ABSTRACT
Background
To evaluate the effectiveness of varicella vaccine (VarV) as post-exposure prophylaxis (PEP) among children during varicella outbreaks.
Material and Methods
A comprehensive literature search was performed in PubMed, Embase, Web of Science, SinoMed, Wanfang and CNKI. Relevant outcomes included the incidence of varicella. Pooled estimates were calculated using a fixed-effects or random-effects model according to the heterogeneity among studies.
Results
A total of 15 studies with 7,474 children that received one or two dosages of VarV as PEP and 183,827 children who received no VarV were included in the meta-analysis. In total, one-dose and two-dose VarV as PEP had 43% (95% confidence interval (CI):27%, 55%) and 60% (95%CI: 35%, 75%) efficacy, respectively. When PEP was applied within 3 days, the pooled VarV as PEP for prevention of varicella was 80% (95%CI: 68%, 88%); when PEP was administered beyond 3 days, the pooled VarV as PEP for the prevention of varicella was 50% (95%CI: 11%, 72%). If the PEP was implemented with a coverage of more than 80%, the VarV could prevent 82% of varicella cases from occurring (95%CI: 15%, 96%); if the PEP covered a maximum of 80% of the susceptible cases, the VarV could prevent 65% of varicella cases from occurring (95%CI: 50%, 76%).
Conclusion
The two-dose VarV had better efficacy than one-dose VarV in the control of varicella outbreaks, especially if PEP was applied within 3 days of an outbreak and in conjunction with a high coverage rate ≥80%.
Introduction
Varicella is a highly infectious disease caused by the varicella-zoster virus, with attack rates in susceptible individuals ranging from 61% to 100% after exposure.Citation1 Although varicella is a self-limited childhood illness, serious complications can arise.Citation2 It is estimated that approximately 420 million cases occur globally annually, resulting in 4.2 million severe complications and 4200 deaths according to the WHO position paper in 2014.Citation3 The disease causes a sizable societal burden for patients and their caregivers due to school absenteeism and work loss.
Fortunately, the varicella vaccine (VarV) has significantly reduced the global varicella-related incidence, morbidity, and mortality since it was developed in 1974.Citation3,Citation4 VarVs were licensed and available throughout the world; however, they were recommended for routine one- or two-dose only in 33 high-income countries and regions in December 2014.Citation5 As we know, it takes an average of 14 days from the infection to the onset of clinical symptoms (range 10–21 days),Citation1,Citation6 which makes using VarV as post-exposure prophylaxis (PEP) possible. Timely receiving VarV as PEP will induce protective immune responses prior to secondary viremia (the last process in the pathogenesis of varicella), which can abort or reduce severity of varicella disease. Therefore, receiving VarV as PEP is an emergency response to the prevention of varicella and outbreak controls in countries where VarVs are not included in their national immunization schedules.
In 1999, the United States Advisory Committee on Immunization Practices (ACIP) recommended that administration a one-dose VarV to persons with no immunization records after exposure to varicella,Citation7 and in 2006 recommended that previously one-dose VarV recipients should also receive a second-dose after exposure.Citation8 Several studies suggested that vaccination as PEP against varicella was effective in hospital, household or school settings.Citation9–12 However, the vaccine effectiveness (VE) of VarV as PEP from these studies varied greatly, ranging from 0.6% to 100%.Citation13–16 Therefore, we conducted a systematic literature review and meta-analysis to provide a more precise estimate of the VE of one-dose or second-dose VarV as PEP for outbreak control.
Material and methods
Literature search
This study was performed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.Citation17 A comprehensive literature search was performed in the PubMed, Web of Science, Web of Science databases, SinoMed (Chinese Bio-Medical Literature Service System, China), Wanfang (China) and CNKI (National Knowledge Infrastructure, China) databases (up to July 15, 2021) for original research articles. The following search terms were used: “Chickenpox,” “varicella,” “chickenpox vaccine,” “varicella vaccine,” “post-exposure prophylaxis,” and “vaccine effectiveness.” The search was limited to human subjects and no imposes on language and publication status. In addition, we also manually searched the reference lists of identified studies to include other potentially eligible trials.
