ABSTRACT
The burden of food contamination and food wastage has significantly contributed to the increased prevalence of foodborne disease and food insecurity all over the world. Due to this, there is an urgent need to develop a smarter food traceability system. Recent advancements in biosensors that are easy-to-use, rapid yet selective, sensitive, and cost-effective have shown great promise to meet the critical demand for onsite and immediate diagnosis and treatment of food safety and quality control (i.e. point-of-care technology). This review article focuses on the recent development of different biosensors for food safety and quality monitoring. In general, the application of biosensors in agriculture (i.e. pre-harvest stage) for early detection and routine control of plant infections or stress is discussed. Afterward, a more detailed advancement of biosensors in the past five years within the food supply chain (i.e. post-harvest stage) to detect different types of food contaminants and smart food packaging is highlighted. A section that discusses perspectives for the development of biosensors in the future is also mentioned.
1. Introduction
Ensuring access to sufficient amount of safe and nutritious food yet environmentally friendly have been a growing attention since the last decades. It is notorious that the increasing prevalence of foodborne diseases has contributed significantly to the global burden of disease and mortality. This results in public health problems as well as economic and social concerns worldwide. According to the World Health Organization (WHO), there are 600 million (i.e. about 1 in 10 people in the world) cases of people becoming ill after eating contaminated food. This number contributes to the global deaths of 420,000 and the loss of 33 million healthy years of life in 2010 [Citation1]. Data from the annual report on food security and nutrition further stated that nearly 8.9% of the total population, or 690 million people, in the world are hungry, although there is sufficient food to feed the world’s population [Citation2,Citation3].
The burden of foodborne diseases and food insecurity as part of food sustainability issues have influenced both developed and developing countries. However, the highest burden occurs in low- and middle-income countries (i.e. developing countries) that have a high level of poverty and pollution. Rapid urbanization, changes in consumer habits, globalization, and climate change have been known to underpin greater challenges to ensuring food safety and security [Citation4]. According to the global estimates, there are 31 foodborne hazards causing 32 diseases, with the most prominent cases being caused by bacteria, viruses, parasites, or chemical substances through contaminated food [Citation4]. Meanwhile, food waste and loss are strongly linked to food insecurity and a high carbon footprint [Citation5].
Contamination of food, along with food loss and waste, may occur at any stage throughout the food supply chain (i.e. the process from farm to fork, including manufacturing, packaging, distribution, storing, and further processing or cooking for consumption). This is because the process inherently deals with the uncertainty of safety and quality aspects [Citation6,Citation7]. Due to this, traceability across the supply chain must be maintained and continually developed by all sectors (i.e. government, researchers, food industry, and consumers). For instance, government should strengthen the requirement of legislation and certification in the food industry. Meanwhile scientific and industry sectors should cooperate in developing better food traceability system for ensuring food safety and quality. Consumers, at the end, should demanding more food information.
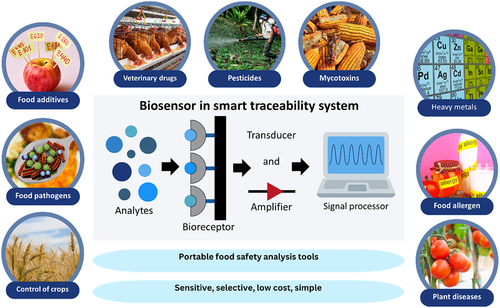
Smart food traceability has been known to significantly help overcome the global challenges related to food omics (i.e. the food fingerprint, which covers the nutritional values, quality, and authenticity of foods, as well as their safety and security) [Citation8]. Biosensors have been known to be reusable and able to replace conventional analytical techniques by giving rapid, accurate, reliable, and multiple analyses [Citation9,Citation10]. Many significant advancements of biosensors for food safety and analysis have been explored, including portable detections of foodborne disease agents in contaminated food. The main principle of detection by biosensors is the combination of a bioreceptor (i.e. biological recognition element) with a transducer (i.e. sensing element), generating a measurable signal proportional to the concentration of analytes. Different types of biosensors have been discovered based on the bioreceptor type (e.g. enzymes, antibodies, microbes, etc.), yet the significance is usually based on the interaction with analytes (i.e. the need to be highly specific) [Citation9]. Alternately, the most common type of biosensor based on its transducer type is electrochemical, while others include optical and mass-sensitive biosensors [Citation11].
Although there are many review articles discussing recent developments of biosensor in food system [Citation12–17], yet it is still limited to found one that discuss applications of biosensor in a whole complex system of food supply chain. The presence review article aims to combine previous studies of biosensors in food safety and analysis from pre-harvest to post-harvest stage. Highlights on the advantages of different biosensors developed within the past 5 years in correlation with smart traceability system are discussed. In general, there are three sections in this review. In the first section, general concepts and the development of food traceability systems and application of biosensors as traceability tools from pre-harvest to post-harvest are discussed. In the second section, information regarding mechanisms and applications of biosensors in food safety and security is mentioned. Lastly, the challenges and future perspectives of recent developments are mentioned.
2. Food traceability and biosensor
2.1. Food traceability
The International Organization for Standardization (ISO) and Codex Alimentarius Commission (CAC) define food traceability as the ability to follow or track the movement or progress of a product (i.e. feed or food) through the food chain, including production, processing, and distribution [Citation18]. Another extent to which the definition of food traceability relates to assurance of food safety is made by the American Production and Inventory Control Society (APICS) [Citation18]. The drivers or motivating factors determining the necessity of food traceability often differ depending on the specific information needed along the supply chain. A review by Islam & Cullen [Citation19] classified the drivers into five categories, including (1) legislation and certification, (2) safety and quality, (3) customer satisfaction, (4) sustainability, and (5) value and efficiency.
It is notable that the urgency for assurance and transparency of food safety within the food supply chain underlined those five driver categories. For instance, certain legislation and certification of a reliable traceability system are required to ensure fair practices in food trade and facilitate the free movement of safe food products within the region [Citation20]. Study by [Citation21] and [Citation22] to assess consumer preferences and willingness to pay for traceable food further proves the statement that food traceability can provide customer satisfaction. The rationale behind this is the significant number of potentially substantial disruptions (e.g. food pathogens, climate, etc.) during the supply chain and the occurrence of food fraud for economic gain that results in unsafe and unsuitable food for consumption [Citation23]. As a result, more people are becoming more knowledgeable about food and demand food credibility or food supply chain transparency.
