ABSTRACT
Vaccine hesitancy (VH) has emerged as a recognized threaten to contain the COVID-19 pandemic. Historically, low vaccine acceptance rates had been described among patients with rheumatic diseases (RMDs). The study objective was to determine COVID-19 VH among Mexican outpatients with RMDs and validate the COVID-19 VH questionnaire. This cross-sectional study was developed in three steps. Step 1 consisted of translation/cultural adaptation of the Oxford-COVID-19-VH questionnaire. Step 2 consisted of pilot testing and questionnaire feasibility, content, construct and criterion validity, reliability (internal consistency and temporal stability) and questionnaire sensitivity to change. Step 3 consisted of VH phenomenon quantification in patients from two metropolitan tertiary-care-level centers. Step 1 followed ISPOR-task-force recommendations. Patients who participated in step 2 (n = 50 for pilot testing/feasibility and n = 208 for questionnaire validation [91 in test–retest and 70 in questionnaire-sensitivity to change]) and step 3 (n = 600) were representative outpatients with RMDs. The seven-item COVID-19 VH questionnaire was found feasible, valid (experts’ agreement ≥80%; a 1-factor structure accounted for 60.73% of the total variance; rho = 0.156, p = .025 between COVID-19 VH questionnaire and score from the Spanish version of the Vaccine Hesitancy Scale; and lower questionnaire scores in patients who reported 5 years-previous influenza vaccination), reliable (Cronbach’s ɑ = 0.889, intra-class correlation coefficient = 0.933 and 95% confidence interval = 0.898–0.956) and sensitive to change (effect size = 1.17 and 0.86, respectively, in patients who decreased [n = 34] and increased [n = 31] questionnaire-score after intervention). VH phenomenon was 35.5%. VH phenomenon was present in a substantial number of Mexican patients with RMDs. The COVID-19 VH questionnaire showed good psychometric properties to assess COVID-19 VH in our population.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has imposed an enormous disease burden worldwide, and there are currently limited treatment options.Citation1,Citation2 The risk of developing severe complications or death is more significant among older patients and those with comorbidities than their counterparts.Citation3 There are conflicting data concerning the actual incidence and severity of SARS-CoV-2 infection in patients with rheumatic diseases (RMDs). Registry data and meta-analyses have shown that the incidence is greater among patients with RMDs and that there might be an association with a prednisone dose of >10 mg/day.Citation4–6 Meanwhile, recent viewpoints suggest overall a similar risk and similar course to those of the general population, with some rheumatic diagnoses, disease activity statuses, and disease-specific treatments associated with worse outcomes.Citation2
Developing a safe, effective, and affordable vaccine has been recognized as the most promising strategy to contain the COVID-19 pandemic. However, vaccine hesitancy (VH) confers a different and unique hurdle to researchers, scientists, governments, and community leaders.Citation7 VH is the term used to define refusal or reluctance in accepting vaccination despite the availability of vaccination services.Citation8 Before the pandemic, the World Health Organization (WHO) identified VH as a top global health threat in 2019.Citation9 More recently, this phenomenon has been defined as an imminent threat in the battle against COVID-19 because achieving herd immunity depends on the population’s willingness to accept vaccination.Citation10,Citation11 A 32-country survey in 26,758 individuals, conducted between October 21 and December 16, 2020, confirmed significant variations among countries of potential acceptance of COVID-19 vaccines.Citation12
Moreover, results from additional surveys overall seem to suggest that willingness to vaccinate against COVID-19 has declined globally between the early months of the pandemic and December 2020.Citation13–19 However, rates tend to fluctuate and might be associated with COVID-19 risk perception,Citation20 and vaccine safety-related issues.Citation21 In addition, vaccination campaigns are becoming part of the political debates. At the same time, there is considerable scientific uncertainty. Available vaccines are limited, particularly in developing countries, and politicians – rather than experts – are the public face of crisis management, all of whom might impact VH rates.Citation15
Currently, there are limited studies that assess COVID-19 VH among patients with RMDs.Citation22–27 Previous studies highlight that vaccine uptake in general, is deficient among specific rheumatic diagnoses such as Rheumatoid Arthritis (RA), although with significant variations among countries.Citation28,Citation29 People with RMDs may have specific concerns on how their underlying disease and their immunomodulatory therapies affect the benefit and safety of receiving COVID-19 vaccination, in addition to fear about potential RMD´s flares after vaccination, that might be related or not to holding antirheumatic medications.Citation6,Citation30 A recent international survey assessed patient perception and outcomes related to COVID-19 vaccines among 2860 vaccinated adult patients with RMDs (mean age 55.3 years, 86.7% female, 86.3% white, and 42.3% with RA).Citation30 The study showed that adverse events affected up to 48% of the participants (primarily fatigue, headache, and widespread muscle/joint pains), while flares of the underlying rheumatic disease that required medication changes, occurred in less than 5% of the population. Finally, recommendations from healthcare professionals are strong drivers of vaccine acceptance among individuals and patients with RMDs,Citation22,Citation23,Citation31 and international organizations currently agreed that rheumatic patients might benefit from COVID-19 vaccination.Citation32–34 Moreover, among the vaccinated adult patients with RMDs previously described, 82% had discussed COVID-19 vaccination with their healthcare provider, and 96% reported that vaccination was recommended.Citation30 Nonetheless, when it comes from VH, what matters most varies across time and communities, highlighting the relevance of addressing the topic from a cultural perspective, which has other ethical implications.
The study’s primary objective was to determine COVID-19 VH among Mexican outpatients from two national referral centers for RMDs. As a secondary objective, we aimed to adapt and validate the Oxford COVID-19 Vaccine Hesitancy ScaleCitation19 in Mexican outpatients with RMDs.