Inclusion criteria
All clinical trials that evaluated the VE of VarV as PEP were considered eligible for analysis. The following inclusion criteria were applied: (1) study design: randomized controlled trial (RCT), cohort study, case-control, or comparative study; (2) population: children: (3) intervention: one-dose or two-dose VarV; (4) control: one-dose VarV or non-vaccination; (4) outcomes: original data on dose-specific VarV VE.
Data extraction and quality assessment
For each study, the following information was extracted using a standardized Excel file: first author, year of publication, study design, study setting, case definition, disease severity, VarV immunization status, number of VarV cases in each group, and outcomes. Data extraction was performed by two independent investigators and checked by a third investigator. Any disagreements between the investigators were resolved by discussion and consensus.
We used the modified Newcastle-Ottawa (NOS)Citation18 scale to assess the methodological quality of non-RCTs included in this meta-analysis. This method comprised of three items in the report of a non-RCT study: patient selection, comparability of the intervention/ control group, and outcome assessment.Citation18 The total NOS score is 9 points, and any study with a score greater than 5 points is regarded as high quality.
Statistical analysis
The incidence of varicella cases was regarded as dichotomous outcome, thus it was expressed as RR with 95% confidence interval (95%CI). Before the original data was synthesized, Cochrane Q chi-square test and I2 statisticCitation19 were used to test the heterogeneity across the included studies, in which A P value < .1 or I2 > 50% was defined to have significant heterogeneity. A random-effects modelCitation20 was used to pool the estimate when substantial heterogeneity was identified; otherwise, a fixed-effects modelCitation21 was used. We also conducted a sensitivity analysis to explore the potential source of heterogeneity by excluding one study at a time when heterogeneity was substantial or obvious. We also performed subgroup analysis based on the vaccination coverage and the PEP time to explore whether these factors had impact on the VE. To calculate the pooled VE estimates, we first obtained the RR for each study, using the formula VE = 1-RR.Citation22 We also performed cumulative meta-analysis to assess the evolution of evidence on VarV effectiveness over time. The studies were sorted out year-wise and the effect estimates of the studies were added to the pooled estimates of the studies that have accrued till that date. A random-effect model was used for the cumulative meta-analysis. Publication bias was assessed by EggerCitation23 and Bgger’s test.Citation24 We used STATA software version 12.0 (Stata Corporation, College Station, TX, USA) for data analysis. A P value less than 0.05 was judged as statistically significant.
Results
Identification of eligible studies
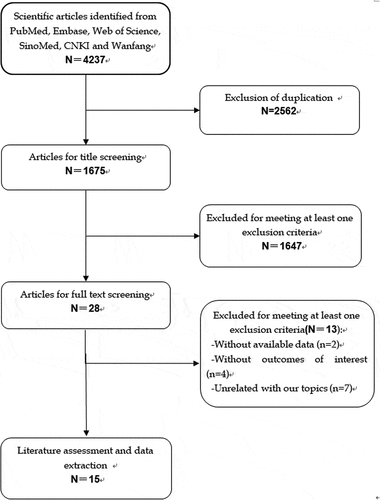
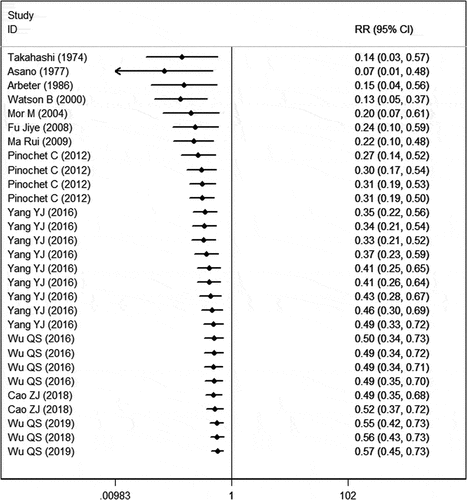
The initial search yielded 4237 relevant publications, of which 2562 were excluded because of duplicate records. Then, 1675 studies were screened for title and abstract, and 1647 of them were excluded because of various reasons (case report, animal experiments, reviews, editors, letters, comments), leaving 28 studies for full-text information review. Of the potential 28 studies, 13 were excluded because 2 did not provide available data, 4 did not provide outcome of our interest, and 7 were unrelated with our topics. Finally, 15 studiesCitation4,Citation9,Citation12,Citation13,Citation15,Citation16,Citation25–33 met the inclusion criteria and were included in this meta-analysis ().
