ABSTRACT
In Italy, SARS-CoV-2 vaccination campaign prioritized healthcare workers (HCWs) to receive two doses of BNT162b2 vaccine, irrespective of a previous SARS-CoV-2 infection. In this real-life study, we compared the humoral response to BNT162b2 vaccine in HCWs with and without a previous SARS-CoV-2 infection. Of the 407 HCWs enrolled, 334 (82.1%) were SARS-CoV-2-naive and 73 (17.9%) SARS-CoV-2-experienced. Post-vaccine humoral response was detectable in more than 98% of HCWs. Overall, the median level of anti-S IgG in SARS-COV-2-experienced HCWs was twice as high as those of SARS-CoV-2-naive subjects (24641.0 AU/mL [IQR: 15273.0–>40000.0] versus 13053.8 [IQR: 7303.3–20105.8]; p < .001), irrespective of the time elapsed from SARS-CoV-2 previous infection. In a subgroup of SARS-CoV-2-naive and -experienced subjects who received only one dose of the vaccine, the latter showed 32 times higher levels of anti-S IgG compared to the former. Although no serious adverse events have been reported, mild to moderate side effects occurred more frequently after the first dose in the SARS-CoV-2-experienced than in naive subjects (67% versus 42%, respectively; p < .001). Notably, post-vaccination anti-SARS-CoV-2 spike IgG levels ≥20,000 AU/mL were independently associated with the risk of fever ≥38°C (adjusted odds ratio [aOR] 5.122, 95% CI 2.368–11.080, p < .0001).
Our study showed high responsiveness of BNT162b2 vaccine and a relationship between levels of antibody response and reactogenicity. It suggests that a single dose of mRNA vaccine might evoke effective protection in SARS-CoV-2-experienced subjects.
Introduction
The pandemic of the coronavirus disease 19 (COVID-19) due to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that started in China in late 2019 has rapidly become an unprecedented global health challenge.Citation1 With continuing cases and deaths from the pandemic, researchers worldwide raced to develop COVID-19 vaccines. The application of new vaccine techniques mainly based on mRNA and adenoviral vector platforms has led to the rapid development of anti-SARS-CoV-2 vaccine candidates. At the end of 2020, the results of phase 3 clinical trials on vaccines targeting the spike (S) proteins of SARS-CoV-2 demonstrated their ability to induce robust humoral and cellular immune responses and showed 95% efficacy at preventing Covid-19 illness, including severe disease.Citation2–7 Since then, the rapid implementation of mass SARS-CoV-2 vaccination has become a global healthcare priority for curbing the epidemic.
COVID-19 vaccination campaign in Italy started on 27 December 2020 as soon as the BNT162b2 (Pfizer-BioNTech) vaccine, an mRNA-based COVID-19 vaccine, was approved for emergency use.Citation8 Initial targeted population included healthcare workers (HCW) to whom two doses of BNT162b2 vaccine were recommended, irrespectively of a previous SARS-CoV-2 infection and regardless of some concerns on the indication of prioritizing vaccination in subjects with preexisting natural immunity.Citation9
The main objective of the study was to compare the humoral response to BNT162b2 vaccine in HCWs with and without a previous SARS-CoV-2 infection. As a secondary objective, we explored the association between previous SARS-CoV-2 infection and the frequency and severity of post-vaccination symptoms.
Methods
Study population
The study was conducted at the Luigi Sacco University Hospital (LSUH) that has served as a main COVID-19 referral center for the metropolitan area of Milan, Italy, since the start of the pandemic.Citation10
From 28 December 2020, LSUH offered the BNT162b2 mRNA-based vaccine to all its 1500 healthcare workers (HCWs) including nurses, doctors, healthcare technicians, health service assistants, cleaners, laboratory staff, and administrative staff. HCWs enrolled into the vaccination program were invited to participate in a pre- and post-vaccination serosurvey on a voluntary basis, and those who agreed gave the written informed consent to the storage of their anonymized data in a protected database. The study was approved by the ethical committee of the University of Milan (Comitato Etico Università degli Studi di Milano, n. 23/21) and conducted in compliance with Good Clinical Practice guidelines and the Declaration of Helsinki.
