 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
South Korea’s National Immunization Program administers the quadrivalent influenza vaccine (QIV) to manage seasonal influenza, with a particular focus on the elderly. After reviewing the safety and immune response triggered by the adjuvanted QIV (aQIV) in individuals aged 65 and older, the Ministry of Food and Drug Safety in Korea approved its use. However, the extensive impact of aQIV on public health is yet to be fully understood. This study assessed the cost-effectiveness of replacing QIV with aQIV in South Korean adults aged 65 years and older. A dynamic transmission model, calibrated with national influenza data, was applied to compare the influence of aQIV and QIV on older adults and the broader population throughout a single influenza season. This study considered both the direct and indirect effects of vaccination on the elderly. We derived the incremental cost-effectiveness ratios (ICERs) from quality-adjusted life-years (QALYs) and costs incurred, validated through a probabilistic sensitivity analysis with 5,000 simulations. Findings suggest that transitioning to aQIV from QIV in the elderly would be cost-effective, particularly if aQIV’s efficacy reaches or exceeds 56.1%. With an ICER of $29,267/QALY, considerably lower than the $34,998/QALY willingness-to-pay threshold, aQIV presents as a cost-effective option. Thus, implementing aQIV with at least 56.1% efficacy is beneficial from both financial and public health perspectives in mitigating seasonal influenza in South Korea.
Introduction
Influenza poses a significant challenge to global health, particularly affecting older adults aged ≥65 years. Annually, influenza affects 5–10% of adults and 20–30% of children worldwide,Citation1 causing 3 − 5 million severe clinical cases and 290,000 to 650,000 deaths.Citation2 In South Korea, influenza causes approximately 400,000 outpatient visits and 7,000 hospital admissions annually,Citation3,Citation4 with older adults experiencing a mortality rate of 46.98 per 100,000, significantly higher than the overall rate of 5.97 per 100,000.Citation5
The influenza vaccination program under the Korean National Immunization Program (NIP) focuses on children under 13 years old and seniors aged 65 years and above, prioritizing vaccination for high-risk populations.Citation6 These high-risk groups include children under 5 years old, adults aged 50 years and older, pregnant women, and individuals with chronic illnesses.Citation6 Currently, the national influenza vaccination coverage is approximately 40%, with coverage levels reaching 80% for older adults aged 65 years and above, as well as children aged 13 years and below.Citation7
Vaccine effectiveness varies based on factors such as demographics, health status, and vaccine match with circulating viral strains. Conventional trivalent and quadrivalent vaccines may offer suboptimal protection for older adults due to reduced immune response with aging.Citation8 Consequently, adjuvanted vaccines such as the MF59-adjuvanted quadrivalent influenza vaccine (aQIV) have been developed, demonstrating enhanced immunogenicity in older adults.Citation9 Several countries, including the U.S., the U.K., Germany, Italy, Spain, and Australia, have begun administering aQIV to older adults.Citation10 In 2022, Korea’s Ministry of Food and Drug Safety approved the use of aQIV for individuals aged 65 and older,Citation11 further supported by the Korean Society of Infectious Diseases in 2023.Citation12
Studies have emphasized the superior efficacy of adjuvanted vaccines in both children and older adults.Citation13 For instance, a longitudinal study conducted in the U.S. across the 2017–2019 influenza seasons reported the enhanced clinical efficacy of the adjuvanted trivalent influenza vaccine (aTIV) in reducing influenza-related medical events among older adults.Citation14 While the benefits of MF59-aQIV are well-established, further research is needed to assess their economic impact. Preliminary analyses conducted in Europe indicate potential health and economic benefits. In Spain, aQIV was found to be more cost-effective than the high-dose quadrivalent influenza vaccine (QIV-HD) for older adults, potentially saving over €61 million.Citation15,Citation16 A Belgian economic analysis indicated that aQIV is cost-effective for older adults compared to the standard-dose quadrivalent influenza vaccine (QIV-SD) and QIV-HD.Citation17 Utilizing aQIV leads to a decrease in influenza-related illnesses, with an incremental cost-effectiveness ratio (ICER) of €15,227 per quality-adjusted life year (QALY) compared to QIV.Citation17 Moreover, it has the potential to generate cost savings over QIV-HD, given its 56% efficacy against infection.Citation17
Recent evaluations in South Korea indicates that switching from QIV to aQIV for individuals aged ≥65 years could potentially reduce influenza-related illnesses and deaths.Citation18 This shift is projected to lead to a 9.52% decrease in influenza cases, 709 fewer hospital admissions, and 145 fewer deaths among the older adults annually.Citation18 However, the study focused solely on older adults and did not consider the broader impact of the vaccine on other age groups.Citation18 Additionally, their analysis employed a static decision tree model, which did not account for the indirect effects of vaccination.