Study characteristics and quality assessment
The main characteristics of included studies are presented in , and the vaccination status of children in each study is presented in . These studies were published between 1974 and 2019. The sample size varied greatly, ranging from 24 to 8427. All the included studies used a prospective or retrospective cohort study design except one,Citation16 which used a randomized, double-blind, placebo-controlled study design. The populations studied included children in different settings such as kindergarten, primary school, middle school and high school. The live attenuated vaccine was the most commonly examined vaccine type, including Oka-Merck vaccine, Varicella-zoster virus vaccine, and live Varicella vaccine (Baike, China). The NOS scale of 14 cohort studies was all greater than 5 points, which indicated that they were of high quality.
Table 1. Key study characteristics
Table 2. Information of the vaccination status in each study
One-dose VE
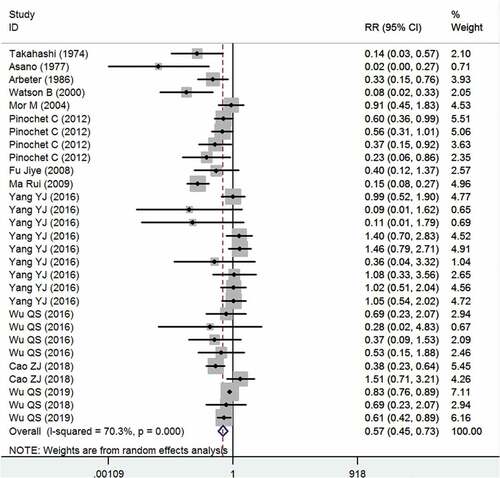
Fourteen studies reported the data of one-dose VarV as PEP after exposure.Citation4,Citation9,Citation12,Citation13,Citation15,Citation16,Citation25–30,Citation32,Citation33 The aggregated results showed that, one-dose VarV significantly reduced the incidence of rate of varicella infection as compared with non-vaccinations (RR = 0.57, 95%CI: 0.45, 0.73; P < .001) (). The pooled one-dose VarV as PEP for prevention of varicella was 43% (95%CI: 27%, 55%). There was significant heterogeneity among the included studies. Therefore, we performed sensitivity analysis (I2=70.3%, P < .001). When the trial with small sample sizeCitation26 was excluded, the overall estimate did not change substantially (RR = 0.59, 95%CI: 0.44, 0.78; P < .001), but the heterogeneity was still present (I2 = 72.2%, P < .001). Then, we excluded the trial with outlier,Citation9 the overall estimate (RR = 0.59, 95%CI: 0.46, 0.74; P < .001) and heterogeneity (I2 = 68.9%, P < .001) did not alter slightly. We then further excluded any single study; however, the heterogeneity was still present (data not shown). Subgroup analysis based on country showed that one-dose VarV significantly reduced the incidence rate of varicella infection in Japan (RR = 0.07, 95%CI: 0.01, 0.48; P = .007), USA (RR = 0.18, 95%CI: 0.04, 0.74; P = .018), Chile (RR = 0.52, 95%CI: 0.37, 0.72; P < .001) and China (RR = 0.69, 95%CI: 0.52, 0.91; P = .008), but not in Israel (RR = 0.91, 95%CI: 0.45, 1.83; P = .789).
