends Microbiol. 2015;23(10):653–65. doi:10.1016/j.tim.2015.07.005.
- Chiu ML, Goulet DR, Teplyakov A, Gilliland GL. Antibody structure and function: the basis for Engineering Therapeutics. Antibodies (Basel). 2019:8. doi:10.3390/antib8040055.
- Wang P, Gajjar MR, Yu J, Padte NN, Gettie A, Blanchard JL, Russell-Lodrigue K, Liao LE, Perelson AS, Huang Y, et al. Quantifying the contribution of Fc-mediated effector functions to the antiviral activity of anti-HIV-1 IgG1 antibodies in vivo. Proc Natl Acad Sci USA. 2020;117(30):18002–09. doi:10.1073/pnas.2008190117.
- van Erp EA, Luytjes W, Ferwerda G, van Kasteren PB. Fc-mediated antibody effector functions during respiratory syncytial virus infection and disease. Front Immunol. 2019;10:548. doi:10.3389/fimmu.2019.00548.
- Junker F, Gordon J, Qureshi O. Fc gamma receptors and their role in antigen uptake, presentation, and T cell activation. Front Immunol. 2020;11:1393. doi:10.3389/fimmu.2020.01393.
- Hilchey SP, Hyrien O, Mosmann TR, Livingstone AM, Friedberg JW, Young F, Fisher RI, Kelleher RJ, Bankert RB, Bernstein SH. Rituximab immunotherapy results in the induction of a lymphoma idiotype-specific T-cell response in patients with follicular lymphoma: support for a “vaccinal effect” of rituximab. Blood. 2009;113(16):3809–12. doi:10.1182/blood-2008-10-185280.
- DiLillo DJ, Ravetch JV. Differential Fc-receptor engagement drives an anti-tumor vaccinal effect. Cell. 2015;161(5):1035–45. doi:10.1016/j.cell.2015.04.016.
- Ahangarzadeh S, Payandeh Z, Arezumand R, Shahzamani K, Yarian F, Alibakhshi A. An update on antiviral antibody-based biopharmaceuticals. Int Immunopharmacol. 2020;86:106760. doi:10.1016/j.intimp.2020.106760.
- Chames P, Van Regenmortel M, Weiss E, Baty D. Therapeutic antibodies: successes, limitations and hopes for the future. Br J Pharmacol. 2009;157(2):220–33. doi:10.1111/j.1476-5381.2009.00190.x.
- Forthal DN, Moog C. Fc receptor-mediated antiviral antibodies. Curr Opin HIV AIDS. 2009;4(5):388–93. doi:10.1097/COH.0b013e32832f0a89.
- Jegaskanda S, Vanderven HA, Wheatley AK, Kent SJ. Fc or not Fc; that is the question: antibody Fc-receptor interactions are key to universal influenza vaccine design. Hum Vaccin Immunother. 2017;13(6):1–9. doi:10.1080/21645515.2017.1290018.
- Yu X, Cragg MS. Engineered antibodies to combat viral threats. Nature. 2020;588(7838):398–99. doi:10.1038/d41586-020-03196-2.
- IgG BS. Fc receptors: evolutionary considerations. Curr Top Microbiol Immunol. 2019;423:1–11. doi:10.1007/82_2019_149.
- Bournazos S, Corti D, Virgin HW, Ravetch JV. Fc-optimized antibodies elicit CD8 immunity to viral respiratory infection. Nature. 2020;588(7838):485–90. doi:10.1038/s41586-020-2838-z.
- Gunn BM, Alter G. Modulating antibody functionality in infectious disease and vaccination. Trends Mol Med. 2016;22(11):969–82. doi:10.1016/j.molmed.2016.09.002.
- Burton DR. Antibodies, viruses and vaccines. Nat Rev Immunol. 2002;2(9):706–13. doi:10.1038/nri891.
- Bournazos S, Ravetch JV. Diversification of IgG effector functions. Int Immunol. 2017;29(7):303–10. doi:10.1093/intimm/dxx025.
- Yang C, Gao X, Gong R. Engineering of Fc fragments with optimized physicochemical properties implying improvement of clinical potentials for Fc-based therapeutics. Front Immunol. 2018;8:1860. doi:10.3389/fimmu.2017.01860.
- Saunders KO. Conceptual approaches to modulating antibody effector functions and circulation half-life. Front Immunol. 2019;10:1296. doi:10.3389/fimmu.2019.01296.
- Ducancel F, Muller BH. Molecular engineering of antibodies for therapeutic and diagnostic purposes. MAbs. 2012;4(4):445–57. doi:10.4161/mabs.20776.
- Garber K. Hunt for improved monoclonals against coronavirus gathers pace. Nat Biotechnol. 2021;39(1):9–12. doi:10.1038/s41587-020-00791-6.
- Morris AB, Farley CR, Pinelli DF, Adams LE, Cragg MS, Boss JM, Scharer CD, Fribourg M, Cravedi P, Heeger PS, et al. Signaling through the Inhibitory Fc receptor FcγRIIB induces CD8+ T cell apoptosis to limit T cell immunity. Immunity. 2020;52(1):136–150.e6. doi:10.1016/j.immuni.2019.12.006.
- Chauhan AK, Chen C, Moore TL, DiPaolo RJ. Induced expression of FcγRIIIa (CD16a) on CD4+ T cells triggers generation of IFN-γhigh subset. J Biol Chem. 2015;290(8):5127–40. doi:10.1074/jbc.M114.599266.
- Bournazos S, Gupta A, Ravetch JV. The role of IgG Fc receptors in antibody-dependent enhancement. Nat Rev Immunol. 2020;20(10):633–43. doi:10.1038/s41577-020-00410-0.