Two blood samples for each participant were obtained: the first within the 7 days preceding the administration of the first vaccine dose (baseline sample), and the second after 28–30 days from the first dose of vaccine (post-vaccination sample), regardless of the receipt of the second dose. Participants were asked to complete a questionnaire that, in addition to demographic and occupational data, included questions concerning whether they had experienced symptoms possibly related to SARS-CoV-2 infection (fever, myalgia, fatigue, sore throat, conjunctivitis, gastrointestinal symptoms, anosmia/dysgeusia, cough, and dyspnea) and/or they have ever had a PCR test on nasopharyngeal swab and/or a serological test positive SARS-CoV-2. At the post-vaccination survey participants were also asked to report any new symptoms or diagnosis of SARS-CoV-2 infection and any side effects that occurred after the first and/or the second vaccination dose. HWCs were asked to define their side effect(s) as follows: mild (negligible symptom(s)), moderate (symptom(s) not requiring specific medical intervention), intense (symptom(s) requiring medications or impacting on the daily activities) and referred them at the high intensity experienced from the first dose to the post-vaccination questionnaire. In the case of fever or hypertension, specifically, HCWs were asked to indicate if fever was <38°C (moderate) or ≥38°C (intense) and weather the diastolic blood pressure was <100 mmHg (moderate) or ≥100 mmHg (intense).
New SARS-CoV-2 infections diagnosed between the two observation time points were considered to be pre-vaccination if they occurred within 5 days from first dose of vaccine administration.Citation11
Serological assays
Plasma samples were stored at −20°C until serological tests were performed.
In order to capture possible asymptomatic infections and to assess the persistence of specific antibodies in previously infected subjects, baseline samples were screened for antibodies against the SARS-CoV-2 nucleocapsid protein (anti-N IgG) using the Abbott chemiluminescent microparticle immunoassay (Abbott, Abbott Park, Illinois, USA) and for IgG antibodies against the receptor-binding domain (RBD) of the viral S1 spike protein (anti-S IgG), which levels showed a strong correlation with neutralizing activity,Citation12,Citation13 using the SARS-CoV-2 IgG II Quant assay (Abbott, Abbott Park, Illinois, USA). The assay cutoff is ≥50 AU/ml, with linear quantification of detected results from 50 to 40,000 AU/ml reported by the manufacturer.Citation14
This same assay was also used to quantify the magnitude of anti-S IgG response in post-vaccination samples.
Definitions
For the purpose of our analyses the study population was divided in two groups: a) HCWs without a prior history of SARS-CoV-2 infection and resulted negative at the baseline anti-N IgG and anti-S IgG screening test (SARS-CoV-2-naive), and b) HCWs with a previous infection documented by a positive PCR test on a nasopharyngeal (NF) swab and/or a positive serologic test or resulted positive to our baseline N/S-IgG screening test (SARS-CoV-2-experienced).
Statistical analysis
The descriptive statistics include proportions for categorical variables, and median values and interquartile range (IQR) for continuous variables. The baseline characteristics of SARS-CoV-2 – naive and SARS-CoV-2-experienced HCWs were compared using the χ2 or the Fisher’s exact test where necessary for categorical variables and the nonparametric Mann–Whitney test for continuous variables.
The Mann–Whitney test or the Kruskal-Wallis with Dunn’s test for pairwise multiple-comparison procedure were used to compare post-vaccination anti-S IgG levels between HCWs according to the following characteristics: a) being SARS-CoV-2 – naive and SARS-CoV-2 – experienced; b) having been diagnosed with SARS-CoV-2 infection for less or more than 6 months, c) having received one or two vaccine doses, and d) being male and female. Differences in anti-S IgG levels according to age strata was also evaluated by using the Cuzick’s trend test. Non-parametric analysis of the correlation between pre- and post-vaccination anti-S IgG levels in SARS-CoV-2-experienced subjects was assessed by Spearman’s rank correlation coefficient rs.
The frequency of post-vaccination symptoms, overall and grade 3 systemic events, was compared between SARS-CoV-2–naive and SARS-CoV-2-experienced HCWs. In case of statistically significant difference in the frequency of grade 3 systemic events between the two groups, we applied multivariable logistic regression model, adjusting for age, sex and post-vaccination anti-S IgG levels. All statistics were conducted using SAS, version 9.4 and p-values < .05 were considered to be statistically significant.