Two-dose versus one-dose VE
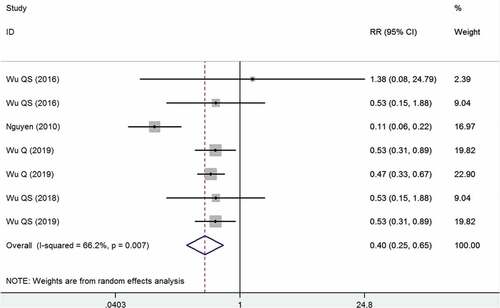
Five studies reported the data of two-dose versus one-dose VarV as PEP after exposure.Citation15,Citation30–33 The pooled estimate showed that the two-dose VarV as PEP significantly reduced the incidence rate of varicella infection as compared with one-dose VarV as PEP (RR = 0.40, 95%CI: 0.25, 0.65; P < .001) (). Compared with students who had previously received one-dose VarV, the VE for students who received two-dose VarV as PEP was 60% (95%CI: 35%, 75%). The test for heterogeneity was significant, then sensitivity analysis was performed. When we excluded the trial with outlier,Citation31 the overall estimate altered slightly (RR = 0.50, 95%CI: 0.39, 0.64; P < .001); however, the heterogeneity was not present (I2 = 0.0%, P = .983). This indicated that the trial conducted by Nguyen et al. contributed to the heterogeneity.
Subgroup analysis
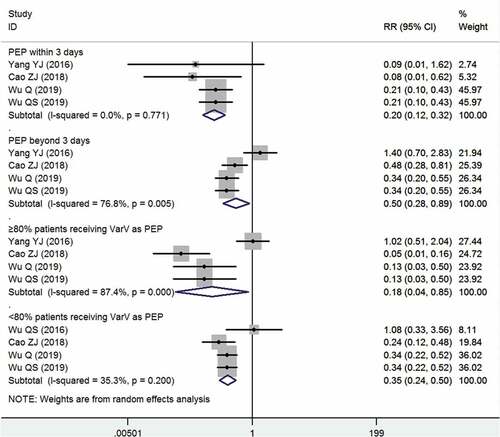
Five studies investigated the factors that might influence the effectiveness of VarV as PEP.Citation13,Citation15,Citation29,Citation30,Citation33 All these studies used a prospective or retrospective cohort study design. The pooled results showed that the PEP remained effective in all the stratified samples (). When PEP was applied within 3 days, as recommended in the implementation guidelines,Citation29 the pooled VarV as PEP for prevention of varicella was 80% (95%CI: 68%, 88%); when the PEP was administered beyond 3 days, the pooled VarV as PEP for prevention of varicella was 50% (95%CI: 11%, 72%). If the PEP was implemented with a coverage of more than 80%, the VarV could prevent 82% of varicella cases from occurring (95%CI: 15%, 96%); if the PEP covered a maximum of 80% of the susceptible cases, the VarV could prevent 65% of varicella cases from occurring (95%CI: 50%, 76%).
Cumulative meta-analysis
Year-wise cumulative meta-analysis was performed for the VE of one-dose VarV as PEP after exposure. We observed that the evidence for the effect of VarV is available from the year 1974 and further studies just shortened the CI of overall effect size without change in the magnitude or direction of the estimates (). From the year 2012, with the addition of estimate from new studies, the RR value remained relatively stable, which ranged from 0.27 to 0.57. This confirmed the reliable and significant effect of one-dose VarV as PEP in the prevention of varicella.
Publication bias
The assessment of publication bias showed that, no evidence of significant publication bias was identified among the included studies (Egger’s test: t = −0.52, P = .672; Begg test: Z = 0.41, P = .475).
Discussion
The present meta-analysis of 15 studies provided evidence for the effectiveness of VarV as PEP among children during varicella outbreaks. Our study showed that one-dose and two-dose VarV as PEP had 43% and 60% efficacy, respectively. When PEP was applied within 3 days, the pooled VarV as PEP for prevention of varicella was 80%; when the PEP was administered beyond 3 days, the pooled VarV as PEP for prevention of varicella was 50%. If the PEP was implemented with a coverage of more than 80% or less than 80%, the VarV could prevent 82% and 65% of varicella cases from occurring, respectively. Our study confirmed that the two-dose VarV had better efficacy than one-dose VarV in the control of varicella outbreaks, especially if PEP was applied within 3 days of an outbreak and in conjunction with a high coverage rate ≥80%.