Given the possibility of introducing the aQIV into South Korea’s NIP,Citation19 it is crucial to understand its broader public health implications through dynamic modeling. Therefore, our study utilized a dynamic influenza transmission and vaccination model to evaluate the cost-effectiveness and impact on disease burden across all age groups, not just the elderly. This study evaluated the cost-effectiveness of aQIV versus QIV among South Korean adults aged ≥65 years from a healthcare perspective using a dynamic mathematical model. Our aim is to investigate how shifting from QIV to aQIV for this age group could lessen the impact of influenza-related illnesses across all age groups in South Korea. These findings could provide valuable insights into the health and economic benefits of incorporating aQIV, thereby informing policymaking for this vulnerable population.
Materials and methods
This study examined the incremental costs and benefits associated with transitioning from QIV to aQIV for individuals aged ≥65 years in South Korea. Two scenarios were explored: 1) administering QIV to all eligible age groups, and 2) providing aQIV, assumed to be 56.1% effective, solely to seniors aged 65 and above, while other age groups received QIV. To assess and compare the cost-effectiveness of this transition for individuals aged ≥65 years, we developed an age-specific dynamic model for seasonal influenza transmission by expanding the susceptible-vaccinated-infected-recovered framework.
Demographic, behavioral, and epidemiological model
To assess the financial and health outcomes of influenza vaccination in South Korea over a single season, we developed an age-structured dynamic model incorporating two vaccine types: QIV and aQIV (). The population was divided into 17 age groups: 0 − 5 months, 6 months −4 years, 5 − 9 years, 10 − 14 years, … ,70 − 74 years, and ≥75 years. In this model, individuals were classified according to their health status into various groups: susceptible (), vaccinated (
for QIV,
for aQIV), exposed (
), infected (
for asymptomatic,
for symptomatic), outpatient (
), hospitalized (
), recovered (
), and deceased (
), with the subscript
indicating specific age group (
= 1, 2, … , 17). The population size for each age group k, Nk(t), is defined by
Figure 1. Model diagram of the seasonal influenza transmission and vaccination.
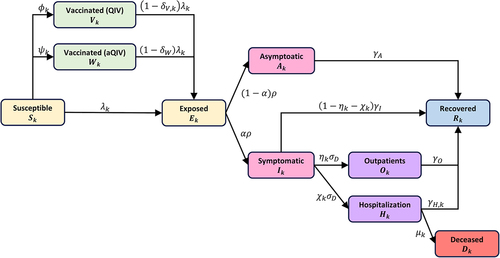
Our model incorporated age-dependent vaccination rates for both QIV () and aQIV (
) in age group
, with vaccine efficacies represented by
for QIV and
for aQIV, aligning with the monthly vaccination rates across all age groups in South Korea.Citation20 Individuals aged ≥6 months were deemed eligible for the influenza vaccine in our model according to South Korea’s existing influenza vaccination guidelines (
and
).
The infection rate for susceptible individuals in age group , denoted as
, was calculated considering factors such as susceptibility (
), contact rates (
), and the relative infectiousness of asymptomatic and outpatient cases (
and
) compared to symptomatic individuals who have not sought outpatient care. Specifically, the force of infection,
, is calculated as
. Here,
indicates the contact rate at which individuals in age group
encounter those in age group
. Of note, within this framework,
denotes the number of individuals in the hospital who pose no infection risk to others due to their isolation.
The average duration of the latent period and the delay in reporting are assumed to be represented by 1/ and
, respectively. The likelihood of symptomatic presentation among those infected is denoted by
. It is also assumed that asymptomatic infected individuals can recover without seeking hospital care. Symptomatic individuals may opt for outpatient care (
or be admitted to a hospital (
) if their symptoms are severe. Additionally, the probabilities of seeking outpatient services and hospital admission for those showing symptoms are indicated by
and
, respectively. To forecast the scenario for the current influenza season, data from Korea’s 2014–2019 National Health Insurance claims were utilized to fit our model and estimate
and
(Supplementary Figure S1).Citation21 Recovery rates for individuals in different health states – asymptomatic (
), outpatient (
), and symptomatic (
) – were also incorporated. Additionally, the model included age-specific recovery rates for hospitalized patients (
) and mortality rates (
).