Food traceability enables whole process monitoring of the uncertainty and complexity of the food supply chain. Thus, ensuring food safety and quality that prevent food waste and the possibility of food contamination, causing foodborne illness [Citation24]. With rapid technological advancements, traceability systems have progressed to a smarter or more intelligent system (). The main principles of smart food traceability are to leverage portable sensors and indicators to collect more comprehensive, traceable, and timely data about food products. The leading group of technologies developed includes portable detection devices, smart indicators and sensors incorporated into food packages, data-assisted whole genome sequencing, and other new digital technologies (e.g. Internet-of-Things (IoT) and cloud computing) [Citation8]. summarizes the advantages and disadvantages of current portable technologies.
Figure 1. Development stages of food traceability system. Reprinted from [Citation18] with permission from Elsevier.
![Figure 1. Development stages of food traceability system. Reprinted from [Citation18] with permission from Elsevier.](/cms/asset/a93bc6d3-f88a-4eb3-b7f7-34b6022412eb/kbie_a_2310908_f0001_oc.jpg)
Table 1. Advantages and disadvantages of portable traceability technologies.
2.2. Biosensors in supporting smart food traceability
7In a similar direction with the development of smart food traceability, research surrounding biosensors has attracted researchers’ attention. In fact, the current development of biosensors has further surpassed the disadvantages of different portable traceability technologies. Biosensors are well-known in the food supply chain for meeting the critical demand for onsite and immediate diagnosis and treatment of food quality control. This is because biosensors enable rapid yet selective, sensitive, and cost-effective detection of targeted analytes. Its ability to be easy-to-use without the need for complicated and expensive sample preparation has been one of the key features to be applied to point-of-care (POC) technology [Citation34]. POC in the food supply chain usually revolves around the concerns of nutrient monitoring, food safety and security, and food production environment control [Citation35].
Within the pre-harvest stage, food crops might be exposed to microbial infestation due to aflatoxin contamination, deficiency of nutrients, extreme weather conditions (drought and floods), and others. Thus, early detection and routine control (i.e. traceability) are urgently needed to prevent pre-harvest loss and further contamination in the supply chain. Many of the biosensors developed have been focusing on the detection of crop pathogens. For instance, a gold nanoparticle (AuNP)-based lateral flow biosensor integrated with universal primer-mediated asymmetric polymerase chain reaction (UP-APCR) was developed for rapid visual detection of Phytophthora infestans, the casual late blight disease in potatoes and tomatoes [Citation36]. The visual detection was done using sandwich-type hybridization assays with a detection limit of 0.1 pg/μL genomic DNA and high specificity within 1.5 hours. presents the mechanisms of the developed biosensor for rapid detection of P. infestans [Citation36].
Figure 2. Mechanism of AuNP-based biosensor based on UP-APCR for rapid detection of P. infestans. A region of P. infestans-specific repetitive DNA sequence was amplified to generate large amounts of ssDNA using APCR. The ssDNA was then applied to the lateral flow biosensor, giving a characteristic red band when there is AuNPs accumulation. Reprinted from [Citation36] with permission from Elsevier.
![Figure 2. Mechanism of AuNP-based biosensor based on UP-APCR for rapid detection of P. infestans. A region of P. infestans-specific repetitive DNA sequence was amplified to generate large amounts of ssDNA using APCR. The ssDNA was then applied to the lateral flow biosensor, giving a characteristic red band when there is AuNPs accumulation. Reprinted from [Citation36] with permission from Elsevier.](/cms/asset/8d509865-d593-4d5b-96b7-bd9b6bafcc67/kbie_a_2310908_f0002_oc.jpg)
Other biosensors were developed to minimize abiotic stress-mediated crop loss based on phytohormone responses, the production of small molecules, free radicals, etc. An electrochemical biosensor to monitor phytohormones, such as salicylic acid, was developed by utilizing microneedle-based electrodes. The electrodes are known to be functionalized with a layer of salicylic acid-selective magnetic molecularly imprinted polymers. The biosensor showed a detection limit of 2.74 μM in both in vitro and in vivo [Citation37]. More examples of biosensor application and comparison with conventional techniques within the pre-harvest stage (i.e. in the agriculture) are summarized in as well as elaborately described elsewhere [Citation58–60].
Table 2. Recent advancement of biosensors as smart food traceability system.
On the other side, the post-harvest stage usually consists of a more aggregate and complex process, including harvesting, sorting, storage, processing, packaging, distribution, and consumption. Application of biosensor in the post-harvest stage generally deals with food safety and authentication analysis which are mainly done during production and processing to ensure the food’s suitability. The analysis might include internal (e.g. nutrients, taste, pH, acidity, enzymes, etc.) and external (e.g. color, odor, texture, etc.) qualities [Citation58]. As food is still constantly moved from one process to another until it is bought by consumers, the need for smart traceability is still urgent. This is because, after being packaged, the food is still prone to contamination and deterioration due to changes in the surrounding environment. While biosensors are known for their great ability to conduct onsite food safety and analysis, the recent development of biosensors as part of smart food packaging has further shown the great potency of biosensors in food traceability systems. As an active and intelligent system, smart food packaging enables manufacturers and consumers to trace the product’s conditions during storage and distribution while extending and maintaining the shelf-life and quality of the food [Citation61,Citation62]. This smart packaging has been incorporated into perishable products such as dairy, meat, seafood, fruits and vegetables, as well as bakery and confectionery products in recent years. The schematic mechanism of active and intelligent food packaging is shown in [Citation63].
Figure 3. General schematic diagram of active and intelligent food packaging. Reprinted from [Citation63] with permission from frontiers (CC-BY 4.0 license).