There has been one published meta-analysis in 2016, which included 3 RCTs to assess the effect of vaccine for PEP against varicella in children and adults.Citation34 Our study expands on the prior study in providing more evidence for the effect of VarV. First, the previous meta-analysis found the evidence to support VarV as PEP in those children after household exposure to varicella. However, their results were limited due to the small sample size (110 patients).Citation34 Whereas, in this meta-analysis, we included a total of 191,301 participants, which enhanced the statistical power to assess the effect of VarV. Second, although RCT is the optimal design to minimize bias and confounding, it is not ethical in population for whom vaccination is routinely recommended. This is why no placebo-controlled RCTs have been performed over the past 15 years. Third, the test-negative design has been widely used in evaluating the VE of influenza vaccines in recent years.Citation35 Thus, we included prospectively or retrospectively observational studies that used the clinical or laboratory diagnosis as the outcome. These studies would provide more accurate data for the effect evaluation. Based on data obtained from these studies, we calculated the VE of VarV, the value of which was more precise than that from RCTs. Fourth, in this meta-analysis, we performed subgroup analysis based on the vaccination coverage and PEP time to explore whether these factors had influence on the VE, which had not been discussed in the previous meta-analysis.
Previous studies that evaluated the VE of VarV as PEP was mainly based on household exposure, and they found that the VE of one-dose VarV in unvaccinated individuals within 3–5 days was 50% after household exposure. Varicella secondary attack rate among household contacts was more than 70% in unvaccinated persons and 15% in persons with one-dose VarV history.Citation36,Citation37 In addition, the disease was more severe in secondary cases than in index case, with more number of lesion, higher fever and risk of pneumonia according to one prospective household study.Citation38 Compared with these high-income countries where VarV was included in their national immunization program, the family size and crowding are higher in most low-income countries. Therefore, we recommend that these countries can reduce the disease burden by implementing VarV as PEP after household exposure.
In the present study, we found that the VE among unvaccinated individuals who received one-dose VarV as PEP was 43%, while the VE of second-dose VarV as PEP among those who had been vaccinated one-dose VarV prior exposure was 60%. Our results were in consistent with that of the previous studies,Citation15,Citation30 but were contrast to that reported by Wu QS et al.Citation32 In that study, the authors performed a retrospective cohort study in 191 students, and found that the VE of first dose as PEP recipients was 100% and of the second dose as PEP recipients was 60%. The authors stated that the higher VE of the first dose of PEP might be explained by the small sample size and the late start of PEP campaign (>12 days after exposure).Citation32 In that study, no varicella was identified in 10 vaccinated students who received the first dose of VarV as PEP during the outbreak; thus, the VE of PEP in the first-dose students was 100%. The time from exposure to vaccination was more than 12 days, and 91% of students received one dose of VarV as PEP at 19 days after exposure, which was longer than that in other studies (within 3–5 days).Citation32 Therefore, the sample size of vaccinated students was small and the VE of first-dose PEP may have been overestimated.Citation32
In another retrospective cohort study,Citation30 the VE of one-dose VarV as PEP was 49.62%, which was similar to that of our study. Nguyen MD, et al. investigated the effectiveness of second-dose VarV for outbreak control at an elementary school.Citation31 They reported that the adjusted incremental second-dose VE was 76% (95%CI: 44–90%) for students with classroom exposure. They concluded that second-dose VarV substantially reduced the varicella incidence for students with classroom expose, and thus, it should be recommended as an appropriate intervention to decrease disease transmission in institution-based outbreaks.Citation31