Due to the relatively brief timeframe of this study, natural birth and death rates were excluded, and the total population was assumed to be asymptotically constant. Similarly, the aging process was not integrated into the model. The equations governing our mathematical model, considering the utilization of both QIV and aQIV vaccines, are as follows:
Epidemiological inputs
We implemented a total influenza vaccination coverage of 40%, anticipating South Korea’s influenza season to mirror the pre-coronavirus disease 2019 (COVID-19) pandemic season of 2014–2019, during which an approximate 40% vaccination rate was achieved.Citation22 Specifically, the coverage levels for children under 14 years and seniors aged 65 years and above, both of which are included in the NIP for seasonal influenza, were 77.5% and 84.3%, respectively.Citation20 Thus, we incorporated these specific coverage levels into our model for these age groups. For age groups not included in the NIP, we adjusted the vaccination levels to align with the overall target of 40%. To mirror actual vaccination trends, our model assumed that within the first two months, 72% of older adults, 69% of children, and 31% of those ineligible for the NIP were vaccinated, with the remaining individuals vaccinated over the following five months to meet our coverage goal.Citation20 The vaccination coverage was assumed to remain consistent across different strategies.
Our model was fitted to an annual attack rate of 7.26% for all influenza cases in South Korea, including both symptomatic and asymptomatic cases. This estimation was based on 2014–2019 data from the National Health Insurance Service (NHIS) Database in South Korea,Citation21 which also provides the age distribution of influenza cases. To accurately model the number of infections and outpatient visits across various age groups, we adjusted the susceptibility parameter () and the proportion of hospital visits (
), ensuring alignment with the observed age distribution of influenza cases ( and Supplementary Figures S1–S3).
Table 1. Description of epidemiological parameters and baseline values.
Vaccine effectiveness
While QIV is currently used for seasonal influenza vaccination in South Korea, its efficacy varies by age, exhibiting lower efficacy observed among the older population.Citation28,Citation29 Data regarding the efficacy of aQIV compared to no vaccine are not yet available; thus, it was deduced from QIV-HD’s estimated efficacy against no vaccine, adjusted by aQIV’s relative efficacy to QIV-HD, employing the method employed in a prior study.Citation17 A recent meta-analysis compared the relative efficacy of aTIV to high-dose trivalent influenza vaccine (TIV-HD).Citation30 This analysis revealed that aTIV’s efficacy was nearly equivalent to that of TIV-HD, presenting a marginal and statistically insignificant superiority of 3.2% (95% CI: −2.5% to 8.9%).Citation30 In our analysis, we assumed that the efficacy of quadrivalent vaccines (aQIV and QIV-HD) would mirror that of their trivalent counterparts (aTIV and TIV-HD), leading to the relative superiority of aQIV vs. QIV-HD as 3.2%.Citation30 Given that QIV-HD’s efficacy is estimated at 54.7%,Citation17 the resultant vaccine efficacy for aQIV was calculated as 56.1%:
The efficacies of the vaccines used in this study are listed in . A sensitivity analysis was conducted, assuming that aQIV’s efficacy was 60.0% and 65.0%.
Utility inputs
Quality of life measures, expressed as health utilities, were assigned to individuals based on their age and health condition, with QALYs specific to the severity level ().Citation32 The utility values for various age groups were derived using the EQ-5D obtained from the Seventh Korea National Health and Nutrition Examination Survey.Citation31 We assumed that the QALY for uninfected individuals, as well as those who have recovered and asymptomatically infected individuals, would be equivalent to that of a general healthy population. The mean reduction in the quality of life during illness with seasonal influenza was obtained from other studies.Citation32–36 For infected individuals receiving medical treatment or hospitalized, utility values of 0.81 and 0.59, respectively, were used to represent the diminished quality of life during illness.Citation32 summarizes these utility and disutility values.
Table 2. Input data for cost, utilities, and disutilities used in the cost-effectiveness analysis.
Costs inputs
The cost of the QIV was $8.10, reflecting the average domestic procurement price for the 2023–2024 influenza season.Citation39 Additionally, the administration fee amounted to $15.08, aligning with the implementation fee set by the Korea Disease Control and Prevention Agency.Citation40 As aQIV is not part of the NIP, its cost data is not readily available. However, an Irish study, considering average prices in the European market, estimated the cost of aQIV to be 1.8 times higher than that of QIV, totaling $29.66, inclusive of the administration fee, in our analysis.Citation23
Treatment expenses, sourced from the NHIS Sample Cohort,Citation31 differ between complicated and uncomplicated cases. However, our model does not classify patients as complicated or uncomplicated cases. Instead, we calculated these costs using a weighted average, considering the proportion of complicated cases by age group. As a result, outpatient treatment costs ranged from $59.7 to $80.8, while hospitalization costs varied from $564.9 to $1,095.2, depending on age. Further details are provided in .