- De Taeye SW, Rispens T, Vidarsson G. The ligands for human IgG and their effector functions. Antibodies (Basel). 2019:8. doi:10.3390/antib8020030.
- Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520. doi:10.3389/fimmu.2014.00520.
- Ben Mkaddem S, Benhamou M, Monteiro RC. Understanding Fc receptor involvement in inflammatory diseases: from mechanisms to new therapeutic tools. Front Immunol. 2019;10:811. doi:10.3389/fimmu.2019.00811.
- Guilliams M, Bruhns P, Saeys Y, Hammad H, Lambrecht BN. The function of Fcγ receptors in dendritic cells and macrophages. Nat Rev Immunol. 2014;14(2):94–108. doi:10.1038/nri3582.
- Herter S, Birk MC, Klein C, Gerdes C, Umana P, Bacac M. Glycoengineering of therapeutic antibodies enhances monocyte/macrophage-mediated phagocytosis and cytotoxicity. J Immunol. 2014;192(5):2252–60. doi:10.4049/jimmunol.1301249.
- Tay MZ, Wiehe K, Pollara J. Antibody-dependent cellular phagocytosis in antiviral immune responses. Front Immunol. 2019;10:332. doi:10.3389/fimmu.2019.00332.
- Araki N, Hatae T, Furukawa A, Swanson JA. Phosphoinositide-3-kinase-independent contractile activities associated with Fcγ-receptor-mediated phagocytosis and macropinocytosis in macrophages. J Cell Sci. 2003;116(2):247–57. doi:10.1242/jcs.00235.
- Huang Z-Y, Barreda DR, Worth RG, Indik ZK, Kim M-K, Chien P, Schreiber AD. Differential kinase requirements in human and mouse Fc-gamma receptor phagocytosis and endocytosis. J Leukoc Biol. 2006;80(6):1553–62. doi:10.1189/jlb.0106019.
- Mantegazza AR, Magalhaes JG, Amigorena S, Marks MS. Presentation of phagocytosed antigens by MHC class I and II. Traffic. 2013;14(2):135–52. doi:10.1111/tra.12026.
- Parcina M, Wendt C, Goetz F, Zawatzky R, Zähringer U, Heeg K, Bekeredjian-Ding I. Staphylococcus aureus-induced plasmacytoid dendritic cell activation is based on an IgG-mediated memory response. J Immunol. 2008;181(6):3823–33. doi:10.4049/jimmunol.181.6.3823.
- Asselin-Paturel C, Brizard G, Chemin K, Boonstra A, O’Garra A, Vicari A, Trinchieri TG. Type I interferon dependence of plasmacytoid dendritic cell activation and migration. J Exp Med. 2005;201(7):1157–67. doi:10.1084/jem.20041930.
- Jego G, Palucka AK, Blanck J-P, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19(2):225–34. doi:10.1016/s1074-7613(03)00208-5.
- Worley MJ, Fei K, Lopez-Denman AJ, Kelleher AD, Kent SJ, Chung AW. Neutrophils mediate HIV-specific antibody-dependent phagocytosis and ADCC. J Immunol Methods. 2018;457:41–52. doi:10.1016/j.jim.2018.03.007.
- Gao R, Sheng Z, Sreenivasan CC, Wang D, Influenza LF. A virus antibodies with antibody-dependent cellular cytotoxicity function. Viruses. 2020:12. doi:10.3390/v12030276.
- Salk HM, Haralambieva IH, Ovsyannikova IG, Goergen KM, Poland GA. Granzyme B ELISPOT assay to measure influenza-specific cellular immunity. J Immunol Methods. 2013;398-399:44–50. doi:10.1016/j.jim.2013.09.007.
- Borsos T, Rapp HJ. Hemolysin titration based on fixation of the activated first component of complement: evidence that one molecule of hemolysin suffices to sensitize an erythrocyte. J Immunol. 1965;95:559–66.
- Diebolder CA, Beurskens FJ, de Jong RN, Koning RI, Strumane K, Lindorfer MA, Voorhorst M, Ugurlar D, Rosati S, Heck AJ, et al. Complement is activated by IgG hexamers assembled at the cell surface. Science. 2014;343(6176):1260–63. doi:10.1126/science.1248943.
- Kellner C, Derer S, Valerius T, Peipp PM. Boosting ADCC and CDC activity by Fc engineering and evaluation of antibody effector functions. Methods. 2014;65(1):105–13. doi:10.1016/j.ymeth.2013.06.036.
- Bournazos S, DiLillo DJ, Ravetch JV. The role of Fc–FcγR interactions in IgG-mediated microbial neutralization. J Exp Med. 2015;212(9):1361–69. doi:10.1084/jem.20151267.
- Bournazos S, Ravetch JV. Fcγ receptor function and the design of vaccination strategies. Immunity. 2017;47(2):224–33. doi:10.1016/j.immuni.2017.07.009.
- Bournazos S, Ravetch JV. Fcγ receptor pathways during active and passive immunization. Immunol Rev. 2015;268(1):88–103. doi:10.1111/imr.12343.
- Igietseme JU, Eko FO, He Q, Black CM. Antibody regulation of T-cell immunity: implications for vaccine strategies against intracellular pathogens. Expert Rev Vaccines. 2004;3(1):23–34. doi:10.1586/14760584.3.1.23.
- Regnault A, Lankar D, Lacabanne V, Rodriguez A, Théry C, Rescigno M, Saito T, Verbeek S, Bonnerot C, Ricciardi-Castagnoli P, et al. Fcγ receptor–mediated induction of dendritic cell maturation and major histocompatibility complex class i–restricted antigen presentation after immune complex internalization. J Exp Med. 1999;189(2):371–80. doi:10.1084/jem.189.2.371.