Patients and methods
Setting and study population
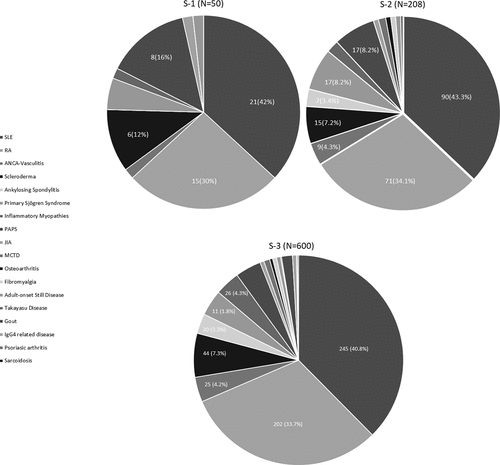
This cross-sectional study was performed between March 1st and August 13th, 2021, and two tertiary-care level and academic centers for RMDs located in Mexico City, contributed with participants to assess VH phenomenon (ClinicalTrials.gov Identifier: NCT04775563): The Instituto Nacional de Ciencias Médicas y Nutrición Salvador-Zubirán (INCMyN-SZ) and the Hospital General de México Dr. Eduardo Liceaga (HGM). Before the pandemic, around 5,000 and 13,000 patients were attending each outpatient clinic and suffered from a variety of RMDs. The most frequent diagnoses were Systemic Lupus Erythematosus (SLE) and RA ().
Table 1. No (%) of patients with at least one visit to the outpatient clinic with the ten most frequent diagnoses specified (January 2019 to December 2019)
During the study period, consecutive outpatients with a definite rheumatologic diagnosis according to the attendant rheumatologist criteria (among those included in ) were invited to participate. Exclusion criteria included patients on palliative care.
Study design
This cross-sectional study was performed in three phases.
Phase 1 consisted of the translation and cross-cultural adaptation to Mexican patients with RMDs of the Oxford COVID-19 Vaccine Hesitancy Scale, a 1-factor predominant, validated seven-items instrument that assesses VH in adult individuals.Citation19 Briefly, the original scale was tested and validated in 5114 adult individuals from the UK; gender, age, ethnicity, income, and region quotas were considered. Initially, a pool of 15 items was developed, and feedback from public involvement groups, including ethnic minority health groups representatives, was obtained. Exploratory and confirmatory factor analyses were used to derive a final seven-item scale, with higher scores indicating a higher level of VH. Principles of good practice for the translation and cultural adaptation process for patient-reported-outcomes measures from the ISPOR task force were followed,Citation35 and the primary author of the original scale agreed to collaborate.
Phase 2 consisted of validating the translated and adapted to patients with RMDs version, performed after pilot testing in 50 outpatients with RMDs to examine questionnaire feasibility (). Judgment experts determined COVID-19 VH questionnaire content validity. The expert committee was integrated by six rheumatologists and four specialists in infectious diseases. Experts were directed to evaluate items and their scale responses according to the following criteria: Relevance, good wording, and appropriate language and meaning for the target population; they were also directed to evaluate the questionnaire face validity. Construct validity was evaluated using factorial analysis. Criterion validity was examined according to the ten-item Likert scale from the Spanish version for Guatemala of the Vaccine Hesitancy Scale (convergent validity)Citation36 and according to self-referred previous 5-years influenza vaccination (divergent validity). COVID-19 VH questionnaire reliability was assessed with internal consistency and temporal stability (test-retest), which was tested after the questionnaire was applied to (at least) 50 patients, twice in the same day, with (mean) 5 hours between both applications; this short lag-time was chosen due to daily intensive information related to COVID-19 vaccines safety in the social media, while considering recall bias. Finally, questionnaire sensitivity to change was examined in (at least) 20 patients per group, those who increased and those who decreased VH score after an informative/educative intervention. The questionnaire was applied twice, at baseline and two weeks apart, after patients received COVID-19 vaccine infographics and were allowed to communicate with dedicated healthcare professionals to resolve doubts. The information was locally adapted from the Center for Disease Control and Prevention COVID-19 vaccine.Citation37 The two-week lag time was considered convenient to ensure patient review of the educative material and comfortable communication with experts.
Table 2. COVID-19 VH questionnaire feasibility
Phase 3 was designed to assess the VH phenomenon in the target population and consisted of the application of the validated version of the COVID-19 VH questionnaire to consecutive outpatients with RMDs, which is a seven-items instrument, based on a five-point Likert scale from one to five, with higher scores translating into a higher level of VH (Appendix 1). Standardized formats were used to assess in all the patients, the current level of disease activity (without disease activity and low, moderate, and high disease activity level), disease activity control (Yes/No), and (direction of) treatment recommendations at the end of the consultation (no changes, treatment intensification, treatment reduction, treatment changes because of adverse events and treatment re-initiation after withdrawal).Citation38
Relevant sociodemographic variables (sex, years of age and formal education, occupation, living with a partner, and universal access to health care), disease-related variables (specific rheumatic diagnosis, years of disease duration, comorbid conditions based on the Rheumatic Diseases Comorbidity Index,Citation39 one year-previous hospitalization and number), and treatment-related variables (immunosuppressive treatment and number of immunosuppressive drugs/patient, and corticosteroid use) were obtained in standardized formats after a careful chart review and patient interview, during phases 2 and 3.
Questionnaires application
Consecutive patients with a definite rheumatic diagnosis were invited to participate. Patients were identified at the outpatient clinic while waiting for a scheduled consultation. Upon patient´s agreement to participate, interviews were performed in a designated area for research purposes, and questionnaires and scales were administered to patients, who were instructed to fill them. Instruments were applied in a specific and standard order, with the COVID-19 VH questionnaire first applied. If necessary, patients could be assisted to fill questionnaires by trained personnel.