Cost-effectiveness analysis
We conducted a cost-effectiveness analysis to evaluate the potential adoption of aQIV as a vaccination option for individuals aged ≥65 years in South Korea, comparing it with the conventional QIV. Our approach involved calculating the net present values of costs and effectiveness to determine the ICER from a healthcare perspective. To account for age-varying life expectancies affected by influenza-related deaths, a discounted rate of 4.5% was applied.
In evaluating the cost-effectiveness of different vaccination strategies, we considered costs related to influenza infection and vaccination, health utilities, and reductions in QALYs. The willingness-to-pay (WTP) threshold was set at $34,998, reflecting South Korea’s GDP per capita, a standard measure in the country’s cost-effectiveness analyses.Citation41,Citation42 The ICER was calculated by dividing the additional discounted costs associated with a strategy by its health benefits (i.e., QALYs gained). QALYs gained represent the QALYs accrued by the population across all age groups transitioning from QIV to aQIV for older adults, while costs encompass the total expenditure required for each vaccination strategy. This assessment spanned a single influenza season, from October 2023 to April 2024.
Results
Our simulation results provide comprehensive insights into South Korea’s NIP concerning seasonal influenza vaccination among older adults. We evaluated the cost-effectiveness of transitioning from conventional QIV to aQIV in this demographic by computing the ICER across different levels of aQIV efficacy in our sensitivity analysis. This assessment projected outcomes such as outpatient visits, hospital admissions, fatalities, QALY improvements, shifts in total costs attributable to differing vaccine efficacies, and the consequent economic ramifications.
Population-level impact
At a baseline efficacy of 56.1% for aQIV against infection, transitioning from QIV to aQIV for individuals aged ≥65 years could lead to a reduction of 5,559 outpatient visits, 1,818 hospitalizations, and 46 deaths in a single influenza season (). With higher efficacy levels for aQIV, greater reductions in cases are anticipated. At 60.0% and 65.0% efficacy, the aQIV strategy may prevent an additional 7,921 − 10,939 outpatient visits, 2,570 − 3,530 hospitalizations, and 68 − 93 deaths, respectively, compared with an all-QIV scenario.
Table 3. Health outcomes and overall costs (USD, $) for each seasonal influenza vaccination strategy.
Cost-effectiveness
presents the total costs and QALY losses for a single influenza season, analyzed from a healthcare perspective. This encompasses vaccination expenses, including administration fees, and disease treatment costs. The overall vaccination cost for all vaccinated individuals receiving QIV exceeded $501.3 million, while vaccinating older adults with aQIV incurred an additional $35.2 million compared to a non-adjuvanted vaccine strategy.
Furthermore, costs associated with outpatient and hospitalized patients were taken into account. Notably, the aQIV strategy resulted in fewer infections and reduced treatment expenses compared to the QIV strategy. While QIV administration incurred a treatment-disease cost of $1,073.0 million, aQIV reduced costs by $13.4 million. However, the combined expenses for aQIV administration to older adults exceeded those for QIV across all age groups by $21.9 million. Despite its higher cost, the aQIV strategy proved more effective in preventing infections than the QIV strategy.
In particular, administering aQIV to older adults was more effective in reducing infections and improving QALYs compared to QIV. It resulted in QALY gains of 750 compared to QIV administration. When comparing the ICER to the WTP threshold of $34,998 per QALY to assess the cost-effectiveness of switching vaccines for older adults, aQIV with an efficacy of 56.1% exhibited an ICER of $29,267 per QALY, below the WTP threshold. Thus, transitioning from QIV to aQIV among the older adults in South Korea would be cost-effective.
Sensitivity analysis
We assessed the cost-effectiveness of the aQIV at two additional efficacy levels in the sensitivity analysis: 60.0% and 65.0% (). With a 60.0% efficacy, aQIV could potentially lower disease treatment costs by approximately $19.0 million compared to QIV. This would result in total costs of over $1,599.5 million, $16.3 million less than using QIV for all vaccinated individuals, including older adults. Furthermore, an additional 1,066 QALYs were gained in this scenario (). At 65.0% efficacy, aQIV could reduce disease treatment costs by $26 million, resulting in an overall cost of $1,592.5 million. Thus, at 65.0% efficacy, switching to aQIV would decrease costs and yield 1,471 QALYs gained, making it a cost-effective approach.
Table 4. Effects of vaccinating all individuals with QIV versus aQIV administration for adults ≥65 years on incremental costs (USD, $), incremental QALYs, and ICERs ($/QALY).
