?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
As of September 2021, 117 COVID-19 vaccines are in clinical development, and 194 are in preclinical development as per the World Health Organization (WHO) published draft landscape. Among the 117 vaccines undergoing clinical trials, the major platforms include protein subunit; RNA; inactivated virus; viral vector, among others. So far, USFDA recognized to approve the Pfizer-BioNTech (Comirnaty) COVID-19 vaccine for its full use in individuals of 16 years of age and older. Though the approved vaccines are being manufactured at a tremendous pace, the wealthiest countries have about 28% of total vaccines despite possessing only 10.8% of the total world population, suggesting an inequity of vaccine distribution. The review comprehensively summarizes the history of vaccines, mainly focusing on vaccines for SARS-CoV-2. The review also connects relevant topics, including measurement of vaccines efficacy against SARS-CoV-2 and its variants, associated challenges, and limitations, as hurdles in global vaccination are also kept forth.
1. Introduction
The immune system (IS) protects our body against infectious diseases and maintains a key role in health and pathogenesis.Citation1 Our IS includes innate as well as acquired immunity types. Innate immunity is developed in an individual since birth. However, acquired immunity is gained or acquired by the body over time. It is further divided into two subtypes, active and passive immunity. Active immunity develops when one is in contact with the pathogen or its antigen. It is mediated by humoral or cell-mediated responses provoked by our body.Citation2 Nevertheless, acquired immunity can be harmonized passively by using vaccines or antibodies from outside and is called passive immunity. Vaccines are made of antigens derived from pathogens that are known to cause disease. Although these antigens have lower virulence than the original, they may provoke the immune system to recognize them and develop antibodies against those antigens. Thus, it protects the body from the disease caused by that actual pathogen in the future. This process of vaccinating our body deliberately to produce a similar immune response for the original disease is called artificially acquired immunity.Citation3 In a similar context, a virus, SARS-CoV-2, identified for the first time on December 31, 2019, has created havoc with associated mortality across the globe. Although, earlier, the SARS-CoV-2 was thought to have a natural origin,Citation4 a piece of equivocal evidence suggesting artificial manipulation of the virus is also coming up recently.Citation5,Citation6 Keeping the debate aside, the primary concern is the novelty that this virus possesses, leading it to escape from the alert immune system of the human body. Once it invades the human body, it primarily targets the lower respiratory system and induces a rapid local immune response, cascading a series of events that eventually damage vital organs and fragile body parts.Citation7,Citation8 Therefore, the current knowledge related to COVID-19 suggests that the IS plays a dual role in elevating or decreasing the severity of this disease. Thus, to prevent the progression of this disease, the immune system needs to be revisited and may be targeted or modulated by the vaccines alongside therapeutic drugs repurposed against COVID-19 for better outcomes.
The current review is a holistic approach to congregate information on the history of vaccines, mainly focusing on vaccines against SARS-CoV-2, those approved by regulatory agencies. The comprehensive review also covers approaches on vaccine design, their mechanistic insights to decipher how they activate the immune system and induce immunity to the host. The review forth sees the numerous platform(s), including DNA (e.g., nCoV vaccine by Zydus Cadila and INO-4800); RNA (e.g., Moderna COVID-19 vaccine, Comirnaty, and CVnCoV); virus-like (e.g., CoVLP and RBD-HBsAg-VLPs-Covid vaccine); viral vector (e.g., COVID-19 Vaccine AstraZeneca, Convidecia, and Sputnik V); pathogen in an inactive form (e.g., protein subunit (e.g., NVX-CoV2373 and SCB-2019); nasal drip, and attenuated virus type employed for the current vaccines’ development against SARS-CoV-2. Moreover, the review is expanded to touch upon relevant topics on measurement of vaccines efficacy, efficacy against the mutant strains, the toxicological analysis conducted so far, and their outcomes, and the evolving paradigm on equitable distribution of vaccines, international collaboration(s) and upcoming IPR issues as hurdles in vaccination of the Globe during the current pandemic. The review is expected to enrich the biologists, immunologists, biotechnologists, chemists, or researchers working in similar and allied areas with an updated insight on the topic of extreme relevance in the current scenario.
2. History of vaccines: imagining the breakthroughs before the SARS-CoV-2 pandemic
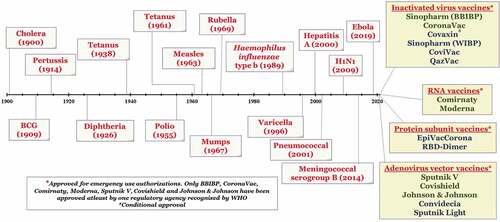
The history of vaccine development goes back to the era of infectious diseases acquired by the human population. The first evidence was witnessed by the work of a Persian physician against combating measles. Additional evidence suggests that the Chinese utilized smallpox inoculation as early as 1000 CE and used them for use against smallpox infection in humans. Research by Edward Jenner in 1796 on cowpox material to develop smallpox vaccines revolutionized this area of vaccine development. This was followed by the immense contribution of Louis Pasteur for the development of the rabies vaccine in 1885. This led to the dawn of bacteriology developments providing the world antitoxins and vaccines. The world saw a crucial revolution in developing effective vaccines against tetanus, anthrax, diphtheria, tuberculosis, plague, typhoid, and cholera until the late 1930s. The mid-20th century has witnessed a tremendous rise in vaccine development, including the vaccines for polio, measles, mumps, and rubella.Citation9,Citation10 The timelines for the discovery of essential vaccines (1900 till date) have been represented in .
Figure 1. Timeline showing some important vaccines developed from 1900 till date. So far, 15 vaccines have got emergency use approval for SARS-CoV-2.
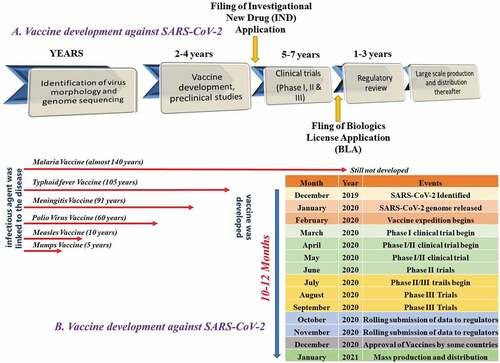
The vaccines development process for SARS-CoV-2 has witnessed rapidness, unlike traditional vaccine development.Citation7 Traditional methods for most vaccine development may take about 15 years to complete. It proceeds with the preliminary work of vaccine design, followed by their in vivo studies on animals to understand safety and efficacy parameters taking almost 6 to 7 long years. This is further followed by preclinical toxicological studies lasting again for approximately 2–4 years. Suppose the efficacy is maintained with no concerning toxic effect. In that case, IND application is filed, and vaccine candidates proceed to clinical trials to determine dosing and study immunogenicity. Suppose things proceed exceedingly well with prerequisite efficacy. In that case, regulatory agencies are approached for filing a biologics license after phase 3. The traditional development is both a costly and timely affair with a significantly less probability of success. However, with the COVID-19 pandemic, the World has witnessed a tremendous improvement in vaccine development in a short period. The major underlying factors accounted for are the progress in biological sciences in previous outbreaks of the Middle East respiratory syndrome coronavirus (MERS-CoV) and SARS-CoV infections, which possess a similar invasion and replication mechanism in humans as SARS-CoV-2 (mechanism has dealt in details in reviewCitation7,Citation8). Typically, antibodies that interact and bind with spike proteins (receptor-binding domains) of coronaviruses and prevent their attachment to human receptors (Angiotensin-converting enzyme or ACE2 in case of SARS-CoV-2) are identified for vaccine development against them. So, during the outbreak of COVID-19 much information was generated about spike protein binding and immune system response that generated antibodies plausibly neutralizing the virus. Unlike traditional vaccine development, where each clinical trial-phase completion is preceded by another, vaccine development against SARS-CoV-2 involves simultaneous overlapping of phases for rapid vaccine development. Moreover, the rapid development of COVID-19 vaccines was also the result of CEPI (Coalition for Epidemic Preparedness Innovation) that came into existence in 2015 with the Ebola outbreak. CEPI ensures the acceleration for vaccine development against emerging pathogens via a close collaboration between public, private, and civic society. Before COVID-19 vaccines, only mumps vaccine was developed within a span of 4 years. No vaccines were even developed against SARS-CoV after 17 years genome sequencing (2003) was done. The same applies to MERS, where no vaccine is developed in a span of 6 years after the genome of MERS was sequenced in the year 2013. However, we have already seen 15 vaccines are launched against SARS-CoV-2 disease.Citation11 The genome was sequenced in January 2020, and the first vaccine batch (mRNA-1273) was put into testing in February 2020, and in March 2020, the first clinical trial was launched.Citation12 So, in a nutshell, the advancement of understanding of previous outbreaks like MERS-CoV and SARS-CoV and their high similarity with SARS-CoV-2 led to rapid vaccine developments against COVID-19. A typical vaccine development illustrated protocol is outlined in .
Figure 2. The illustration represents the process for (a) traditional vaccine development and (b) accelerated vaccine development in the COVID-19 pandemic against SARS-CoV-2 shown as compared to the traditional vaccine development timeline for malaria, typhoid, meningitis, polio, measles, and mumps.
3. Approaches for vaccine design
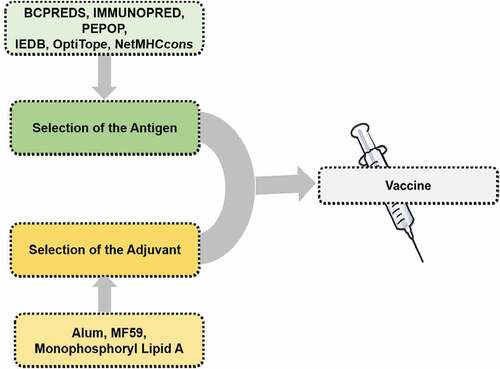
The composition of the rationally designed vaccine includes antigen, adjuvants, and delivery system. A successful vaccine design is often not always easy, as sufficient knowledge of the protection mechanism is always known. Our understanding of the immune mechanism to fight against the pathogen is broadly established.Citation13 However, specific contributions of different effectors and detailed mechanisms are known only for a few pathogens. Moreover, a particular pathogen also contains many antigenic entities. Among these, the selection of the best possible epitopes is still a challenge in vaccine development.Citation14 Therefore, the vaccine development strategies still lack universally acceptable methods, and we generally rely on empirical approaches to vaccine development. These processes are tedious, time-consuming, and costlier, which require lots of infrastructure and human efforts. However, the general direction toward vaccine development is as discussed below.Citation15
Antigen selection is the most essential and critical step in vaccine development. In the modern era, because of several advancements, the traditional and time-consuming methodology of antigen selection has now been replaced by modern approaches, viz., high throughput screenings, in silico, and genomics/proteomics related profiling techniques.Citation15 Most importantly, the antigenic protein selected must contain an appropriate epitope for B cell receptors (BCRs), should also be suitable for MHC complex within T cell receptors (TCRs) entities. In this case, sometimes synthetic peptides are cost-effective and can be considered an important starting point in vaccine design. Such a form of the peptide-based vaccine is handy in the pandemic situation. With this approach, large-scale production is quite possible.Citation15 Moreover, this approach facilitates the exposure of only a limited and required antigen, excluding unnecessary exposure of allergens and thus enabling us to avoid side effects and autoimmune responses. Identifying a perfect antigenic peptide can be easily facilitated by computational prediction methods and in silico approaches. Moreover, some automated tools for synthesizing such a long-chain peptide are still considered as an approach in vaccine development.Citation15 The perfect fit of the BCR epitope is also considered very important, and for that scientific approaches are again challenging. It can be realized from the fact that in the majority of the cases, the crucial part that is associated with the epitope is fully grown 3D protein is represented by the distinctively situated fractions of the primary structure of the protein. Therefore, the peptide chain and its proper folding are crucial for such a form of peptide-based vaccine development approach. However, concerning this scenario, some prediction tools, such as BCPREDS, IMMUNOPRED, and PEPOP are the unique approaches concerning such vaccine-related research and development.Citation16 Moreover, extensive data handling and automatization seem very important in vaccine development. Therefore, machine learning (ML) and artificial intelligence (AI) have now emerged as essential tools for the development of vaccines against diverse pathogens, including COVID-19 disease. ML/AI has provided numerous methodologies, such as gradient boosting decision tree, deep neural network, and artificial neural network, that may assist in predicting the most appropriate epitopes in the vaccine development process.Citation17,Citation18 One of its successful examples is the work of Fast et al.Citation19 that disclosed the use of artificial neural networks, namely MARIA and NetMHCPan4 and identified the B-cell and T-cell epitopes of SARS-CoV-2 virus. Using this method, the research group registered success in identifying 405 T-cell epitopes with a very promising presentation score for MHC-I/II and a couple of neutralizing B-cell epitopes located on the S protein. In another study by Ong et al.Citation20 the group disclosed the bioinformatics tools to explore the prospective application of the non-structural protein as a vaccine candidate for developing preventive measures against COVID-19 disease. Similarly, according to Yang et al., DeepVacPred,Citation21 i.e., the deep neural network-based approach also seems very promising in the prediction of multi-epitope for the development of a vaccine against the COVID-19 disease. Considering these facts, ML/AI is also a fascinating technique that seems very rationale and can facilitates the development of a vaccine against several infections, including COVID-19 disease.
Next, generating and controlling the immunological response is not a simple phenomenon, and the role of MHC is also considered very critical in this case. Herein, the protein or peptide that has been designed and used in vaccine development should also display some sequence that works best with the MHC. Therefore, the immunogenic peptide selection according to MHC is still challenging. Herein, in vitro assay-based, TCR epitope identification approach is a critical approach; however, it faces highly time-consuming, cost-related difficulties.Citation15 Therefore, in this case, several in silico strategies are developed to facilitate such research and development within a reasonable time and cost-effectively. Some of the actual databases and tools used herein include IEDB,Citation22 OptiTope,Citation23 and NetMHCcons.Citation24
To further enhance the protective immunity of the vaccine, adjuvants are essential starting points for the development of the COVID-19 vaccine. There are two significant effects of using adjuvants, viz boosting immunogenicity and reducing the vaccine protein per dose, that we can witness with the usage of adjuvants in vaccine-based formulations.Citation25 The examples of the adjuvants are diverse that include microbial products, saponins, microparticles, liposomes, mineral salts, and many more. In this context, two different categories of vaccine adjuvants are immunostimulants and delivery agents; herein, former categories include cytokines, saponins, Toll-like receptor (TLR) agonists, and the latter category adjuvants include emulsions, microparticles, mineral salts, and other similar entities.Citation26 The central role of immunostimulants is to activate the antigen-presenting cells and cytokines secretions. In contrast, delivery agents facilitate the proper delivery of antigen and the controlled release of the antigen for producing an appropriate immune stimulations response. Saponins, steroids, triterpenoid glycosides from the plant, animal, and marine sources are the most critical example, shows significant immunostimulatory properties.Citation25 So far, adjuvants like alum, MF59, AS03, CPG 1018 have been used for the development of COVID-19 vaccines. These are known to provoke distinctive immunological profiles, thus eliciting the synergistic effect. The primary molecular targets affected by the adjuvants licensed vaccines so far may be grouped under three heads, namely, Toll-like receptors (TLR), Cytosolic pattern recognition receptors (PRRs), and C-type lectin receptors (CLRs).Citation27 They control the quantity and quality of humoral response along with a cellular response by producing a high amount of INFγ and IL-12 in case of TH1 and no production of IL-12 in case of TH2 response.Citation28,Citation29 In addition to containing the genetic information of immunogenic proteins, DNA and RNA vaccine also act as adjuvant and are recognized by various PRRs (Pattern recognition receptors) which initiate signaling transduction to activate the immune response.Citation30 Immunological ligands present on antigen molecules are identified by PRRs to be presented on antigen-presenting dendritic cells in the antigenic milieu and internalized for antigen processing and presentation and associated with adaptive immune response. PRRs such as TLRs, nucleotide-binding oligomerization domain (NOD) like receptor (NRLs), and retinoic acid-inducible gene −1 like receptor (RLRs), which may be cytosolic (endomembrane) and present on the cell surface of APCs bind to microbial origin PAMPs such as proteins, lipids, nucleic acids, and carbohydrates. Interaction between PRRs and PAMPs triggers complex cascades of intracellular signaling, resulting in various chemokines, cytokines, and type 1 interferons (INFs).Citation31 Maturation of dendritic cell after antigen recognition leads to its internalization stimulated by chemokines and cytokines signals and finally drain them to the lymph node. The dendritic cells in the lymph node further present antigen to naive T cells via MHC-I and MHC-II molecules and also activate B-lymphocytes. Recognition of processed antigen with MHC by T cell Receptor (TCR) and associated with other costimulatory molecules CD28 and B7 induced activation and proliferation of T cell. The cytokines secretion pattern by dendritic cells decides the subset of T cells as secretion of IL-12 produce TH1 cell which secretes INFγ and is effective against intracellular viral and bacterial pathogens. IL-4 production leads to TH2 response, and IL-4 along with other cytokines leads to humoral response. Activation of B cell taking directly as B cell receptor (BCR) bind to epitopes on antigenic site of vaccine and start to proliferate and converts to antibody-secreting plasma cell. B-cell activation also takes place with T cell’s help that induced effector function and memory response.Citation32,Citation33 Hence, along with antigen, adjuvant selection is also considered critical in vaccine development. Some of the adjuvants their immunogenic impacts and utility in current COVID-19 vaccines are presented in .
Table 1. Adjuvants their immunogenic impacts and utility in present COVID-19 vaccines
A brief outline of vaccine development is provided in .
Once the vaccines are developed, their production initiates. Vaccines are regulated primarily as biologics. In the United States, the regulation is governed by USFDA (U.S. Food and Drug Administration) and CBER (Center for Biologics Evaluation and Research). In European countries, it is governed by the EMA (European Medicines Agency). Harmonization of licensing and regulating between the FDA and EMA ensures safe and effective vaccine delivery to the market. Vaccine manufacturing, in general, is a complex process.Citation34 Once the vaccine is approved by regulators firm, and there are 11 long essential steps taking between 6 and 36 months overall, out of which 70% of the time is dedicated to quality control.Citation35 The process initiates via a. antigen development; b. harvesting; c. purification; d. inactivation; e. vaccines are assembled; f. formulation; g. filling; h. freeze-drying; i. packaging; j. batch release; and k. transport.