Strategies for quality control
Before the study initiation, its design and objectives were shared with the physicians involved in outpatients’ care, and agreement on terminology and concepts was achieved. Also, patients with RMDs participated in items adaptation to RMDs and pilot testing to avoid bias in question design and pretest bias.Citation40 In all the cases, questionnaires and scales were applied by trained personnel not involved in patient care, on the same day that patients visited their primary rheumatologists, and in a particular location within the outpatient clinic that was suitable for clinical research, to facilitate confidentiality and to reduce bias associated to questionnaire administration.Citation40 Finally, questionnaires and scales were reviewed to identify missing information.
Description of sample and sample size calculation
Three different convenience samples of consecutive outpatients were included, and quotes were considered to represent the distribution of the other rheumatic diagnoses for each outpatient clinic. In addition, nine outpatients participated in phase 1.
The first sample (S-1) included 50 outpatients from center 1. The number of patients had followed the recommendations for pilot testing.Citation41
The second sample (S-2) included (at least) 200 outpatients from center one and was used for the validation process. The sample size was based on published methodological recommendations.Citation41 The sample size for questionnaire sensitivity to change was estimated in (at least) 20 randomized patients per group (with post-intervention increase and decrease COVID-19 VH questionnaire score), to detect a moderate effect size (Cohen’s d = 0.5), 90% of power, and alpha = 0.05, one-tailed.Citation42,Citation43 Similarly, (at least) 50 additional patients were randomly selected for test-retest.
The patient’s randomization process was conducted through a computer-generated randomization scheme using randomly permuted blocks (software available at website www.randomization.com). A priori, an independent collaborator generated the random allocation sequence. The list was divided into two groups of patients, those allocated to sensitivity to change maneuver and those allocated to test-retest; it also included a consecutive number and the assigned group.
The third sample (S-3) included (at least) 600 outpatients derived from both centers. Together, outpatient clinics registered 18,000 patients with RMDs in 2019. We estimated the sample size to detect VH considering a prevalence of 30%.Citation20 We obtained 577 patients with a 95% confidence level and 4% precision.
All sample size calculations were performed using G power 3.1.9.2 version.
Statistical analysis
The COVID-19 VH questionnaire score was calculated as the mean of individual items scores, and the “Do not know” option response was excluded from scoring.Citation19 In addition, we agreed that to provide a score, at least six out of the seven items should be scored, which led that (mean) score ranged from 0.86 to 7.00. VH was defined if the score assigned was ≥ 1.86. This cutoff was based on the 75-percentile data logarithmic transformation.Citation41
Phases 1–3: Descriptive statistics were performed to describe the variables of the patients included in the three samples, with frequencies and percentages for categorical variables or the mean/median and standard deviation (SD)/Q25-Q75 for continuous variables with normal/non-normal distribution.
Phase 2: Content and face validity by experts were examined with agreement percentages. Construct validity was evaluated using exploratory factorial analysis (principal components). Criterion validity (convergent) was analyzed using Spearman rank correlation coefficient (rho) to determine the strength of the relationship between the COVID-19 VH questionnaire score and the ten-item-Likert-scale score from the Spanish version for Guatemala of the Vaccine Hesitancy Scale;Citation36 the strength of the correlation was interpreted according to published recommendations.Citation41 Divergent validity was examined using the Mann Whitney U test to compare the COVID-19 VH questionnaire score between patients who self-reported five years of Influenza vaccination and those who denied it. Cronbach’s α was used to assess the internal consistency of the questionnaire. For temporal stability/test-retest, intra-class correlation coefficients (ICC) and their 95% confidence intervals (CI) were calculated using a single measurement, absolute-agreement, 2-way mixed-effects model. Cronbach’s α, ICC, and 95% CI interpretations followed published recommendations.Citation42,Citation44,Citation45 COVID-19 VH questionnaire sensitivity to change was defined with the distribution-based indicator effect size.Citation42,Citation43 Finally, floor and ceiling effects of the questionnaire were determined as the percentage of patients who achieved the lowest and highest score of the scale, respectively.
Missing data were below 1%, and no imputation was performed.
All statistical analyses were performed using Statistical Package for the Social Sciences version 21.0 (SPSS Chicago IL). A value of p < .05 was considered statistically significant.
Ethics
The Internal Review Board from each Institution approved the study. Written informed consent was obtained from all the patients who participated.
Results
Phase 1. Translation to Spanish and adaptation to patients with RMDs of the Oxford COVID-19 vaccine hesitancy scale
The process included ten steps, which are summarized in . The resultant COVID-19 VH questionnaire was subjected to psychometric validation.
Table 3. Summary of the translation and adaptation to RMDs of the Oxford COVID-19 vaccine hesitancy scale
Phase 2. Validation of the translated and adapted to patients with RMDs version: the COVID-19 VH questionnaire
Samples’ description ()
Table 4. Description of the samples´ characteristics
S-1 and S-2 included patients which data were used for the validation process. Twenty-three patients scored <six items, and their data were excluded from analysis, although their characteristics were similar to those of the patients analyzed. The final number of patients included in each sample was 50 in S-1 and 208 in S-2.
Patients from both samples were representative of outpatients with RMDs from center 1. Briefly, they were primarily middle-aged females, with (median) 12 years of formal education; patients had substantial disease duration, and the underlying RMD was under control; the majority of them were on immunosuppressive drugs, and almost half of them on corticosteroids.
summarizes the most frequent diagnoses.
Pilot testing for COVID-19 VH questionnaire feasibility
The questionnaire was found feasible by 50 patients with RMDs, as summarized in .