In this study, we found that when PEP was applied with 3 days, the effect of VarV was 80%; when VarV was administered beyond 3 days, the VarV as PEP for prevention of varicella was 50%. This indicated that VarV would achieve better efficacy when it was administered early. Our results were in consistent with that of previous studies.Citation15 Wu QS, et al. performed a prospective cohort study of 6762 students to evaluate the effectiveness of two-dose VarV as PEP in Shanghai, China. Their results showed that the VE of two-dose VarV within 3 days of exposure was higher than that beyond 3 days (adjusted VE: 77% vs. 64%).Citation15 The VarV coverage rate was another factor that might affect the VE of VarV. In the present study, the pooled data suggested that the VE was higher among subjects with a coverage rate ≥80% than those with a coverage rate <80% (82% vs. 65%). Our results were accordance with that of previous studies.Citation13,Citation33 Cao ZJ et al. collected three-year surveillance data of 3524 susceptible students to assess the effectiveness of VarV as PEP.Citation13 Their results showed that the VE was 95.2% if the PEP was administered with a coverage rate of ≥80% and the VE was 59.4% if the PEP was administered with a coverage rate of <80%.Citation13
We found that the VE of the pooled one-dose and second-dose VarV as PEP were 43% and 60%, respectively. However, it was lower than the pooled VE of routine one-dose and two-dose VarV (one-dose VarV, 81%; two-dose VarV, 92%).Citation38 The lower VE of VarV as PEP may be explained by the following three reasons. Firstly, some individuals infected with varicella after PEP may be due to too late to induce immune responses, because it takes 7–10 days to induce protective antibodies after vaccination.Citation39 This is why we have observed that administration VarV within 3 days of exposure conferred higher VE than receiving beyond 3 days. Secondly, history of exposure to varicella is one of risk factors of breakthrough infection.Citation40 The estimate of VE of routine VarV during outbreaks was lower than that reported by RCTs and case-control studies.Citation41 The weight of the data from outbreaks in our study was high, which may underestimate the VE of receiving VarV as PEP. Thirdly, a varicella-like rash was one of the most common adverse events followed by vaccination (6%),Citation3 and 86% occurred within the first 21 days of receiving the vaccine.Citation42 These individuals who developed varicella-like rashes after PEP might be highly misdiagnosed as varicella cases due to their contact history, thus causing an underestimation of the VE of PEP.
This systematic review and meta-analysis has several limitations. Firstly, the time span of studies included was nearly 40 years, and the effect of vaccination varieties on the VarV was not considered. Secondly, the majority of studies included in this study used clinically diagnosed cases rather than laboratory confirmed cases to assess the VE of VarV, which may result in misdiagnosis bias. Thirdly, due to the insufficient data of the included studies, we did not assess VE of VarV for prevention of severe varicella, which was another potential benefit for receiving VarV as PEP. Fourthly, there were few household studies being done after 2000 and the weight of data from outbreaks was too large. Lastly, most of the included studies were performed in China, which might prevent a generalization of our results to other areas with different demographic and epidemiologic characteristics.
In conclusion, our results suggest that the use of VarV as PEP is a potential strategy for the prevention of morbidity from varicella infection. The VE of receiving second-dose VarV as PEP was higher than that of one-dose VarV as PEP. Further studies are needed to investigate whether the routine two-dose VarV schedule is more effective in the reducing varicella incidence and in controlling the size of outbreaks.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Heininger U, Seward JF. Varicella. Lancet (London, England). 2006;368:1365–76. doi:10.1016/s0140-6736(06)69561-5.
- Bozzola E, Bozzola M. Varicella complications and universal immunization. J Pediatr (Rio J). 2016;92:328–30. doi:10.1016/j.jped.2016.05.001.
- Twh O. Varicella and herpes zoster vaccines: WHO position paper, June 2014. Releve Epidemiologique Hebd. 2014;89:265–87.
- Takahashi M, Otsuka T, Okuno Y, Asano Y, Yazaki T. Live vaccine used to prevent the spread of varicella in children in hospital. Lancet (London, England). 1974;2:1288–90. doi:10.1016/s0140-6736(74)90144-5.
- Wutzler P, Bonanni P, Burgess M, Gershon A, Sáfadi MA, Casabona G. Varicella vaccination - the global experience. Expert Rev Vaccines. 2017;16:833–43. doi:10.1080/14760584.2017.1343669.