- Munroe ME, Bishop GA. A costimulatory function for T cell CD40. J Immunol. 2007;178(2):671–82. doi:10.4049/jimmunol.178.2.671.
- Chan KR, Ong EZ, Mok DZ, Ooi EE. Fc receptors and their influence on efficacy of therapeutic antibodies for treatment of viral diseases. Expert Rev Anti-Infect Ther. 2015;13(11):1351–60. doi:10.1586/14787210.2015.1079127.
- Lev A, Sigal L. Getting in front and behind the enemy lines to counter virus infection. Cell Host Microbe. 2013;13(2):121–22. doi:10.1016/j.chom.2013.01.013.
- den Dunnen J, Vogelpoel LT, Wypych T, Muller FJ, De Boer L, Kuijpers TW, Zaat SA, Kapsenberg ML, de Jong EC. IgG opsonization of bacteria promotes Th17 responses via synergy between TLRs and FcγRIIa in human dendritic cells. Blood. 2012;120(1):112–21. doi:10.1182/blood-2011-12-399931.
- Boonnak K, Dambach KM, Donofrio GC, Tassaneetrithep B, Marovich MA. Cell type specificity and host genetic polymorphisms influence antibody-dependent enhancement of dengue virus infection. J Virol. 2011;85(4):1671–83. doi:10.1128/JVI.00220-10.
- Mallery DL, McEwan WA, Bidgood SR, Towers GJ, Johnson CM, James LC. Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21). Proc Natl Acad Sci USA. 2010;107(46):19985–90. doi:10.1073/pnas.1014074107.
- Foss S, Watkinson RE, Grevys A, McAdam MB, Bern M, Høydahl LS, Dalhus B, Michaelsen TE, Sandlie I, James LC, et al. TRIM21 immune signaling is more sensitive to antibody affinity than its neutralization activity. J Immunol. 2016;196(8):3452–59. doi:10.4049/jimmunol.1502601.
- McEwan WA, Tam JC, Watkinson RE, Bidgood SR, Mallery DL, James LC. Intracellular antibody-bound pathogens stimulate immune signaling via the Fc receptor TRIM21. Nat Immunol. 2013;14(4):327–36. doi:10.1038/ni.2548.
- Clift D, So C, McEwan WA, James LC, Schuh SM. Publisher Correction: acute and rapid degradation of endogenous proteins by Trim-Away. Nat Protoc. 2019;14(8):2596. doi:10.1038/s41596-018-0092-8.
- Caddy SL, Vaysburd M, Papa G, Wing M, O’Connell K, Stoycheva D, Foss S, Terje Andersen J, Oxenius A, James LC. Viral nucleoprotein antibodies activate TRIM21 and induce T cell immunity. EMBO J. 2021;40(5):e106228. doi:10.15252/embj.2020106228.
- Hangartner L, Zinkernagel RM, Hengartner H. Antiviral antibody responses: the two extremes of a wide spectrum. Nat Rev Immunol. 2006;6(3):231–43. doi:10.1038/nri1783.
- Mackness BC, Jaworski JA, Boudanova E, Park A, Valente D, Mauriac C, Pasquier O, Schmidt T, Kabiri M, Kandira A, et al. Antibody Fc engineering for enhanced neonatal Fc receptor binding and prolonged circulation half-life. MAbs. 2019;11(7):1276–88. doi:10.1080/19420862.2019.1633883.
- Bai Y, Ye L, Tesar DB, Song H, Zhao D, Björkman PJ, Roopenian DC, Zhu X. Intracellular neutralization of viral infection in polarized epithelial cells by neonatal Fc receptor (FcRn)-mediated IgG transport. Proc Natl Acad Sci USA. 2011;108(45):18406–11. doi:10.1073/pnas.1115348108.
- Baker K, Rath T, Pyzik M, Blumberg RS. The role of FcRn in antigen presentation. Front Immunol. 2014;5:408. doi:10.3389/fimmu.2014.00408.
- Ward ES, Devanaboyina SC, Ober RJ. Targeting FcRn for the modulation of antibody dynamics. Mol Immunol. 2015;67(2):131–41. doi:10.1016/j.molimm.2015.02.007.
- Qiao S-W, Kobayashi K, Johansen F-E, Sollid LM, Andersen JT, Milford E, Roopenian DC, Lencer WI, Blumberg RS. Dependence of antibody-mediated presentation of antigen on FcRn. Proc Natl Acad Sci USA. 2008;105(27):9337–42. doi:10.1073/pnas.0801717105.
- Baker K, Rath T, Flak MB, Arthur JC, Chen Z, Glickman JN, Zlobec I, Karamitopoulou E, Stachler MD, Odze RD, et al. Neonatal Fc receptor expression in dendritic cells mediates protective immunity against colorectal cancer. Immunity. 2013;39(6):1095–107. doi:10.1016/j.immuni.2013.11.003.
- Rath T, Baker K, Pyzik M, Blumberg RS. Regulation of immune responses by the neonatal Fc receptor and its therapeutic implications. Front Immunol. 2014;5:664. doi:10.3389/fimmu.2014.00664.
- Richardson SI, Moore PL. Targeting Fc effector function in vaccine design. Expert Opin Ther Targets. 2021;25(6):467–77. doi:10.1080/14728222.2021.1907343.
- Barrett JR, Belij-Rammerstorfer S, Dold C, Ewer KJ, Folegatti PM, Gilbride C, Halkerston R, Hill J, Jenkin D, Stockdale L, et al. Author Correction: phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat Med. 2021;27(6):1113. doi:10.1038/s41591-021-01372-z.