![Figure 3. General schematic diagram of active and intelligent food packaging. Reprinted from [Citation63] with permission from frontiers (CC-BY 4.0 license).](/cms/asset/77b69a7a-5de9-4426-9cc5-e30737c44ca8/kbie_a_2310908_f0003_oc.jpg)
The underlying mechanism of active packaging to prolong shelf-life, maintain nutritional and organoleptic quality, inhibit microbial contamination or growth, and prevent the contaminants’ migration is known through the interaction of the product, package, and environment through the absorption of oxygen, ethylene, moisture, carbon dioxide (CO2), and odors, as well as the release of CO2, ethanol, flavor, and antimicrobial agents [Citation61]. Meanwhile, intelligent packaging systems play roles to detect, record, trace, or communicate information about the food products within the food chain by perceiving information concerning the initial food composition and storage condition, headspace composition, and microbial growth through three principal systems: indicators, sensors, and radio frequency identification systems (i.e. data carriers) [Citation64].
A biosensor incorporated with nanomaterials (i.e. nanosensors) has been extensively researched for their excellent prospects in food safety analysis and smart packaging. Nanomaterials have been explored for their great antimicrobial, mechanical, optical, and thermal properties to indicate the freshness, period for safe consumption, storage temperature, and others of food [Citation65]. Current discoveries of the combined integration of biosensors with nanomaterials include the development of a microfluidic colorimetric biosensor using gold nanoparticles (AuNPs) for rapid detection of Escherichia coli O157:H7 concentrations in chicken samples with color change output [Citation66]. This combined integrated biosensor and nanomaterials have shown a breakthrough in the challenges of smart food packaging, where gold nanoparticles provide an excellent platform for fast, low-cost, portable, and on-site food safety biosensors through their hydrogen bonding, nucleic acid hybridization, aptamer-target binding, antigen-antibody recognition, enzyme inhibition, and enzyme mimicking activity [Citation67]. Another great bio-based material employed as biosensors for food packaging is chitosan-based hydrogels that have antimicrobial, antioxidant, and biodegradability qualities [Citation67]. summarizes the present development of biosensors in smart food traceability system from pre-harvest stage (i.e. agriculture) to post-harvest stage (i.e. to determine food quality and safety).
3. Biosensors in food safety and security
Increased food demand because of exponential population growth have prompted the need to frame the food security challenge and solution through food system transformation. The food system should adopt a multidimensional approach at all stages of the food supply chain (from production to consumption) to be environmentally, economically, and socially sustainable, resilient, and efficient [Citation68,Citation69]. As mentioned by [Citation70], technologies such as remote sensing, tracing and tracking, active packaging, etc. are promising tools to tackle food security issues. This is because it could reduce the demand trajectory, fill the production gap, and avoid production losses. Biosensors represent a cutting-edge frontier in food traceability systems, enabling smart food safety and quality management tools. Thus, food security issues due to food contamination and the deterioration of nutrients and qualities could also be easily traced and prevented.
There have been many types of biosensors developed around food safety and quality tools, yet the main classification of biosensors in foodborne applications is based on their transducers. Optical biosensors, whose output signal is light emission, usually allow direct (label-free) detection of foodborne pathogens. The basic detection principle is usually found when cells bind to receptors or become immobilized on the transducer surface, causing changes that can be detected by the sensors. Electrochemical biosensor detection, on the other hand, is primarily relate to the ability to detect specific molecules (e.g. DNA-binding drugs, glucose, hybridized DNA). The principle is based on the measurable electrons or ions that are produced or suppressed by different types of chemical reactions [Citation71].
The applications of biosensors within food safety and security include the detection of foodborne pathogens, toxins, veterinary drugs, pesticides, and other chemical contaminants (i.e. food allergen, heavy metals, etc.) as described next. summarizes the development of biosensors to detect contaminants that concern food safety and quality.
Table 3. Recent development of biosensors in food safety and quality field.
3.1. Detection of foodborne pathogens
The majority of foodborne disease outbreaks are caused by various forms of pathogenic bacteria, viruses, and parasites. Among the severe and fatal bacterial infections, Salmonella, Campylobacter, and enterohaemorrhagic E. coli are the most common pathogens, affecting millions of people annually. The symptoms might include fever, headache, nausea, vomiting, abdominal pain, and diarrhea. Outbreaks of salmonellosis are usually linked with eggs, poultry, and other products of animal origin, while foodborne cases caused by Campylobacter are mainly caused by raw milk, raw or undercooked poultry, and drinking water. Enterohaemorrhagic E. coli, on the other hand, is usually associated with unpasteurized milk, undercooked meat, and contaminated fresh fruits and vegetables. Other bacteria that have caused foodborne diseases are Listeria infections from unpasteurized dairy products and various ready-to-eat foods, and V. cholerae, which mainly contaminate rice, vegetables, millet gruel, and various types of seafood [Citation4].
Recently, a ratiometric electrochemical biosensor based on the combination of SRCA (Saltatory Rolling Circle Amplification) and the CRISPR/Cas12a (CRISPR associated with system 12a) system for ultrasensitive and specific detection of Salmonella in food was developed. The basic principle of detection lies in the self-calibration of ratiometric electrochemical measurement to reduce internal or external disturbances, along with specific signal amplification using rapid SRCA amplification technology and the trans-cleavage capabilities of Cas12a. The biosensor displayed a detection limit as low as 2.08 fg/μL of Salmonella in pure culture and 100% sensitivity, 97.8% specificity, and 98% accuracy in the actual sample [Citation122]. Another low-field NMR (Nuclear Magnetic Resonance) biosensor based on a high-density carboxyl polyacrylate targeting gadolinium (Gd) probe was developed to rapidly detect Salmonella in milk. presents the schematic diagram of the principle of the NMR biosensor for detecting Salmonella in milk samples. At first, the target probe was obtained through an amide reaction resulting in activated polyacrylic acid and streptavidinylated polyacrylic acid (SA-PAA), which further undergoes a chelating adsorption reaction for gadolinium. The target probe of SA-PAA-Gd was then used to capture Salmonella through antigen-antibody interaction. This biosensor has shown a detection limit of 3.3 × 103 CFU/mL within 1.5 hours [Citation123].