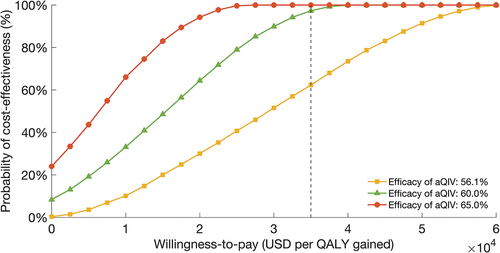
illustrates cost-effectiveness acceptability curves from probabilistic sensitivity analysis, depicting the probability of each vaccination strategy being cost-effective at different WTP thresholds. The analysis evaluated the probability of cost-effectiveness associated with transitioning from QIV to aQIV among older adults, considering three efficacy levels (56.1%, 60.0%, and 65.0%). At a WTP threshold of $34,998/QALY, all strategies demonstrated cost-effectiveness, with aQIV exhibiting a 60% probability of cost-effectiveness at 56.1% efficacy. At efficacy levels of 60.0% and 65.0%, the probability of aQIV being cost-effective approached nearly 100% at the same WTP threshold.
Figure 2. Cost-effectiveness acceptability curves depicting the cost-effective probability of aQIV versus QIV based on the efficacy of aQIV.
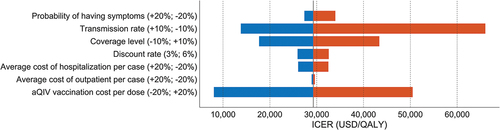
Furthermore, a one-way sensitivity analysis was conducted on key elements of the cost-effectiveness study (), considering various factors such as the probability of symptom manifestation post-infection, transmissibility, vaccination coverage, discount rate, influenza illness treatment costs, and aQIV vaccine cost per dose. The results indicated that using aQIV for vaccination remained cost-effective, with a 10% higher transmission rate. However, it was not cost-effective if the transmission rate was 10% lower, vaccination coverage was 10% higher, or aQIV cost was 20% higher per dose at a WTP threshold of $34,998/QALY. Notably, variations in aQIV costs had the greatest impact on the study’s outcomes, while outpatient treatment costs had minimal influence.
Figure 3. One-way deterministic sensitivity analysis.
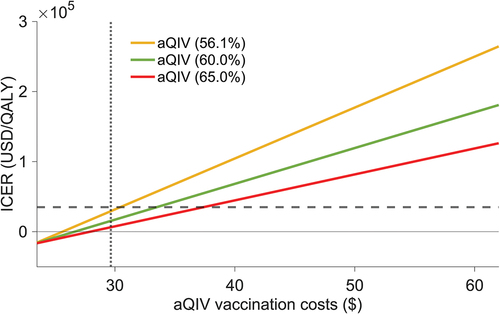
For each aQIV efficacy level, we determined the maximum cost at which switching to this vaccine remained cost-effective (). Baseline vaccine prices, including administration fees, were $23.18 for QIV and varied between $25 and $60 for aQIV. The results showed that the transition to aQIV for older adults remained cost-effective even if the vaccination cost per person, including administration costs, reached $30.4. Moreover, at efficacies of 60.0% and 65.0%, aQIV remained cost-effective up to vaccination costs of $33.4 and $37.3, respectively.
Figure 4. Incremental cost-effectiveness ratios based on the total vaccination costs.
Discussion
In South Korea, discussions are ongoing regarding the inclusion of adjuvant vaccines in the NIP for individuals aged 65 and older, supported by growing evidence of the effectiveness and safety of adjuvanted trivalent vaccines. This study evaluated the cost-effectiveness of replacing QIV with aQIV in South Korea’s NIP for seasonal influenza among older adults. Our findings suggest that aQIV vaccination becomes cost-effective when the vaccination cost per individual remains below $30.4, assuming a baseline efficacy of 56.1%. Transitioning from QIV to aQIV for seasonal influenza among older adults was projected to prevent an additional 15,676 symptomatic infections.
To our knowledge, this is the first evaluation of the cost-effectiveness of aQIV in older adults in South Korea. Previous studies from various countries have consistently demonstrated the cost-effectiveness of aQIV compared to QIV-SD for this demographic.Citation16–46 For instance, a Belgian study confirmed aQIV’s cost-effectiveness for adults over 65, consistent with findings.Citation17 In Ireland, aQIV was considered cost-effective when its efficacy exceeded QIV by more than 3%.Citation23 The ICER for administering aQIV was found to be €2,420/QALY and €12,970/QALY from societal and healthcare perspectives, respectively, figures which fall below the WTP threshold of €45,000.Citation23 Although variations in cost-effectiveness were observed based on the vaccine’s pricing, its significant cost-benefit ratio primarily stemmed from aQIV’s improved efficacy in older populations. Given the increased risk of severe influenza complications and mortality among older populations, the adoption of more efficacious vaccines such as aQIV could lead to substantial reductions in healthcare utilization.