The first step, i.e., antigen development, is achieved by growing the viruses (or other infectious pathogens) primarily via using continuous cell lines or chicken fibroblasts. Thereafter, the desired antigens are harvested, and the purification of specific antigens is done. The next step involves inactivation, where the pathogenicity is suppressed, and the immunological properties of antigens are retained. Furthermore, all the antigenic components are combined as a single unit, and the formulation is achieved using desired adjuvants. Once formulated, the vaccines are filled, and lyophilization is achieved for better stability. The lyophilized vials are packed, and the batches are released after stringent quality control before final transportation. Packaging and transportation are also critical steps, particularly for the vaccines that maintain their stability at shallow temperatures. Among COVID-19 vaccine candidates launched so far, Moderna COVID-19 vaccine requires storage conditions of 20°C, Comirnaty requiring a temperature of −70°C, whereas Covishield and Sputnik V require standard refrigeration.
4. A mechanistic overview of vaccines in development immunity
Developing a safe and efficacious vaccine against infectious pathogens prevents disease incidence. It prolongs life expectancy by decreasing morbidity and mortality. A highly immunized population provides herd immunity and helps eradicate infectious disease globally.Citation36 Live attenuated, inactivated, subunit, recombinant, DNA, RNA, and conjugated vaccines are used clinically to prevent many infectious diseases. The understanding of mechanistic vaccination insights, i.e., the interplay between innate and adaptive immune response by vaccine molecules, immensely assist in developing safe and efficacious futuristic vaccines.Citation37 The main goal is to initiate an early innate immune response and develop both humoral and cellular responses very similar to our immune system to fight against infectious pathogens. The mechanism of the vaccine is to develop immunity include processing, presentation, and activation of both B and T lymphocytes.Citation29 The Live attenuated and Inactivated vaccine contains the whole antigen and the subunit vaccine (protein-based) PAMP and other immune receptors/epitopes. In contrast, other vaccines may or may not have PAMP.Citation37 Vaccines injected inside the body by various routes bring the cellular and soluble components, such as neutrophil, microphage, dendritic cell, cytokines, chemokines, and other inflammatory responses to interplay and initiate and activate an innate immune response. Further, the vaccines reported (platforms discussed in the next section) against SARS-CoV-2 also trigger similar mechanism(s) as discussed in the subsections. RNA vaccines mechanistically act by introducing an mRNA sequence, which is further encoded by host transcriptional machinery to a specific antigen. mRNA vaccines instruct the host cell for protein synthesis that is further utilized in immunity development. Protein-based vaccines mimic the virus proteins once inside the host cells and are utilized therefore for antibodies development, conferring immunity.Citation38 The PS vaccine development has utilized recombinant technology for synthesizing the most suitable protein antigens capable of eliciting strong immune responses in the host. PS vaccines incorporate harmless S protein derived from SARS-CoV-2, which is recognized by the immune system. It allows the immune system to create antibodies and upgrades the B and T cells to assist them during actual infection. In contrast, RNA or mRNA vaccines contain the codon-optimized sequences or RNA of a pathogen encoding for the desired protein, in general S-protein in the case of COVID-19 vaccines. This vaccine utilizes newer nucleic acid-based technology that applies a predefined amount of the antigen to the vaccine. Once vaccinated, it delivers instructions to the translatory machinery to synthesize and develop S protein fragments and, in the process, get itself degraded and never enters the nucleus of the cell. The S protein is further recognized by the immune system and prepares them to invade the possible infection with the original strain of SARS-CoV-2.Citation39 Viral vector vaccines employ genetically engineered virus DNA that instructs the host cells to produce proteins that are further deployed for immunity development. The last category, i.e., inactivated or weakened virus vaccine, utilizes inactive or weak virus that is further recognized by our immune system and confers immunity.
5. Platforms explored for the vaccine development in COVID-19
Broadly, vaccines are categorized into two main types: live (attenuated) or non-live (inactivated), concerned with the replicating strains of the pathogenic organism or only a component of the pathogen or whole pathogen in dead or killed form. The live vaccines are typically restricted to the population with immunocompromised status (HIV patients or those on immunosuppressive drugs), since these vaccines can elevate the immune response in an uncontrolled manner via their replication. At the same time, inactivated vaccines pose no threat to immunocompromised individuals.Citation40
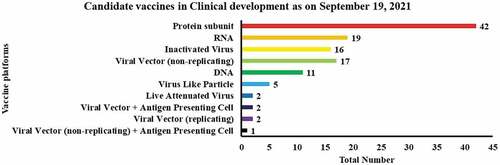
The vaccines against SARS-CoV-2 are developed using, a. using Receptor Binding Domain (RBD) of spike protein (also includes recombinant spike protein and RBD-based vaccine); b. inactivated vaccines are grown in a cell culture containing SARS-CoV-2 (chemically inactivated); c. attenuated vaccine from the weakened genome of the virus; d. Virus-like particles (VLPs) displaying spike protein on their surface with no genome; e. Replication-incompetent vector vaccine that does not propagate within the vaccinated cells but only expresses spike proteins; f. Replication competent vector vaccines that can be propagated within the vaccinated cells and express spike proteins; g. Inactivated virus vaccines with copies of spike protein and chemically inactivated; h. DNA vaccines containing the DNA plasmid that could encode spike genes by interacting with the mammalian promoter; i. RNA vaccines have RNA that could encode spike protein within mammalian cells.Citation41 As of September 2021, 117 COVID-19 vaccines are in clinical development, and 194 are in preclinical development as per the World Health Organization (WHO) published draft landscape. Among the 117 vaccines undergoing clinical trials, the major platforms include protein subunit (42); RNA-based (19); Inactivated virus-based (16); Viral vector-based (17), among others ().
Figure 4. The graph highlights the vaccines platforms currently in clinical trial utilized in the quest for SARS-CoV-2 vaccine development.
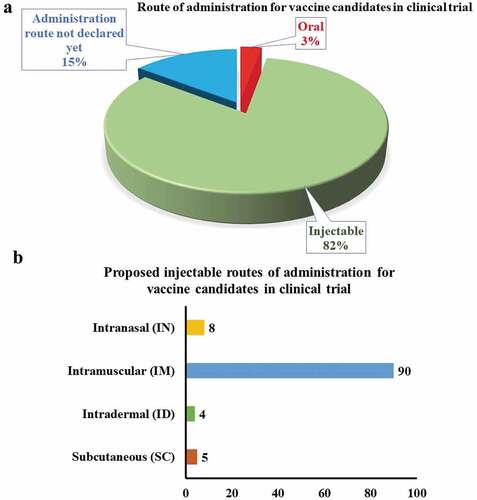
The proposed vaccines are intended to be administered via oral (3%) or injectable routes (82%) (). The central route for the latter category () includes the intradermal, intramuscular, intranasal, or subcutaneous route.
6. Current COVID-19 vaccines and their status so far
This section includes a discussion on COVID-19 Vaccines developed using platforms like RNA, DNA; Protein subunit; Viral Vector; Virus-like particles, and Live-attenuated type.
6.1. RNA vaccines
As RNA encodes for the genetic information for protein synthesis and, RNA-based immunization is therefore also anticipated the expression of proteins involved in the immunization process. In 1990, the proof of concept was provided while experimenting with an RNA, i.e., once the murine model was inoculated with the mRNA, the desired immunogenic response was reflected in the same.Citation42,Citation43 Since then, several studies have demonstrated the ability of RNA to initiate both T-cell mediated and antibody-mediated immune responses.Citation44 In contrast to DNA, RNA does not require the transcription process, and therefore, directly reaches the cytoplasm to induce translation of the desired antigenic protein.Citation45 Additionally, RNA molecules do not show host genome integration, which is a significant drawback of DNA-based formulations.Citation46
Additionally, RNA-vaccine formulation also presents an intrinsic adjuvant property, but the relative stability of the formulation is a significant issue in development. Therefore, several strategies have been utilized to improve the RNA delivery in a stable form, including lipid encapsulation of RNA molecules or polymer-based nanoparticle formulation,Citation47 microinjections, protamine condensation, RNA adjuvants, RNA patches, and in vitro transcribed (IVT) mRNA in a complexing agent.Citation48 Moreover, being a versatile platform, RNA can be inoculated via different routes such as intramuscular, intravenous, subcutaneous, intranodal, intradermal, and intrasplenic routes, and gene gun method.Citation44 In general, RNA vaccines are of two types: (i) Conventional mRNA or non-amplifying mRNA (simplest form), and (ii) RNA replicons or self-amplifying mRNA containing viral positive-stranded RNA vaccine. The conventional mRNA vaccine consists of a desired, smaller-sized RNA molecule, usually processed through gene sequencing followed by synthesis, cloning into a DNA plasmid, and in vitro transcription. Once vaccinated with non-amplifying mRNA, it immediately undergoes cellular internalization and initiates protein translation using the host machinery to produce desired, encoded antigenic protein.
On the other hand, replicons comprise engineered RNA (~10 kb) that encodes for the factors (e.g., RNA polymerase) necessary for the amplification of RNA within a target cell. In general, replicons are more effective as compared to the non-amplifying mRNA, as replicons can produce a high level of antigen expression via the encoded sub-genomic RNA.Citation49 These replicons can produce virus-like particles when provided in trans cell culture.Citation50 Interestingly, replicons can be designed to deliver multiple RNA vectors simultaneously, say gene encoding for the antigen of interest and replicate in a single formulation.Citation51 It offers efficient manufacturability, ease of optimization, and a better safety profile. Considering the self-amplifying nature, replicons can exhibit a prolonged and higher antigen expression following a lower dose.Citation52 However, the manufacturing and stability of the replicons is a significant problem in this type of vaccine too.
Several pieces of research have aimed to enhance the stability and delivery of both the RNA vaccine formulations. For conventional mRNA vaccines, the incorporation of 5ʹ-cap or 3ʹpoly-A sequences, the derivatization of RNA with pseudouridine, and decontamination by chromatography has shown improved vaccine stability and delivery of the vaccine.Citation44 Nevertheless, considering the greater flexibility in antigen manipulation and rapidness in manufacturing, RNA presents itself as a potential platform for developing promising COVID-19 vaccine candidates. As evident, the Moderna COVID-19 vaccine was the first vaccine candidate to enter clinical trials within just 10 weeks following the genome sequencing of SARS-CoV-2. As of September 19, 2021, a total of 24 out of 194 and 19 out of 117 vaccine candidates based on the RNA platform are undergoing pre-clinical and clinical trials, respectively.
RNA-based vaccines have advantages, such as rapid synthesis (thus suited for modified vaccination), preclusion of integration with the host genome, potent activation of innate immune response employing various cellular pathways, including endosomal and cytosolic receptor/sensor pathways (such as TLR3, TLR7, NLRP3, NOD2), possible potentiation of B-cell mediated immunity, and intrinsic adjuvant effect.Citation44 However, NLRP3 activation could precipitate various disease conditions that include diabetes (Type 2), Alzheimer’s, Prion, including other infectious diseases. The specific activation mechanism of NLRP3 inflammasome is still unequivocal, but the stimulus associated DAMPs (danger-associated molecular patterns) which includes uric acid, silica, and PAMPs are commonly associated with its activation.Citation53
It is also suggested that the RNA vaccine imitates acute infection concerning antigen-specific rapid immune responses that recede fast.Citation54 By virtue of intrinsic immunostimulatory properties, RNA vaccine acts as an optimal adjuvant (in addition to pathogen-specific immunogen) that activates innate immunity without causing systemic inflammation, thus lacking severe side effects. Current RNA vaccines constitute purified in vitro-transcribed ss-mRNA with altered nucleotides that results in minimized binding to TLR/immune sensors, consequently restricting unnecessary or extreme production of type I interferon.Citation39
6.1.1. Representative RNA vaccines
6.1.1.1. Moderna COVID-19 vaccine or mRNA-1273
This vaccine was developed as a result of a joint venture between the National Institute of Allergy and Infectious Diseases (NIAID) and Moderna, Inc. Moderna COVID-19 vaccine (earlier mRNA-1273), which was the first candidate to enter the clinical trials for the assessment of safety and immunogenicity profile.Citation44 This vaccine contains the genes encoding for the sequence of pre-fusion stabilized S protein that provokes the production of antigenic proteins to induce an immune response within the host. Peak antibody response (usually dose-dependent) was observed on day 15 following the first dose.Citation55 Neutralizing antibodies were found to be in the detectable range, particularly in half of the recipients after the first dose and in all recipients after the second injection. It inferred the necessity of a two-dose regimen. CD4+ response was observed with 25 μg and 100 μg dose cohort, and a reduced CD8+ response with the subsequent vaccination with 100 μg dose.Citation55 On May 11, 2020, mRNA-1273 was granted with fast-track designation by the FDA.Citation44
Available information on the profile of Moderna COVID-19 vaccine, a ready for use vaccine, suggests it remains stable at −20°C (−4°F) for up to 6 months during shipping or for long-term storage. However, once thawed, the vaccine will remain stable under standard refrigeration conditions (2°C to 8°C) will keep the product stable for ~30 days with a shelf life of 6 months.Citation44
For administration purposes, recent stability data (published on April 1, 2021) recommends keeping the vaccine at room temperature for 24 hours (previously 12 hours) once removed from the refrigeration. Furthermore, a punctured vial can be used up to 12 hours (earlier 6 hours). Moreover, the Moderna vaccine is not required to be kept at ultra-cold temperature owing to its specific lipid nanoparticles formulation, properties, and structure. The qualitative composition of Moderna vaccine formulated as mRNA-lipid nanoparticles (LNPs) follow as SM-102 (ionizable lipid), PEG2000-DMG = 1-monomethoxypolyethyleneglycol-2, 3-dimyristylglycerol with polyethylene glycol of average molecular weight 2000 1, 2-Distearoyl-sn-glycero-3 phosphocholine cholesterol with Tris (tromethamine) buffer and sodium acetate, sucrose, and water.Citation56 The FDA now authorized the Moderna COVID-19 vaccine in two multi-dose vial presentations (i.e., 11 or 15 doses in a single vial).Citation44 Nevertheless, evidence of long-term immunogenicity and safety concerns of the vaccine is anticipated.
6.1.1.2. Comirnaty or BNT162b2
A collaborative approach from BioNTech and Pfizer led to the development of four mRNA-based vaccines (viz. BNT162a1, BNT162b1, BNT162b2, and BNT162c2) containing separate mRNA that encodes for a distinct antigenic protein.Citation44 Among these, BNT162b2 comprising of LNP-encapsulated nucleoside-modified mRNA encodes for the viral S-protein was considered for further development, owing to its satisfactory immunogenicity and tolerability profile.Citation57
In a placebo-controlled, randomized, phase 2/3 trial (NCT04368728), BNT162b2 was found to be ~95% effective among individuals with a history of no previous or existing infection.Citation58 As evident from the large trials, excellent results of BNT162b2 (brand name: Comirnaty) led to the granting of EUA in the UK.Citation59 Later on 21st December 2020, the European Commission (EC) approved a conditional marketing authorization (CMA) for immunization against COVID-19 in the population of ≥16 years of age.Citation59 It has now received CMA, EUA, or temporary approval in over 40 countries worldwide, including all the states of the EU.Citation59 Recent data from a pivotal phase 3 trial revealed the vaccine to be 100% and 95.3% effective against severe COVID-19 cases as described by the CDC and USFDA, respectively. Comirnaty remains stable for 6 months in an ultra-freeze condition (i.e., −80°C to −60°C) but shows short stability of 5 days when refrigerated. Despite this, BioNTech and Pfizer claimed to design dry ice-containing thermal shippers (temperature-controlled) with controlled temperatures within −70° to +10°C.
6.1.1.3. CVnCoV
With assistance from the German Federal government, CureVac has developed an mRNA vaccine known as CVnCoV to prevent COVID-19. The vaccine consists of non-chemically modified nucleotides,Citation60 which elicits desirable immune responses in the mice and hamster models. Phase 1 trial (NCT04449276) from Belgium and Germany showed CVnCoV to be safe, well-tolerated, and immunogenic in the participants.Citation61 A multicentre, phase 2a trial is enrolling in Peru and Panama to determine the safety and immunogenicity in 691 volunteers (age ranging from 18 to 60 years or >60 years) (NCT04515147). Similarly, a randomized, multicentre, placebo-controlled, phase 2b/3 trial is currently undergoing in Latin America and Europe to assess the efficacy and safety of CVnCoV in adults (NCT04652102). Additionally, another randomized, phase 3 trial evaluates the immunogenicity and safety of CVnCoV (2-dose schedule) in Germany (NCT04674189). Notably, the vaccine is expected to remain stable for a minimum of 3 months under refrigeration (+5°C or +41°F) and for ~24 hours at 25°C in ready-to-use form.
6.2. DNA vaccines
DNA vaccine involves the use of DNA plasmids as a vector to deliver fragments of the gene that are transcribed to encode immunogenic antigens within the host cells.Citation62 These vaccines importantly enable antigen presentation toward MHC molecules that further assist in T-cell recognition. The DNA vaccines development against COVID-19 is thought to strengthen the immune system responses comprising both including cellular and humoral. They are also thought not to interfere or arouse any imbalance of T cells or Tregs.Citation63
6.2.1. Representative DNA vaccines
In total, there are 10 DNA vaccines designed to combat the pandemic, primarily considering the S protein of the virus in the vaccine development. Among them, the nCoV vaccine by Zydus Cadila is in phase 3 trial.Citation64 INO-4800+electroporation by Inovio pharmaceuticals is another DNA vaccine that has undergone phase 2/3 trial stage.Citation65 Moreover, AG0301-COVID19 is yet another vaccine designed based on the two-immunization scheme in which two doses should be administered intramuscularly within a two-week interval.Citation65 Similarly, the Covigenix VAX-001 vaccine was developed by Entos Pharmaceuticals Inc. in collaboration with the Canadian Institute of Health Research.Citation66 To evaluate the efficacy of this vaccine, a clinical trial was performed on males and non-pregnant females of the age group 18 to <55 and 65 to <85 years. Like GX-19, this is also a phase 1/2 placebo-controlled, randomized, observer-blind, and dose-ranging. INO-4800 was designed by Inovio Pharmaceuticals.