Judgment experts’ questionnaire content validity
Experts generally agreed on the item and scale response evaluation (≥90% agreement for item relevance and appropriate language and meaning for the target population and ≥80% agreement for good wording), but item 6, which reached 70% agreement for the “good wording” section; after modifications, 90% of the experts approved item 6. In addition, the questionnaire showed 100% face validity.
COVID-19 VH questionnaire construct validity
summarizes results from factor analysis. The KMO measure was 0.886, and a significant result (X2 = 744.956, p ≤ 0.001) for the Bartlett sphericity test confirmed the adequacy of the sample. A 1-factor structure was extracted, which accounted for 60.73% of the total variance. The post-validation structure of the questionnaire did not differ from that of the original scale.
Table 5. Item loadings for the 1-factor of the C19-VH questionnaire
COVID-19 VH questionnaire criterion validity
Convergent validity
There was a low but significant correlation between the questionnaire score and the ten-item-Likert-scale score from the Spanish version for Guatemala of the Vaccine Hesitancy Scale,Citation36 rho = 0.156, p = .025.
Divergent validity
Patients who reported five years of previous influenza vaccination had lower questionnaire scores than those who denied previous vaccination: 1.6 (1.1–1.9) vs. 1.9 (1.4–2.3), p ≤ .001.
COVID-19 VH questionnaire reliability: internal consistency and temporal stability
Results of internal consistency (Cronbach’s α) and temporal stability/test-retest (ICC and 95% CI) for individual items scores and global questionnaire score are presented in , which additionally includes median (Q25-Q75) scores and floor and ceiling effects. Test-retest was performed in 91 patients randomly selected from S-2.
Table 6. Psychometric characteristics of the C19-VH questionnaire
COVID-19 VH questionnaire sensitivity to change
There were 70 patients from S-2 (excluding 91 patients randomized for test-retest) who were randomly selected for sensitivity to change analysis. They all received infographics as previously described, and 29 patients (41.4%) communicated with experts. Following the educative intervention, 34 patients decreased the COVID-19 VH questionnaire score (change of 76%) and 31 patients increased the score (change of 50%), and effect size values were 1.17 and 0.86, respectively. Five patients maintained the same score.
Phase 3. Covid-19 VH phenomenon
S-3 description
summarizes patients’ characteristics. Briefly, the 600 patients included were primarily middle-aged (46 years [35–56]), females (86.5%), with (median) 12 years of formal education; almost half were married or living with a partner (52.3%), while 42% referred having a job (formal or non-formal). The most frequent diagnoses were SLE (40.8%) and RA (33.7%), and additional diagnoses are presented in . Patients had (median) 9 years of disease duration and Rheumatic Disease Comorbidity Index score of 0. The majority of them had adequate control of the disease activity status (75.6%) according to the physician evaluation and were on immunosuppressive drugs (88.2%), while 43.2% received corticosteroids.
VH phenomenon
There were 19 questionnaires with less than six items scored, and they were discarded from the analysis. Median (Q25-Q75) COVID-19 VH questionnaire score in the 581 questionnaires left was 1.57 (1.29–2). Based on the cutoff proposed (≥1.86, which corresponds to 75-percentile data logarithmic transformation), 206 patients (35.5%) had VH.
Discussion
The present study revealed that VH was present in 35.5% of Mexican outpatients with RMDs, from two academic centers in Mexico City. To our knowledge, this is the first study that uses a validated questionnaire/scale to assess COVID-19 VH among patients with RMDs. In them, VH-related knowledge has been conceived based on online independent survey applications,Citation22–25 phone survey applications,Citation26 and face-to-face interview/survey application,Citation27 all of whom lack a solid development and validation process. Meanwhile, the use of self-report or poorly designed instruments can result in misclassification bias. VH is a complex theoretical construct, which measurement should ideally involve its operationalization in a defined variable and the development and application of an instrument to its adequate quantification. Key indicators of the quality of a measuring instrument are its reliability and validity; in addition, the responsiveness of the measure to change is of interest in the VH phenomenon, where its modification in the appropriated direction as a result of healthcare professional’s intervention is a primary target.Citation46
The COVID-19 VH questionnaire development and validation processes were rigorous methodological.Citation19,Citation35 Relevant healthcare professionals (considering the target population), primarily rheumatologists, infectious disease specialists and psychologists, and patients with representative RMDs contribute to reframing the construct under validation. Clinicians may be the best observers for certain aspects of RMDs, but only patients can report on more subjective elements such as the expressed intent to accept a COVID-19 vaccine.Citation47 The wording for single items was optimized through a process of face validity testing with a multidisciplinary group of healthcare providers. Meanwhile, instruction’s wording and clarity were submitted to patient’s evaluations, as was the final version of the questionnaire, before its formal validation process; the changes subsequently made were crucial for enhancing acceptance by the intended population. Importantly, different samples of consecutive outpatients with RMDs, representative of real-world outpatients attending two tertiary-care-level centers, were used for analysis. Accordingly, our results could be generalized to populations of Spanish-speaking patients with similar characteristics.