Figure 4. Schematic diagram of NMR biosensor to detect salmonella in milk. Firstly, the target probe was prepared (a) followed with detection of salmonella in milk (b). Reprinted from [Citation123] with permission from Elsevier.
![Figure 4. Schematic diagram of NMR biosensor to detect salmonella in milk. Firstly, the target probe was prepared (a) followed with detection of salmonella in milk (b). Reprinted from [Citation123] with permission from Elsevier.](/cms/asset/3be40f12-f6f7-42af-b202-5f8104c3a4a1/kbie_a_2310908_f0004_oc.jpg)
E. coli O157:H7, as part of the Shiga-toxin-producing E. coli (STEC), and C. jejuni infections have also presented an alarming challenge in food safety. [Citation124] developed a microfluidic chemiluminescence biosensor based on multiple signal amplification of a combined CHA with H2-Au NP-catalyzed CL reaction for rapid and ultrasensitive detection of E. coli O157:H7. A label-free, specific, rapid, and cost-effective electrochemical biosensor has also been successfully developed using phage EP01 as the recognition agent for detection of E. coli O157:H7 GXEC-N07 in fresh milk and raw pork [Citation125]. For Campylobacter detection, a whole-cell V. harveyi-based biosensor assay developed to accurately quantify and observe the interspecies signaling molecule of C. jejuni called autoinducer-2 (AI-2) has shown great prospect in complex food matrices of food production [Citation126]. A paper-based DNA biosensor based on an enhanced chemiluminescence signal on a DNA dot blot and a silica nanoparticle was also developed to monitor Campylobacter in naturally contaminated chicken meat without pre-amplification [Citation127].
In viral pathogens, norovirus (NoV) is one of the most common foodborne infections, causing nausea, explosive vomiting, watery diarrhea, and abdominal pain [Citation4]. Given that NoV causes over 200,000 deaths each year, [Citation128] created a photoelectrochemical biosensor coupled with a novel custom-made monoclonal antibody as a convenient POC system for diagnosing NoV infection and detecting NoV-contaminated food samples. An electrochemical biosensor based on specific binding peptides coated onto the gold electrode has also exhibited highly specific detection of NoV from oysters [Citation129]. The schematic illustration of the biosensor to detect NoV is shown in [Citation129]. Similarly, [Citation130] also developed a 3D electrochemical aptasensor for NoV detection in spiked oysters based on phosphorene-gold nanocomposites.
Figure 5. Detection of Norovirus using peptide-coated electrochemical biosensor. Reprinted from [Citation129] with permission from Elsevier.
![Figure 5. Detection of Norovirus using peptide-coated electrochemical biosensor. Reprinted from [Citation129] with permission from Elsevier.](/cms/asset/def30bc7-8425-438f-900d-0c9d0e805927/kbie_a_2310908_f0005_oc.jpg)
3.2. Detection of mycotoxins
Mycotoxins, the secondary metabolites produced by fungi, have been known to have a significant toxicity effect on human and animal health, economies, and international trade. Mycotoxin contamination might occur on crops either during harvesting or storage. There have been about 300 mycotoxins identified and reported to contaminate 30 to 100% of food and feed samples in the world. Five major mycotoxin groups that are commonly found are aflatoxins (AF), ochratoxins (OTA), fumonisins, zearalenone (ZEN), and deoxynivalenol/nivalenol (DON) [Citation131]. As multiple mycotoxin contamination in foodstuffs poses synergistic effects that cause a more significant threat to human health, portable chemiluminescence optical fiber aptamer-based biosensors for ultrasensitive onsite assay of multiplex mycotoxins in food are developed [Citation132]). With a LOD of 0.015–0.423 pg/mL, the biosensor demonstrated sensitive and multiple analysis of mycotoxins in infant cereals. The selective and multiple analysis of the biosensor is mainly based on optical fibers that have specific recognition of single-stranded binding proteins (SSB) and mycotoxin aptamers (Jia et al., 2022).
Another portable chemiluminescence biosensor for rapid on-field screening and quantification of OTA in wine and coffee samples was developed [Citation133]. The user-friendly smartphone-based biosensor was developed using the combination of low-cost, disposable analytical cartridges that contain a lateral flow immunoassay (LFIA) strip with the chemiluminescence detection system of the smartphone camera as a light detector. The biosensor showed a LOD of 0.3 and 0.1 μg/L for wine and coffee, respectively. On the other hand, [Citation6] successfully developed an electrochemical biosensor based on E. coli as the signal recognition element, p-benzoquinone as the mediator, and a two-step reaction procedure. The biosensor showed detection limits of 1 and 6 ng/mL for Aflatoxin B1 (AFB1) and ZEN, enabling a promising tool for toxicity evaluation in corn and peanut oils. Moreover, a colorimetric biosensor with a wide detection range for dual mycotoxins detection was developed using a Fe3O4/GO-based platform for AFB1 detection and a Fe3O4@Au based platform for OTA detection [Citation134]. The detection principle of a colorimetric biosensor is shown in [Citation134].
Figure 6. Detection principle of a colorimetric biosensor to detect AFB1 and OTA. Formerly, the two platforms of Fe3O4/GO and TP-GO and Fe3O4@Au and Au NPs were formed through the combination of an AFB1 aptamer and the complementary strands of an OTA aptamer and probe, respectively. The absence and presence of both AFB1 and OTA will result in platform separation and a colorless supernatant. The addition of an alkaline solution to magnetically separated solids and the usage of Au NPs in the supernatant, on the other hand, results in a dark blue-colored solution. Reprinted from [Citation134] with permission from Elsevier.