6.3. Protein subunit (PS) vaccines
The “protein-based” or “subunit” vaccine comprises technologies to develop viral antigenic parts or fragments eliciting an effective immune response.Citation67 The PS vaccine development has used recombinant technology for synthesizing protein antigens. This approach has advantages over other platforms regarding the robust immune response, less severe adverse effects, less demanding in terms of production, storage, and transportation. Still, it demands optimization of adjuvant required for a more vigorous immune response.Citation67 Since recombinant PS vaccines are non-replicating, they are considered a safer approach. The principle lies in the uptake of recombinant viral antigen by the adjuvant-stimulated antigen-presenting cells (APCs) with the subsequent presentation to adaptive immune cells. This technology has been utilized and tested widely. The first example of the PS vaccine was anthrax protective antigen made in the 1960s and licensed in 1970; however, the PS vaccines for influenza remain famous.Citation67 Currently, the COVID-19 PS vaccines account for 33% of all vaccine candidates against COVID-19. Out of 28 PS vaccine candidates against COVID-19, six have already entered Phase 3 clinical trials.Citation68 These PS COVID-19 vaccine candidates are using different antigenic parts, mainly different versions of full-length structural spike protein or fragment of S protein (S1 domain) that mediates viral binding with angiotensin-converting enzyme 2 (ACE2) receptor of host cells or the receptor-binding domain (RBD). The S protein contains three domains: N-terminal outer domain with subunit S1 and subunit S2, C-terminal cytoplasmic domain, and a third transmembrane domain. The S protein has a trimeric structure with three S1 subunits (for viral attachment to host cells) placed on the top of the stem of three S2 subunits (for virus-cell membrane fusion). Receptor-binding motif (RBM) of RBD (residues 331–524) in the S1 subunit is precisely required for initial docking to ACE2. Following binding to the target cell surface ACE2 receptor and subsequent catalysis/priming through cellular transmembrane protease serine 2 (TMPRSS2) protease that leads to cleavage at a specific site, the S protein prefusion conformation is changed into a stable post-fusion conformation leading to the S2-mediated fusion of viral particle and the cell membrane.Citation69 The prefusion forms are usually more immunogenic, and therefore act as more attractive vaccine targets and are the strategic core of the COVID-19 vaccine.
6.3.1. Representative PS vaccines
6.3.1.1. NVX-CoV2373
Novavax, a USA-based biotechnology company, and the department of health and human services have developed a COVID-19 PS vaccine “NVX-CoV2373.” NVX-CoV2373 contains a stabilized trimeric full-length purified protein in a prefusion state engineered using the SARS-CoV-2 coronavirus spike (S) protein genetic sequence. A modified spike gene was inserted into baculovirus, allowed to infect Sf9 moth cells, which then expressed spike proteins spontaneously joined to form spikes. These spike proteins were then harvested from Sf9 cells and assembled into nanoparticles using Novavax-patented Matrix-MTM adjuvant and recombinant nanoparticle technology.Citation70 NVX-CoV2373 is stored at 2°C to 8°C, which makes it easier to distribute and store as compared to other vaccines, which need to be kept frozen. Results from Phase 1/2 study demonstrated that the vaccine candidate “NVX-CoV2373” formulated using Matrix-M induced a Th1-biased immune response when two inoculations on day 0 and 21 of two dosages, 5 and 25 μg, were performed, showing high antibody titers. Results demonstrated that NVX-CoV2373 is well-tolerated and can generate highly neutralizing antibodies against the virus.Citation71 Phase 3 clinical trial involving 15,000 volunteers aged between 18 and 84 (including 27% over the age of 65) in the United Kingdom (UK) was launched in September 2020. On December 28, 2020, the PREVENT-19 (PRE-fusion protein subunit vaccine Efficacy Novavax Trial-COVID-19), a randomized placebo-controlled observer-blinded Phase 3 study entered a large-scale clinical trial of 30,000 subjects 18 years of age and older. Phase 3 trial carried out in the UK released the interim results; the trial-tested two vaccine doses administered 3 weeks apart and reported 62 COVID-19 symptomatic cases of which 56 belonged to the placebo group, whereas six were in the vaccine group; and only one in the placebo group displayed severity, and 32 were with the UK strain. On January 28, 2021, it was announced that NVX-CoV2373 had met the primary endpoint with 89.3% vaccine efficacy in UK trials.Citation70 On March 11, 2021, Novavax reported that the UK trial demonstrated a 95.6% efficacy rate as effective against the WT SARS-CoV-2 strain, which is the highest among all vaccines with efficacy data so far.Citation72 NVX-CoV2373 has also initiated the pediatric expansion of the phase 3 trial, where its safety, efficacy, and immunogenicity will be evaluated in up to 3000 adolescents from 12 to 17 years in the US.Citation73 Till now, clinical data analysis has shown that minor occurrences of severe and medically attended adverse events advocate that Novavax is still better than other vaccines currently available.
6.3.1.2. SCB-2019
Another PS-based COVID-19 vaccine that has advanced into Phase 3 trial is “SCB-2019” by Clover Biopharmaceuticals Inc, a China-based biotechnology company.Citation74 SCB-2019 comprises of stabilized recombinant trimeric SARS-CoV-2 Spike protein (S) developed using a patented Trimer-Tag® technology (Clover Biopharmaceuticals, Chengdu, China) and a rapid mammalian cell-culture-based expression system (Chinese hamster ovary cells). The S-Trimer protein subunit vaccine has used the full-length WT SARS-CoV-2 spike protein (subunits S1 and S2) as the antigen and is formulated using two different adjuvants, ASO3 (oil-in-water emulsion) and CpG (TLR9 agonist) plus Alum, and it resembles the natural trimeric viral spike proteins configuration.Citation74
These formulations are stable at 2–8°C. Its clinical trials were started on June 20, 2020 (NCT04405908). The interim results from Phase 1, randomized, double-blind placebo-controlled trial reported that as compared to non-adjuvanted S-trimer protein, the vaccine containing S-Trimer protein formulated using ASO3 or CpG/Alum adjuvants, when given as two doses 21 days apart, stimulates robust cellular and humoral immune responses against SARS-CoV-2 that directly correlates with the high viral neutralizing activity. The results from this study advocate the use of 9 μg AS03-adjuvanted SCB-2019 and 30 μg CpG/Alum-adjuvanted SCB-2019 as preferred options to be suitable for phase 2/3 trials.Citation74
6.3.1.3. RBD-Dimer (ZF2001)
ZF2001 is a joint venture of the Institute of Microbiology of the Chinese Academy of Sciences (IMCAS), and Anhui Zhifei Longcome Biopharmaceutical has jointly developed a COVID-19 PS vaccine “ZF2001” using a tandem repeat dimeric RBD of the SARS-CoV-2 S protein as the antigen (residues 319–537), manufactured in CHOZN CHO K1 cell line. It is known that RBD is responsible for the engagement of ACE2 receptors, and therefore, targeting it would stimulate immune responses focusing on blocking receptor binding. Previously, many RBD-based vaccines have shown efficacy in animal models, and evaluation of various COVID-19 RBD-based vaccines is in progress.Citation75 To assess the safety and immunogenicity profile of ZF2001, two randomized, double-blind, placebo-controlled phase 1 and phase 2 trials began in China on June 22, 2020 and July 12, 2020, respectively. No vaccine-associated serious adverse events were reported in the Phase 1 trial that involved 50 participants. In Phase 2 trial involving 900 participants, 7 reported severe adverse events, viz. one in the 25 and 50 μg dose in two-dose schedule, one and two in the 25 and 50 μg dose in three-dose schedule, respectively, and two in the placebo group in the three-dose schedule; however, none were considered vaccine-associated. Thus, phase 1 and phase 2 trials observed that 25 and 50 μg doses of vaccination in two-dose or three-dose schedules are well-tolerated. Three-dose schedule performed at days 0, 30, and 60 demonstrated 93–100% seroconversion rate of neutralizing antibodies (97% in the 25 µg group and 93% in the 50 µg group), with the geometric mean titers (GMTs) exceeding the magnitude of convalescent serum samples obtained from RT-PCR-confirmed COVID-19 participants. Also, T-helper 1 and T-helper 2 cell-associated cytokines were found to be produced in a balanced proportion that advocates for a vaccine-mediated cellular immune response. Data from these trials indicated the use of the 25 μg dose in a three-dose schedule in an ongoing phase 3 clinical trial (NCT04646590).Citation76 A study by An et al. reported that ZF2001 protects mice and nonhuman primates (NHPs) by reducing viral RNA and relieving lung injury via inducing increased levels of RBD-binding and SARS-CoV-2 neutralizing antibody, as well as eliciting balanced T-helper 1 and T-helper 2 cell-mediated cellular responses.Citation76 In a small-sample lab study involving 12 serum samples from recipients of ZF2001, it was observed that ZF2001 retained neutralizing activity against the B.1.351 variant. However, its activity was weaker than compared with the original strain.Citation77 Now, the safety and efficacy of ZF2001 are being evaluated in the ongoing randomized, double-blind, placebo-controlled Phase 3 trials comprising of 29,000 participants aged 18 years and above, which were started on November 18, 2020. Out of the 29,000 participants, 750 participants aged 18–59 years and 250 participants aged 60 years and above, are scheduled to be enrolled in China; whereas 21,000 participants aged 18–59 years and 7000 participants aged 60 years and above, will be enrolled outside China. Safety and immunogenicity will be assessed among the Chinese participants, and efficacy, immunogenicity, and safety will be assessed among the participants outside China. To determine the immunogenicity, the IgG levels of SARS-CoV-2 neutralizing antibody, as well as RBD-binding antibody, will be analyzed by blood sampling prior to vaccination, 14 days, and 6 months following the entire vaccination procedure. The estimated primary completion date and study completion date is April 2021.Citation78
6.3.1.4. EpiVacCorona
Apart from the viral vector vaccine “Sputnik-V,” “EpiVacCorona” is Russia’s second vaccine developed by the Federal Budgetary Institution of Science Vektor State Research Center of Virology and Biotechnology. EpiVacCorona is a synthetic peptide vaccine that contains synthetic peptide antigens of the SARS-CoV-2 S protein conjugated to a carrier protein adsorbed on aluminum hydroxide adjuvant. Phase 1 and phase 2 clinical trials began on July-August 2020, and they consisted of two stages: stage 1 involved 14 participants aged 18–30 years, and it evaluated the safety, reactogenicity, and immunological activity; Stage 2 as single-blind, comparative, randomized placebo-controlled involved 86 participants aged 18–60 years using two intramuscular vaccine administrations spaced 21–28 days. A two-dose vaccine regimen elicited antibody production in 100% of the participants, and no signs of local or systemic adverse reactions were observed.Citation79 As per media reports, participants developed sufficient protective antibodies to last up to 6 months. Post-registration phase 3 clinical trials of EpiVacCorona began in November-December 2020. In a pre-clinical non-human study (adult male and female rats, including pregnant ones), it was demonstrated that EpiVacCorona does not possess embryotoxic properties and does not affect the offspring’s survival rate. The study has shown that EpiVacCorona, when administered twice at 260 μg dose, spaced 14 days apart, protects against the SARS-CoV-2 virus in hamsters, ferrets, and non-human primates (African green monkeys and rhesus macaques) by inducing virus-specific antibodies and accelerating the elimination of virus from upper respiratory tract.Citation79
6.3.1.5. UB-612
Apart from utilizing the native full-length S-protein or RBD as antigen, another approach is engineering multiepitope vaccines synthesized from peptides. US-based company COVAXX/Vaxxinity has developed a COVID-19 vaccine candidate, UB-612 that includes explicitly multiple epitopes, such as regions mimicking the SARS-CoV-2 virus to stimulate B-cell and T-cell responses. Instead of focusing only on the Spike (S) protein, COVAXX/Vaxxinity’s UB-612 is rationally developed to target antigen from the S protein, the RBD, and other epitopes of viral structural proteins enough to stimulate B-cell and CD8+ T-cell memory responses. It contains S1 subunit RBD genetically fused to a single-chain Fc domain of human IgG1, i.e., S1-RBD-sFc fusion protein, integrated with peptides representing T helper and cytotoxic T-cell epitopes on S2 subunit, and Membrane and Nucleocapsid protein parts of SARS-CoV-2. UB-612 is formulated with CpG1 and aluminum phosphate (AdjuPhos®) to induce a broad T cell response, and it is stable at 2–8°C.Citation80 A preclinical study by Guirakhoo et al. reported that UB-612 is immunogenic, shows protection in Adeno-associated virus (AAC) hACE2 mice, and lacks immunopathology in lungs. Interim data from Phase I clinical trial evaluating the safety, tolerability, and immunogenicity profiles of two inoculations of UB-612 across three dose levels viz. 10, 30, and 100 µg in 60 healthy adults aged 20–55 revealed that UB-612 stimulated robust antibody responses that were well-tolerated and safe. Following two doses of 100 µg of UB-612, anti-S1-RBD and virus-specific neutralizing antibodies were observed to be induced in 100% of the participants.Citation81 UB-612 is currently in a multi-center, placebo-controlled, randomized, observer-blind Phase 2 clinical trial to examine further the immunogenicity, safety, and tolerability in three distinct cohorts viz. 12–18 years old, 19–64 years old, and 65 years and older.
6.4. Viral vector vaccines
Viral vectors are usually developed by substituting the viral gene with the desired antigen or pathogenic transgene, expressing immune responses within the host for the targeted pathogen.Citation82 Being a dynamic platform for large-scale manufacturing, it also allows a broad spectrum of viral vectors to be used in vaccine development; e.g., in the case of COVID-19, vectors like adenovirus, Sendai viruses, rabies viruses, influenza viruses, parainfluenza viruses, MVA, and Newcastle viruses.Citation83 Generally, this platform includes both replicating (attenuated) and non-replicating viral vectors. The non-replicating vectors tend to infect the host cells to produce the desired antigens without necessarily generating the new virus particles, whereas replicating vectors produces both new virus particles and the antigen of choice.Citation84 The lessons learned from the past coronavirus pandemics (MERS and SARS) have provided the viral vectors a high benefit for the rapid development. Currently, two replicating and 16 non-replicating (total 18) viral vector-based candidates are under clinical trials, another 40 candidates are undergoing preclinical evaluation.
6.4.1. Representative viral vector vaccines
6.4.1.1. COVID-19 vaccine AstraZeneca (former AZD1222)
COVID-19 Vaccine AstraZeneca is a non-replicating viral vector vaccine that has been developed by AstraZeneca and the University of Oxford. It is an isolated Y25 derivative of the replication-deficient chimpanzee viral vector-based candidate containing the full-length viral S-protein. The phase 1/2 trial of AZD1222 demonstrated acceptable safety data, immunogenicity, and tolerability levels.Citation85 The phase 2/3 trials were conducted across countries, including the UK (COV001 in phase 1/2; and COV002 in phase 2/3), Brazil (COV003 in phase 3), and South Africa (COV005 in phase 1/2). The interim data on safety and efficacy in the above-mentioned four trials demonstrated significant efficacy (~70·4%) after administering two doses and 64.1% protection after at least one standard dose, with no safety-related issues.Citation85 On December 30, 2020, the vaccine (in 4 to 12 weeks dosing interval) was approved by the UK Medicines and Healthcare products Regulatory Agency (MHRA) for emergency supply for active immunization in adults (age ≥18 years). In partnership with the Serum Institute of India (SII), the vaccine has been authorized for emergency use in India. It has also been approved for emergency use in Argentina, El Salvador, the Dominican Republic, Morocco, Mexico, and the European Union (EU) for active immunization of adults. On February 15, 2021, the WHO has also granted Emergency Use Listing (EUL) to the COVID-19 Vaccine AstraZeneca (Covishield in India) for active immunization in individuals aged ≥18 years to protect from COVID-19, including the new South African B1.351 variant. Overall, the vaccine has been granted to either the EUA or CMA in more than 50 countries. AstraZeneca’s COVID-19 vaccine is expected to remain stable for at least 6 months in refrigerated conditions during storage and transportation.Citation86 A randomized, double-blind, placebo-controlled, multicentre, phase 3 trial (D8110C00001) conducted in the US was found to be 76%, 85%, and 100% efficacious against symptomatic COVID-19 cases, in severe/acute disease (or hospitalized) and in symptomatic COVID-19 subjects aged ≥65 years, respectively. However, scrutiny of these data is still awaited.Citation87
6.4.1.2. Convidecia (earlier Ad5-nCoV)
CanSino Biologics Inc. and Beijing Institute of Biotechnology have co-developed an Adenovirus Type 5 (Ad5) Vector vaccine, Ad5-nCoV (trade name: Convidecia), which is a replication-defective Ad5 vector that encodes for the viral S-protein.Citation88 A non-randomized, open-label, phase 1 study showed the vaccine candidate to be immunogenic, safe, and tolerable in healthy subjects. However, a high dose of the vaccine was found to be effective. Still, it exhibited higher adverse events, such as fatigue, fever, dyspnea, arthralgia, and myalgia.Citation89 It was further supported by a randomized, placebo-controlled, double-blind, phase 2 study, which demonstrated higher tolerability and reduced immune response among the older subjects. A single-dose vaccine-elicited rapid immune response (within 14 days) and significant antibody-mediated and cellular-mediated immunity (within 28 days). The interim data from the phase 3 trial of a single-dose convidecia suggested an overall efficacy of 68.83% after 14 days and 65.28% after 28 days at preventing the symptomatic disease. In addition, it has an efficacy of 90.07% and 95.47% at preventing severe disease after 28 days and 14 days, respectively. In June 2020, the convidecia received approval from the Health Bureau of the Logistics Support Department of the Central Military Commission. Convidecia is expected to be stable in refrigerated conditions. On February 25, 2021, the National Medical Products Administration of China (NMPA) granted CMA for Convidecia in China.