Our findings align with recent assessments in South Korea, anticipate favorable outcomes, including reduced influenza incidents and associated adversities, by replacing QIV with aQIV in older adults.Citation18 These projections underscore the potential of aQIV in alleviating the burden of influenza-related diseases among South Korea’s elderly.
However, the study is not without limitations. Due to the lack of available data on aQIV’s efficacy, adjustments were made based on the relative vaccine efficacy of aTIV against TIV. This approach was taken to mitigate the uncertainty surrounding aQIV’s performance. Moreover, since aQIV has yet to be included in South Korea’s NIP, there is an ambiguity regarding its procurement costs. To address these uncertainties, we drew cost parallels between QIV and aQIV from European modelsCitation23 and recommend future research to establish the precise cost-efficacy breakpoints based on varying levels of aQIV’s efficacy.
In conclusion, transitioning to aQIV for the elderly in South Korea promises not only to mitigate influenza’s impact but also to uphold cost-effectiveness, especially when efficacy surpasses 56.1%. This research offers pivotal insights for decision-makers on the advantages of switching from QIV to aQIV in South Korea’s aged demographic. The broad-spectrum benefits of diminishing disease prevalence, extending beyond the vaccinated elderly to the wider community, underscore the strategic advantage of adopting aQIV within national healthcare frameworks.
Author contributions
Conceptualization: ES
Methodology: YS, ES
Investigation: YS, ES
Validation: YS, ES
Writing – original draft: YS, ES
Writing – review & editing: YS, ES
All authors attest they meet the ICMJE criteria for authorship.
Sponsor
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Supplementary material.docx
Download MS Word (9.4 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2348124.
Additional information
Funding
References
- WHO. Influenza. Geneva (Switzerland): WHO. https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccine-standardization/influenza.
- (WHO) WHO. Influenza (Seasonal); 2023. https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal).
- Yun JW, Choi MJ, Shin GS, Lim JO, Noh JY, Kim YK, Song JY, Kim WJ, Choi S-E, Cheong HJ, et al. Cost-effectiveness of influenza vaccine strategies for the elderly in South Korea. PLOS ONE. 2019;14(1):e0209643. doi:10.1371/journal.pone.0209643.
- Iuliano AD, Roguski KM, Chang HH, Muscatello DJ, Palekar R, Tempia S, Cohen C, Gran JM, Schanzer D, Cowling BJ, et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391:1285–9. doi:10.1016/S0140-6736(17)33293-2.
- Park M, Wu P, Goldstein E, Joo Kim W, Cowling BJ. Influenza-associated excess mortality in South Korea. Am J Prev Med. 2016;50:111–119. doi:10.1016/j.amepre.2015.09.028.
- Seo J, Lim J. The impact of free vaccination policies under the Korean influenza national immunization program: trends in influenza vaccination rates in South Korea from 2010 to 2019. PLOS ONE. 2022;17(1):e0262594. doi:10.1371/journal.pone.0262594.
- Kim J, Dorgan JF, Kim H, Kwon O, Kim Y, Kim Y, Ko KS, Park YJ, Park H, Jung S. Association between use of nutrition labels and risk of chronic kidney disease: the Korean National Health and Nutrition Examination Survey (KNHANES) 2008–2019. Nutrients. 2022 Apr 21;14(9):1731. doi:10.3390/nu14091731.
- Izurieta HS, Chillarige Y, Kelman J, Wei Y, Lu Y, Xu W, Lu M, Pratt D, Chu S, Wernecke M, et al. Relative effectiveness of cell-cultured and egg-based influenza vaccines among elderly persons in the United States, 2017–2018. J Infect Dis. 2018;220:1255–1264. doi:10.1093/infdis/jiy716.
- Morse K, Cleveland KW. Efficacy of the adjuvanted influenza vaccine compared with the high-dose for older people. Sr Care Pharm. 2023;38(4):156–160. doi:10.4140/TCP.n.2023.156.
- Seqirus C. CSL seqirus products. www.csl.com.
- Dae-Yeol N. CSL seqirus “Immune-boosting quadrivalent flu vaccine for the elderly, approved in Korea”. Hit News; 2022. http://www.hitnews.co.kr/news/articleView.html?idxno=41557.
- KSID. 2023 adult immunization recommendations. Influenza vaccine; 2023. www.ksid.or.kr.