Results
Participants characteristics
Of the 1550 HCWs who were offered COVID-19 vaccination, 407 participated in the study: 334 (82.1%) SARS-CoV-2-naive and 73 (17.9%) SARS-CoV-2-experienced HCWs. The baseline characteristics of SARS-CoV-2-naive and SARS-CoV-2-experienced HCWs are shown in . SARS-CoV-2-experienced HCWs were more frequently males than SARS-CoV-2-naive, but there were no between-groups differences according to age and occupational category. SARS-CoV-2-experienced HCWs included 21 individuals without a history of infection who resulted positive to baseline anti-S IgG test but had no report of positive PCR on a NF swab. Overall, of the 73 SARS-CoV-2-experienced HCWs, 93.2% resulted positive for anti-SARS-CoV-2 spike IgG at baseline, while in 49.3% of them baseline anti-N IgG were negative. The median interval between SARS-CoV-2 diagnosis and enrollment resulted significantly longer in these subjects compared to those who tested anti-N IgG positive (258 days [IQR 166–289] versus 75 days [IQR 70–90], respectively, p = .007).
Table 1. Demographic, occupational, and serological characteristics of the study population
Vaccination serological response
All participants received the first dose of BNT162b2 mRNA COVID-19 vaccine. After 28–30 days from the first dose administration, 326 (97.6%) of SARS-CoV-2-naive HCWs had completed their 2-dose vaccination schedule, while 8 (2.4%) had the second dose delayed for personal reasons (4 were unwell and 4 had familiar commitments and were rescheduled). Among SARS-CoV-2-experienced HCWs, 60 (82.2%) had received the second dose, while 13 (17.8%) declined it.
At this post-vaccination time point, anti-S IgG were detectable in more than 98% of HCWs. Overall, the median levels of anti-S IgG in SARS-CoV-2-experienced HCWs increased from 871.9 AU/mL [IQR: 233.9–2767.9] at baseline () to 24641.0 AU/mL [IQR: 15273.0->40000.0] at the post-vaccination sampling when they were twice as high as those of SARS-CoV-2-naive subjects (13053.8 [IQR: 7303.3–20105.8]; p < .001). A significant correlation was found in SARS-CoV-2-experienced persons between baseline and post-vaccination (2 doses) anti-S IgG levels (Spearman Rank Correlation rs = 0.45; p < .001). When the humoral response was compared between SARS-CoV-2-experienced and -naive subjects who had received only one dose of the vaccine, the former showed 32 times higher levels of anti-S IgG compared to the latter (15273.0 AU/mL [IQR: 4107.3–31200.5] and 469.6 AU/mL [IQR: 1.7–7976.3], respectively) (). Moreover, anti-S IgG levels among SARS-CoV-2-experienced HCWs who had completed a 2-dose course of vaccination did not significantly differ between subjects who had been diagnosed with SARS-CoV-2 infection for less than 6 months (n = 40) and those who had been diagnosed for more than 6 months (n = 20) ().
Figure 1. a) Anti-SARS-CoV-2 spike antibody levels after one and two doses of the BNT162b2 mRNA COVID-19 vaccine in SARS-CoV-2-experienced and SARS-CoV-2-naive HCWs. b) Anti-SARS-CoV-2 spike antibody levels after two doses of vaccine in SARS-CoV-2-experienced HCWs who had been diagnosed with SARS-CoV-2 infection for less than 6 months and those who had been diagnosed for more than 6 months at the time of vaccination. Horizontal bars express the median values.
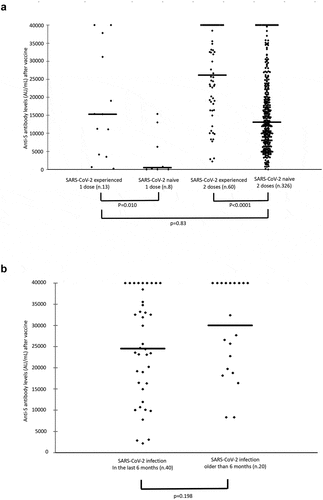
No statistically significant difference was found in post-vaccination anti-S IgG levels according to sex, while a significant decreasing trend was shown with increasing of age (p = .021) among SARS-CoV-2-naive subjects.