The COVID-19 VH questionnaire showed adequate internal consistency with a good Cronbach’s α coefficient for the total scale.Citation44 The same researcher assessed the test-retest reliability in 91 patients, and we observed an ICC and 95% CI indicating good reliability.Citation45 The construct validity was demonstrated by KMO sampling and Bartlett's test of sphericity, both confirming the adequacy of the sample size for conducting factor analysis,Citation48 and a single factor structure was extracted, accounting for 60.73% of the variance. Face and content validity were examined by a multidisciplinary group of experts involved in RMDs and COVID-19 management (during the pandemic, the Institution was declared a dedicated COVID-19 hospital). Divergent validity was documented by significant differences in VH COVID-19 scores between those who reported five years-previous Influenza vaccination and their counterparts. Also, the COVID-19 VH global score showed neither floor nor ceiling effect, defined when more than 15% of the patients achieved the lowest or highest score, respectively; floor and ceiling effects can reduce the possibility of detecting change over time. The questionnaire showed sensitivity to change, was feasible based on patients’ evaluation, and was suitable for low-literacy patients. A more significant percentage of change was detected in the patients who improved the score compared to those who deteriorated the score. Surprisingly, a substantial number of patients increased the score and indicated that the educative maneuver was unsuccessful in them, probably related to personal solid anti-vaccine beliefs; also, in these patients, the educative intervention might have been insufficient to compensate anti-vaccine feedback from social media, friends and/or family. Finally, the COVID-19 VH questionnaire had a single score which ensures simplicity in routine clinical practice, while a short time was required to fill the questionnaire (mean 1.6 minutes). Also, we propose that at least six out of seven items be scored to guarantee that the COVID-19 VH construct is comprehensively assessed.
Our rate of COVID-19 VH found (35.5%) was within the range of previous descriptions in different populations of patients with RMDs,Citation22–27,Citation49–51 all of whom were survey-based. Four studies were performed in Italy,Citation24,Citation26,Citation49,Citation50 and patient’s willingness to potentially receive a SARS-CoV-2 vaccine ranged from 54.9%Citation24 to 82%.Citation49 Gauer et al.Citation27 did face-to-face structured interviews with 280 Indian patients with RMDs and 102 controls, using a questionnaire previously tested in 20 patients; they found a vaccine acceptance of 54% among the patients. Yurttas et al.Citation25 conducted a web-based questionnaire survey in three groups of participants from Istanbul (Turkey), among whom 732 had RMDs; the online survey was designed and executed following published recommendationsCitation52 and was tested in 22 hospital workers; authors reported that 29.2% of the patients with RMDs were willing to get vaccinated, 19.0% were unwilling, and 51.8% were undecided. Boekel et al.Citation22 reported data from an online survey answered by 1361 patients with RMDs, 366 patients with multiple sclerosis, and 682 controls from the Netherlands; the proportion of patients and controls who would be willing to get vaccinated against SARS-CoV-2 was similar, 61% in the patient group and 65% in the control group. Felten et al.Citation23 applied 57 web-based questions regarding COVID-19 vaccination to 1266 patients from 56 countries with a self-reported diagnosis of systemic autoimmune or inflammatory rheumatic disease, and healthcare professionals; they found that 54.2% of the patients were willing to get vaccinated against SARS-CoV-2, 32.2% reported uncertainty and 13.6% refused vaccination. A cluster analysis of the previous results, showed that there were 53 Mexican patients with RMDs, further characterized in cluster 1 (“Most willing to get vaccinated,” n = 5), cluster 2 (“More hesitant,” n = 25) and cluster 3 (“Mostly opposed to getting vaccinated,” n = 23).Citation53 Finally, Ko et al.Citation51 applied an online de-identified survey to 3451 adult Australian patients from rheumatology clinics, to describe the SARS-CoV-2 VH rate and factors influencing it; the survey response rate was 18.6%, and 34.4% of the patients were vaccine-hesitant (unwilling or undecided); survey content validity and feasibility were explored with the participation of four specialist clinicians and three patients research partners. As shown, a wide range of COVID-19 VH rates had been described across different populations of patients with RMDs, which might be associated with factors related to population characteristics, the timing of the VH phenomenon measurement, and how the COVID-19 VH construct was defined and measured. Importantly, none of the studies considered Mexican patients, but the cluster analysis international study included 53 Mexican patients with RMDs, although specific diagnoses distribution was not detailed.Citation53 Recent local and vaccine-related scientific literature highlights that 79.8% of Mexican patients with RMDs were vaccinated for influenza at least once during 2019; the study addressed knowledge and attitudes about influenza vaccination in 223 patients with RMDs, primarily integrated with RA patients (64.8%).Citation54
Some limitations of the study need to be addressed. At first, the COVID-19 VH questionnaire validation process was conducted at a single academic center. At the same time, the VH phenomenon rate was established in two tertiary-care-level centers located in a metropolitan area, where attending RMDs patients might have particular characteristics which might affect the VH phenomenon rate. Second, most of the patients included had SLE and RA diagnoses, while other diagnoses were mildly represented in our patients. Third, the study was performed in Spanish-speaking Mexican patients, which might affect the generalizability of the results to all Spanish-speaking patients with RMDs; nonetheless, the seven-item questionnaire was found feasible in low literacy patients, and we consider it can be generalized to Spanish speaking patients from the Latin-American region. Forth, we did determine a limited number of psychometric characteristics of the questionnaire, those considered necessary for a first approach. Also, alternative and sophisticated strategies for judging the quality of a measuring instrument, such as Rasch models and item-response theory or latent-trait models, were not used. Fifth, patients included might have responded socially acceptable or in line with the impression they want to create, which might be particularly true in the context where the COVID-19 VH questionnaire was applied. Sixth, it is relevant to note that reported intentions may not always translate into vaccine uptake,Citation55 and the VH phenomenon rate obtained should be considered cautiously.
Conclusions
We presented the rationale and the methods for the translation and cultural adaptation and validation process of a questionnaire allowing for assessing the COVID-19 VH phenomenon in Spanish-speaking patients with RMDs. The questionnaire was valid and reliable and can be easily implemented in the outpatient setting and for research purposes, particularly in low literacy patients. Documenting VH rates is the first step and should be followed by collecting and analyzing data on the reasons for patients (and physicians) VH, which is critical for informing the design of effective vaccine messaging in the community of patients with RMDs.Citation53 We are currently performing a mixed-methods design study to address the topic comprehensively.