- Tregoning JS, Russell RF, Kinnear E. Adjuvanted influenza vaccines. Hum Vaccines Immunother. 2018;14(3):550–564. doi:10.1080/21645515.2017.1415684.
- Boikos C, Fischer L, O’Brien D, Vasey J, Sylvester GC, Mansi JA. Relative effectiveness of adjuvanted trivalent inactivated influenza vaccine versus egg-derived quadrivalent inactivated influenza vaccines and high-dose trivalent influenza vaccine in preventing influenza-related medical encounters in US adults >/= 65 years during the 2017–2018 and 2018–2019 influenza seasons. Clin Infect Dis. 2021;73(5):816–823. doi:10.1093/cid/ciab152.
- Ruiz-Aragon J, Marquez-Pelaez S, Gani R, Alvarez P, Guerrero-Luduena R. Cost-effectiveness and burden of disease for adjuvanted quadrivalent influenza vaccines compared to high-dose quadrivalent influenza vaccines in elderly patients in Spain. Vaccines. 2022;10(2):176. doi:10.3390/vaccines10020176.
- Fochesato A, Sottile S, Pugliese A, Márquez-Peláez S, Toro-Diaz H, Gani R, Alvarez P, Ruiz-Aragón J. An economic evaluation of the adjuvanted quadrivalent influenza vaccine compared with standard-dose quadrivalent influenza vaccine in the Spanish older adult population. Vaccines. 2022;10(8):1360. doi:10.3390/vaccines10081360.
- Marbaix S, Dauby N, Mould-Quevedo J. Cost-effectiveness of the adjuvanted quadrivalent influenza vaccine in the elderly Belgian population. Expert Rev Vaccines. 2023;22(1):608–619. doi:10.1080/14760584.2023.2229917.
- Choi MJ, Yun JW, Song JY, Ko K, Mould JF, Cheong HJ. A comparative analysis of influenza-associated disease burden with different influenza vaccination strategies for the elderly population in South Korea. Vaccines. 2022;10(9):1387. doi:10.3390/vaccines10091387.
- Han-Joo K. Seqirus eyes swift introduction of influenza vaccines into S.Korea. Seoul (Korea): Yonhap News Agency; 2022. https://en.yna.co.kr/view/AEN20220216008100320?section=search.
- KDCA. Status of national influenza vaccination support project for the 2018–2019 season. In: Department V, editor; 2019. www.kdca.go.kr.
- Hwang SH, Lee H, Jung M, Kim SH, Sung HK, Oh MD, Lee JY. Incidence, severity, and mortality of influenza during 2010–2020 in Korea: a nationwide study based on the population-based national health insurance service database. J Korean Med Sci. 2023;38(8). doi:10.3346/jkms.2023.38.e58.
- KDCA. Annual influenza vaccination rate. www.chs.kdca.go.kr.
- Nguyen VH, Ashraf M, Mould-Quevedo JF. Cost-effectiveness of the use of adjuvanted quadrivalent seasonal influenza vaccine in older adults in Ireland. Vaccines. 2023;11(5):933. doi:10.3390/vaccines11050933.
- Longini IM Jr., Halloran ME, Nizam A, Yang Y. Containing pandemic influenza with antiviral agents. Am J Epidemiol. 2004;159(7):623–633. doi:10.1093/aje/kwh092.
- Bolge SC, Kariburyo F, Yuce H, Fleischhackl R. Predictors and outcomes of hospitalization for influenza: real-world evidence from the United States medicare population. Infec Dis Ther. 2021;10(1):213–228. doi:10.1007/s40121-020-00354-x.
- Galvani AP, Reluga TC, Chapman GB. Long-standing influenza vaccination policy is in accord with individual self-interest but not with the utilitarian optimum. Proc Natl Acad Sci. 2007;104(13):5692–5697. doi:10.1073/pnas.0606774104.
- Hong TH, Lee HS, Kim NE, Lee KJ, Kim YK, An JN, Kim J-H, Kim HW, Park S. Recent increases in influenza-related hospitalizations, critical care resource use, and In-hospital mortality: a 10-year population-based study in South Korea. J Clin Med. 2022;11(16):4911. doi:10.3390/jcm11164911.
- KDCA. Guidelines for the management of the NIP for influenza in the 2023–2024 season; 2023. https://nip.kdca.go.kr/irhp/infm/goRefcBrdView.do.
- Beyer WE, McElhaney J, Smith DJ, Monto AS, Nguyen-Van-Tam JS, Osterhaus AD. Cochrane re-arranged: support for policies to vaccinate elderly people against influenza. Vaccine. 2013;31(50):6030–6033. doi:10.1016/j.vaccine.2013.09.063.