- Grose C. Varicella vaccination of children in the United States: assessment after the first decade 1995-2005. J Clin Virol. 2005;33:89–95;discussion 6–8. doi:10.1016/j.jcv.2005.02.003.
- Prevention of varicella. Update recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommendations Rep. 1999;48:1–5.
- Marin M, Güris D, Chaves SS, Schmid S, Seward JF. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommendations Rep. 2007;56:1–40.
- Asano Y, Nakayama H, Yazaki T, Kato R, Hirose S. Protection against varicella in family contacts by immediate inoculation with live varicella vaccine. Pediatrics. 1977;59:3–7.
- Asano Y, Hirose S, Iwayama S, Miyata T, Yazaki T, Takahashi M. Protective effect of immediate inoculation of a live varicella vaccine in household contacts in relation to the viral dose and interval between exposure and vaccination. Biken J. 1982;25:43–45.
- Naganuma Y, Osawa S, Takahashi R. Clinical application of a live varicella vaccine (Oka strain) in a hospital. Biken J. 1984;27:59–61.
- Arbeter AM, Starr SE, Plotkin SA. Varicella vaccine studies in healthy children and adults. Pediatrics. 1986;78:748–56.
- Cao Z, Chen D, Yang Y, Zhang D. Effectiveness of post-exposure prophylaxis during varicella outbreaks among primary and middle school students in Shanghai: an analysis of three-year surveillance data. Vaccine. 2018;36:5754–59. doi:10.1016/j.vaccine.2018.08.004.
- Yang Y. Effectiveness of vaccination following varicella outbreaks among schools in Hongkou district, Shanghai. Zhonghua Yu Fang Yi Xue Za Zhi [Chin J Preventive Med]. 2016;50:91–93. doi:10.3760/cma.j.0253-9624.2016.01.016.
- Wu Q, Liu J, Wang Y, Zhou Q, Wang X, Xuan Z, Zhang L, Gao Y, Chen B, Hu Y, et al. Effectiveness of second-dose varicella vaccination as post-exposure prophylaxis: a prospective cohort study. Clin Microbiol Infect. 2019;25:872–77. doi:10.1016/j.cmi.2018.11.013.
- Mor M, Harel L, Kahan E, Amir J. Efficacy of postexposure immunization with live attenuated varicella vaccine in the household setting–a pilot study. Vaccine. 2004;23:325–28. doi:10.1016/j.vaccine.2004.06.004.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clinical Research Ed). 2009;339:b2535. doi:10.1136/bmj.b2535.
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the as sessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical Research Ed). 2003;327:557–60. doi:10.1136/bmj.327.7414.557.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi:10.1016/0197-2456(86)90046-2.
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48.
- Greenwood M, Yule GU. The statistics of anti-typhoid and anti-cholera inoculations, and the interpretation of such statistics in general. Proc R Soc Med. 1915;8:113–94. doi:10.1177/003591571500801433.
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Research Ed). 1997;315:629–34. doi:10.1136/bmj.315.7109.629.
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. doi:10.2307/2533446.
- Watson B, Seward J, Yang A, Witte P, Lutz J, Chan C, Orlin S, Levenson R. Postexposure effectiveness of varicella vaccine. Pediatrics. 2000;105:84–88. doi:10.1542/peds.105.1.84.
- Pinochet C, Cerda J, Hirsch T, Mieres J, Inostroza C, Abarca K. Effectiveness of varicella vaccine as post exposure prophylaxis in Chilean children. Revista Chilena de Infectologia: Organo Oficial de La Sociedad Chilena de Infectologia. 2012;29:635–40. doi:10.4067/s0716-10182012000700008.
- Fu JY, Dun Z, Sun PY, Xu RH, Dong SL. Evaluation of emergency immunization effect of an varicella outbreak in a primary school. Chin J Nat Med. 2008;10:448–49.
- Ma R, Sun MP, Sun M, Hou WJ, Jiang GY, Peng XH, Wu J. Effectiveness on post-exposure vaccination of varicella and its influencing factors in elementary schools in Beijing. Chin J Epidemiol. 2009;30:559–63.