![Figure 6. Detection principle of a colorimetric biosensor to detect AFB1 and OTA. Formerly, the two platforms of Fe3O4/GO and TP-GO and Fe3O4@Au and Au NPs were formed through the combination of an AFB1 aptamer and the complementary strands of an OTA aptamer and probe, respectively. The absence and presence of both AFB1 and OTA will result in platform separation and a colorless supernatant. The addition of an alkaline solution to magnetically separated solids and the usage of Au NPs in the supernatant, on the other hand, results in a dark blue-colored solution. Reprinted from [Citation134] with permission from Elsevier.](/cms/asset/39e7fc29-4172-46e0-9612-99b1aeec6e75/kbie_a_2310908_f0006_oc.jpg)
3.3. Detection of veterinary drug
Antibiotics, such as chloramphenicol, sulfadiazine, neomycin, and kanamycin, are the major group of veterinary drugs used in food-producing animals to prevent or cure disease. The misuse of veterinary drugs (i.e. antibiotics) often leads to the deposition of drug residues in the tissues and organs of food animals. This will then induce serious health hazards (e.g. allergies, antimicrobial resistance) when accumulated in the human body. [Citation109] have successfully fabricated an ultrasensitive label-free biosensor based on aptamer-functionalized 2D photonic crystal (SiO2-Au-ssDNA 2D PC) to detect kanamycin in milk. With the combination of the negatively charged AuNPs and sulfhydryl-modified ssDNA, the biosensor has resulted in excellent performance with a LOD of 1.10 pg/mL [Citation135]. A more recent development of an electrochemical biosensor with a LOD of 0.6 pM to detect kanamycin in milk was also manufactured based on exonuclease III-assisted dual-recycling amplification [Citation136]. The ultrasensitive and catalytic signal amplification of the biosensor were constructed using high-conductive MXene/VS2 and high-activity CeCu2O4 bimetallic nanoparticles as the electrode surface and nanozyme, respectively. Meanwhile, the accurate detection of the biosensor was fabricated from the dual supplementary recycling of primer DNA and hairpin DNA ().
Figure 7. Schematic diagram of electrochemical biosensor based on exonuclease-III-assisted dual-recycling amplification for rapid, sensitive, and accurate detection of kanamycin in milk. Reprinted from [Citation136] with permission from Elsevier.
![Figure 7. Schematic diagram of electrochemical biosensor based on exonuclease-III-assisted dual-recycling amplification for rapid, sensitive, and accurate detection of kanamycin in milk. Reprinted from [Citation136] with permission from Elsevier.](/cms/asset/f3b6d18a-02f1-48f4-9003-271989274256/kbie_a_2310908_f0007_oc.jpg)
Another ultrasensitive and selective colorimetric biosensor based on G-quadruplex DNAzyme was also developed to detect residues of tetracyclines in foods [Citation137]. Tetracycline antibiotics (e.g. tetracycline (TET), oxytetracycline (OTC), chlortetracycline (CTC), and doxycycline (DOX)) are widely used in the field of livestock husbandry, and when they are misused, their residues are often found in animal-derived foods such as milk, honey, and pork [Citation138]. The buildup in the human body can lead to serious diseases such as liver damage, tooth yellowing, allergic disorders, intestinal flora disorders, and bacterial resistance. The underlying mechanism of the biosensor to give results of tetracycline detection that could be determined even by the naked eye is based on the reaction between tetracycline and DNAzyme, which is composed of hemin and G-quadruplex and has peroxidase-like activity to form a stable complex and reduce catalytic activity. This reaction will then cause the solution’s color to change from yellow to green [Citation137]. A more recent similar colorimetric biosensor to detect tetracycline antibiotics was constructed with a LOD of 0.333 ng/mL [Citation139].
Furthermore, ampicillin (AMP), as one of the most used β-lactam antibiotics with antibacterial activity against gram-negative and positive bacteria, is also extensively used in agriculture, livestock, poultry, aquaculture, etc. There have been a number of severe environmental and food safety concerns recorded due to the overdose of this antibiotic, including endocarditis, membranitis, intestinal infection, and irritability. Yadav et al. [Citation140] have successfully fabricated a label-free electrochemical immunosensor based on molybdenum disulfide nanoparticles modified disposable indium tin oxide (ITO) with a LOD of 0.028 µg/mL in different food samples (milk, orange juice, and tap water). Detection of sulfamethazine, which is the most widely used and detected sulfonamide in animal-derived foods, was also studied with an antibody-antigen-aptamer sandwich electrochemical biosensor [Citation141].
3.4. Detection of pesticides
Organophosphate (OPP) and carbamate pesticides have had a positive impact on insect pest control and crop production globally. However, the indiscriminate and widespread use may result in impending toxicity to the environment and human health [Citation142]. The progressive research on the development of various biosensors has ranged from the use of conventional immobilizing supports to more advanced hybrid or composite nanomaterials [Citation143]. Previous reviews by [Citation144] and [Citation145] have summarized different enzymatic electrochemical biosensors for pesticide detection in foods. In particular, the inhibition-based biosensors that utilize the acetylcholinesterase (AChE) enzyme are shown to be mostly preferred. This is because the toxicity of organophosphorus pesticides will result in the formation of covalent bonding and the permanent inactivity of the AChE enzyme ().
Figure 8. (a) inhibition activity of AChE by OP and carbamate pesticides. Reprinted from [Citation146] with permission from hibiscus Publisher (CC by 4.0 license); (b) schematic diagram of zinc oxide (ZnO)-based biosensor to detect OP. Reprinted from [Citation147] with permission from MDPI (CC by 4.0 license).
![Figure 8. (a) inhibition activity of AChE by OP and carbamate pesticides. Reprinted from [Citation146] with permission from hibiscus Publisher (CC by 4.0 license); (b) schematic diagram of zinc oxide (ZnO)-based biosensor to detect OP. Reprinted from [Citation147] with permission from MDPI (CC by 4.0 license).](/cms/asset/d05d9326-d08e-4fbc-83a5-860a2c6881d7/kbie_a_2310908_f0008_oc.jpg)
For instance, a low cost and highly sensitive biosensor which immobilized the AChE enzyme on zinc oxide (ZnO) demonstrated excellent performance with the detection limit range from 0.5 nM–5 µM [Citation147] (). A graphene/chitosan/parathion multi-residue electrochemical biosensor was also fabricated to detect 11 types of OP pesticides through an indirect competitive method [Citation148]. The biosensor was prepared by combining the formation of phosphorylated AChE between organophosphorus molecules and AChE as well as the excellent conductivity of graphene. Likewise, a portable electrochemical biosensor was constructed by integrating a laser-induced graphene (LIG) electrode on polyimide (PI) foil and MnO2 nanosheets loaded on the paper [Citation149]. With a ‘sign-on’ electrochemical response of OPs determination in vegetables, the detection principle of the biosensor is known to rely on AChE-catalyzed hydrolytic product-triggered disintegration of MnO2 nanosheets. Another type of biosensor, which is voltametric with confirmed reusability after 90 days, was also executed based on enzyme activity inhibition of fungal laccase and bacterial catalase [Citation150].