Published evidence regarding the RMDs-related COVID-19 VH phenomenon has been carried out mainly in developed countries. There is a need to address the research gap and consider data from Latin-American region patients, where highly represented low- and middle-income countries. As recently published,Citation56 a higher willingness to take a COVID-19 vaccine was found in low-middle income countries compared with the United States and Russia, in the general population. At the same time, health workers were the most trusted sources of guidance about COVID-19 vaccines. Finally, vaccination campaigns should focus on enhancing vaccine acceptance among patients with RMDs and translating the levels of stated acceptance into actual uptake.
Supplemental Material
Download ()Acknowledgments
We acknowledge the participation of Daniel Freeman, Gabriel Medrano, José Francisco Moctezuma, Conrado García, Julio Casasola, Janitzia Vázquez-Mellado, Gabriela Huerta, Roberto Cruz, Alfonso Gastélum-Strozzi, Lexli D. Pacheco-Santiago and Vivian A. Estrada-González.
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.2003649.
Disclosure statement
No potential conflict of interest was reported by theauthor(s).
Additional information
Funding
References
- Chang AY, Cullen MR, Harrington RA, Barry M. The impact of novel coronavirus COVID-19 on noncommunicable disease patients and health systems: a review. J Intern Med. 2021;289(4):450–62. doi:10.1111/joim.13184.
- Robinson PC, Yazdany J, Machado PM. Global research collaboration in a pandemic-challenges and opportunities: the COVID-19 global rheumatology alliance. Curr Opin Rheumatol. 2021;33(2):111–16. doi:10.1097/BOR.0000000000000783.
- Sarzi-Puttini P, Marotto D, Caporali R, Montecucco CM, Favalli EG, Franceschini F, Fredi M, Balduzzi S, Bazzani C, Bongiovanni S, et al. Prevalence of COVID infections in a population of rheumatic patients from Lombardy and Marche treated with biological drugs or small molecules: a multicentre retrospective study. J Autoimmun. 2021;116:102545. doi:10.1016/j.jaut.2020.102545.
- Gianfrancesco M, Hyrich KL, Al-Adely S, Carmona L, Danila MI, Gossec L, Izadi Z, Jacobsohn L, Katz P, Lawson-Tovey S, et al. Characteristics associated with hospitalization for COVID-19 in people with rheumatic disease: data from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis. 2020;79(7):859–66. doi:10.1136/annrheumdis-2020-217871.
- Akiyama S, Hamdeh S, Micic D, Sakuraba A. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheum Dis. 2020;13:218946. doi:10.1136/annrheumdis-2020-218946.
- Furer V, Rondaan C, Agmon-Levin N, van Assen S, Bijl M, Kapetanovic MC, de Thurah A, Mueller-Ladner U, Paran D, Schreiber K, et al. Point of view on the vaccination against COVID-19 in patients with autoimmune inflammatory rheumatic diseases. RMD Open. 2021;7(1):e001594. doi:10.1136/rmdopen-2021-001594.
- Coustasse A, Kimble C, Maxik K. Letter to the editor: COVID-19 and vaccine hesitancy a challenge the United States must overcome. J Ambulatory Care Manage. 2021;44(1):71–75. doi:10.1097/JAC.0000000000000360.
- MacDonald NE; SAGE Working Group on Vaccine Hesitancy. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33(34):4161–64. doi:10.1016/j.vaccine.2015.04.036.
- World Health Organization. Ten threats to global health in 2019. Geneva (Switzerland): WHO; 2012.
- D’Souza G, Dowdy D. What is herd immunity and how can we achieve it with COVID-19? Johns Hopkins Bloomberg school of public health expert insights. [accessed 2021 January 15]. https://www.jhsph.edu/covid-19/articles/achieving-herd-immunity-with-covid19.html.
- Lin C, Tu P, Beitsch LM. Confidence and receptivity for COVID-19 vaccines: a rapid systematic review. Vaccines (Basel). 2020;9(1):16. doi:10.3390/vaccines9010016.
- Wouters OJ, Shadlen KC, Salcher-Konrad M, Pollard AJ, Larson H, Teerawattananon Y, Jit M. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet. 2021;397(10278):1023–34. doi:10.1016/S0140-6736(21)00306-8.
- Thigpen CL, Funk C. Most Americans expect a COVID-19 vaccine within a year; 72% say they would get vaccinated. [accessed 10 August 2020]. https://www.pewresearch.org/fact-tank/2020/05/21/mostamericans-expect-a-covid-19-vaccine-within-a-year-72-say-theywould-get-vaccinated/.
- King’s College London. Who’s least likely to say they’ll get a COVID-19 vaccine? [accessed 2021 February 6]. https://www.kcl.ac.uk/news/whos-least-likely-to-say-they%26#x2019;ll-get-a-covid-19-vaccine.
- Peretti-Watel P, Seror V, Cortaredona S, Launay O, Raude J, Verger P, Fressard L, Beck F, Legleye S, L’Haridon O; COCONEL Group. A future vaccination campaign against COVID-19 at risk of vaccine hesitancy and politicisation. Lancet Infect Dis. 2020;20(7):769–70. doi:10.1016/S1473-3099(20)30426-6.
- Szilagyi PG, Thomas K, Shah MD, Vizueta N, Cui Y, Vangala S, Kapteyn A. National trends in the US public’s likelihood of getting a COVID-19 vaccine - April 1 to December 8, 2020. JAMA. 2021;325(4):396–98. doi:10.1001/jama.2020.26419.
- Lazarus JV, Ratzan SC, Palayew A, Gostin LO, Larson HJ, Rabin K, Kimball S, El-Mohandes A. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. 2021;27(2):225–28. doi:10.1038/s41591-020-1124-9.