- Yang Y. Effectiveness of vaccination following varicella outbreaks among schools in Hongkou district Shanghai. Chin J Prev Med. 2016;50:91–93.
- Wu QS, Liu M, Wu AQ, Zhou Q, Shen H, Zhang JJ, Liu JY. The vaccine protection effect of a public health emergency caused by varicella in a junior high school in Shanghai. J Prev Med Inf. 2016;32:289–93.
- Nguyen MD, Perella D, Watson B, Marin M, Renwick M, Spain CV. Incremental effectiveness of second dose varicella vaccination for outbreak control at an elementary school in Philadelphia, Pennsylvania, 2006. Pediatr Infect Dis J. 2010;29:685–89. doi:10.1097/INF.0b013e3181d9f657.
- Wu QS, Liu JY, Wang X, Chen YF, Zhou Q, Wu AQ, Wang L. Effectiveness of varicella vaccine as post-exposure prophylaxis during a varicella outbreak in Shanghai, China. Int J Infect Dis. 2018;66:51–55. doi:10.1016/j.ijid.2017.10.016.
- Wu QS, Wang X, Liu JY, Chen YF, Zhou Q, Wang Y, Sha J-D, Xuan Z-L, Zhang L-W, Yan L, et al. Varicella outbreak trends in school settings during the voluntary single-dose vaccine era from 2006 to 2017 in Shanghai, China. Int J Infect Dis. 2019;89:72–78. doi:10.1016/j.ijid.2019.09.009.
- McDonald J. Vaccines for postexposure prophylaxis against varicella (chickenpox) in children and adults. Paediatr Child Health. 2016;21:91–92. doi:10.1093/pch/21.2.91.
- Belongia EA, Simpson MD, King JP, Sundaram ME, Kelley NS, Osterholm MT, McLean HQ. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis. 2016;16:942–51. doi:10.1016/s1473-3099(16)00129-8.
- Seward JF, Zhang JX, Maupin TJ, Mascola L, Jumaan AO. Contagiousness of varicella in vaccinated cases: a household contact study. Jama. 2004;292:704–08. doi:10.1001/jama.292.6.704.
- Ceyhan M, Tezer H, Yildirim I. Secondary attack rate of hepatitis A, varicella and mumps in household settings and reliability of family history to detect seronegative children for necessity of vaccination. Scand J Infect Dis. 2009;41:501–06. doi:10.1080/00365540902968027.
- Poulsen A, Cabral F, Nielsen J, Roth A, Lisse IM, Vestergaard BF, Aaby P. Varicella zoster in Guinea-Bissau: intensity of exposure and severity of infection. Pediatr Infect Dis J. 2005;24:102–07. doi:10.1097/01.inf.0000151034.15747.4a.
- Watson B, Rothstein E, Bernstein H, Arbeter A, Arvin A, Chartrand S, Clements D, Kumar ML, Reisinger K, Blatter M, et al. Safety and cellular and humoral immune responses of a booster dose of varicella vaccine 6 years after primary immunization. J Infect Dis. 1995;172:217–19. doi:10.1093/infdis/172.1.217.
- Zhang X, Yan Y, Liu F, Luo Y, Zhang J, Peng X, Zhang Y, Han S, Zhao J, He Y. The epidemiology and risk factors for breakthrough varicella in Beijing Fengtai district. Vaccine. 2012;30:6186–89. doi:10.1016/j.vaccine.2012.07.062.
- Yin M, Xu X, Liang Y, Ni J. Effectiveness, immunogenicity and safety of one vs. two-dose varicella vaccination: ameta-analysis. Expert Rev Vaccines. 2018;17:351–62. doi:10.1080/14760584.2018.1433999.
- Sharrar RG, LaRussa P, Galea SA, Steinberg SP, Sweet AR, Keatley RM, Wells ME, Stephenson WP, Gershon AA. The postmarketing safety profile of varicella vaccine. Vaccine. 2000;19:916–23. doi:10.1016/s0264-410x(00)00297-8.