- Chaudhury S, Ockenhouse CF, Regules JA, Dutta S, Wallqvist A, Jongert E, Waters NC, Lemiale F, Bergmann-Leitner E. The biological function of antibodies induced by the RTS,S/AS01 malaria vaccine candidate is determined by their fine specificity. Malar J. 2016;15(1):301. doi:10.1186/s12936-016-1348-9.
- Kurtovic L, Atre T, Feng G, Wines BD, Chan J-A, Boyle MJ, Drew DR, Hogarth PM, Fowkes FJI, Bergmann-Leitner ES, et al. Multifunctional antibodies are induced by the RTS,S malaria vaccine and associated with protection in a phase 1/2a trial. J Infect Dis. 2020. doi:10.1093/infdis/jiaa144.
- Coler RN, Day TA, Ellis R, Piazza FM, Beckmann AM, Vergara J, Rolf T, Lu L, Alter G, Hokey D, et al. The TLR-4 agonist adjuvant, GLA-SE, improves magnitude and quality of immune responses elicited by the ID93 tuberculosis vaccine: first-in-human trial. Npj Vaccines. 2018;3(1):34. doi:10.1038/s41541-018-0057-5.
- Bournazos S, DiLillo DJ, Goff AJ, Glass PJ, Ravetch JV. Differential requirements for FcγR engagement by protective antibodies against Ebola virus. Proc Natl Acad Sci USA. 2019;116(40):20054–62. doi:10.1073/pnas.1911842116.
- Chan CEZ, Seah SGK, Chye H, Massey S, Torres M, Lim APC, Wong SKK, Neo JJY, Wong PS, Lim JH, et al. The Fc-mediated effector functions of a potent SARS-CoV-2 neutralizing antibody, SC31, isolated from an early convalescent COVID-19 patient, are essential for the optimal therapeutic efficacy of the antibody. PLOS ONE. 2021;16(6):e0253487. doi:10.1371/journal.pone.0253487.
- Tauzin A, Nayrac M, Benlarbi M, Gong SY, Gasser R, Beaudoin-Bussières G, Brassard N, Laumaea A, Vézina D, Prévost J, et al. A single dose of the SARS-CoV-2 vaccine BNT162b2 elicits Fc-mediated antibody effector functions and T cell responses. Cell Host Microbe. 2021;29(7):1137–1150.e6. doi:10.1016/j.chom.2021.06.001.
- Suscovich TJ, Fallon JK, Das J, Demas AR, Crain J, Linde CH, Michell A, Natarajan H, Arevalo C, Broge T, et al. Mapping functional humoral correlates of protection against malaria challenge following RTS,S/AS01 vaccination. Sci Transl Med. 2020;12(553):eabb4757. doi:10.1126/scitranslmed.abb4757.
- Nelson CS, Huffman T, Jenks JA, Cisneros de la Rosa E, Xie G, Vandergrift N, Pass RF, Pollara J, Permar SR. HCMV glycoprotein B subunit vaccine efficacy mediated by nonneutralizing antibody effector functions. Proc Natl Acad Sci USA. 2018;115(24):6267–72. doi:10.1073/pnas.1800177115.
- Perez LG, Martinez DR, deCamp AC, Pinter A, Berman PW, Francis D, Sinangil F, Lee C, Greene K, Gao H, et al. V1V2-specific complement activating serum IgG as a correlate of reduced HIV-1 infection risk in RV144. PLOS ONE. 2017;12(7):e0180720. doi:10.1371/journal.pone.0180720.
- Excler J-L, Ake J, Robb ML, Kim JH, Plotkin SA, Papasian CJ. Nonneutralizing functional antibodies: a new “old” paradigm for HIV vaccines. Clin Vaccine Immunol. 2014;21(8):1023–36. doi:10.1128/CVI.00230-14.
- Tomaras GD, Plotkin SA. Complex immune correlates of protection in HIV-1 vaccine efficacy trials. Immunol Rev. 2017;275(1):245–61. doi:10.1111/imr.12514.
- Sondermann P, Szymkowski DE. Harnessing Fc receptor biology in the design of therapeutic antibodies. Curr Opin Immunol. 2016;40:78–87. doi:10.1016/j.coi.2016.03.005.
- Wang X, Mathieu M, Brezski RJ. IgG Fc engineering to modulate antibody effector functions. Protein Cell. 2018;9(1):63–73. doi:10.1007/s13238-017-0473-8.
- Stavenhagen JB, Gorlatov S, Tuaillon N, Rankin CT, Li H, Burke S, Huang L, Johnson S, Bonvini E, Koenig S, et al. Fc optimization of therapeutic antibodies enhances their ability to kill tumor cells in vitro and controls tumor expansion in vivo via low-affinity activating Fcγ receptors. Cancer Res. 2007;67(18):8882–90. doi:10.1158/0008-5472.CAN-07-0696.
- Nordstrom JL, Gorlatov S, Zhang W, Yang Y, Huang L, Burke S, Li H, Ciccarone V, Zhang T, Stavenhagen J, et al. Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcγ receptor binding properties. Breast Cancer Res. 2011;13(6):R123. doi:10.1186/bcr3069.
- Bang YJ, Giaccone G, Im SA, Oh DY, Bauer TM, Nordstrom JL, Li H, Chichili GR, Moore PA, Hong S, et al. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann Oncol. 2017;28(4):855–61. doi:10.1093/annonc/mdx002.
- Mimoto F, Katada H, Kadono S, Igawa T, Kuramochi T, Muraoka M, Wada Y, Haraya K, Miyazaki T, Hattori K. Engineered antibody Fc variant with selectively enhanced Fc RIIb binding over both Fc RIIaR131 and Fc RIIaH131. Protein Eng Des Sel. 2013;26(10):589–98. doi:10.1093/protein/gzt022.