Finally, in the timespan between the two plasma samples we did not observe any symptomatic infection except one subject who resulted positive to nasopharyngeal swab one day after the first dose and was considered a pre-vaccine infection, thus included in SARS-CoV-2 experienced-HCWs.
Vaccine-associated side effects
At least one side effect was reported by 245 (73.4%) and 63 (86.3%) of SARS-CoV-2-naive and SARS-CoV-2-experienced subjects (p = .019), respectively, none requiring hospitalization (). Symptoms after both first and second dose of vaccine were reported by 98 and 23 SARS-CoV-2-naive and – experienced participants, respectively. Overall, a higher number of SARS-CoV-2 naive HCWs reported side effects after the second vaccine dose than after the first dose, while no significant difference emerged in the number of SARS-CoV-2-experienced subjects reporting side effects after the first or second vaccine dose.
Table 2. Frequency of HCWs reporting vaccine-associated side effects according to SARS-CoV-2 infection status
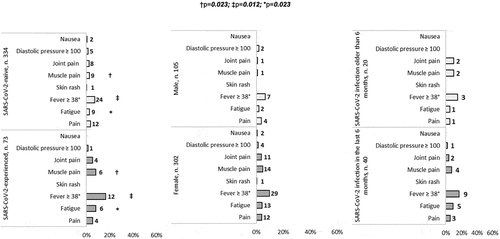
The most common side effect was localized injection-site pain, followed by fatigue, fever, muscle pain, joint pain, nausea and very rarely skin rash and blood hypertension. Systemic intense (grade 3) events were more frequent in SARS-CoV-2-experienced than in SARS-CoV-2-naive vaccine recipients, but no significant association between the frequency of grade 3 events and the time elapsed from the diagnosis of SARS-CoV-2 infection was seen ().
Figure 2. Relative frequency of vaccine-associated side effects (grade 3) compared between SARS-CoV-2-experienced and -naive participants, between male and females and between HCWs with SARS-CoV-2 infection within the 6 months preceding vaccination and HCWs with a SARS-CoV-2 infection past this time.
shows the multivariable logistic regression analysis of the factors associated with the odds of developing a systemic grade 3 event after vaccine exposure. Post-vaccination anti-SARS-CoV-2 spike IgG levels ≥20,000 AU/mL was independently associated with the risk of fever ≥38°C (adjusted odds ratio [aOR] 5.122, 95% CI 2.368–11.080, p < .0001).
Table 3. Multivariable logistic regression analysis of factors associated with the risk of developing fever ≥38°C, intense myalgias and fatigue after COVID-19 vaccination.*
Moreover, to be SARS-COV-2-naive was independently associated with a reduced likelihood of presenting fatigue (adjusted odds ratio [aOR] 0.281, 95% CI 0.084–0.935, p = .038).
Discussion
In this study we have assessed the neutralizing antibody response to BNT162b2 mRNA COVID-19 vaccine in previously infected and uninfected HCWs. The vaccine resulted effective and well tolerated, with no severe side effects reported.
In accordance with previous reportsCitation15–20 vaccination of individuals with previous SARS-CoV-2 infection lead to anti-S IgG levels at least twice as high as SARS-CoV-2 -naive subjects, irrespective of the loss of SARS-CoV-2 N antibodies and of the time elapsed from infection diagnosis. Notably, in patients with a confirmed date of SARS-CoV-2 infection, over a median of 80 days [IQR, 72–257] from the diagnosis, 49.3% of subjects resulted negative for anti-N IgG though 86% of them still had anti-SARS-CoV-2 spike IgG antibodies. Participants with anti-N IgG loss had an older SARS-CoV-2 infection compared with subjects with detectable N IgG. This finding confirms previous observation on SARS-CoV-2 antibody kineticCitation21 that showed IgG titers to RBD, S1 and N antigens to start their decrease in patients after the first month from the onset of symptoms, particularly in asymptomatic infection or mild disease. We also confirmed anti-N-antibodies to have a shorter post-infection persistence than anti-S-antibodiesCitation22, thus making the N-specific antibodies detection to estimate SARS-CoV-2 seroprevalence of low utility.