3.5. Detection of other contaminants
Although it is not a common food allergen, there has been a growing incidence of mustard allergies. Therefore, a disposable electrochemical PCR-free biosensor was generated for the selective detection of protein Sin a 1, the most potent allergen in yellow mustard [Citation151]. The detection principle was done through the formation of DNA/RNA heterohybrid-specific antibodies by sandwich hybridization, resulting in simple and fast detection with a LOD of 3 pM. In a similar direction, [Citation152] have successfully fabricated an aptameric biosensor using graphene oxide to detect the alarming shrimp allergy due to tropomyosin. The advancement of biosensors that allow allergen detection and evaluation of allergy drugs was also studied. Jeong et al. [Citation153] constructed a bioelectronic sensor based on nanovesicles combined with anti-immunoglobulin E (anti-IgE) antibody receptors for signal amplification. The sensing system showed that it was sensitive and selectively able to detect the peanut allergen Arachis hypogaea 2 (Ara h 2) with a LOD of 0.1 fM in real food samples such as peanut and egg white.
Heavy metals (e.g. mercury, lead, cadmium, and arsenic) are known to pose a serious threat to food safety if consumed above the weekly allowable intake. [Citation154] developed a cell-free paper-based biosensor for on-site detection of Hg2+ and Pb2+ in water using a combination of in vitro transcription (IVT) technology with allosteric transcription factors (aTFs). The detection principle mainly relied on the aTFs specific affinity characteristic toward metal ions that cause dissociation from DNA and result in a measurable signal of transcribed fluorescent RNA (). Copper is a heavy metal that is also classified as an essential micronutrient for performing various bodily functions for plant, animal, and human health (e.g. production of red blood cells, collagen, energy, etc.). Copper should be monitored on a regular basis to avoid toxicity and health problems caused by over- and underconsumption [Citation155]. Žunar et al. [Citation156] have successfully transformed the native copper response of yeast S. cerevisiae into a whole-cell eukaryotic whole-cell copper biosensor to evaluate copper bioavailability.
Figure 9. Schematic diagram of a cell-free paper-based biosensor for on-site detection of heavy metals Hg2+ and Pb2+ in water based on aTfs. Reprinted from [Citation154] with permission from Elsevier.
![Figure 9. Schematic diagram of a cell-free paper-based biosensor for on-site detection of heavy metals Hg2+ and Pb2+ in water based on aTfs. Reprinted from [Citation154] with permission from Elsevier.](/cms/asset/e31b6e4b-d51d-4ea2-a33c-7f84f89d30cf/kbie_a_2310908_f0009_oc.jpg)
Food additives, in particular hydrogen peroxide (H2O2), are strong oxidizing agents that are often used in food processing as a bleaching agent in wheat flour, an antimicrobial agent in milk, or a sterilizing agent for food packaging materials. As high ingestion of hydrogen peroxide could result in significant health hazards, Vasconcelos et al. [Citation157] developed a chemiluminescence biosensor using a hydroxyethylcellulose-based membrane to detect hydrogen peroxide (H2O2) in different types of milk (i.e. fresh-raw, whole, semi-skimmed, and skimmed milk). The biosensor proved to be a quick, environmentally friendly, and low-cost method for detecting H2O2 in milk, with a LOD of 1.0 × 10−3 % w/w for fresh, raw, skim, and whole milk, and 2.0 × 10−3 % w/w for semi-skimmed milk. Moreover, synthetic colorants that are often used to enhance the sensory properties of foods are known to contain azo compounds, which pose hazards to human health. A study by Manjunatha [Citation158] developed a sensitive and selective cyclic voltammetric sensing system that utilized a poly (glycine) modified carbon paste electrode to determine tartrazine with a LOD of 2.83 × 10−7 mol/L.
4. Challenges and future perspective
Despite the necessity of traceability system within the complex food supply chain, not all food companies have sufficient economic value or scale to invest in. It is claimed by many studies that biosensors are cost-effective, yet the cost to manufacture biosensors still needs to be reduced as commercial applications are still lacking. Currently, self-powered biosensors based on biofuel cells have attracted great interest as they could advance the cost-efficiency of biosensors to another extent while being user-friendly and highly suitable for miniaturization, portability, and wearability [Citation159]. Lack of uniformity in the systems, coordination, allocation of costs and benefits for research and development, as well as globalization pace, climate, geographical location, and natural resources between each country have pose further challenges for implementing efficient application of biosensor for food system. [Citation160] have successfully developed a highly thermal and storage stable electrochemical biosensor for facilitating rapid pesticide detection of fruits and vegetables in a variety of climates. Another biosensor developed with long shelf life after 40 days also further supported the advancement of biosensor to tackle challenges in food safety and analysis technologies [Citation161].
Furthermore, as nanomaterials could be toxic, the fabrication of biosensor using this material might rise other challenges related to health. Therefore, further study on green synthesis and incorporation of biocompatible materials have grasped the insurgencies along with enhancing the sustainability value of biosensors in the food system (i.e. repurpose, reuse, degradable, or recyclable material) [Citation162]. Development of biosensors using microorganisms as the bioreceptor also have attracted many researchers’ attention. This is because the regulatory genes and proteins of microorganisms possess various responsive mechanisms to cope with environmental stress, pollutants, and heavy metals [Citation163,Citation164]. This then could be useful for the development of biosensor in agriculture as well as environmental monitoring. By having minimum requirements of electricity, water, gas, and energy from biosensor, minimal generation of carbon footprints could also be achieved.