6.4.1.3. Sputnik V (Gam-COVID-Vac)
Gam-COVID-Vac was developed by the Gamaleya Research Institute, which contains two recombinant adenovirus vectors (rAD26 and rAd5) that encodes for the viral S-protein. Dose one comprises 0.5 ml rAD26, whereas the second dose consists of 0.5 ml rAD5. A Russian phase 1/2 trial assessed the safety and immunogenicity of lyophilized, frozen vaccine formulation in healthy subjects (18–60 years). The vaccine candidate showed good tolerability and elicited strong cell-mediated and antibody-mediated immunity in the participants without precipitating any significant adverse effects.Citation90 Currently, several phase 3 trials are undergoing in different countries, including India (NCT04640233), Venezuela (NCT04642339), the United Arab Emirates (NCT04656613), Russian Federation (NCT04741061), and Belarus (NCT04564716). In a Russian phase 3 trial (NCT04530396), Gam-COVID-Vac demonstrated an efficacy of 91·6% against COVID-19 disease, which was found to be well-tolerated in a large cohort.Citation90 Currently, Sputnik V has been approved in about 59 countries, and two trials (NCT04713488 and NCT04741061) are evaluating a single-dose Sputnik V formulation (Sputnik Light). The vaccine can be kept in refrigerated conditions for easy distribution globally, including hard-to-reach regions. However, a major drawback that is associated with Sputnik V is the manufacturing of a second dose. This is owing to the reason that the rAD5 virus takes a longer time to grow and may hamper the vaccine supply and availability during the second jab.
6.4.1.4. Janssen COVID-19 vaccine (Ad26.COV2.S)
Janssen Pharmaceutical Companies of Johnson and Johnson has leveraged the AdVac® platform (used earlier for Ebola vaccine development), particularly the recombinant adenovirus type 26 or Ad26, to design the vaccine candidate, Ad26.COV2.S for delivery of the desired antigen encoding for the viral S-protein. The interim result of the placebo-controlled, randomized, phase 1/2a trial (NCT04436276) supported the desired immunogenicity and safety profile of Ad26.COV2-S in the participants.Citation91 Furthermore, a single-dose immunization with the vaccine successfully elicited neutralizing and binding antibody responses as well as cell-mediated immune responses.Citation92 Safety Data from the phase 3 trial (n = 43,783) revealed the vaccine to be well tolerable, having mild-to-moderate side effects, such as myalgia, headache, fatigue, nausea, and injection site pain. On the other hand, effectiveness data from 39,321 participants showed the vaccine to be 67% effective in protecting from moderate to severe or critical diseases occurring at least 14 days after immunization and 66% effective in protecting from mild to severe or critical diseases occurring at least 28 days following vaccination. In addition, it was ~77% and 85% effective in protecting people from severe or critical diseases occurring at least 14 days and 28 days following the vaccination, respectively. Based on these data, on 27th February 2021, the USFDA issued EUA to the single-dose Janssen COVID-19 Vaccine for use in ≥18 year’s individuals. On March 11, 2021, EMA also granted CMA for the Janssen COVID-19 Vaccine for ≥18 years of age. This single-dose COVID-19 vaccine is likely to remain stable for about 24 months at −20°C (−4°F) and at least 3 months under standard refrigeration.
6.5. Virus-like particles (VLPs) vaccines
Virus-like particles (VLPs) are multiprotein virus-like structures that resemble the conformation and organization of native virus particles but are a deficit of viral genetic material. As a result, they are noninfective toward host cells, and thus are safe and suitable vaccine candidates. VLPs are a kind of modification of protein subunit vaccines that constitute viral capsid proteins that on recombinant expression within a host cell, self assemble into a capsid-like structure lacking viral genome as well as other non-structural virus proteins. As VLPs lack viral genetic material, they cannot replicate in the host but can stimulate humoral and cellular immune responses. These noninfective VLPs act as a scaffold to which various copies of an epitope can be chemically attached, and these clustered epitopes on the surface of VLPs lead to amplified B-cell activation and subsequent antibody responses. VLPs vaccines have been successful for various viral pathogens, viz. hepatitis B virus and human papilloma virus, by virtue of their potential to deliver the targeted antigens to the immune system effectively, leading to initiation of both humoral and cellular immune responses.Citation84,Citation93,Citation94 There are two COVID-19 VLPs vaccines currently in clinical assessment; a. CoVLP vaccine adjuvanted with AS03 from Medicago Inc., Canada, and b. RBD SARS-CoV-2 HBsAg VLP vaccine from SpyBiotech/Serum Institute of India.
6.5.1. Representative virus-like particles vaccines
6.5.1.1. Coronavirus-like particle COVID-19 vaccine candidate (CoVLP)
The CoVLP vaccine (NCT04636697) by Medicago Inc. was developed using plant-based technology that utilizes transient transfection of a non-transgenic plant, Nicotiana benthamiana, and a disarmed Agrobacterium tumefaciens transfer vector to produce VLPs. CoVLP is the first plant-derived COVID-19 vaccine. CoVLP, a self-assembled VLP, comprises recombinant spike (S) glycoprotein trimers embedded into the nanoparticles’ lipid bilayer. The CoVLP vaccine can be stored at 2–8°C and is administered with the GlaxoSmithKline (GSK) adjuvant AS03 system composed of α-tocopherol, squalene, and poly-sorbate 80 in an oil-in-water emulsion. This adjuvant system has been reported to stimulate a transient innate immune response at the inoculation site in animal models and in human peripheral blood, and this innate immune response strengthens the adaptive response toward vaccine antigen, eliciting high response magnitude, durability, and antibody avidity.Citation95,Citation96 Results from Medicago’s Phase 1 study showed that AS03 notably magnified both cellular and humoral responses to CoVLPs, and the vaccine was found to be safe and tolerable. The phase 1 study was initiated in July 2020 to assess the safety, tolerability, and immunogenicity profiles of two doses (21 days apart) of 3.75 µg, 7.5 µg, or 15 µg of CoVLP vaccine; all formulations were found to be well-tolerated with mild-to-moderate adverse events. Following the booster dose, neutralizing antibodies in the CoVLP+AS03 groups were observed tenfold higher than titers in Covid-2019 convalescent sera. Also, both S protein-specific IFN-γ and IL-4 cellular responses were increased.Citation97 Based on Phase 1 trial results, a two-dosage schedule of adjuvanted CoVLP (3.75 μg) entered into Phase 2/3 clinical trials in November 2020. None of the peer-reviewed data from the interim report of phase 2 of the ongoing Phase 2/3 randomized, placebo-controlled trial regulated at multiple sites in Canada and the USA showed that CoVLP+AS03 was well-tolerated and adverse events were mild or moderate in healthy adults aged 18–64 (“Adults”) and in older adults aged 65+ (“Older Adults”). CoVLP+AS03 stimulated an excellent humoral response in “Adults” than “Older Adults” after the first dose, but this effect was subjugated in both age groups following the second dose. In both age groups, a single dose of CoVLP+AS03 stimulated significant IFN-γ and IL-4 responses, and the booster dose resulted in a further increase in significant IFN-γ and IL-4 responses. However, the IFN-γ and IL-4 cellular responses were more assertive in “Adults” than “Older Adults.” CoVLP + AS03 has been in Phase 3 clinical trial since mid-March and is being conducted in North America, Latin America, and Europe. The data analysis for the third population, i.e., “Adults with Comorbidities,” and assessment of the efficacy is currently ongoing, and will be released once available.Citation98
6.5.1.2. RBD-HBsAg-VLPs-Covid vaccine
The RBD-HBsAg-VLPs-Covid vaccine (ACTRN12620000817943) is based on the RBD domain of SARS-CoV-2 conjugated to the hepatitis B surface antigen (HBsAg) VLPs. Currently, it is in Phase 1 and 2 clinical trials.Citation99
6.6. Attenuated live vaccine
Live attenuated vaccines are obtained by cultivating a living microbe under laboratory conditions and generate a weakened form of the virus that is incapable of causing disease in a healthy individual. The attenuated microbe’s inherent tendency to stimulate the immune system by triggering toll-like receptors (TLRs), CD4, CD8 T, and B cells develop a robust and long-lasting immunological response that is effective in preventing infection.Citation100 Various live attenuated vaccines, such as Bacillus Calmette-Guerin (BCG), measles vaccine, Rubella vaccine, and polio vaccine (OPV) have proven protection against several infections.Citation101,Citation102 Recent studies show that COVID-19, the suppressed immune system plays a crucial role in disease occurrence.Citation103 Thus, a live-attenuated vaccine that innates the immune response may increase resistance to infection caused by SARS CoV-2. The COVID-19 live attenuated vaccine produces by deleting or mutating the SARS-CoV virulence gene, which hindered replication to a limited extent, such as the deletion of structural E protein, targeting Non-structural protein (nsp1, nsp16), deletion of 2’-O-methylase gene, and codon deoptimization.Citation104–106 Currently, many live-attenuated vaccine projects are undergoing COVID-19 disease. The most critical example includes the vaccine developed by the Serum Institute of India with Codagenix, Inc., i.e., the COVI-VAC (CDX-005) vaccine. There are many other examples, but none of these have yet entered clinical trials.Citation107–109
A brief compilation of different platforms including RNA, DNA, and PS. Viral vector and VLP vaccine undergoing advanced phase clinical trials are compiled in . The data is compiled as per the information provided in the COVID-19 vaccine tracker and landscape last assessed on September 19, 2021.
Table 2. A brief compilation of different platforms including, RNA, DNA, PS. Viral vector and VLP vaccine undergoing advanced phase clinical trials (Phase 2/3 and 3) as per information provided in COVID-19 vaccine tracker and landscape last assessed on September 19, 2021
6.7. Bacillus Calmette-Guerin (BCG) vaccine
The BCG vaccine is an attenuated live strain of Mycobacterium bovis used to protect against tuberculosis infection.Citation109 Many studies have shown that the BCG vaccine has positive nonspecific effects (NSEs) on the immune system in addition to treatment against tuberculosis.Citation110 The NSE’s effect of BCG is primarily elicited by potentiating both innate and adaptive immune responses.Citation111 This enhanced immune response offers protection against various respiratory viral infections, such as Salmonella, Shigella, malaria, and respiratory syncytial viruses, and forms the basis of its use in certain types of bladder cancer, melanoma, etc. These live attenuated vaccines cause metabolic and epigenetic changes in the immune system, resulting in an enhanced immunological response known as trained immunity.Citation112,Citation113 Recently, there have been several published articles on the global ubiquity of COVID-19 that indicated that countries with BCG vaccination programs have a less COVID-19 mortality risk than countries without such a policy. Miller et al. and Hegarty et al. reported an epidemiological report that indicated a correlation between BCG vaccination policy and reduced COVID-19 morbidity and mortality.Citation114,Citation115 Further, Dayal et al. compared the case fatality rates (CFR) between countries with a significant effect of COVID-19 and countries where BCG revaccination policies promote a defensive immune response in the population against severe COVID-19. The data obtained from the findings further support the countries with a mandatory BCG vaccination program that offers protection against COVID-19, probably avoiding progression.Citation116 The numerous epidemiological correlations hypothesized that the impact of the BCG vaccine against COVID-19 infection is currently generating much buzz. Nevertheless, there is still no evidence that the BCG vaccine policy protects against the COVID-19 virus. Two clinical trials have been registered, with several more are in the pipeline to see whether Bacillus Calmette-Guerin might reduce the occurrence and seriousness of COVID-19.Citation117 Furthermore, Fu et al. corroborate the current evidence on BCG’s vaccine defense against COVID-19. Various clinical scenarios and model specifications using data obtained from Johns Hopkins University Coronavirus Resource Center and BCG program data from the World Atlas BCG Policies and WHO/UNICE for analysis. The study revealed a preventive effect of the BCG vaccine in the early phases of the pandemic, but no such data in the latter stages. They also found that in the early stages of a pandemic, vaccinated young people may have a protective effect, while the older population may not. Thus, clinical trials conclusively confirm BCG’S defense against COVID-19.Citation118
6.8. Intranasal vaccine
Mucosal, as well as systemic immune response, varies upon usual infection and one that is induced via vaccine injection. The protection mechanism of the lower human respiratory tract mainly includes IgG, whereas IgG1 has a prominent role. The natural protection of the upper respiratory tract is also achieved by the secretory IgA1 (sIgA1) also. A further dominating factor for the systemic immune response after the natural infection is IgG1, and for the mucosal immune response, it is by sIgA1.Citation119 However, IM vaccination tends to facilitate only the serum IgG and ignoring the trigger of mucosal IgA-based immunity response, leaving the individual vulnerable to upper respiratory tract infections. Therefore, the Intranasal vaccination seems highly useful, providing scope to trigger mucosal antibody responses that are ignored by the IM vaccination route. Still, systemic immune responses are often found not that optimum after this type of vaccination. However, considering these facts, we can say that mucosal immunity is very critical in controlling the SARS-CoV-2 infection and transmission rate. Moreover, intranasal vaccination is noninvasive (needless) with high ease of administration, ideally suited for children and adults and has the flexibility of scalable manufacturing, and may be able to meet global vaccine demand, particularly in developing countries with high populations.Citation119 There are currently six vaccines in the clinical phase administered via the intranasal route (). One recent example is BBV154, developed by Bharat Biotech as an intranasal vaccine. The vaccine utilizes the Adenovirus vector platform and has proven its protective efficacy against SARS-CoV-2 (ChAd) in mice, hamsters, and rhesus macaques, suggesting a high viral clearance on respiratory airways (both upper and lower). The vaccine is currently at Phase 1.Citation120 The details of other vaccines to be administered via nasal route are compiled in .
Table 3. Intranasal vaccines are currently undergoing clinical trials. The data is compiled as per information provided in the COVID-19 vaccine tracker and landscape last assessed on September 19, 2021
7. Vaccine’s efficacy and SARS-CoV-2 variants
Vaccine efficacy (VE) is concerned with relative risk reduction. It was first designed by Yule and Greenwood in the year 1915 to elucidate the efficacy of typhoid and cholera vaccines.Citation121 Herein VE is an important parameter that is the percentage representation given by the following mathematical equatio:n
In this case, the nearer the value to 100, the greater the efficacy/effectiveness of the vaccine.
VE is frequently calculated on a set of a population, and usually, its value differs among the different population(s) under study. Thus, it does not allow the calculation of the efficacy of the same vaccine among the different populations across the globe, thus probably giving misinterpreted results. The best way to measure VE is in clinical trials, particularly randomized type and double-blinded. For example, the Sinopharm (BBIBP) has shown a VE of 78.1% tested in the United Arab Emirates and Bahrain. A VE of 65.3% has been reported for CoronaVac tested in USA and Indonesia populations. While Covishield reported the VE of 74.2% when tested in the UK population. Further, to improve the VE, the majority of COVID-19 vaccines are proposed to be given in more than 1 dose. A closer look at the WHO vaccine landscape () for COVID-19 vaccine candidate revealed that only 15 vaccines (14%) had been reported with a single-dose regimen, 70 vaccines (65%) with 2 doses, and one vaccine (1%) with 3 doses have been reported so far. More than one dose of the vaccine is prescribed owing to the development of incomplete immunity after the first and possible wear-off in acquired immunity after a certain time lapse hence a booster dose is recommended.Citation122,Citation123 Protective immunity to COVID-19 gets plateaued with a single vaccine dose, and the second dose helps to boost it. The first dose of vaccine primes the immune system to recognize and fight against COVID-19 infection, but after a specific time period, when it is rechallenged with a second dose, it reinforces the protection by generating a large number of antibodies (cell-mediated immunity) and stimulating memory cells to remember and help to produce antibodies in case of reinfection. Clinical trial results indicate that the second dose significantly increases the production of antibodies and thus, provides a more robust immune response against COVID-19 infection.Citation124 Data from the Pfizer/BioNTech vaccine trial found 52% effectiveness against symptomatic COVID-19 from 12 days following the first vaccine dose, which subsequently increased to 95% after administering the second dose.Citation58 A similar pattern was observed with the Oxford/AstraZeneca vaccine where 76% protection was achieved against symptomatic COVID-19 from 22 days after the first vaccine dose, which raised to 81% following a second dose that was given 12 weeks after the first dose.Citation125 Also, a recent study observed that from 21 days after the administration of the first dose of Pfizer/BioNTech and Oxford/AstraZeneca vaccine, COVID-19 infections reduced to 66% and 61%, respectively; however, in individuals who had received second vaccine doses, these figures further declined to 80% and 79%, respectively.Citation126 All of these reports suggest that getting a second dose reinforces the immune response and is critical for maximum protection against COVID-19 infections. Even after this, limitations remain; first, the antibody response wanes over time, and secondly, the emergence of variants renders the original vaccine less effective, which also indicates toward the need for consideration of booster shot. Recently, a preprint reported that the Pfizer/BioNTech vaccine’s efficacy apexed at 96.2% at 7 days to 2 months following the second vaccine dose and then reduced to 83.7% at 4 months.Citation127 Another recent preprint from Israel suggests that the third vaccine dose is effective in reducing the risk of confirmed infection and severe illness by 11.4 and >10-fold, respectively, and also indicates the possibility of reducing delta variant infection employing booster doses.Citation128
Although the recommending booster doses have equivocal feedbacks both by the FDA and CDC, more research data is still required to make an unbiased decision. However, both have presented their recommendation for booster shots among the immunocompromised population.
At the present time, there are numerous reports that SARS-CoV-2 is evolving at a rapid pace, and the vaccines might become inactive or ineffective for its variants.Citation129 Scientists suspect the variants in the S-Protein of SARS-CoV-2 may increase virus shedding and further may enhance the affinity toward the ACE2 receptor. This, on the other hand, could impair or damage the virus-neutralizing antibodies (NAbs) binding sites of S-protein, thus compromising the vaccine’s efficacy. As reported, the virus undergoes evolution at a rate of ~1.1 × 10−3 substitutions/site/year (i.e., one substitution every 11 days).Citation130 Recently variants, namely, B.1.526; B.1.526.1; B.1.525; P.2 (B.1.1.28.2); B.1.617; B.1.617.1; B.1.617.2; B.1.617.3; B.1.1.7; P.1 (B.1.1.28.1); B.1.351; B.1.427; B.1.429 among other have been reported in various countries across the Globe. These variants are formed owing to single-point mutations in the RBD domain of S-protein. D614G is a prevalent mutation found in all these reported strains. Whereas L452R is the one other mutation identified in B.1.526.1, B.1.427, and B.1.429, whereas E484K is found in B.1.525, P.2, P.1, and B.1.351, B.1.526, and B.1.1.7 variants.Citation131
Among the reported variants so far, significant attention has shifted to the delta variant (B.1.617.2), which is characterized by numerous point mutations within the spike protein.Citation132 The significant mutations identified so far include T19R, L452R, D950N, D614G, P681R, T478K, and one deletion (Δ157-158). The mutation L452R and T478K so far have been associated with a muted antigenic response toward the neutralizing antibodies.Citation133 Mutation P618R has been further associated with the cleavage of S1 and S2 subunits of the spike protein, allowing the enhanced interaction with host cells, leading to increased replication, consequently causing higher viral load and increased transmissibility.Citation134,Citation135 As per the reports of the CDC, the delta variant is considered to be two times more contagious than its previous counterparts. Unvaccinated individuals are more prone to being affected by the delta variant, but at the same time, the efficacies of the reported vaccines drastically drop against this reported strain.Citation133 Further, the viral load is reported to be a thousand times much higher than the original variant. The studies so far have pointed to the need for at least two doses of vaccines that could be effective against hospitalization once infected by the delta strain. As far as the literature is concerned, data on vaccine efficacy against delta variants are limited.