- Coleman BL, Sanderson R, Haag M, McGovern I. Effectiveness of the MF59-adjuvanted trivalent or quadrivalent seasonal influenza vaccine among adults 65 years of age or older, a systematic review and meta-analysis. Influenza Other Respir Viruses. 2021;15:813–823. doi:10.22541/au.161933909.92394260/v1.
- Shin G, Kang D, Cheong HJ, Choi SE. Cost-effectiveness of extending the national influenza vaccination program in South Korea: does vaccination of older adults provide health benefits to the entire population? Vaccines. 2022;10(6):932. doi:10.3390/vaccines10060932.
- Wu DBC, Chaiyakunapruk N, Pratoomsoot C, Lee KKC, Chong HY, Nelson RE, Smith PF, Kirkpatrick CM, Kamal MA, Nieforth K, et al. Cost-utility analysis of antiviral use under pandemic influenza using a novel approach – linking pharmacology, epidemiology and heath economics. Epidemiol Infec. 2018;146(4):496–507. doi:10.1017/S0950268818000158.
- Khazeni N, Hutton DW, Garber AM, Owens DK. Effectiveness and cost-effectiveness of expanded antiviral prophylaxis and adjuvanted vaccination strategies for an influenza a (H5N1) pandemic. Ann Intern Med. 2009;151(12):840. doi:10.7326/0000605-200912150-00156.
- Turner DA, Wailoo AJ, Cooper NJ, Sutton AJ, Abrams KR, Nicholson KG. The cost-effectiveness of influenza vaccination of healthy adults 50–64 years of age. Vaccine. 2006;24(7):1035–1043. doi:10.1016/j.vaccine.2004.12.033.
- Angus DC, Musthafa AA, Clermont G, Griffin MF, Linde-Zwirble WT, Dremsizov TT, Pinsky MR. Quality-adjusted survival in the first year after the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163(6):1389–1394. doi:10.1164/ajrccm.163.6.2005123.
- Macario A, Chow JL, Dexter F. A Markov computer simulation model of the economics of neuromuscular blockade in patients with acute respiratory distress syndrome. BMC Med Inform Decis Mak. 2006;6(1):15. doi:10.1186/1472-6947-6-15.
- WHO. The global health observatory; 2020. www.who.int.
- KOSIS. Population census(sex- and age-specific). Seoul (Korea): KOSIS; 2021. https://gsis.kwdi.re.kr/statHtml/statHtml.do?orgId=338&tblId=DT_1IN0503&conn_path=I2.
- KDCA. North Chungcheong Province (South Korea). 2023. https://www.kdca.go.kr/board/board.es?mid=a20501010000&bid=0015&act=view&list_no=722739#.
- KDCA. North Chungcheong Province (South Korea). 2023. https://www.kdca.go.kr/board/board.es?mid=a20505010000&bid=0017&act=view&list_no=721582.
- Baltussen RM, Adam T, Tan-Torres Edejer T, World Health O. Making choices in health - WHO guide to cost effectiveness analysis: WHO guide to cost-effectiveness analysis. Vol. 78. Albany: World Health Organization Albany; 2004. doi:10.1590/s1135-57272004000300012.
- Bank TW. GDP per capita (current US$) - Korea, Rep. https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=KR.
- Ruiz-Aragón J, Márquez-Peláez S, Gani R, Alvarez P, Guerrero-Luduena R. Cost-effectiveness and burden of disease for adjuvanted quadrivalent influenza vaccines compared to high-dose quadrivalent influenza vaccines in elderly patients in Spain. Vaccines. 2022;10(2):176. doi:10.3390/vaccines10020176.
- Calabrò GE, Boccalini S, Panatto D, Rizzo C, Di Pietro ML, Abreha FM, Ajelli M, Amicizia D, Bechini A, Giacchetta I, et al. The new quadrivalent adjuvanted influenza vaccine for the Italian elderly: a health technology assessment. Int J Environ Res Public Health. 2022;19(7):4166. doi:10.3390/ijerph19074166.
- Kohli MA, Maschio M, Mould-Quevedo JF, Drummond M, Weinstein MC. The cost-effectiveness of an adjuvanted quadrivalent influenza vaccine in the United Kingdom. Hum Vaccin Immunother. 2021;17(11):4603–4610. doi:10.1080/21645515.2021.1971017.
- Kohli MA, Maschio M, Cartier S, Mould-Quevedo J, Fricke FU. The cost-effectiveness of vaccination of older adults with an MF59-adjuvanted quadrivalent influenza vaccine compared to other available quadrivalent vaccines in Germany. Vaccines. 2022;10(9):1386. doi:10.3390/vaccines10091386.