Moreover, SARS-CoV-2 neutralizing antibody levels measured after one dose of vaccine in a subgroup of previously infected individuals were similar to those measured after the second dose in naive subjects, confirming a single dose of the vaccine to be likely sufficient for the induction of an effective response in previously infected individuals.Citation16–20 This approach might be helpful in widening the vaccine supply, particularly in countries with limited availability and is supported by the recent Italian national recommendations that indicate a one-dose shot in subjects who were diagnosed with SARS-CoV-2 infection between 3 and 6 months before vaccination.Citation23
We did not observe a different response in neutralizing antibody levels between males and females; however, and consistently with the findings by Abu Jabal, et al.Citation15 significantly lower antibody levels were observed with increasing age in SARS-CoV-2-naive subjects.
Although no serious adverse events have been reported in our vaccine recipients, in agreement with previous reports, a high number of mild to moderate side effects occurred, particularly after the second dose.Citation24,Citation25 In agreement with Krammer et al.,Citation16 symptoms like muscle pain and fatigue were significantly more pronounced in individuals with past SARS-CoV-2 infection than in subjects SARS-CoV-2-naive.
Interestingly, we demonstrated a direct relationship between the onset of fever ≥38°C and high SARS-CoV-2 IgG antibody levels elicited by the vaccination. This finding confirms a strong vaccine reactogenicity to be strictly correlated with antibody response levels.Citation16
The main limitation of the study is represented by the lack of an available standardized serological test which makes challenging the comparison of our results with those of other studies and to interpret the clinical meaning of quantitative serology response to vaccination and its effectiveness in avoiding infection. Indeed, although anti-spike antibodies levels have been associated with neutralizing activity,Citation12,Citation13,Citation26 our study was not designed to determine their effects on protection from infection and its clinical consequences. Nevertheless, the incidence decrease of COVID-19 from pre- to post-vaccination has been well established, although positive tests in asymptomatic individuals have been observed following vaccination.Citation27–30 However, reliable immune markers like antibody levels that correlate with protection from SARS-CoV-2 are still under investigation. Although several studies to date, including ours, showed that most of vaccinated people develop specific antibodies against SARS-CoV-2, it is not yet defined the range of antibody levels above which individuals are protected from severe COVID-19 and death, or even from infection and transmission. Recently, a threshold of IgG(S-RBD) levels at or above 4,160 AU/mL have been proposed as a surrogate measure of antibody neutralization on a plaque reduction neutralization test,Citation31 but no association with clinical outcomes have been provided. Moreover, humoral response to SARS-CoV-2 vaccination do not give any insight into other important aspects of immunity, including cellular immune response and further analyses are needed to reliably assess the effectiveness and duration of humoral and cellular immune response.
Other limitations of the study are: 1) despite the large number of participants, the study is limited to HCWs who volunteered to be tested and vaccinated, thus some selection bias might not be excluded, i.e. HCWs with a previous diagnosis of SARS-CoV-2 infection who willingly postpone vaccination; 2) as elderly individuals are under-represented in a working setting like the one described, although statistically significant, the lower antibody levels observed with increasing age may be underestimated and not representative of general population, as well as the lack of extreme ages and of information on co-morbidities.
Finally, we observed a relatively lower seroprevalence of anti-S-IgG antibodies at baseline in our cohort of HCWs, compared to other reports.Citation32 In a recent metanalysisCitation33 the overall seroprevalence of SARS-CoV-2 antibodies among HCWs reported by August 2020 was 8.7% with a wide range (from 0% to 45.3%). Our results confirmed the relatively low seroprevalence observed in HCWs at LSUH between March and May 2020Citation10 and is likely the result of a deep re-organization of our hospital that closed down its routine activities as soon as the number of COVID-19 cases raised in Milan, being a referral Hospital for Infectious Diseases with highly trained personnel to infection control measures.
In conclusion, the BNT162b2 mRNA COVID-19 vaccine induced a high rate of serological response in a large cohort of HCWs with antibody levels higher in HCWs with past COVID-19 diagnosis in whom the levels after the first dose was comparable to the complete vaccination in naive controls. This suggests that a single dose of mRNA vaccine might evoke effective protection in prior SARS CoV-2 infected subjects, although these results need to be confirmed on elderly and fragile population.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- World Health Organization (WHO). Coronavirus disease (COVID-19) weekly epidemiological update and weekly operational update. [accessed 2021 May 1]. https://covid19.who.int/.