Comirnaty (BNT162b2 vaccine) and Covishield (ChAdOx1 nCoV-19 vaccine) have been reported to be effective () against delta variants. Few other vaccines are also undergoing phase trials to evaluate their efficacy against the delta variant. In this league, Janssen (Ad26.COV2.S) was found to reduce its efficacy by only 1.6 folds when tested against delta variants possessing L452R and T478K mutation in the RBD domain.Citation134 A study conducted in Qatar to evaluate the vaccine efficacy (phase 3) of Moderna (mRNA-1273 vaccine) against delta variant found 57.4% (≥14 days after the first dose and no second dose) and 80.2% (≥14 days after the second dose).Citation136 Novavax (NVX-CoV2373 vaccine), in its phase II study disclosed that when given as booster dose, it increased the neutralizing antibodies titer value by four folds after the initial vaccination. A six-fold increase in cross-reactive functional antibodies in comparison to primary vaccines was reported against the delta variant.Citation137 Further studies conducted on the Sputnik-V vaccine efficacy against delta variant found it to be 83% efficacious.Citation138 Furthermore, a recent study highlighted that delta variants are approximately six-fold less sensitive toward neutralizing antibodies (in vitro) obtained from recovered individuals and eight folds less sensitive toward the antibodies elicited during post-vaccination in comparison to wild-type SARS-CoV-2.Citation139
Table 4. Efficacy of launched COVID-19 vaccines against the delta variant strain of SARS-CoV-2
Many studies are currently on the way to test the efficacy of approved vaccines against these variants, although they are not quite as effective with the original wild type Wuhan strain.Citation140 The efficacy of the vaccines reported so far against some variants of SARS-CoV-2 is compiled in .
Table 5. Efficacy of approved COVID-19 vaccines against the variants of SARS-CoV-2 in comparison to original wildtype Wuhan strain
8. Side effects and adverse events associated with the COVID-19 vaccines candidates
Apart from possessing antigenic components, vaccines carry various adjuvants that may elicit some of the side reactions or toxicological effects in the host. To ensure the safety of vaccines, they undergo rigorous preclinical toxicological studies. The primary evaluation studies include a. assessing the dose-effect (single or repeat doses); b. effect on reproductive and developmental for ensuring safety, particularly in pregnant women and neonatal; c. mutagenicity testing; d. carcinogenicity evaluation; and e. safety assessments.Citation141 This is further ensured by some vital factors but not only limited to study designs, which include the suitable models for in vivo and in vitro assays required for the optimum establishment of safety, purity, and potency of the vaccine candidates. However, COVID-19 vaccines have been launched in a short span of time. All of them had undergone rigorous assessment in terms of safety and toxicity before their recommendation for emergency approval (15 in total) or received approval by regulatory agencies. However, no long-term studies have been conducted for the toxicity analysis so far. The six approved vaccines so far by the WHO or other regulatory agencies include Sinopharm (BBIBP); CoronaVac, Comirnaty; Moderna; Sputnik V; Covishield, and Johnson & Johnson. The assessment of the three major used COVID-19 vaccines (Comirnaty, Covishield, and Moderna) by EMA disclosed no significant toxicities during their repeated doses in the non-clinical animal. The study was conducted for a short time span and the parameters that were assessed included toxicity, genotoxicity, genetic and reproductive developmental toxicity. However, the adverse reactions reported for vaccines against COVID-19 so far may be categorized into local reactions, which include sore arm, red arm, erythema, and swelling that usually occurs at the site of injection.Citation142 The second category includes nonspecific systemic effect arousing as a result of activation of the immune system. The reactogenicity causes headaches, fever, chills, myalgia, diarrhea, and/or fatigue. These are associated with the majority of the approved vaccines and are considered of low concern. Furthermore, some fatal severe adverse reactions are also reported (although rare) with the majority of the approved vaccines. The significant adverse severe reaction includes anaphylaxis, myocarditis, and thrombocytopenia.Citation143,Citation144 As per the meta-analysis report of Wu and group pain at the injection site and tenderness are the most common local reactions whereas fatigue and headaches are chiefly associated with the systemic reactions. The meta-analysis classified the local and systemic reactions on the type of platforms used that portrayed the RNA (including mRNA), and virus-like particle vaccines are primarily associated with these reactions.Citation144
Considering the severe reactions, anaphylaxis which is a severe life-threatening allergic reaction, can be caused by the administration of any vaccine due to hypersensitivity reactions of the immune system. The majority of anaphylaxis cases have been reported by the administration of mRNA vaccines, BNT162b2 and mRNA-1273, with 4.7- and 2.5-times higher chances, respectively.Citation145 The data is expected to increase as the immunization rate increases. Sixty-six cases (63 of 66 were women) of anaphylaxis were reported among 17,524,676 mRNA vaccinations. The majority of these cases are thought to be linked to the adjuvant polyethylene glycol (PEG) utilized in their production. The severe subsequent reaction that is reported with COVID-19 vaccines is myocarditis associated with heart inflammation. This is associated again with the mRNA vaccines, BNT162b2 and mRNA-1273.Citation146 As per the reports (latest September 1, 2021) of the Vaccine Adverse Event Reporting System (VAERS), 1,404 reports of myocarditis have been reported in the age group of 30 or fewer years, particularly after the second dose.Citation147
Next, one of the rare but fatal adverse reaction thrombosis with thrombocytopenia syndrome (TTS) is reported.Citation148 The cases were initially reported by the EMA following the adenovirus vaccine, AZD1222 (ChAdOx1) or Covisheild vaccine leading to 30 deaths as of March 2021.Citation149 Similar incidents were noticed in the US following the administration of another adenovirus vaccine, Ad26.COv2-s or Janssen (Johnson & Johnson’s) that reported six deaths among 6.8 million vaccinated individuals. This led the USFDA to pause the administration of Ad26.COv2-s in April 2021.Citation150,Citation151 The analyses of vaccine-induced immune thrombotic thrombocytopenia (VITT) cases on individuals in Germany revealed that thrombocytopenia was associated with heparin degradation, a critical biological that prevents blood from clotting inside the body. The immunochemical tests confirmed the detectable levels of heparin–platelet factor 4 (PF4), which is a chemokine (CXCL4) and a positively charged tetrameric protein that binds polyanionic and negatively charged molecule heparin and promotes blood coagulation.Citation152 As of September 2021, 45 confirmed cases of TTS upon administration of Janssen vaccines had been reported, particularly in females younger than 50 years. The heparin-induced thrombocytopenia earlier was thought to be linked with adenovirus DNA platform that induces the PF4 linked antibodies, but the report of two confirmed cases of TTS after the administration of Moderna vaccine (356 million doses) dampens this possibility, leaving a quest for new research in this area. Further, death is rarely associated with post-vaccination, US has reported 7,439 deaths (0.0020% of the total US population) as of September 7, 2021, after the administration of 375 million COVID-19 vaccine doses.Citation153
Further, the long-term safety data on the vaccine is limited for individuals with comorbid conditions or for pregnant and lactating women. However, long-term studies are underway for the approved vaccines to assess their safety in all groups since adverse effects mediated by an immune or nonimmune mechanism cannot be ruled out. Additionally, the vaccine produces different titer concentrations of antibodies in the vaccinated individuals. However, the individuals having a suppressed or compromised immune system by use of certain immunosuppressant or prevailing diseases conditions like HIV or cancer (leukemia and lymphoma in particular) or autoimmune disease or those recently underwent organ transplant may not be protected after vaccination.Citation154,Citation155 A research by Brian and the group disclosed the efficacy of BNT162b2 (Pfizer-BioNTech) and mRNA-1273 vaccine on solid organ transplant recipients. The group reported that 436 transplant recipients (median age 55.9 years) received BNT162b2 (52%) and mRNA-1273 (48%) vaccine. Among 436 vaccines, recipients have prescribed combination or single doses of immunosuppressants drugs, which include tacrolimus received by 83% of recipients, corticosteroids by 54%, mycophenolate by 66%, azathioprine by 9%, sirolimus by 4%, and everolimus by 2% of the total recipients. The group further disclosed that only 76 out of 436 individuals were able to produce the detectable antibodies after a median of 20 days. The mRNA-1273 vaccine was found to be more efficacious (69%) than those who received BNT162b2 (31%). The current study indeed points to the high vulnerability of organ transplant recipients toward COVID-19 infection, despite vaccination. The study also overlays the need for antibody testing and thorough immunophenotyping of vulnerable populations.Citation156
Further, the same group analyzed the effect of the second vaccine (mRNA-1273) dose on 658 participants. The study revealed that 357 individuals were found with detectable levels of antibodies (median titer value 142.1 U/mL) after the second dose.Citation157 Although the study showed significant improvement in neutralizing antibodies production but cannot deny the fact that this population is still vulnerable to acquire the infection. Therefore, considering this there is a need to administer the booster doses to such a vulnerable population or the use of monoclonal antibodies may be considered. The attenuated live viruses’ vaccine should be avoided for these compromised populations. Further, for efficient use of vaccines to the vulnerable population, there is a need to identify the neutralization titer, estimate the protective neutralization level, and analyze the duration of immune defense post-vaccination.Citation158 Still, the research is undergoing to understand the effectiveness of the vaccine in immunocompromised patients, and favorable results are still awaited.Citation155
9. Challenges, limitations, and future direction for COVID-19 vaccines
Any claim on safety or toxicity for current vaccines will be a premature statement.Citation159 However, in order to issue an EUA, the FDA seeks the benefit-risk assessment and considers the authorization of those products that prove to have potential benefits in the treatment and which at the same time outweigh their potential risks. To conclude safety and efficacy, a two-month median follow-up and assessment is done to improve the confidence level. Although 2 months of follow-up is insufficient to thoroughly analyze the outcome of the vaccine in the long term but may provide factual data to authorize their use in pandemics where saving lives becomes an utmost priority.Citation160 It is, therefore, worth waiting for long-term safety data and, at the same time, recognize the benefits and risks of COVID-19 vaccines without considering the unmotivated biases and solely on the basis of available scientific shreds of evidence to date.Citation142
Moreover, there are additionally a few challenges associated with the current COVID-19 vaccines. The significant challenges for current COVID-19 vaccines may be broadly grouped under three categories: a. Vaccine policies associated challenges;Citation161 b. challenges associated with vaccine production;Citation162 and c. anti-vaccine attitude. The challenges associated with the policies include but may not limit to a. consideration of research and development initiatives by innovative financing and funds grants including the open market for vaccines; b. requirement of coordinated uniformity in clinical trials to address the diversity in the population (race, age, sex, comorbidity, etc.) around the globe for efficacious outcomes; c. regulating the transparency in the vaccine outcomes from different trials further influenced by the variants; d. thorough monitoring of vaccine efficacy and potential adverse effects post-vaccination; e. ensuring the vaccine equity and distribution; f. sufficient vaccine production for the underdeveloped and developing countries and their safe transportation and storage; g. overcoming the vaccine hesitancy; h. alternative research plan to be set up for the population most vulnerable even after vaccination (e.g., immunocompromised population); i. International collaboration; and j. Global immunization. Moreover, some of the challenges associated with vaccine production include a. vaccine safety in a larger population with diversity and long-term safety and efficacy establishment; b. high-end research to monitor the vaccine platform from DNA, RNA, or mRNA (newer platform) as these platforms did not display much success in the past infections; mass production of vaccines; c. efficient quality control; d. expanded safety analysis; and e. regulatory approvals.
Apart from these, another major challenge includes anti-vaccine attitude or vaccine hesitancy. Due to overwhelming cases of unwillingness and uncertainty among individuals to receive the vaccination, it is further adding hurdles to manage the pandemic in the long term. The primary reasons for these are attributed to miscommunication, unawareness, distrust in health agencies, and safety hesitancy among individuals.Citation163,Citation164
Next, the WHO established the COVID-19 Vaccines Global Access (COVAX) facility to facilitate procurement and equitable distribution of COVID-19 vaccines among every country affected by the virus irrespective of their income repute. The COVAX was itself collaborated with ACT-Accelerator vaccine partners, which includes CEPI and GAVI (Global Alliance for Vaccines and Immunization), and UNICEF shares the distribution responsibilities. The core goal for the setup of the Vaccine Alliance was to relieve the economic burden and at the same time ensure protecting the health system and population of the countries. This is thought to be achieved by a. accelerating the development of vaccines; b. provides financial support to the promiscuous vaccine candidate; c. mitigate the financial risks of investors via push and pull financing mechanisms; d. ensuring equal access to vaccines around the Globe, and e. ensuring the transparent allocation and use.Citation165,Citation166
The COVAX initially identified ‘prioritized groups,’ which include health care workers, older adults, and those with severe conditions. The COVAX initially received enough vaccines to vaccinate 20% of the total population of the country belonging to the mentioned groups during their Phase 1. Phase 2 was concerned with sending doses to prioritized countries with high risk. This includes countries with a high COVID-19 positivity rate, having vulnerable health systems, and maintaining a humanitarian buffer that includes vulnerable populations, including refugees, asylum workers, and workers employed therein.Citation166 It is also proposed that COVAX will cover 92 low and middle economic countries through its Advance Market Commitment initiative. To ensure the procurement of vaccines by these countries, they should be in possession of the National Deployment and Vaccination Plan (NDVP). NDVP is proposed to be an operational plan to ensure implementing and monitoring COVID-19 vaccinating strategy in that particular country (“one-country plan”). Once an NDVP plan is submitted by a country, it is reviewed and recommended by COVAX Facility, including WHO, UNICEF, and regulating partners to ensure the proposed plan is good with well-equipped facilities including trained technical hands as it will help in mass immunization, with low wastage and at the least possible time. The significant points that will be foreseen for the implementation of NDVP include a. Regulatory preparedness; b. planning and coordination between governance and management; c. costing and funding providing a realistic budget; d. strategy for vaccinations, target population in the order of priority; e. plan for supply and strategy for waste management; f. allocation of technical humans for immunization and their training; g. strategy and proper layout vaccine acceptance and demand accordingly; h. safety assessment of vaccines; i. monitoring of immunization data and disease surveillance.Citation167 COVAX is principally funded by Western countries and private groups and has led to a revenue gathering of 6268 million USD as per the last reports. In a recent report by the WHO, 172 countries and numerous COVID-19 vaccine candidates are part of the vaccine Global Access Facility. India, through the GAVI alliance, has also joined COVAX.Citation130 India will procure 145 million vaccine doses (previous target 240 million) via the COVAX NVDF protocol. The dose numbers were affected likely due to the ban of vital raw materials by the US and pertaining IPR issues.Citation168,Citation169 However, the US Government, in its latest statement of May 5, 2021, announced its intention to support the WTO (World Trade Organization) in waiving off the IPR’s on COVID-19 vaccines. This if implemented, could do wonders in overcoming inequity in the global distribution of COVID-19 vaccines. This could essentially assist in, a. saving countless lives by involving a high amount of COVID-19 vaccine production and fast distribution; b. manufacturers would be kept on a check to ensure they do not block the production of raw material ((glass vials, resin, tubing, filters, and disposable bags), or other goods required for vaccine production; c. less competitive production ensuring the vaccines at affordable prices by all, and; d. ensure transparent negotiations among various countries.Citation170 Moreover, the WHO has also urged the member states and current vaccine manufacturers to collaborate with the WHO in sharing their intellectuals through the WHO’s COVID-19 Technology Access Pool (C-TAP) and the mRNA technology transfer hub”. These hubs formed by the WHO are meant to ensure transparent and voluntary sharing of the license, technology data, and trade secrets. However, no pharmaceutical companies have come up with the initiative and did not sign up the memorandum.Citation171
Other limitations that are coming up include whether or not developed antibodies after COVID-19 infection or immunization by vaccines could provide long-lasting immunity to the individuals? The answer to this was a mere NO! It was further understood that no evidence was there to support once a person infected will be immune to the subsequent infections. The durability of immunity, antibody titers vary from individual to individual post-infection and may further alter with time leading to the waning of antibodies at disproportionate rates. Moreover, antibody titers also vary with antigen isotype, emerging variants, and disease severity. The severe cases are correlated with elevated antibody titers; however, the kinetics of their waning remain the same. It has been estimated that IgA and IgM levels begin to wane after post-60 days, and IgG levels have been seen to degrade after 90 days of the COVID-19 infections. Although the humoral immunity may be measured by antibody testing, there exists no reliable method to determine the T-cell mediated cellular response that plays an integral role in maintaining the immunity. Moreover, considering nonscientific parameters, antibody tests could have numerous statistical limitations, could give inaccurate or false-positive results.
Besides the drawback, antibody testing could immensely assist in identifying and managing the patient care as various evidence of medical sequelae, multisystem inflammatory syndrome, systemic hyper inflammation (cytokine storm), coagulation defects (micro clots), and neurological damages, and neurocognitive deficits have been associated with mild to severe COVID-19 infections. The antibody testing could also immensely assist in determining the immune responses to the immunization by different vaccines. Of particular note is an antibody-dependent enhancement (ADE) that is generally associated with the low antibody levels that could enhance the possibility of reinfections via binding to non-neutralizing antibodies and thus enhancing the SARS-CoV-2 interaction and entry within host cells. The authors are of the opinion that antibody testing should be solely limited to a tool for assessing overall public health in spite of the individuals. The antibody testing should be used to a. analyze whether the sufficient titer value of antibody has been formed after vaccination; b. analyses whether the titer concentration is maintained up to what duration or time span; c. understanding how the variants are invading the immune response elicited by the vaccination and how it is influencing the herd immunity thereof.