- Rugo HS, Im SA, Cardoso F, Cortes J, Curigliano G, Pegram MD, Musolino A, Bachelot T, Wright GS, De Laurentiis M, et al. Phase 3 SOPHIA study of margetuximab plus chemotherapy vs trastuzumab plus chemotherapy in patients with HER2+metastatic breast cancer after prior anti-HER2 therapies: second interim overall survival analysis. Cancer Res. 2020;80:Abstract nr GS1–02.
- Shields RL, Namenuk AK, Hong K, Meng YG, Rae J, Briggs J, Xie D, Lai J, Stadlen A, Li B, et al. High resolution mapping of the binding site on human IgG1 for FcγRI, FcγRII, FcγRIII, and FcRn and design of IgG1 variants with improved binding to the FcγR. J Biol Chem. 2001;276(9):6591–604. doi:10.1074/jbc.M009483200.
- Irvine EB, Alter G. Understanding the role of antibody glycosylation through the lens of severe viral and bacterial diseases. Glycobiology. 2020;30(4):241–53. doi:10.1093/glycob/cwaa018.
- Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 2007;25(1):21–50. doi:10.1146/annurev.immunol.25.022106.141702.
- Walker MR, Lund J, Thompson KM, Jefferis R. Aglycosylation of human IgG1 and IgG3 monoclonal antibodies can eliminate recognition by human cells expressing FcγRI and/or FcγRII receptors. Biochem J. 1989;259(2):347–53. doi:10.1042/bj2590347.
- Jefferis R. Glycosylation of antibody therapeutics: optimisation for purpose. Methods Mol Biol. 2009;483:223–38. doi:10.1007/978-1-59745-407-0_13.
- Umaña P, Jean–Mairet J, Moudry R, Amstutz H, Bailey JE. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat Biotechnol. 1999;17(2):176–80. doi:10.1038/6179.
- Ferrara C, Grau S, Jäger C, Sondermann P, Brünker P, Waldhauer I, Hennig M, Ruf A, Rufer AC, Stihle M, et al. Unique carbohydrate–carbohydrate interactions are required for high affinity binding between FcγRIII and antibodies lacking core fucose. Proc Natl Acad Sci USA. 2011;108(31):12669–74. doi:10.1073/pnas.1108455108.
- Shields RL, Lai J, Keck R, O’Connell LY, Hong K, Meng YG, Weikert SH, Presta LG. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277(30):26733–40. doi:10.1074/jbc.M202069200.
- Ito A, Ishida T, Yano H, Inagaki A, Suzuki S, Sato F, Takino H, Mori F, Ri M, Kusumoto S, et al. Defucosylated anti-CCR4 monoclonal antibody exercises potent ADCC-mediated antitumor effect in the novel tumor-bearing humanized NOD/Shi-scid, IL-2Rgamma(null) mouse model. Cancer Immunol Immunother. 2009;58(8):1195–206. doi:10.1007/s00262-008-0632-0.
- Beck A, Reichert JM. Marketing approval of mogamulizumab: a triumph for glyco-engineering. MAbs. 2012;4(4):419–25. doi:10.4161/mabs.20996.
- Evans JB, Syed BA. From the analyst’s couch: next-generation antibodies. Nat Rev Drug Discov. 2014;13(6):413–14. doi:10.1038/nrd4255.
- Liu R, Oldham RJ, Teal E, Beers SA, Cragg MS. Fc-engineering for modulated effector functions-improving antibodies for cancer treatment. Antibodies (Basel). 2020:9. doi:10.3390/antib9040064.
- Hiatt A, Bohorova N, Bohorov O, Goodman C, Kim D, Pauly MH, Velasco J, Whaley KJ, Piedra PA, Gilbert BE, et al. Glycan variants of a respiratory syncytial virus antibody with enhanced effector function and in vivo efficacy. Proc Natl Acad Sci USA. 2014;111(16):5992–97. doi:10.1073/pnas.1402458111.
- Ackerman ME, Moldt B, Wyatt RT, Dugast A-S, McAndrew E, Tsoukas S, Jost S, Berger CT, Sciaranghella G, Liu Q, et al. A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples. J Immunol Methods. 2011;366(1–2):8–19. doi:10.1016/j.jim.2010.12.016.
- Boesch AW, Miles AR, Chan YN, Osei-Owusu NY, Ackerman ME. IgG Fc variant cross-reactivity between human and rhesus macaque FcγRs. MAbs. 2017;9(3):455–65. doi:10.1080/19420862.2016.1274845.
- Jung ST, Kelton W, Kang TH, Ng DT, Andersen JT, Sandlie I, Sarkar CA, Georgiou G. Effective phagocytosis of low Her2 tumor cell lines with engineered, aglycosylated IgG displaying high FcγRIIa affinity and selectivity. ACS Chem Biol. 2013;8(2):368–75. doi:10.1021/cb300455f.
- Richards JO, Karki S, Lazar GA, Chen H, Dang W, Desjarlais JR. Optimization of antibody binding to FcgammaRIIa enhances macrophage phagocytosis of tumor cells. Mol Cancer Ther. 2008;7(8):2517–27. doi:10.1158/1535-7163.MCT-08-0201.
- Lazar GA, Dang W, Karki S, Vafa O, Peng JS, Hyun L, Chan C, Chung HS, Eivazi A, Yoder SC, et al. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci USA. 2006;103(11):4005–10. doi:10.1073/pnas.0508123103.
- Smith P, DiLillo DJ, Bournazos S, Li F, Ravetch JV. Mouse model recapitulating human Fcgamma receptor structural and functional diversity. Proc Natl Acad Sci USA. 2012;109(16):6181–86. doi:10.1073/pnas.1203954109.