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–16. doi:10.1056/NEJMoa2035389. PMID: 33378609.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–15. doi:10.1056/NEJMoa2034577. PMID: 33301246.
- Walsh EE, Frenck RW Jr, Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383:2439–50. doi:10.1056/NEJMoa2027906.
- Ewer KJ, Barrett JR, Belij-Rammerstorfer S, Sharpe H, Makinson R, Morter R, Flaxman A, Wright D, Bellamy D, Bittaye M, et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat Med. 2020;27:270–78. doi:10.1038/s41591-020-01194-5.
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:9–111. doi:10.1016/S0140-6736(20)32661-1.
- Sahin U, Muik A, Vogler I, Derhovanessian E, Kranz LM, Vormehr M, Quandt J, Bidmon N, Ulges A, Baum A, et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. 2021;595(7868):572–77. doi:10.1038/s41586-021-03653-6.
- https://www.aifa.gov.it/documents/20142/823882/Comunicato_AIFA_620_EN.pdf
- Anichini G, Terrosi C, Gandolfo C, Gori Savellini G, Fabrizi S, Miceli GB, Cusi MG. SARS-CoV-2 antibody response in persons with past natural infection. N Engl J Med. 2021;385:90–92. doi:10.1056/NEJMc2103825.
- Milazzo L, Lai A, Pezzati L, Oreni L, Bergna A, Conti F, Meroni C, Minisci D, Galli M, Corbellino M, et al. Dynamics of the seroprevalence of SARS-CoV-2 antibodies among healthcare workers at a COVID-19 referral hospital in Milan, Italy. Occup Environ Med. 2021;78(8):541–47. oemed-2020-107060. doi:10.1136/oemed-2020-107060. Online ahead of print.
- Wassie GT, Azene AG, Bantie GM, Dessie G, Aragaw AM. Incubation period of severe acute respiratory syndrome novel Coronavirus 2 that causes Coronavirus disease 2019: a systematic review and meta-analysis. Curr Ther Res Clin Exp. 2020;93:100607. doi:10.1016/j.curtheres.2020.100607.
- Fujigaki H, Inaba M, Osawa M, Moriyama S, Takahashi Y, Suzuki T, Yamase K, Yoshida Y, Yagura Y, Oyamada T, et al. Comparative analysis of antigen-specific anti-SARS-CoV-2 antibody isotypes in COVID-19 patients. J Immunol. 2021;206:2393–401. doi:10.4049/jimmunol.2001369.
- Poh CM, Carissimo G, Wang B, Amrun SN, Lee CY, Chee RS, Fong SW, Yeo NK, Lee WH, Torres-Ruesta A, et al. Two linear epitopes on the SARS-CoV-2 spike protein that elicit neutralizing antibodies in COVID-19 patients. Nat Commun. 2020;11:2806. doi:10.1038/s41467-020-16638-2.
- Bryan A, Pepper G, Wener MH, Fink SL, Morishima C, Chaudhary A, Jerome KR, Mathias PC, Greninger AL. Performance characteristics of the Abbott architect SARS-CoV-2 IgG assay and seroprevalence in Boise, Idaho. J Clin Microbiol. 2020;58:e00941–20. doi:10.1128/JCM.00941-20.
- Abu Jabal K, Ben-Amram H, Beiruti K, Batheesh Y, Sussan C, Zark S, Edelstein M. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill. 2021;26:2100096. doi:10.2807/1560-7917.ES.2021.26.6.2100096.
- Krammer F, Srivastava K, Alshammary H, Amoako AA, Awawda MH, Beach KF, Bermúdez-González MC, Bielak DA, Carreño JM, Chernet RL, et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384(14):1372–74. doi:10.1056/NEJMc2101667.
- Saadat S, Rikhtegaran Tehrani Z, Logue J, Newman M, Frieman MB, Harris AD, Sajadi MM. Binding and neutralization antibody titers after a single vaccine dose in health care workers previously infected with SARS-CoV-2. JAMA. 2021;325(14):1467–69. doi:10.1001/jama.2021.3341.
- Prendecki M, Clarke C, Brown J, Cox A, Gleeson S, Guckian M, Randell P, Dalla Pria A, Lightstone L, Xu XN, et al. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet. 2021;397:1178–81. doi:10.1016/S0140-6736(21)00502-X.