Moreover, the induction of specific memory T and B cells is foremost crucial for the long-term protection against SARS-CoV-2 infection. It is noteworthy that T cells, specifically CD4+ T cells are essential to recognize the specific viral epitopes and may provide an everlasting resistance against reinfection. They also allow in provoking potent B cell responses allowing the antibody affinity maturation and maintaining the serum levels IgG and IgA. However, stimulation and proliferation of B cells will come to a halt once the viral clearance has been done.Citation172 Currently, no significant research has been done to elucidate whether infection with SARS-CoV-2 ensures durable immunity or protects against reinfections.Citation173 It is also not sure whether reinfection can be asymptomatic. Moreover, antibody titers (memory status) also vary with antigen isotype and disease severity. The severe cases are correlated with elevated antibody titers leading to the production of more neutralizing antibodies comprising chiefly of IgM, IgG, and IgA. However, the kinetics of their waning remains the same. It has been estimated that IgA and IgM levels begin to wane after post-60 days, and IgG levels have been seen to degrade after 90 days of the COVID-19 infections.Citation174 The neutralizing antibodies may not be in detectable quantities, but the humoral immunity is found to remain intact, and that could prevent the severity of the reinfection but could not wholly safeguard against it. Many recent studies have pointed toward some level of protection against reinfection, but the durability of the developed immunity is what needs to be sought. Further, the receptor binding domain (RBD) of SARS-CoV-2 is the primary site for the interaction of neutralizing antibodies developed via immunization, whereas the infection with SARS-CoV-2 induces neutralizing antibodies to RBD and against the spike or nucleocapsid proteins. Available data suggest that, in recovered COVID-19 patients, a single dose can significantly increase and develop antibody and memory B-cells, but after the second dose, no significant increase in antibodies and memory B-cells was observed. Still, the studies are ongoing to determine whether the vaccines could provide everlasting immunity, or whether they will wane with time, giving the possibility of the booster doses.Citation175 Recently, booster doses of Janssen (Johnson and Johnson) and Pfizer-BioNTech COVID-19 Vaccine have been authorized by USFDA for certain population(s). Furthermore, the effects of innate or passive immunity against the SARS-CoV-2 variants are debatable. Thus, considering the current scenario, it is recommended to get vaccinated after recovery from COVID-19 as a single dose is sufficient to develop memory B-cells.
Another significant limitation to be sought is the comorbidity status. It is pretty evident that older adults, especially those living with comorbidities and who are frail have been affected by COVID-19 and even in the post-vaccination state. A case-control study reportedCitation176 that frailty was associated with the first dose of vaccination, mainly among the older subjects (above 60 years) and in the individuals living in highly deprived regions. On the other hand, individuals with BMI <30 kg/m2 (without obesity) had shown a lower rate of infection following the first dose vaccination. In the disease profile analysis, this study reported that comorbidities, including heart diseases, lung diseases, and kidney diseases, were significantly associated with the increased odds of post vaccination infection (especially after the first dose). From the vantage point of the impact of age on vaccination, a study reported that the ChAdOx1 nCoV-19 vaccine (now COVID-19 Vaccine AstraZeneca) is better tolerated in older participants than younger adults. For instance, subjects receiving two standard doses of the ChAdOx1 nCoV-19 reported that 88% (n = 49) participants aged 18–55 years, 73% (n = 30) participants aged 56–69 years, and 61% (n = 49) participants aged ≥70 years experienced at least one local reaction including pain at injection-site and tenderness; and 86% (n = 49) subjects in 18–55 year group, 77% (n = 30) in the 56–69 year group, and 65% (n = 49) in the ≥70 years experienced at least one systemic reaction including headache, fatigue, fever, and myalgias.Citation64 However, the significant limitations of the study include single-blind design, and its inclusion (few subjects above 80 years of age), and exclusion (particularly in people with substantial underlying chronic disease and frailty) criteria. On the contrary, another study suggests a significantly lower absolute mean titer in elderly participants (>80 years) as compared to the younger group (<60 years). After the second dose of vaccine, about 31.3% of the elderly subjects showed no detectable neutralizing antibodies as compared to the 2.2% in the younger group.Citation177
Moreover, cross-reactivity, the ability to react with structurally similar heterologous antigens, remains a debatable topic in context with the vaccination and emerging SARS-CoV-2 variants. Variants are allegedly capable of escaping the host immunity even after vaccination. Of note, the B.1.351 variant is capable of escaping the immunity obtained from earlier infection and immunization with certain COVID-19 vaccines (e.g., COVID-19 Vaccine AstraZeneca).Citation76 Also, the Comirnaty vaccine and the Moderna COVID-19 vaccine demonstrated a reduction in antibody neutralization by two-thirdsCitation178 and six-foldCitation179 respectively, with the B.1.351 variant compared to prior variants. Although most of the vaccines are effective against the B.1.1.7 variant, antibody neutralization has been observed to be modestly reduced with the mRNA vaccines.Citation179 A study examined the IgG antibody levels elicited by the AstraZeneca and Pfizer/BioNTech vaccine against S-protein from the wild type (Wuhan), B.1.1.7, B.1.351, P.1., and B.1.617.2. Interestingly, the results suggest 85% and 100% pan specificity to all the S-variants by the AstraZeneca and Pfizer/BioNTech vaccine, respectively.Citation180 Even though the mutation profile of the P.1 variant is almost similar to B.1.351, there are no specific studies conducted to evaluate the efficacy of vaccines against the P.1 variant. Extensive studies are anticipated to determine the effectiveness of these vaccines against the emerging variants and establish a robust “one-for-all” platform.
Undoubtedly, there are likely to be many more circulating SARS-CoV-2 variants that require robust genomic surveillance to detect them, mainly to detect the infected jurisdiction. Genomic sequencing and initiatives like ‘New Variant Assessment Platform’ is anticipated to be helpful in detecting SARS-CoV-2 variants.Citation181 Nevertheless, the frontline vaccine producers, including Moderna, AstraZeneca, and Pfizer/BioNTech, are planning to produce booster doses of vaccines to protect against variants of concern, including B.1.351.Citation182 Apart from these, the regulatory authorities are also adapting swiftly to facilitate rapid regulatory vaccine pipelines, for instance, the guidance and implemented policy laid by the FDA to streamline the vaccine regulation.Citation183 Optimistically, contributions from all these sectors will garner resources at an expeditious pace for the good of humanity.
Further, if a variant of the present SARS-CoV-2 becomes more severe in the future, it could again put a burden on vaccine development and the need to get vaccinated (or booster doses) each year, similar to the influenza vaccine. This further put us in the situation to ask, ‘Where do we go?’ we as a unit need to analyze and come up with solutions. The critical consideration to ponder will include but is only limited to A. Will, the long-term safety and efficacy studies on vaccines, will be favorable? If not, are we prepared with ‘Plan B’? B. What will be the fate of antibody stability and durability, including their half-life, in the long run? Will they get worn out? C. What will be the fate of current vaccines against variants of SARS-CoV-2? Will they maintain their efficacy? D. How would the mixing of two different vaccines or vaccine cocktails impact efficacy, or will it elicit a toxic response; E. Should we need to be concerned over unnatural sequences in the genome of SARS-CoV-2, could they initiate a new pandemic altogether? F. How to streamline the vaccine equity? G. Do we value money more than human lives? Considerations should be made to make IPRs transparent during such pandemics.
There is no doubt about the concern for global immunization. Each and every individual needs to be immunized at a rapid pace to control the pandemic irrespective of equity and IPR issues. Failing to which restoring the social and economic health is back on track will become an intimidating task.
10. Conclusion
As of September 2021, 117 COVID-19 vaccines are in clinical development, and 194 are in preclinical development, as per the World Health Organization (WHO) published draft landscape. Among the 117 vaccines undergoing clinical trials, the major platforms include protein subunit (42); RNA-based (19); Inactivated virus-based (16); Viral vector-based (17), among others. A closer look at the WHO vaccine landscape revealed that 15 vaccines (15%) had been reported with a single-dose regimen, 74 vaccines (63%) with 2 doses, and one vaccine (1%) with 3 doses intended to be administered via Oral (3%) or injectable routes (85%). So far, 13 vaccines have been approved for early or limited use, while 8 have been approved for full use against SARS-CoV-2 by at least one of the stringent regulatory authorities (SRA) recognized by WHO. To date, USFDA recognized to approve the Pfizer-BioNTech (Comirnaty) COVID-19 vaccine for its full use in individuals of 16 years of age and older. Comirnaty is the only vaccine that is available under the EUA for the age group between 12 and 15 years, along with the approval to be used as a booster or third dose in specific immunocompromised individuals.
However, vaccines are developing at a tremendous pace, but still, the wealthiest countries (27) have 28% of the total vaccines and possess only 10.8% of the total world population, suggesting uneven access to vaccines or vaccines inequity. Considering vaccination with income status, 54.8% population residing in high-income countries, 49.5% population residing in upper-middle countries, followed by 10.3% population of lower-middle-income countries and 0.6% for low-income countries population has been fully vaccinated. To overcome the disparity, the WHO has set up COVAX to facilitate procurement and equitable distribution of COVID-19 vaccines irrespective of their income status, and has also initiated NDVP still the total of 60% contributions have been made only by three countries that include the USA, China, and India. Further, there are unequivocal concerns over the license, technology data, and trade secrets of big vaccine manufacturers and high-income countries. Though WHO has urged member states and current vaccine manufacturers to collaborate with the WHO in sharing their intellectuals through the WHO’s COVID-19 Technology Access Pool (C-TAP) and the mRNA technology transfer hub”. However, no pharmaceutical companies have come up with the initiative and did not sign up the memorandum, which is a sign of concern again.
Apart from this, safety and efficacy data for all the approved vaccines are based on a two-month median follow-up and assessment that overlooks the benefit-risk assessment and considers the authorization of those products that prove to have potential benefits in the treatment and which at the same time outweigh their potential risks. However, 2 months of follow-up is insufficient to thoroughly analyze the outcome of the vaccine in the long term but may provide factual data to authorize its use in pandemics where saving lives becomes an utmost priority. It is, therefore, recommended to wait for long-term safety data and, at the same time, ascertain the benefits and risks of COVID-19 vaccines without considering the unmotivated biases and solely on the basis of available scientific shreds of evidence to date. However, our current evacuation plan should be to vaccinate the entire globe to bring the current pandemic to a halt. However, this seems to be a distant dream so far, as only 13.7% global population has been vaccinated that covers the countries with the highest incomes that are getting vaccinated 30 times faster than lower-income countries. The vaccine, in conjunction with COVID-19 appropriate behavior, may yield speedy control of the pandemic. Otherwise, vaccines alone may decrease the severity of the disease but may not decrease the number of new cases of infection. How long the antibodies are generated in response to vaccines will further determine how soon the booster dose(s) are needed.
In the last 21 months since the declaration of the pandemic due to SARS-CoV-2, our understanding of COVID-19 and emerging variants of the virus, and the rapid development of COVID-19 vaccines (including mRNA vaccine introduction for the first time in vaccine history) resulted in a positive impact. With the constant efforts of interdisciplinary collaboration of scientific and policy-making bodies of the whole world will eventually find solutions to the challenges and restore normal conditions.
Author contributions
The manuscript has been prepared and drafted with the understanding and contributions of all the authors.
Abbreviations
| ACE2 | = | Angiotensin-converting enzyme 2 |
| Ads-nCoV | = | Adenovirus type-5 vectored COVID -19 |
| BCRs | = | B cell receptors |
| BCG | = | Bacillus Calmette-Guerin |
| CEPI | = | Coalition for Epidemic Preparedness Innovations |
| CoVLP | = | Coronavirus-like particle COVID-19 vaccine candidate |
| CFRs | = | Case fatality rates |
| COVAX | = | COVID-19 Vaccines Global Access Facility |
| C-TAP | = | COVID-19 Technology Access Pool |
| CBER | = | Centre for Biologics Evaluation and Research |
| EMA | = | European Medicines Agency |
| EUA | = | Emergency Use Authorization |
| GAVI | = | Global Alliance for Vaccines and Immunisation |
| H-CoV-229E | = | Human coronavirus 229E |
| MERS-CoV | = | Middle East respiratory syndrome coronavirus |
| ML | = | Machine learning |
| mTOR | = | mammalian target of rapamycin |
| NIAID | = | National Institute of Allergy and Infectious Diseases; |
| NDVP | = | National Deployment and Vaccination Plan |
| NAbs | = | virus-neutralizing antibodies |
| PS | = | Protein subunit |
| RBD | = | Receptor-binding domain |
| SARS-CoV-2 | = | Severe Acute Respiratory Syndrome Coronavirus 2 |
| TCRs | = | T cell receptors |
| TTS | = | Thrombosis with Thrombocytopenia Syndrome |
| TLRSs | = | Toll-like Receptors |
| USFDA | = | U.S. Food and Drug Administration |
| VE | = | Vaccine efficacy |
Acknowledgments
All the authors thank their respective affiliated working institutes and universities for providing the necessary infrastructure and support to carry out the current work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Parkin J, Cohen B. An overview of the immune system. Lancet. 2001;357:1777–89. doi:10.1016/S0140-6736(00)04904-7.
- Baxter D. Active and passive immunity, vaccine types, excipients and licensing. Occup Med (Lond). 2007;57:552–56. doi:10.1093/occmed/kqm110.
- Heffernan JM, Keeling MJ. Implications of vaccination and waning immunity. Proc Biol Sci. 2009;276:2071–80. doi:10.1098/rspb.2009.0057.
- Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Med. 2020;26:450–52. doi:10.1038/s41591-020-0820-9.
- Yee S, Tan CS, Khan A, Lee KS, Goh BH, Ming LC. SARS-COV-2 as an artificial creation: scientific arguments and counterarguments. J Med Life. 2021;14:118–20. doi:10.25122/jml-2020-0175.
- Amanat F, Krammer F. SARS-CoV-2 vaccines: status report. Immunity. 2020;52:583–89. doi:10.1016/j.immuni.2020.03.007.
- Thakur S, Sarkar B, Ansari AJ, Khandelwal A, Arya A, Poduri R, Joshi G. Exploring the magic bullets to identify Achilles’ heel in SARS-CoV-2: delving deeper into the sea of possible therapeutic options in Covid-19 disease: an update. Food Chem Toxicol. 2021;147:111887. doi:10.1016/j.fct.2020.111887.
- Poduri R, Joshi G, Jagadeesh G. Drugs targeting various stages of the SARS-CoV-2 life cycle: exploring promising drugs for the treatment of Covid-19. Cell Signal. 2020;74:109721. doi:10.1016/j.cellsig.2020.109721.
- Plotkin SA, Plotkin SL. The development of vaccines: how the past led to the future. Nat Rev Microbiol. 2011;9:889–93. doi:10.1038/nrmicro2668.
- Rappuoli R, Pizza M, Del Giudice G, De Gregorio E. Vaccines, new opportunities for a new society. Proc Natl Acad Sci USA. 2014;111:12288–93. doi:10.1073/pnas.1402981111.
- Wherry EJ, Jaffee EM, Warren N, D’Souza G, Ribas A. How did we get a COVID-19 vaccine in less than 1 year?. Clin Cancer Res. 2021;27:2136–38. doi:10.1158/1078-0432.CCR-21-0079.
- Funk CD, Laferriere C, Ardakani A. A snapshot of the global race for vaccines targeting SARS-CoV-2 and the COVID-19 pandemic. Front Pharmacol. 2020;11:937. doi:10.3389/fphar.2020.00937.
- Zepp F. Principles of vaccine design—lessons from nature. Vaccine. 2010;28:C14–C24. doi:10.1016/j.vaccine.2010.07.020.
- Soria-Guerra RE, Nieto-Gomez R, Govea-Alonso DO, Rosales-Mendoza S. An overview of bioinformatics tools for epitope prediction: implications on vaccine development. J Biomed Inform. 2015;53:405–14. doi:10.1016/j.jbi.2014.11.003.
- Rueckert C, Guzman CA. Vaccines: from empirical development to rational design. PLoS Pathog. 2012;8:e1003001. doi:10.1371/journal.ppat.1003001.
- Abidi A, Can M. On the accuracies of sequence based linear B cell epitope predictors. Southeast Eur J Soft Comput. 2018;6. doi:10.21533/scjournal.v6i2.142.
- Wang J, Wang H, Wang X, Chang H. Predicting drug-target interactions via FM-DNN learning. Curr Bioinform. 2020;15:68–76. doi:10.2174/1574893614666190227160538.
- Wong KK. Optimization in the design of natural structures, biomaterials, bioinformatics and biometric techniques for solving physiological needs and ultimate performance of bio-devices. Curr Bioinform. 2019;14:374–75. doi:10.2174/157489361405190628122355.
- Fast E, Altman RB, Chen B. Potential T-cell and B-cell epitopes of 2019-nCoV. BioRxiv 2020:2020 02 19 955484. doi:10.1101/2020.02.19.955484.
- Ong E, Wong MU, Huffman A, He Y. COVID-19 coronavirus vaccine design using reverse vaccinology and machine learning. Front Immunol. 2020;11:1581. doi:10.3389/fimmu.2020.01581.
- Yang Z, Bogdan P, Nazarian S. An in silico deep learning approach to multi-epitope vaccine design: a SARS-CoV-2 case study. Sci Rep. 2021;11:3238. doi:10.1038/s41598-021-81749-9.
- Vita R, Mahajan S, Overton JA, Dhanda SK, Martini S, Cantrell JR, Wheeler DK, Sette A, Peters B. The immune epitope database (IEDB): 2018 update. Nucleic Acids Res. 2019;47:D339–D43. doi:10.1093/nar/gky1006.
- Toussaint NC, Kohlbacher O. OptiTope—a web server for the selection of an optimal set of peptides for epitope-based vaccines. Nucleic Acids Res. 2009;37:W617–W22. doi:10.1093/nar/gkp293.