- Schwarzinger M, Watson V, Arwidson P, Alla F, Luchini S. COVID-19 vaccine hesitancy in a representative working-age population in France: a survey experiment based on vaccine characteristics. Lancet Public Health. 2021;6(4):e210–21. doi:10.1016/S2468-2667(21)00012-8.
- Freeman D, Loe BS, Chadwick A, Vaccari C, Waite F, Rosebrock L, Jenner L, Petit A, Lewandowsky S, Vanderslott S, et al. COVID-19 vaccine hesitancy in the UK: the Oxford coronavirus explanations, attitudes, and narratives survey (Oceans) II. Psychol Med. 2020;11:1–15. doi:10.1017/S0033291720005188.
- Caserotti M, Girardi P, Rubaltelli E, Tasso A, Lotto L, Gavaruzzi T. Associations of COVID-19 risk perception with vaccine hesitancy over time for Italian residents. Soc Sci Med. 2021;272:113688. doi:10.1016/j.socscimed.2021.113688.
- Cines DB, Bussel JB. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 2021;384(23):2254–56. doi:10.1056/NEJMe2106315.
- Boekel L, Hooijberg F, van Kempen ZLE, Vogelzang EH, Sw T, Killestein J, Nurmohamed MT, Boers M, Kuijpers TW, van Ham SM, et al. Perspective of patients with autoimmune diseases on COVID-19 vaccination. Lancet. 2021;3:e241–3. doi:10.1016/S2665-9913(21)00037-0.
- Felten R, Dubois M, Ugarte-Gil MF, Chaudier A, Kawka L, Bergier H, Costecalde C, Pijnenburg L, Fort J, Chatelus E, et al. Vaccination against COVID-19: expectations and concerns of patients with autoimmune and rheumatic diseases. Lancet Rheumatol. 2021;3(4):e243–5. doi:10.1016/S2665-9913(21)00039-4.
- Priori R, Pellegrino G, Colafrancesco S, Alessandri C, Ceccarelli F, Di Franco M, Riccieri V, Scrivo R, Sili Scavalli A, Spinelli FR, et al. SARS-CoV-2 vaccine hesitancy among patients with rheumatic and musculoskeletal diseases: a message for rheumatologists. Ann Rheum Dis. 2021;80(7):953–54. doi:10.1136/annrheumdis-2021-220059.
- Yurttas B, Poyraz BC, Sut N, Ozdede A, Oztas M, Uğurlu S, Tabak F, Hamuryudan V, Seyahi E. Willingness to get the COVID-19 vaccine among patients with rheumatic diseases, healthcare workers and general population in Turkey: a web based survey. Rheumatol Int. 2021;41(6):1105–14. doi:10.1007/s00296-021-04841-3.
- Smerilli G, Cipolletta E, Moscioni E, Francioso F, Risa AM, Maccarrone V, Zompa D, Di Matteo A, Di Carlo M, De Angelis R, et al. Correspondence on ‘SARS-CoV-2 vaccine hesitancy among patients with rheumatic and musculoskeletal diseases: a message for rheumatologists.’ Ann Rheum Dis. 2021;80(10):e168–e168. doi:10.1136/annrheumdis-2021-220586.
- Gaur P, Agrawat H, Shukla A. COVID-19 vaccine hesitancy in patients with systemic autoimmune rheumatic disease: an interview based survey. Rheumatol Int. 2021;41(9):1601–05. doi:10.1007/s00296-021-04938-9.
- Boucher VG, Pelaez S, Gemme C, Labbe S, Lavoie KL. Understanding factors associated with vaccine uptake and vaccine hesitancy in patients with rheumatoid arthritis: a scoping literature review. Clin Rheumatol. 2021;40(2):477–89. doi:10.1007/s10067-020-05059-7.
- Figueroa-Parra G, Esquivel-Valerio JA, Santoyo-Fexas L, Moreno-Salinas A, Gamboa-Alonso CM, De Leon-Ibarra AL, Galarza-Delgado DA. Knowledge and attitudes about influenza vaccination in rheumatic diseases patients. Hum Vaccin Immunother. 2021;17(5):1420–25. doi:10.1080/21645515.2020.1816108.
- Sattui SE, Liew JW, Kennedy K, Sirotich E, Putman M, Moni TT, Akpabio A, Alpízar-Rodríguez D, Berenbaum F, Bulina I, et al. Early experience of COVID-19 vaccination in adults with systemic rheumatic diseases: results from the COVID-19 global rheumatology alliance vaccine survey. RMD Open. 2021 Sep;7(3):e001814. doi:10.1136/rmdopen-2021-001814.
- Vergera P, Dubéc E. Restoring confidence in vaccines in the COVID-19 era. Expert Rev Vaccines. 2020;19(11):991–93. doi:10.1080/14760584.2020.1825945.
- ACR COVID-19 vaccine guidance recommends vaccination, addresses immunosuppressant drugs & patient concerns. [accessed 2021 August 19]. https://www.rheumatology.org/About-us/Newsroom/Press-releases/ID/1138.
- EULAR. EULAR view-points on SARS-CoV-2 vaccination in patients with RMDs; 2020 December. Consulted 2021 October 5 in https://www.eular.org/eular_sars_cov_2_vaccination_rmd_patients.cfm.
- Hasseli R, Pfeil A, Krause A, Schulze-Koops H, Müller-Ladner U, Specker C, Hoyer B, Lorenz H-M, Regierer A, Richter J; COVID-19 Task Force of the German Society for Rheumatology (DGRh). A survey to evaluate knowledge, perceptions and attitudes toward COVID-19 vaccinations among rheumatologists in Germany. Rheumatol Int. 2021 Nov;41(11):1949–56. doi:10.1007/s00296-021-04986-1.