- Ahmed AA, Keremane SR, Vielmetter J, Bjorkman PJ. Structural characterization of GASDALIE Fc bound to the activating Fc receptor FcγRIIIa. J Struct Biol. 2016;194(1):78–89. doi:10.1016/j.jsb.2016.02.001.
- Introna M, Golay J. Complement in antibody therapy: friend or foe? Blood. 2009;114(26):5247–48. doi:10.1182/blood-2009-10-249532.
- Lee CH, Romain G, Yan W, Watanabe M, Charab W, Todorova B, Lee J, Triplett K, Donkor M, Lungu OI, et al. Correction: corrigendum: IgG Fc domains that bind C1q but not effector Fcγ receptors delineate the importance of complement-mediated effector functions. Nat Immunol. 2017;18:1173. doi:10.1038/ni1017-1173c.
- Moore GL, Chen H, Karki S, Lazar GA. Engineered Fc variant antibodies with enhanced ability to recruit complement and mediate effector functions. MAbs. 2010;2(2):181–89. doi:10.4161/mabs.2.2.11158.
- Idusogie EE, Wong PY, Presta LG, Gazzano-Santoro H, Totpal K, Ultsch M, Mulkerrin MG. Engineered antibodies with increased activity to recruit complement. J Immunol. 2001;166(4):2571–75. doi:10.4049/jimmunol.166.4.2571.
- de Jong RN, Beurskens FJ, Verploegen S, Strumane K, van Kampen MD, Voorhorst M, Horstman W, Engelberts PJ, Oostindie SC, Wang G, et al. A novel platform for the potentiation of therapeutic antibodies based on antigen-dependent formation of IgG hexamers at the cell surface. PLOS Biol. 2016;14(1):e1002344. doi:10.1371/journal.pbio.1002344.
- Schütze K, Petry K, Hambach J, Schuster N, Fumey W, Schriewer L, Röckendorf J, Menzel S, Albrecht B, Haag F, et al. CD38-specific biparatopic heavy chain antibodies display potent complement-dependent cytotoxicity against multiple myeloma cells. Front Immunol. 2018;9:2553. doi:10.3389/fimmu.2018.02553.
- Ravetch JV, Bournazos S Human IgG Fc domain variants with improved effector function. United States patent WO/2019/125846. 2019 June 27.
- Weitzenfeld P, Bournazos S, Ravetch JV. Antibodies targeting sialyl Lewis A mediate tumor clearance through distinct effector pathways. J Clin Invest. 2019;129(9):3952–62. doi:10.1172/JCI128437.
- Mimoto F, Igawa T, Kuramochi T, Katada H, Kadono S, Kamikawa T, Shida-Kawazoe M, Hattori K. Novel asymmetrically engineered antibody Fc variant with superior FcγR binding affinity and specificity compared with afucosylated Fc variant. MAbs. 2013;5(2):229–36. doi:10.4161/mabs.23452.
- Wang G, de Jong RN, van den Bremer EJ, Beurskens FJ, Labrijn AF, Ugurlar D, Gros P, Schuurman J, Parren PHI, Heck AR. Molecular basis of assembly and activation of complement component C1 in complex with immunoglobulin G1 and antigen. Mol Cell. 2016;63(1):135–45. doi:10.1016/j.molcel.2016.05.016.
- Dall’Acqua WF, Kiener PA, Wu H. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn). J Biol Chem. 2006;281(33):23514–24. doi:10.1074/jbc.M604292200.
- Zalevsky J, Chamberlain AK, Horton HM, Karki S, Leung IW, Sproule TJ, Lazar GA, Roopenian DC, Desjarlais JR. Enhanced antibody half-life improves in vivo activity. Nat Biotechnol. 2010;28(2):157–59. doi:10.1038/nbt.1601.
- Ko S, Jo M, Jung ST. Recent achievements and challenges in prolonging the serum half-lives of therapeutic IgG antibodies through Fc engineering. BioDrugs. 2021;35(2):147–57. doi:10.1007/s40259-021-00471-0.
- Foss S, Watkinson R, Sandlie I, James LC, Andersen JT. TRIM 21: a cytosolic Fc receptor with broad antibody isotype specificity. Immunol Rev. 2015;268(1):328–39. doi:10.1111/imr.12363.
- Ng PML, Kaliaperumal N, Lee CY, Chin WJ, Tan HC, Au VB, Goh AX, Tan QW, Yeo DSG, Connolly JE, et al. Enhancing antigen cross-presentation in human monocyte-derived dendritic cells by recruiting the intracellular Fc receptor TRIM21. J Immunol. 2019;202(8):2307–19. doi:10.4049/jimmunol.1800462.
- Foss S, Bottermann M, Jonsson A, Sandlie I, James LC, Andersen JT. TRIM21—from intracellular immunity to therapy. Front Immunol. 2019;10:2049. doi:10.3389/fimmu.2019.02049.
- Tam JC, Bidgood SR, McEwan WA, James LC. Intracellular sensing of complement C3 activates cell autonomous immunity. Science. 2014;345(6201):1256070. doi:10.1126/science.1256070.
- Bottermann M, Foss S, Caddy SL, Clift D, van Tienen LM, Vaysburd M, Cruickshank J, O’Connell K, Clark J, Mayes K, et al. Complement C4 prevents viral infection through capsid inactivation. Cell Host Microbe. 2019;25(4):617–629.e7. doi:10.1016/j.chom.2019.02.016.
- Clift D, McEwan WA, Labzin LI, Konieczny V, Mogessie B, James LC, Schuh M. A method for the acute and rapid degradation of endogenous proteins. Cell. 2017;171(7):1692–1706.e18. doi:10.1016/j.cell.2017.10.033.