- Karosiene E, Lundegaard C, Lund O, Nielsen M. NetMHCcons: a consensus method for the major histocompatibility complex class I predictions. Immunogenetics. 2012;64:177–86. doi:10.1007/s00251-011-0579-8.
- Sharma R, Palanisamy A, Dhama K, Mal G, Singh B, Singh KP. Exploring the possible use of saponin adjuvants in COVID-19 vaccine. Hum Vaccines Immunother. 2020;16:2944–53. doi:10.1080/21645515.2020.1833579.
- Cox JC, Coulter AR. Adjuvants—a classification and review of their modes of action. Vaccine. 1997;15:248–56. doi:10.1016/s0264-410x(96)00183-1.
- Pulendran B, S. Arunachalam P, O’Hagan DT. Emerging concepts in the science of vaccine adjuvants. Nat Rev Drug Discov. 2021;20:454–75. doi:10.1038/s41573-021-00163-y.
- Awate S, Babiuk LA, Mutwiri G. Mechanisms of action of adjuvants. Front Immunol. 2013;4:114. doi:10.3389/fimmu.2013.00114.
- McKee AS, MacLeod MK, Kappler JW, Marrack P. Immune mechanisms of protection: can adjuvants rise to the challenge? BMC Biol. 2010;8:1–10. doi:10.1186/1741-7007-8-37.
- Zhang C, Maruggi G, Shan H, Li J. Advances in mRNA vaccines for infectious diseases. Front Immunol. 2019;10:594. doi:10.3389/fimmu.2019.00594.
- Federico S, Pozzetti L, Papa A, Carullo G, Gemma S, Butini S, Campiani G, Relitti N. Modulation of the innate immune response by targeting toll-like receptors: a perspective on their agonists and antagonists. J Med Chem. 2020;63:13466–513. doi:10.1021/acs.jmedchem.0c01049.
- Moyle PM, Toth I. Modern subunit vaccines: development, components, and research opportunities. ChemMedChem. 2013;8:360–76. doi:10.1002/cmdc.201200487.
- Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol. 2011;12:509–17. doi:10.1038/ni.2039.
- Gomez PL, Robinson JM. Vaccine manufacturing. Plotkin’s Vaccines. 2018;51–60.e1. doi:10.1016/B978-0-323-35761-6.00005-5.
- Philippidis A. COVID-19: top 60 drug treatments in development: the biopharma industry is ramping up the development of dozens of potential drug therapies and clinical testing in an all-hands effort to combat the pandemic. Genet Eng Biotechnol News. 2020;40:10–13. doi:10.1089/gen.40.04.02.
- Rodrigues CM, Plotkin SA. Impact of vaccines; health, economic and social perspectives. Front Microbiol. 2020;11. doi:10.3389/fmicb.2020.01526.
- Saylor K, Gillam F, Lohneis T, Zhang C. Designs of antigen structure and composition for improved protein-based vaccine efficacy. Front Immunol. 2020;11:283. doi:10.3389/fimmu.2020.00283.
- Heinz FX, Stiasny K. Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines. 2021;6:104. doi:10.1038/s41541-021-00369-6.
- Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–79. doi:10.1038/nrd.2017.243.
- Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol. 2021;21:83–100. doi:10.1038/s41577-020-00479-7.
- Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586:516–27. doi:10.1038/s41586-020-2798-3.
- Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–68. doi:10.1126/science.1690918.
- Borah P, Deb PK, Deka S, Venugopala KN, Singh V, Mailavaram RP, Kalia K, Tekade RK. Current scenario and future prospect in the management of COVID-19. Curr Med Chem. 2021;28:284–307. doi:10.2174/0929867327666200908113642.
- Borah P, Deb PK, Al-Shar’i NA, Dahabiyeh LA, Venugopala KN, Singh V, Shinu P, Hussain S, Deka S, Chandrasekaran B. Perspectives on RNA vaccine candidates for COVID-19. Front Mol Biosci. 2021;8:635245. doi:10.3389/fmolb.2021.635245.
- Liu MA. A comparison of plasmid DNA and mRNA as vaccine technologies. Vaccines (Basel). 2019;7:37. doi:10.3390/vaccines7020037.
- Jensen S, Thomsen AR. Sensing of RNA viruses: a review of innate immune receptors involved in recognizing RNA virus invasion. J Virol. 2012;86:2900–10. doi:10.1128/JVI.05738-11.
- Mukherjee A, Waters AK, Kalyan P, Achrol AS, Kesari S, Yenugonda VM. Lipid–polymer hybrid nanoparticles as a next-generation drug delivery platform: state of the art, emerging technologies, and perspectives. Int J Nanomed. 2019;14:1937. doi:10.2147/IJN.S198353.
- de Queiroz N, Marinho FV, Chagas MA, Leite LCC, Homan EJ, de Magalhaes MTQ, Oliveira SC. Vaccines for COVID-19: perspectives from nucleic acid vaccines to BCG as delivery vector system. Microbes Infect. 2020;22:515–24. doi:10.1016/j.micinf.2020.09.004.
- Maruggi G, Chiarot E, Giovani C, Buccato S, Bonacci S, Frigimelica E, Margarit I, Geall A, Bensi G, Maione D, et al. Immunogenicity and protective efficacy induced by self-amplifying mRNA vaccines encoding bacterial antigens. Vaccine. 2017;35:361–68. doi:10.1016/j.vaccine.2016.11.040.
- Geall AJ, Verma A, Otten GR, Shaw CA, Hekele A, Banerjee K, Cu Y, Beard CW, Brito LA, Krucker T, et al. Nonviral delivery of self-amplifying RNA vaccines. Proc Natl Acad Sci USA. 2012;109:14604–09. doi:10.1073/pnas.1209367109.
- Beissert T, Perkovic M, Vogel A, Erbar S, Walzer KC, Hempel T, Brill S, Haefner E, Becker R, Türeci Ö, et al. A trans-amplifying RNA vaccine strategy for induction of potent protective immunity. Mol Ther. 2020;28(1):119–28. doi:10.1016/j.ymthe.2019.09.009.
- Bloom K, van den Berg F, Arbuthnot P. Self-amplifying RNA vaccines for infectious diseases. Gene Ther. 2021;28:117–29. doi:10.1038/s41434-020-00204-y.
- Yang Y, Wang H, Kouadir M, Song H, Shi F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019;10:128. doi:10.1038/s41419-019-1413-8.
- Dolgin E. The tangled history of mRNA vaccines. Nature. 2021;597(7876):318–24. doi:10.1038/d41586-021-02483-w.
- Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, McCullough MP, Chappell JD, Denison MR, Stevens LJ, et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920–31. doi:10.1056/NEJMoa2022483.
- Crommelin DJA, Anchordoquy TJ, Volkin DB, Jiskoot W, Mastrobattista E. Addressing the cold reality of mRNA vaccine stability. J Pharm Sci. 2021;110:997–1001. doi:10.1016/j.xphs.2020.12.006.
- Walsh EE, Frenck R, Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R, Swanson KA, et al. RNA-based COVID-19 vaccine BNT162b2 selected for a pivotal efficacy study. Medrxiv. 2020. doi:10.1101/2020.08.17.20176651.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–15. doi:10.1056/NEJMoa2034577.
- Lamb YN. BNT162b2 mRNA COVID-19 vaccine: first approval. Drugs. 2021;81:495–501. doi:10.1007/s40265-021-01480-7.
- Mukherjee R. Global efforts on vaccines for COVID-19: since, sooner or later, we all will catch the coronavirus. J Biosci. 2020;45:1–10. doi:10.1007/s12038-020-00040-7.
- Oostvogels L, Kremsner P, Kreidenweiss A, Leroux-Roels I, Leroux-Roels G, Kroidl A, Schunk M, Schindler C, Bosch J, Fendel R, Gabor JJ. Phase 1 assessment of the safety and immunogenicity of an mRNA-lipid nanoparticle vaccine candidate against SARS-CoV-2 in human volunteers. Medrxiv. 2020. doi:10.1101/2020.11.09.20228551.
- Silveira MM, Oliveira TL, Schuch RA, McBride AJA, Dellagostin OA, Hartwig DD. DNA vaccines against leptospirosis: a literature review. Vaccine. 2017;35:5559–67. doi:10.1016/j.vaccine.2017.08.067.
- Duerr GD, Heine A, Hamiko M, Zimmer S, Luetkens JA, Nattermann J, Rieke G, Isaak A, Jehle J, Held SAE, et al. Parameters predicting COVID-19-induced myocardial injury and mortality. Life Sci. 2020;260:118400. doi:10.1016/j.lfs.2020.118400.
- Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, Voysey M, Aley PK, Angus B, Babbage G, Belij-Rammerstorfer S. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396:1979–93. doi:10.1016/S0140-6736(20)32466-1.
- Lee P, Kim CU, Seo SH, Kim DJ. Current status of COVID-19 vaccine development: focusing on antigen design and clinical trials on later stages. Immune Network. 2021;21:e4. doi:10.4110/in.2021.21.e4.
- Coronavirus vaccine tracker. [ accessed 2021 Sep 19]. https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html.
- Bottomley MJ, Rappuoli R, Finco O. Vaccine design in the 21st century. In: Bloom BR, and Lambert P-H, editors. The vaccine book. USA: Academic Press; 2016. p. 45–65.
- Chen J, Gao K, Wang R, Wei GW. Prediction and mitigation of mutation threats to COVID-19 vaccines and antibody therapies. Chem Sci. 2021;12:6929–48. doi:10.1039/d1sc01203g.
- Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, Zhang Q, Shi X, Wang Q, Zhang L. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–20. doi:10.1038/s41586-020-2180-5.
- Callaway E, Mallapaty S. Novavax covid vaccine protects people against variants. Nature. 2021;590:17. doi:10.1038/d41586-021-00268-9.
- Keech C, Albert G, Cho I, Robertson A, Reed P, Neal S, Plested JS, Zhu M, Cloney-Clark S, Zhou H, Smith G. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320–32. doi:10.1056/NEJMoa2026920.
- Mahase E. Covid-19: Novavax vaccine efficacy is 86% against UK variant and 60% against South African variant. Br Med J. 2021. doi:10.1136/bmj.n296.
- Cheng RZ. Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID-19)?. Med Drug Discov. 2020;5:100028. doi:10.1016/j.medidd.2020.100028.
- Richmond P, Hatchuel L, Dong M, Ma B, Hu B, Smolenov I, Li P, Liang P, Han HH, Liang J, Clemens R. Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: a phase 1, randomised, double-blind, placebo-controlled trial. Lancet. 2021;397:682–94. doi:10.1016/S0140-6736(21)00241-5.
- Dai L, Gao GF. Viral targets for vaccines against COVID-19. Nat Rev Immunol. 2021;21:73–82. doi:10.1038/s41577-020-00480-0.
- Madhi SA, Baillie V, Cutland CL, Voysey M, Koen AL, Fairlie L, Padayachee SD, Dheda K, Barnabas SL, Bhorat QE. Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B.1.351 variant. N Engl J Med. 2021;384:1885–98. doi:10.1056/NEJMoa2102214.
- Montastruc F, Thuriot S, Durrieu G. Hepatic disorders with the use of Remdesivir for Coronavirus 2019. Clin Gastroenterol Hepatol. 2020;18:2835–36. doi:10.1016/j.cgh.2020.07.050.
- Fan Q, Zhang B, Ma J, Zhang S. Safety profile of the antiviral drug Remdesivir: an update. Biomed Pharmacother. 2020;130:110532. doi:10.1016/j.biopha.2020.110532.
- Ryzhikov AB, Ryzhikov EА, Bogryantseva MP, Danilenko ED, Imatdinov IR, Nechaeva EA, Pyankov OV, Pyankova OG, Susloparov IM, Taranov OS, Gudymo AS, Immunogenicity and protectivity of the peptide vaccine against SARS-CoV-2. Ann Russ Acad Sci Med Sci. 2021;76:5–19. doi:10.15690/vramn1528.
- Gupta S, Wang W, Hayek SS, Chan L, Mathews KS, Melamed ML, Brenner SK, Leonberg-Yoo A, Schenck EJ, Radbel J, Reiser J, Investigators SC, Association between early treatment with Tocilizumab and mortality among critically Ill patients with COVID-19. JAMA Intern Med. 2021;181:41–51. doi:10.1001/jamainternmed.2020.6252.
- Guirakhoo F, Kuo L, Peng J, Huang JH, Kuo B, Lin F, A novel SARS-CoV-2 multitope protein/peptide vaccine candidate is highly immunogenic and prevents lung infection in an Adeno associated virus human angiotensin-converting enzyme 2 (AAV hACE2) mouse model. BioRxiv. 202010.1101/2020.11.30.399154.
- Bouard D, Alazard-Dany D, Cosset FL. Viral vectors: from virology to transgene expression. Br J Pharmacol. 2009;157:153–65. doi:10.1038/bjp.2008.349.
- Bezbaruah R, Borah P, Kakoti BB, Al-Shar IN, Chandrasekaran B, Jaradat DMM, Al-Zeer MA, Abu-Romman S, Developmental landscape of potential vaccine candidates based on viral vector for prophylaxis of COVID-19. Front Mol Biosci. 2021;8:635337. doi:10.3389/fmolb.2021.635337.
- van Riel D, de Wit E. Next-generation vaccine platforms for COVID-19. Nat Mater. 2020;19:810–12. doi:10.1038/s41563-020-0746-0.
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, Bibi S B, Baillie VL, Barnabas SL, Bhorat QE, Bibi S. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi:10.1016/S0140-6736(20)32661-1.
- Khan Sharun RS, Dhama K. Oxford-AstraZeneca COVID-19 vaccine (AZD1222) is ideal for resource-constrained low-and middle-income countries. Ann Med Surg. 2021. doi:10.1016/j.amsu.2021.102264.
- Knoll MD, Wonodi C. Oxford–AstraZeneca COVID-19 vaccine efficacy. Lancet. 2021;397:72–74. doi:10.1016/S0140-6736(20)32623-4.
- Xi CE, Singh J. A review of the animal and human trials of the Ad5-nCoV vaccine candidate. Int J Stud Res. 2021;10. doi:10.47611/jsr.v10i1.1159.
- Wu S, Zhong G, Zhang J, Shuai L, Zhang Z, Wen Z, Wang B, Zhao Z, Song X, Chen Y, et al. A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nat Commun. 2020;11:4081. doi:10.1038/s41467-020-17972-1.
- Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, Kovyrshina AV, Lubenets NL, Grousova DM, Erokhova AS, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–81. doi:10.1016/S0140-6736(21)00234-8.
- Sadoff J, Gray G, Vandebosch A, Cardenas V, Shukarev G, Grinsztejn B, Goepfert PA, Truyers C, Fennema H, Spiessens B, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187–201. doi:10.1056/NEJMoa2101544.
- Stephenson E, Reynolds G, Botting RA, Calero-Nieto FJ, Morgan MD, Tuong ZK, Bach K, Sungnak W, Worlock KB, Yoshida M, et al. Single-cell multi-omics analysis of the immune response in COVID-19. Nat Med. 2021;27(5):904–16. doi:10.1038/s41591-021-01329-2.
- Roldão A, Mellado MCM, Castilho LR, Carrondo MJ, Alves PM. Virus-like particles in vaccine development. Expert Rev Vaccines. 2010;9:1149–76. doi:10.1586/erv.10.115.
- Nooraei S, Bahrulolum H, Hoseini ZS, Katalani C, Hajizade A, Easton AJ, Ahmadian G. Virus-like particles: preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J Nanobiotechnol. 2021;19:1–27. doi:10.1186/s12951-021-00806-7.
- Morel S, Didierlaurent A, Bourguignon P, Delhaye S, Baras B, Jacob V, Planty C, Elouahabi A, Harvengt P, Carlsen H, Kielland A. C, Elouahabi A, Harvengt P, Carlsen H, Kielland A. Adjuvant system AS03 containing alpha-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine. 2011;29:2461–73. doi:10.1016/j.vaccine.2011.01.011.
- Burny W, Callegaro A, Bechtold V, Clement F, Delhaye S, Fissette L, Janssens M, Leroux-Roels G, Marchant A, van den Berg RA, Garçon N M, Leroux-Roels G, Marchant A, Van den Berg RA, Garçon N. Different adjuvants induce common innate pathways that are associated with enhanced adaptive responses against a model antigen in humans. Front Immunol. 2017;8:943. doi:10.3389/fimmu.2017.00943.
- Ward BJ, Gobeil P, Seguin A, Atkins J, Boulay I, Charbonneau PY, Couture M, D’Aoust M-A, Dhaliwall J, Finkle C, et al. Phase 1 randomized trial of a plant-derived virus-like particle vaccine for COVID-19. Nat Med. 2021;27:1071–78. doi:10.1038/s41591-021-01370-1.
- Gobeil P, Pillet S, Séguin A, Boulay I, Mahmood A, Vinh DC, Charland N, Boutet P, Roman FP, Van Der Most R, Perez MD N, Boutet P, Roman FP, Van Der Most R, Perez MD. Interim report of a phase 2 randomized trial of a plant-produced virus-like particle vaccine for Covid-19 in healthy adults aged 18-64 and older adults aged 65 and older. Medrxiv. 2021. doi:10.1101/2021.05.14.21257248.
- Bakhiet M, Taurin S. SARS-CoV-2: targeted managements and vaccine development. Cytokine Growth Factor Rev. 2021;58:16–29. doi:10.1016/j.cytogfr.2020.11.001.
- Khuroo MS, Khuroo M, Khuroo MS, Sofi AA, Khuroo NS. COVID-19 vaccines: a race against time in the middle of death and devastation! J Clin Exp Hepatol. 2020;10:610–21. doi:10.1016/j.jceh.2020.06.003.
- Tielemans S, de Melker HE, Hahne SJM, Boef AGC, van der Klis FRM, Sanders EAM, van der Sande MAB, Knol MJ. Non-specific effects of measles, mumps, and rubella (MMR) vaccination in high income setting: population based cohort study in the Netherlands. Br Med J. 2017;358:j3862. doi:10.1136/bmj.j3862.
- Plotkin S. History of vaccination. Proc Natl Acad Sci USA. 2014;111:12283–87. doi:10.1073/pnas.1400472111.