- Wild D, Grove A, Martin M, Eremenco S, McElroy S, Verjee-Lorenz A, Erikson P. Principles of good practice for the translation and cultural adaptation process for Patient-Reported Outcomes (PRO) measures: report of the ISPOR task force for translation and cultural adaptation. Value Health. 2005;8(2):94–104. doi:10.1111/j.1524-4733.2005.04054.x.
- Domek GJ, O’Leary ST, Bull S, Bronsert M, Contreras-Roldan IL, Bolaños-Ventura GA, Kempe A, Asturias EJ. Measuring vaccine hesitancy: field testing the WHO SAGE working group on vaccine hesitancy survey tool in Guatemala. Vaccine. 2018;36(35):5273–81. doi:10.1016/j.vaccine.2018.07.046.
- Vacunarse. Su vacunación contra el COVID-19. [accessed 2021 August 19]. https://espanol.cdc.gov/coronavirus/2019-ncov/vaccines/your-vaccination.html.
- Guaracha-Basáñez GA, Contreras-Yáñez I, Hernández-Molina G, González-Marín A, Pacheco-Santiago LD, Valverde-Hernández SS, Peláez-Ballestas I, Pascual-Ramos V. Clinical and bioethical implications of health care interruption during the covid-19 pandemic: a cross-sectional study in outpatients with rheumatic diseases. PLoS One. 2021;16(7):e0253718. doi:10.1371/journal.pone.0253718.
- England BR, Sayles H, Mikuls TR, Johson DS, Michaud K. Validation of the rheumatic disease comorbidity index. Arthritis Care Res (Hoboken). 2015;67(6):865–72. doi:10.1002/acr.22456.
- Choi BC, Pak AW. A catalog of biases in questionnaires. Prev Chronic Dis. 2005;2:A13.
- Steiner DL, Norman GR, and Cairney J. Health measurement scales. A practical guide to their development and use. 3th ed. New York/EUA: Oxford University; 2003.
- Cohen J. Statistical power analysis for the behavioural sciences. New York/EUA: Academic Press; 1977.
- Husted JA, Cook RJ, Farewell VT, Gladman DD. Methods for assessing responsiveness: a critical review and recommendations. J Clin Epidemiol. 2000;53(5):459–68. doi:10.1016/s0895-4356(99)00206-1.
- Bland JM, Altman DG. Statistics notes: Cronbach's alpha. BMJ. 1997;314(7080):572. doi:10.1136/bmj.314.7080.572.
- Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–63. doi:10.1016/j.jcm.2016.02.012.
- Machingaidze S, Wiysonge CS. Understanding COVID-19 vaccine hesitancy. A new study unpacks the complexities of COVID-19 vaccine hesitancy. Nat Med. 2021;27(8):1338–39. doi:10.1038/s41591-021-01459-7.
- Lohr KN, Zebrack BJ. Using patient-reported outcomes in clinical practice: challenges and opportunities. Qual Life Res. 2009;18(1):99–107. doi:10.1007/s11136-008-9413-7.
- Pett MA, Lackey NR, and Sullivan JJ. Making sense of factor analysis: the use of factor analysis for instrument development in health care research. California/EUA: Sage Publications Inc; 2003.
- Campochiaro C, Trignani G, Tomelleri A, Cascinu S, Dagna L; COVID-19 Vaccine Study Group. Potential acceptance of COVID-19 vaccine in rheumatological patients: a monocentric comparative survey. Ann Rheum Dis. 2021:annrheumdis-2020-219811. doi:10.1136/annrheumdis-2020-219811.
- Bugatti S, Balduzzi S, De Stefano L, Manzo A, Xoxi B, Bogliolo L, Monti S, Delvino P, Montecucco C. Correspondence on ‘EULAR December 2020 viewpoints on SARS-CoV-2 vaccination in patients with RMDs.’ Ann Rheum Dis. 2021:annrheumdis-2021-220541. doi:10.1136/annrheumdis-2021-220862.
- Ko T, Dendle C, Woolley I, Morand E, Antony A. SARS-COV-2 vaccine acceptance in patients with rheumatic diseases: a cross-sectional study. Hum Vaccin Immunother. 2021:1–9. doi:10.1080/21645515.2021.1958611.
- Gaur PS, Zimba O, Agarwal V, Gupta L. Reporting survey based studies—a primer for authors. J Korean Med Sci. 2020;35(45):e398. doi:10.3346/jkms.2020.35.e398.
- Felten R, Dubois M, Ugarte-Gil MF, Chaudier A, Kawka L, Bergier H, Costecalde C, Pijnenburg L, Fort J, Chatelus E, et al. Cluster analysis reveals 3 main patterns of behavior towards SARS-CoV-2 vaccination in patients with autoimmune and inflammatory diseases. Rheumatology (Oxford). 2021:keab432. doi:10.1093/rheumatology/keab432.
- Figueroa-Parra G, Esquivel-Valerio JA, Santoyo-Fexas L, Moreno-Salinas A, Gamboa-Alonso CM, De Leon-Ibarra AL, Galarza-Delgado DA. Knowledge and attitudes about influenza vaccination in rheumatic diseases patients. Hum Vaccin Immunother. 2021;17(5):1420–25. doi:10.1080/21645515.2020.1816108.
- McEachan R, Conner M, Taylor N, Lawton R. Prospective prediction of health-related behaviours with the theory of planned behaviour: a meta-analysis. Health Psychol. 2011;5:97–144. doi:10.1080/17437199.2010.521684.
- Solís Arce JS, Warren SS, Meriggi NF, Scacco A, McMurry N, Voors M, Syunyaev G, Malik AA, Aboutajdine S, Adeojo O, et al. COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat Med. 2021;27(8):1385–94. doi:10.1038/s41591-021-01454-y.