As food system has become more complex, it is also urgently needed nowadays to have a more integrated food detection system. Biosensor combined with emerging technologies, such as smartphones, 3D printing, IoT, AI, and blockchain, could lead biosensor into another extend of advancement. For instance, combining biosensor and smartphone could significantly improve detection accuracy and shorten the detection time due to the automation and cloud-data saving from smartphone. With the current globalization where smartphones are being used by almost all people, smartphone-assisted biosensors will give enormous potential for onsite detection of food contaminants. For instance, Abdelbasset et al. [Citation165] discussed that smartphone-based aptasensor offers a semi-automated user interface that can be exploited by an inexpert person, along with fast and wireless data transferability. It could then be a breaking stone for onsite, portable, and simple monitoring in the smart food traceability system. As for sensor array, it can improve the specificity of biosensor due to its ability to accurately identify very similar and wide range of analytes in mixtures for fingerprint identification. This then can be used for detecting any food adulteration. Lastly, biosensor and IoT technologies could lead in the wireless transmission technology [Citation154,Citation166].
5. Conclusion
The increased prevalence of foodborne illness and food insecurity have shown great urgency in developing smart food traceability systems that are rapid, accurate, reliable, low cost, and able to conduct multiple analyses. Many advancements in biosensors over the years have shown great promise in enabling whole process monitoring to tackle the uncertainty and complexity of the food supply chain. The present review highlights different fabrications of biosensors within the pre- and post-harvest stages of food (e.g. agriculture, detecting biological and chemical food contaminants, smart food packaging). Based on the current trends, many biosensors associated with nanoparticles have more advantages, such as a lower detection limit, higher sensitivity, selectivity, and stability over long-term usage. However, there are still, many challenges to be tackled and improved in the future.
Author contributions statement
Catarina Meliana: Writing – original draft, Conceptualization, Design; Jingjing Liu: Conceptualization, Investigation; Pau Loke Show: Resources, Writing- Reviewing and Editing; Sze Shin Low: Writing- Reviewing and Editing, Visualization, Supervision, Funding acquisition.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- WHO. 2015. WHO estimates of the global burden of foodborne diseases. www.who.int
- FAO. The state of food security and nutrition in the world 2020. The State Of Food Security And Nutrition In The World 2020. 2020. doi: 10.4060/CA9692EN
- Mc Carthy U, Uysal I, Badia-Melis R, et al. Global food security – issues, challenges and technological solutions. Trends Food Sci Technol. 2018;77:11–23. doi: 10.1016/J.TIFS.2018.05.002
- WHO. 2022, May 19. Food safety. https://www.who.int/news-room/fact-sheets/detail/food-safety
- UN. n.d. Goal 12: Ensure sustainable consumption and production patterns. [cited 2023 Mar 3]. https://www.un.org/sustainabledevelopment/sustainable-consumption-production/
- Chen S, Brahma S, Mackay J, et al. The role of smart packaging system in food supply chain. J Food Sci. 2020;85(3):517–525. doi: 10.1111/1750-3841.15046
- Reynolds C, Goucher L, Quested T, et al. Review: consumption-stage food waste reduction interventions – what works and how to design better interventions. Food Policy. 2019;83:7–27. doi: 10.1016/J.FOODPOL.2019.01.009
- Yu Z, Jung D, Park S, et al. Smart traceability for food safety. Crit Rev Food Sci Nutr. 2020;62(4):905–916. doi: 10.1080/10408398.2020.1830262
- Ma Z, Meliana C, Munawaroh HSH, et al. Recent advances in the analytical strategies of microbial biosensor for detection of pollutants. Chemosphere. 2022;306:135515. doi: 10.1016/J.CHEMOSPHERE.2022.135515
- Ukhurebor KE, Adetunji CO. Relevance of biosensor in climate smart organic agriculture and their role in environmental sustainability: what has been done and what we need to do? 2021. pp. 115–136. doi: 10.1007/978-3-030-66165-6_7
- Mishra GK, Barfidokht A, Tehrani F, et al. Food safety analysis using electrochemical biosensors. Foods 2018. 2018;7(9):141. doi: 10.3390/FOODS7090141
- Adetunji CO, Nwankwo W, Ukhurebor KE, et al. Application of biosensor for the identification of various pathogens and pests mitigating against the agricultural production: recent advances. In: Pudake RN, Jain U, Kole C, editors. Biosensors in Agriculture: Recent Trends and Future Perspectives. 2021. pp. 169–189. doi:10.1007/978-3-030-66165-6_9.