- Zheng M, Gao Y, Wang G, Song G, Liu S, Sun D, Xu Y, Tian Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17:533–35. doi:10.1038/s41423-020-0402-2.
- Netland J, DeDiego ML, Zhao J, Fett C, Alvarez E, Nieto-Torres JL, Enjuanes L, Perlman S. Immunization with an attenuated severe acute respiratory syndrome coronavirus deleted in E protein protects against lethal respiratory disease. Virology. 2010;399:120–28. doi:10.1016/j.virol.2010.01.004.
- Hou Y, Meulia T, Gao X, Saif LJ, Wang Q, Gallagher T. Deletion of both the tyrosine-based endocytosis signal and the endoplasmic reticulum retrieval signal in the cytoplasmic tail of spike protein attenuates porcine epidemic diarrhea virus in pigs. J Virol. 2019;93. doi:10.1128/JVI.01758-18.
- Menachery VD, Yount BL Jr., Josset L, Gralinski LE, Scobey T, Agnihothram S, Katze MG, Baric RS. Attenuation and restoration of severe acute respiratory syndrome coronavirus mutant lacking 2’-o-methyltransferase activity. J Virol. 2014;88:4251–64. doi:10.1128/JVI.03571-13.
- Zhao J, Zhao S, Ou J, Zhang J, Lan W, Guan W, Wu X, Yan Y, Zhao W, Wu J. COVID-19: Coronavirus vaccine development updates. Front Immunol. 2020;11:602256. doi:10.3389/fimmu.2020.602256.
- Sharun K, Dhama K. India’s role in COVID-19 vaccine diplomacy. J Travel Med. 2021;28. doi:10.1093/jtm/taab064.
- Luca S, Mihaescu T. History of BCG vaccine. Maedica (Bucur). 2013;8:53–58. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3749764/.
- Report on BCG vaccine use for protection against mycobacterial infections including tuberculosis, leprosy, and other nontuberculous mycobacteria (NTM) infections. [ accessed 2021 Sep 10]. https://www.who.int/immunization/sage/meetings/2017/october/1_BCG_report_revised_version_online.pdf .
- Shann F. Nonspecific effects of vaccines and the reduction of mortality in children. Clin Ther. 2013;35:109–14. doi:10.1016/j.clinthera.2013.01.007.
- Netea MG, Dominguez-Andres J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, Joosten LAB, van der Meer JWM, Mhlanga MM, Mulder WJM, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20:375–88. doi:10.1038/s41577-020-0285-6.
- Moorlag S, Arts RJW, Van Crevel R, Netea MG. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect. 2019;25:1473–78. doi:10.1016/j.cmi.2019.04.020.
- Miller A, Reandelar MJ, Fasciglione K, Roumenova V, Li Y, Otazu GH. Correlation between universal BCG vaccination policy and reduced mortality for COVID-19. Medrxiv. 2020. doi:10.1101/2020.03.24.20042937.
- Hegarty PK, Kamat A, Zafirakis H, Dinardo A. BCG vaccination may be protective against Covid-19. Preprint. 2020. doi:10.13140/RG.2.2.35948.10880.
- Dayal D, Gupta S. Connecting BCG vaccination and COVID-19: additional data. Medrxiv. 2020. doi:10.1101/2020.04.07.20053272.
- Bacille Calmette-Guérin (BCG) vaccination and COVID-19: scientific brief, 12 April 2020. [accessed 2021 Sep 10]. https://www.who.int/news-room/commentaries/detail/bacille-calmette-gu%C3%A9rin-(bcg)-vaccination-and-covid-19 .
- Fu W, Ho PC, Liu CL, Tzeng KT, Nayeem N, Moore JS, Wang L-S, Chou S-Y. Reconcile the debate over protective effects of BCG vaccine against COVID-19. Sci Rep. 2021;11:8356. doi:10.1038/s41598-021-87731-9.
- Birkhoff M, Leitz M, Marx D. Advantages of intranasal vaccination and considerations on device selection. Indian J Pharm Sci. 2009;71:729–31. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2846493/ .
- Remy KE, Brakenridge SC, Francois B, Daix T, Deutschman CS, Monneret G, Jeannet R, Laterre P-F, Hotchkiss RS, Moldawer LL. Immunotherapies for COVID-19: lessons learned from sepsis. Lancet Respir Med. 2020;8:946–49. doi:10.1016/S2213-2600(20)30217-4.
- Greenwood M, Yule GU. The statistics of anti-typhoid and anti-cholera inoculations, and the interpretation of such statistics in general. Proc R Soc Med. 1915;8:113–94. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2004181/ .
- Moore JP. Approaches for optimal use of different COVID-19 vaccines: issues of viral variants and vaccine efficacy. JAMA. 2021;325:1251–52. doi:10.1001/jama.2021.3465.
- Goldfarb JL, Kreps S, Brownstein JS, Kriner DL. Beyond the first dose - Covid-19 vaccine follow-through and continued protective measures. N Engl J Med. 2021;385:101–03. doi:10.1056/NEJMp2104527.
- Abbasi J. Study suggests lasting immunity after COVID-19, with a big boost from vaccination. JAMA. 2021;326:376–77. doi:10.1001/jama.2021.11717.
- Hung IF, Poland GA. Single-dose Oxford–AstraZeneca COVID-19 vaccine followed by a 12-week booster. Lancet. 2021;397:854–55. doi:10.1016/S0140-6736(21)00528-6.
- Pritchard E, Matthews PC, Stoesser N, Eyre DW, Gethings O, Vihta KD, Jones J, House T, VanSteenHouse H, Bell I, et al. Impact of vaccination on new SARS-CoV-2 infections in the United Kingdom. Nat Med. 2021;27:1370–78. doi:10.1038/s41591-021-01410-w.
- Thomas SJ, Moreira ED, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Marc GP, Polack FP, Zerbini C, Bailey R. Six month safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. Medrxiv. 2021. doi:10.1101/2021.07.28.21261159.
- Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N, Mizrahi B, Alroy-Preis S, Ash N, Milo R, et al. BNT162b2 vaccine booster dose protection: a nationwide study from Israel. N Engl J Med. 2021;385:1393–400. doi:10.1056/NEJMoa2114255.
- Moore JP, Offit PA. SARS-CoV-2 vaccines and the growing threat of viral variants. JAMA. 2021;325:821–22. doi:10.1001/jama.2021.1114.
- Martin MA, VanInsberghe D, Koelle K. Insights from SARS-CoV-2 sequences. Science. 2021;371:466–67. doi:10.1126/science.abf3995.
- Diurno F, Numis FG, Porta G, Cirillo F, Maddaluno S, Ragozzino A, De Negri P, Di Gennaro C, Pagano A, Allegorico E, Bressy L. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. Eur Rev Med Pharmacol Sci. 2020;24:4040–47. doi:10.26355/eurrev_202004_20875.
- Kupferschmidt K, Wadman M. Delta variant triggers new phase in the pandemic. Science. 2021;372(6549):1375–76. doi:10.1126/science.372.6549.1375.
- Delta variant: what we know about the science. [ accessed 2021 Sep 15]. https://www.cdc.gov/coronavirus/2019-ncov/variants/delta-variant.html .
- Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, Stowe J, Tessier E, Groves N, Dabrera G, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385:585–94. doi:10.1056/NEJMoa2108891.
- Sanderson K. COVID vaccines protect against Delta, but their effectiveness wanes. Nature. 2021. doi:10.1038/d41586-021-02261-8.
- Tang P, Hasan MR, Chemaitelly H, Yassine HM, Benslimane F, Al Khatib HA, AlMukdad S, Coyle P, Ayoub HH, Al Kanaani Z, Al Kuwari E. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the Delta (B. 1.617. 2) variant in Qatar. Medrxiv. 2021. doi:10.1101/2021.08.11.21261885.
- Novavax announces COVID-19 vaccine booster data demonstrating four-fold increase in neutralizing antibody levels versus peak responses after primary vaccination. [ accessed 2021 Sep 15]. https://ir.novavax.com/2021-08-05-Novavax-Announces-COVID-19-Vaccine-Booster-Data-Demonstrating-Four-Fold-Increase-in-Neutralizing-Antibody-Levels-Versus-Peak-Responses-After-Primary-Vaccination .
- Russia’s Sputnik V shot around 83% effective against delta variant, health minister says. [ accessed 2021 Sep 15]. https://www.reuters.com/business/healthcare-pharmaceuticals/russias-sputnik-v-shot-around-83-effective-against-delta-variant-health-minister-2021-08-11/ .
- Mlcochova P, Kemp S, Dhar MS, Papa G, Meng B, Ferreira I, Datir R, Collier DA, Albecka A, Singh S, et al. SARS-CoV-2 B.1.617.2 delta variant replication and immune evasion. Nature. 2021. doi:10.1038/s41586-021-03944-y.
- Rubin R. COVID-19 vaccines vs variants—determining how much immunity is enough. JAMA. 2021;325:1241–43. doi:10.1001/jama.2021.3370.
- Green MD, Al-Humadi NH. Preclinical toxicology of vaccines. In: A comprehensive guide to toxicology in nonclinical drug development; 2017. p. 709–35. doi:10.1016/B978-0-12-803620-4.00027-X.
- Hernandez AF, Calina D, Poulas K, Docea AO, Tsatsakis AM. Safety of COVID-19 vaccines administered in the EU: should we be concerned? Toxicol Rep. 2021;8:871–79. doi:10.1016/j.toxrep.2021.04.003.
- Novak N, Tordesillas L, Cabanillas B. Adverse rare events to vaccines for COVID-19: from hypersensitivity reactions to thrombosis and thrombocytopenia. Int Rev Immunol. 2021;1–10. doi:10.1080/08830185.2021.1939696.
- Wu Q, Dudley MZ, Chen X, Bai X, Dong K, Zhuang T, Salmon D, Yu H. Evaluation of the safety profile of COVID-19 vaccines: a rapid review. BMC Med. 2021;19:173. doi:10.1186/s12916-021-02059-5.
- COVID C, Team R. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14–23, 2020. Morb Mortal Wkly Rep. 2021;70;46. doi:10.1001/jama.2021.0600.
- Myocarditis and pericarditis after mRNA COVID-19 vaccination. [ accessed 2021 Sep 1]. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/myocarditis.html .
- Gargano JW, Wallace M, Hadler SC, Langley G, Su JR, Oster ME, Broder KR, Gee J, Weintraub E, Shimabukuro T. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on immunization practices—United States, June 2021. Morb Mortal Wkly Rep. 2021;70:977. doi:10.15585/mmwr.mm7027e2external.
- Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol. 2021;1:11. doi:10.1038/s41577-021-00592-1.
- Vaxzevria (previously COVID-19 vaccine AstraZeneca): risk of thrombocytopenia and coagulation disorders. [ accessed 2021 Sep 10]. https://www.ema.europa.eu/en/medicines/dhpc/vaxzevria-previously-covid-19-vaccine-astrazeneca-risk-thrombocytopenia-coagulation-disorders#documents-section .
- Joint CDC and FDA statement on Johnson & Johnson COVID-19 vaccine. [ accessed 2021 Sep 7]. https://www.fda.gov/news-events/press-announcements/joint-cdc-and-fda-statement-johnson-johnson-covid-19-vaccine .
- MacIntyre CR, Veness B, Berger D, Hamad N, Bari N. Thrombosis with Thrombocytopenia Syndrome (TTS) following AstraZeneca ChAdOx1 nCoV-19 (AZD1222) COVID-19 vaccination - A risk-benefit analysis for people < 60 years in Australia. Vaccine. 2021;39:4784–87. doi:10.1016/j.vaccine.2021.07.013.
- Alam W. COVID-19 vaccine-induced immune thrombotic thrombocytopenia: a review of the potential mechanisms and proposed management. Sci Prog. 2021;104:368504211025927. doi:10.1177/00368504211025927.
- Selected adverse events reported after COVID-19 vaccination. [ accessed 2021 Sep 7]. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html .
- Forni G, Mantovani A. COVID-19 vaccines: where we stand and challenges ahead. Cell Death Differ. 2021;28:626–39. doi:10.1038/s41418-020-00720-9.
- COVID-19 vaccines may protect many, but not all, people with suppressed immune systems. [ accessed 2021 Sep 10]. https://www.science.org/news/2021/04/covid-19-vaccines-may-protect-many-not-all-people-suppressed-immune-systems .
- Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, Garonzik-Wang JM. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325:1784–86. doi:10.1001/jama.2021.4385.
- Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, Garonzik-Wang JM. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204–06. doi:10.1001/jama.2021.7489.
- Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, Subbarao K, Kent SJ, Triccas JA, Davenport MP. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–11. doi:10.1038/s41591-021-01377-8.
- Radbel J, Narayanan N, Bhatt PJ. Use of Tocilizumab for COVID-19-induced cytokine release syndrome: a cautionary case report. Chest. 2020;158(1):e15–e9. doi:10.1016/j.chest.2020.04.024.
- Krause PR, Gruber MF. Emergency use authorization of covid vaccines - safety and efficacy follow-up considerations. N Engl J Med. 2020;383:e107. doi:10.1056/NEJMp2031373.
- Forman R, Shah S, Jeurissen P, Jit M, Mossialos E. COVID-19 vaccine challenges: what have we learned so far and what remains to be done? Health Policy (New York). 2021;125:553–67. doi:10.1016/j.healthpol.2021.03.013.
- Sharma O, Sultan AA, Ding H, Triggle CR. A review of the progress and challenges of developing a vaccine for COVID-19. Front Immunol. 2020;11:585354. doi:10.3389/fimmu.2020.585354.
- Machingaidze S, Wiysonge CS. Understanding COVID-19 vaccine hesitancy. Nat Med. 2021;27:1338–39. doi:10.1038/s41591-021-01459-7.
- Vaccine hesitancy: what it means and what we need to know in order to tackle it. [ accessed 2021 Sep]. https://www.who.int/immunization/research/forums_and_initiatives/1_RButler_VH_Threat_Child_Health_gvirf16.pdf .
- Eccleston-Turner M, Upton H. International collaboration to ensure equitable access to vaccines for COVID-19: the ACT-accelerator and the COVAX facility. Milbank Q. 2021;99:426–49. doi:10.1111/1468-0009.12503.
- Access and allocation: how will there be fair and equitable allocation of limited supplies? [ accessed 2021 Sep 14]. https://www.who.int/news-room/feature-stories/detail/access-and-allocation-how-will-there-be-fair-and-equitable-allocation-of-limited-supplies .
- Andreakos E, Tsiodras S. COVID-19: lambda interferon against viral load and hyperinflammation. EMBO Mol Med. 2020;12:e12465. doi:10.15252/emmm.202012465.
- Vanden Eynde JJ. COVID-19: a brief overview of the discovery clinical trial. Pharmaceuticals (Basel). 2020;13:65. doi:10.3390/ph13040065.
- Bollyky TJ, Gostin LO, Hamburg MA. The equitable distribution of COVID-19 therapeutics and vaccines. JAMA. 2020;323:2462–63. doi:10.1001/jama.2020.6641.
- Krishtel P, Malpani R. Suspend intellectual property rights for covid-19 vaccines. Br Med J. 2021;373:n1344. doi:10.1136/bmj.n1344.
- Zarocostas J. What next for a COVID-19 intellectual property waiver? Lancet. 2021;397:1871–72. doi:10.1016/s0140-6736(21)01151-x.
- Gaebler C, Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Tokuyama M, Cho A, Jankovic M, Schaefer-Babajew D, Oliveira TY, et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591:639–44. doi:10.1038/s41586-021-03207-w.
- Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, Grifoni A, Ramirez SI, Haupt S, Frazier A, Nakao C. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371. doi:10.1126/science.abf4063.
- Marklund E, Leach S, Axelsson H, Nystrom K, Norder H, Bemark M, Angeletti D, Lundgren A, Nilsson S, Andersson L-M, et al. Serum-IgG responses to SARS-CoV-2 after mild and severe COVID-19 infection and analysis of IgG non-responders. PLoS One. 2020;15(10):e0241104. doi:10.1371/journal.pone.0241104.
- Callaway E. Had COVID? You’ll probably make antibodies for a lifetime. Nature. 2021. doi:10.1038/d41586-021-01442-9.
- Antonelli M, Penfold RS, Merino J, Sudre CH, Molteni E, Berry S, Canas LS, Graham MS, Klaser K, Modat M, et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis. 2021;00460–6. doi:10.1016/S1473-3099(21.
- Muller L, Andree M, Moskorz W, Drexler I, Walotka L, Grothmann R, Ptok J, Hillebrandt J, Ritchie A, Rabl D, et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. Clin Infect Dis. 2021. doi:10.1093/cid/ciab381.
- Liu Y, Liu J, Xia H, Zhang X, Fontes-Garfias CR, Swanson KA, Cai H, Sarkar R, Chen W, Cutler M, et al. Neutralizing activity of BNT162b2-elicited serum. N Engl J Med. 2021;384:1466–68. doi:10.1056/NEJMc2102017.
- Wu K, Werner AP, Moliva JI, Koch M, Choi A, Stewart-Jones GBE, Bennett H, Boyoglu-Barnum S, Shi W, Graham BS, et al. mRNA-1273 vaccine induces neutralizing antibodies against spike mutants from global SARS-CoV-2 variants. BioRxiv. 2021. doi:10.1101/2021.01.25.427948.
- Ebanks D, Faustini S, Shields A, Parry H, Moss P, Plant T, Richter A, Drayson M. Cross reactivity of serological response to SARS-CoV-2 vaccination with viral variants of concern detected by lateral flow immunoassays. J Infect. 2021; 83(4):e18–20. S0163-4453(21)00363-7. doi:10.1016/j.jinf.2021.07.020.
- UK to support rest of the world to find COVID-19 virus variants. [ accessed 2021 Sep 16]. https://www.gov.uk/government/news/uk-to-support-rest-of-the-world-to-find-covid-19-virus-variants .
- Abdool Karim SS, de Oliveira T. New SARS-CoV-2 variants—clinical, public health, and vaccine implications. N Engl J Med. 2021;384:1866–68. doi:10.1056/NEJMc2100362.
- Coronavirus (COVID-19) update: FDA issues policies to guide medical product developers addressing virus variants. [ accessed 2021 Sep 16]. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-policies-guide-medical-product-developers-addressing-virus.