- Manisty C, Otter AD, Treibel TA, McKnight Á, Altmann DM, Brooks T, Noursadeghi M, Boyton RJ, Semper A, Moon JC. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;397:1057–58. doi:10.1016/S0140-6736(21)00501-8.
- Gobbi F, Buonfrate D, Moro L, Rodari P, Piubelli C, Caldrer S, Riccetti S, Sinigaglia A, Barzon L. Antibody response to the BNT162b2 mRNA COVID-19 vaccine in subjects with prior SARS-CoV-2 infection. Viruses. 2021;13:422. doi:10.3390/v13030422.
- Röltgen K, Powell AE, Wirz OF, Stevens BA, Hogan CA, Najeeb J, Hunter M, Wang H, Sahoo MK, Huang C, et al. Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome. Sci Immunol. 2020;5:eabe0240. doi:10.1126/sciimmunol.abe0240.
- Grandjean L, Saso A, Torres Ortiz A, Lam T, Hatcher J, Thistlethwayte R, Harris M, Best T, Johnson M, Wagstaffe H, et al. Long-term persistence of spike antibody and predictive modeling of antibody dynamics following infection with SARS-CoV-2. Clin Infect Dis. 2021;ciab607. Online ahead of print. doi:10.1093/cid/ciab607.
- Ministero della Salute. [accessed 2021 October 25].https://www.trovanorme.salute.gov.it/renderNormsanPdf?anno=2021&codLeg=79033&parte=1%20&serie=null
- Chapin-Bardales J, Gee J, Myers T. Reactogenicity following receipt of mRNA-based COVID-19 vaccines. JAMA. 2021;325:2201–02. doi:10.1001/jama.2021.5374.
- Saita M, Yan Y, Ito K, Sasano H, Seyama K, Naito T. Reactogenicity following two doses of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers in Japan. J Infect Chemother. 2021 Sep 16. S1341-321X(21)00258-0. Online ahead of print. doi:10.1016/j.jiac.2021.09.009.
- Wajnberg A, Amanat F, Firpo A, Altman DR, Bailey MJ, Mansour M, McMahon M, Meade P, Rao Mendu D, Muellers K, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370:1227–30. doi:10.1126/science.abd7728.
- Haas JE, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, Brooks N, Smaja M, Mircus G, Pan K, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–29. doi:10.1016/S0140-6736(21)00947-8.
- Hall VJ, Foulkes S, Saei A, Andrews N, Oguti B, Charlett A, Wellington E, Stowe J, Gillson N, Atti A, et al. COVID-19 vaccine coverage in health-care workers in England and e ectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397:1725–35. doi:10.1016/S0140-6736(21)00790-X.
- Vasileiou E, Simpson CR, Shi T, Kerr S, Agrawal U, Akbari A, Bedston S, Beggs J, Bradley D, Chuter A, et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397:1646–57. doi:10.1016/S0140-6736(21)00677-2.
- North CM, Barczak A, Goldstein RH, Healy BC, Finkelstein DM, Ding DD, Kim A, Boucau J, Shaw B, Gilbert RF, et al. Determining the incidence of asymptomatic SARS-CoV-2 among early recipients of COVID-19 vaccines: a prospective cohort study of healthcare workers before, during and after vaccination [DISCOVER-COVID-19]. Clin Infect Dis. 2021;ciab643. Online ahead of print. doi:10.1093/cid/ciab643.
- Ebinger JE, Fert-Bober J, Printsev I, Wu M, Sun N, Prostko JC, Frias EC, Stewart JL, Van Eyk JE, Braun JG, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med. 2021;27:981–84. doi:10.1038/s41591-021-01325-6.
- Varona JF, Madurga R, Peñalver F, Abarca E, Almirall C, Cruz M, Ramos E, Castellano Vázquez JM. Seroprevalence of SARS-CoV-2 antibodies in over 6000 healthcare workers in Spain. Int J Epidemiol. 2021;50:400–09. doi:10.1093/ije/dyaa277.
- Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers: a systematic review and meta-analysis. J Hosp Infect. 2021;108:120–34. doi:10.1016/j.jhin.2020.11.008.