- Zeng J, Santos AF, Mukadam AS, Osswald M, Jacques DA, Dickson CF, McLaughlin SH, Johnson CM, Kiss L, Luptak J, et al. Target-induced clustering activates Trim-Away of pathogens and proteins. Nat Struct Mol Biol. 2021;28(3):278–89. doi:10.1038/s41594-021-00560-2.
- Gautam R, Nishimura Y, Gaughan N, Gazumyan A, Schoofs T, Buckler-White A, Seaman MS, Swihart BJ, Follmann DA, Nussenzweig MC, et al. A single injection of crystallizable fragment domain–modified antibodies elicits durable protection from SHIV infection. Nat Med. 2018;24(5):610–16. doi:10.1038/s41591-018-0001-2.
- Dall’Acqua WF, Woods RM, Ward ES, Palaszynski SR, Patel NK, Brewah YA, Wu H, Kiener PA, Langermann S. Increasing the affinity of a human IgG1 for the neonatal Fc receptor: biological consequences. J Immunol. 2002;169(9):5171–80. doi:10.4049/jimmunol.169.9.5171.
- Robbie GJ, Criste R, Dall’acqua WF, Jensen K, Patel NK, Losonsky GA, Griffin MP. A novel investigational Fc-modified humanized monoclonal antibody, motavizumab-YTE, has an extended half-life in healthy adults. Antimicrob Agents Chemother. 2013;57(12):6147–53. doi:10.1128/AAC.01285-13.
- Dolgin E. ‘Super-antibodies’ could curb COVID-19 and help avert future pandemics. Nat Biotechnol. 2021;39(7):783–85. doi:10.1038/s41587-021-00980-x.
- Corti D, Purcell LA, Snell G, Veesler VD. Tackling COVID-19 with neutralizing monoclonal antibodies. Cell. 2021;184(12):3086–108. doi:10.1016/j.cell.2021.05.005.
- YG-R AG, Juarez E, Casal MC, Moya J, Falci DR, Sarkis E, Solis J, Hanzhe Z, Scott N, Cathcart AL, et al., for the COMET-ICE Investigators. Early Covid-19 treatment with SARS-CoV-2 neutralizing antibody Sotrovimab. medRxiv. 2021. doi:10.1101/2021.05.27.21257096.
- Dimitrov DS. Engineered CH2 domains (nanoantibodies). MAbs. 2009;1(1):26–28. doi:10.4161/mabs.1.1.7480.
- Kruse RL. Therapeutic strategies in an outbreak scenario to treat the novel coronavirus originating in Wuhan, China. F1000Res. 2020;9:72. doi:10.12688/f1000research.22211.2.
- Wozniak-Knopp G, Bartl S, Bauer A, Mostageer M, Woisetschläger M, Antes B, Ettl K, Kainer M, Weberhofer G, Wiederkum S, et al. Introducing antigen-binding sites in structural loops of immunoglobulin constant domains: Fc fragments with engineered HER2/neu-binding sites and antibody properties. Protein Eng Des Sel. 2010;23(4):289–97. doi:10.1093/protein/gzq005.
- Traxlmayr MW, Faer M, Stadlmayr G, Hasenhindl C, Antes B, Rüker F, Obinger C. Directed evolution of stabilized IgG1-Fc scaffolds by application of strong heat shock to libraries displayed on yeast. Biochim Biophys Acta. 2012;1824(4):542–49. doi:10.1016/j.bbapap.2012.01.006.
- Zhang Y, Zhou Z, Zhu S-L, Zu X, Wang Z, Zhang L-K, Wang W, Xiao G. A novel RSV F-Fc fusion protein vaccine reduces lung injury induced by respiratory syncytial virus infection. Antiviral Res. 2019;165:11–22. doi:10.1016/j.antiviral.2019.02.017.
- Divine R, Dang HV, Ueda G, Fallas JA, Vulovic I, Sheffler W, Saini S, Zhao YT, Raj IX, Morawski PA, et al. Designed proteins assemble antibodies into modular nanocages. bioRxiv. 2020. doi:10.1101/2020.12.01.406611.
- Moldt B, Shibata-Koyama M, Rakasz EG, Schultz N, Kanda Y, Dunlop DC, Finstad SL, Jin C, Landucci G, Alpert MD, et al. A nonfucosylated variant of the anti-HIV-1 monoclonal antibody b12 has enhanced Fc RIIIa-mediated antiviral activity in vitro but does not improve protection against mucosal SHIV challenge in macaques. J Virol. 2012;86(11):6189–96. doi:10.1128/JVI.00491-12.
- Natsume A, Wakitani M, Yamane-Ohnuki N, Shoji-Hosaka E, Niwa R, Uchida K, Satoh M, Shitara K. Fucose removal from complex-type oligosaccharide enhances the antibody-dependent cellular cytotoxicity of single-gene-encoded bispecific antibody comprising of two single-chain antibodies linked to the antibody constant region. J Biochem. 2006;140(3):359–68. doi:10.1093/jb/mvj157.
- Zeitlin L, Pettitt J, Scully C, Bohorova N, Kim D, Pauly M, Hiatt A, Ngo L, Steinkellner H, Whaley KJ, et al. Enhanced potency of a fucose-free monoclonal antibody being developed as an Ebola virus immunoprotectant. Proc Natl Acad Sci USA. 2011;108(51):20690–94. doi:10.1073/pnas.1108360108.
- Lu L, Palaniyandi S, Zeng R, Bai Y, Liu X, Wang Y, Pauza CD, Roopenian DC, Zhu X. A neonatal Fc receptor-targeted mucosal vaccine strategy effectively induces HIV-1 antigen-specific immunity to genital infection. J Virol. 2011;85(20):10542–53. doi:10.1128/JVI.05441-11.
- Jaworski JP, Kobie J, Brower Z, Malherbe DC, Landucci G, Sutton WF, Guo B, Reed JS, Leon EJ, Engelmann F, et al. Neutralizing polyclonal IgG present during acute infection prevents rapid disease onset in simian-human immunodeficiency virus SHIV SF162P3-infected infant rhesus macaques. J Virol. 2013;87(19):10447–59. doi:10.1128/JVI.00049-13.
- Euler Z, Alter G. Exploring the potential of monoclonal antibody therapeutics for HIV-1 eradication. AIDS Res Hum Retroviruses. 2015;31(1):13–24. doi:10.1089/aid.2014.0235.
- Cathcart AL, Havenar-Daughton C, Lempp FA, Ma D, Schmid M, Agostini ML, Guarino B, Di Iulio J, Rosen L, Tucker H, et al. The dual function monoclonal antibodies VIR-7831 and VIR-7832 demonstrate potent in vitro and in vivo activity against SARS-CoV-2. bioRxiv. 2021. doi:10.1101/2021.03.09.434607.
- Ullah I, Prévost J, Ladinsky MS, Stone H, Lu M, Anand SP, Beaudoin-Bussières G, Symmes K, Benlarbi M, Ding S, et al. Live imaging of SARS-CoV-2 infection in mice reveals neutralizing antibodies require Fc function for optimal efficacy. bioRxiv. 2021. doi:10.1101/2021.03.22.436337.
- Winkler ES, Gilchuk P, Yu J, Bailey AL, Chen RE, Chong Z, Zost SJ, Jang H, Huang Y, Allen JD, et al. Human neutralizing antibodies against SARS-CoV-2 require intact Fc effector functions for optimal therapeutic protection. Cell. 2021;184(7):1804–1820.e16. doi:10.1016/j.cell.2021.02.026.
- Liu Z, Xu W, Xia S, Gu C, Wang X, Wang Q, Zhou J, Wu Y, Cai X, Qu D, et al. RBD-Fc-based COVID-19 vaccine candidate induces highly potent SARS-CoV-2 neutralizing antibody response. Signal Transduct Target Ther. 2020;5(1):282. doi:10.1038/s41392-020-00402-5.
- He Y, Li J, Li W, Lustigman S, Farzan M, Jiang S. Cross-neutralization of human and palm civet severe acute respiratory syndrome coronaviruses by antibodies targeting the receptor-binding domain of spike protein. J Immunol. 2006;176(10):6085–92. doi:10.4049/jimmunol.176.10.6085.
- Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8(1):34–47. doi:10.1038/nri2206.
- Du L, Zhao G, Chan CC, Sun S, Chen M, Liu Z, Guo H, He Y, Zhou Y, Zheng B-J, et al. Recombinant receptor-binding domain of SARS-CoV spike protein expressed in mammalian, insect and E. coli cells elicits potent neutralizing antibody and protective immunity. Virology. 2009;393(1):144–50. doi:10.1016/j.virol.2009.07.018.
- Winkler ES, Gilchuk P, Yu J, Bailey AL, Chen RE, Zost SJ, Jang H, Huang Y, Allen JD, Case JB, et al. Human neutralizing antibodies against SARS-CoV-2 require intact Fc effector functions and monocytes for optimal therapeutic protection. bioRxiv. 2020. doi:10.1101/2020.12.28.424554.
- Chenoweth AM, Wines BD, Anania JC, Mark Hogarth P. Harnessing the immune system via FcγR function in immune therapy: a pathway to next-gen mAbs. Immunol Cell Biol. 2020;98(4):287–304. doi:10.1111/imcb.12326.
- Asokan M, Dias J, Liu C, Maximova A, Ernste K, Pegu A, McKee K, Shi W, Chen X, Almasri C, et al. Fc-mediated effector function contributes to the in vivo antiviral effect of an HIV neutralizing antibody. Proc Natl Acad Sci USA. 2020;117(31):18754–63. doi:10.1073/pnas.2008236117.
- Goulet DR, Atkins WM. Considerations for the design of Antibody-Based Therapeutics. J Pharm Sci. 2020;109:74–103. doi:10.1016/j.xphs.2019.05.031.
- Eroshenko N, Gill T, Keaveney MK, Church GM, Trevejo JM, Rajaniemi H. Implications of antibody-dependent enhancement of infection for SARS-CoV-2 countermeasures. Nat Biotechnol. 2020;38(7):789–91. doi:10.1038/s41587-020-0577-1.
- Ramadhany R, Hirai I, Sasaki T, Ono K, Ramasoota P, Ikuta K, Kurosu T. Antibody with an engineered Fc region as a therapeutic agent against dengue virus infection. Antiviral Res. 2015;124:61–68. doi:10.1016/j.antiviral.2015.10.012.
- Correia IR. Stability of IgG isotypes in serum. MAbs. 2010;2(3):221–32. doi:10.4161/mabs.2.3.11788.
- Muhammed Y. The best IgG subclass for the development of therapeutic monoclonal antibody drugs and their commercial production: a review. Immunome Res. 2020;16:173. doi:10.35248/1745-7580.20.16.173.
- Aalberse RC, IgG4 SJ. breaking the rules. Immunology. 2002;105(1):9–19. doi:10.1046/j.0019-2805.2001.01341.x.
- Wang S, Peng Y, Wang R, Jiao S, Wang M, Huang W, Shan C, Jiang W, Li Z, Gu C, et al. Characterization of neutralizing antibody with prophylactic and therapeutic efficacy against SARS-CoV-2 in rhesus monkeys. Nat Commun. 2020;11(1):5752. doi:10.1038/s41467-020-19568-1.