- Ali AA, Altemimi AB, Alhelfi N, et al. Application of biosensors for detection of pathogenic food bacteria: a review. Biosensors (Basel). 2020;10(6):58. doi: 10.3390/BIOS10060058
- Malvano F, Pilloton R, Albanese D. Label-free impedimetric biosensors for the control of food safety–a review. Int J Environ Anal Chem. 2020;100(4):468–491. doi: 10.1080/03067319.2019.1667096
- Patel G, Pillai V, Bhatt P, et al. Application of nanosensors in the food industry. In: Han B, Tomer VK, Nguyen TA, Farmani A, Kumar Singh P, editors. Nanosensors for smart cities. Netherlands: Elsevier; 2020. pp. 355–368 doi:10.1016/B978-0-12-819870-4.00021-9
- Thakur M, Wang B, Verma ML. Development and applications of nanobiosensors for sustainable agricultural and food industries: recent developments, challenges and perspectives. Environ Technol Innov. 2022;26:102371. doi: 10.1016/j.eti.2022.102371
- Zhang Z, Lou Y, Guo C, et al. Metal–organic frameworks (MOFs) based chemosensors/biosensors for analysis of food contaminants. Trends Food Sci Technol. 2021;118:569–588. doi: 10.1016/j.tifs.2021.10.024
- Qian J, Ruiz-Garcia L, Fan B, et al. Food traceability system from governmental, corporate, and consumer perspectives in the European Union and China: a comparative review. Trends Food Sci Technol. 2020;99:402–412. doi: 10.1016/J.TIFS.2020.03.025
- Islam S, Cullen JM. Food traceability: a generic theoretical framework. Food Control. 2021;123:107848. doi: 10.1016/J.FOODCONT.2020.107848
- ASEAN. 2016. ASEAN Food Safety Policy. www.asean.org
- Liu R, Gao Z, Nayga RM, et al. Consumers’ valuation for food traceability in China: does trust matter? Food Policy. 2019;88:101768. doi: 10.1016/J.FOODPOL.2019.101768
- Dima A, Arvaniti E, Stylios C, et al. Adapting open innovation practices for the creation of a traceability system in a meat-producing industry in northwest Greece. Sustainability. 2022;14(9):5111. doi: 10.3390/su14095111
- Griesche C, Baeumner AJ. Biosensors to support sustainable agriculture and food safety. Trends Analyt Chem. 2020;128:115906. doi: 10.1016/J.TRAC.2020.115906
- Zhou C, Zou H, Sun C, et al. Recent advances in biosensors for antibiotic detection: selectivity and signal amplification with nanomaterials. Food Chem. 2021;361:130109. doi: 10.1016/J.FOODCHEM.2021.130109
- da Paixao Teixeira JL, dos Santos Carames ET, Baptista DP, et al. Vibrational spectroscopy and chemometrics tools for authenticity and improvement the safety control in goat milk. Food Control. 2020;112:107105. doi: 10.1016/j.foodcont.2020.107105
- Kunyaboon S, Thumanu K, Park JW, et al. Evaluation of lipid oxidation, volatile compounds and vibrational spectroscopy of silver carp (hypophthalmichthys molitrix) during ice storage as related to the quality of its washed mince. Foods. 2021;10(3):495. doi: 10.3390/foods10030495
- Singh H, Singh G, Kaur N, et al. Pattern-based colorimetric sensor array to monitor food spoilage using automated high-throughput analysis. Biosens Bioelectron. 2022;196:113687. doi: 10.1016/j.bios.2021.113687
- An X, Zuo P, Ye BC. A single cell droplet microfluidic system for quantitative determination of food-borne pathogens. Talanta. 2020;209:120571. doi: 10.1016/j.talanta.2019.120571
- Xiang X, Shang Y, Ye Q, et al. A salmonella serogroup rapid identification system for food safety based on high throughput microfluidic chip combined with recombinase aided amplification. Sensors And Actuat B Chem. 2022;357:131402. doi: 10.1016/j.snb.2022.131402
- Balamurugan S, Ayyasamy A, Joseph KS. IoT-blockchain driven traceability techniques for improved safety measures in food supply chain. Int j Inf Tecnol. 2022;14(2):1087–1098. doi: 10.1007/s41870-020-00581-y
- Lu Y, Li P, Xu H. A food anti-counterfeiting traceability system based on blockchain and internet of things. Procedia Comput Sci. 2022;199:629–636. doi: 10.1016/j.procs.2022.01.077
- Wang X, Wang Y, Guo C, et al. A pattern-free paper enzyme biosensor for one-step detection of fish freshness indicator hypoxanthine with a microfluidic aggregation effect. Food Chem. 2022;405:134811. doi: 10.1016/J.FOODCHEM.2022.134811
- Lin K, Chavalarias D, Panahi M, et al. Mobile-based traceability system for sustainable food supply networks. Nature Food. 2020;1(11):673–679. doi: 10.1038/s43016-020-00163-y
- Kaya HO, Cetin AE, Azimzadeh M, et al. Pathogen detection with electrochemical biosensors: advantages, challenges and future perspectives. J Electroanal Chem. 2021;882:114989. doi: 10.1016/J.JELECHEM.2021.114989
- Neethirajan S, Ragavan V, Weng X, et al. Biosensors for sustainable food engineering: challenges and perspectives. Biosensors 2018. 2018;8(1):23. doi: 10.3390/BIOS8010023
- Zhan F, Wang T, Iradukunda L, et al. A gold nanoparticle-based lateral flow biosensor for sensitive visual detection of the potato late blight pathogen, phytophthora infestans. Anal Chim Acta. 2018;1036:153–161. doi: 10.1016/J.ACA.2018.06.083
- Bukhamsin A, Ait Lahcen A, Filho JDO, et al. Minimally-invasive, real-time, non-destructive, species-independent phytohormone biosensor for precision farming. Biosens Bioelectron. 2022;214:114515. doi: 10.1016/J.BIOS.2022.114515
- Olias LG, Otero AR, Cameron PJ, et al. A soil microbial fuel cell-based biosensor for dissolved oxygen monitoring in water. Electrochimica Acta. 2020;362:137108. doi: 10.1016/J.ELECTACTA.2020.137108
- Ma Z, Liu J, Li H, et al. A fast and easily parallelizable biosensor method for measuring extractable tetracyclines in soils. Environ Sci Technol. 2019;54(2):758–767. doi: 10.1021/ACS.EST.9B04051/SUPPL_FILE/ES9B04051_SI_001.PDF
- Bae JW, Seo HB, Belkin S, et al. An optical detection module-based biosensor using fortified bacterial beads for soil toxicity assessment. Anal Bioanal Chem. 2020;412(14):3373–3381. doi: 10.1007/S00216-020-02469-Z/METRICS
- Liu Y, Guo M, Du R, et al. A gas reporting whole-cell microbial biosensor system for rapid on-site detection of mercury contamination in soils. Biosens Bioelectron. 2020;170:112660. doi: 10.1016/J.BIOS.2020.112660
- Kumar V, Arora K. Trends in nano-inspired biosensors for plants. Mater Sci Energy Technol. 2020;3:255–273. doi: 10.1016/J.MSET.2019.10.004
- Wang X, Cheng M, Yang Q, et al. A living plant cell-based biosensor for real-time monitoring invisible damage of plant cells under heavy metal stress. Sci Total Environ. 2019;697:134097. doi: 10.1016/J.SCITOTENV.2019.134097
- Mohtar LG, Aranda P, Messina GA, et al. Amperometric biosensor based on laccase immobilized onto a nanostructured screen-printed electrode for determination of polyphenols in propolis. Microchem J. 2019;144:13–18. doi: 10.1016/J.MICROC.2018.08.038
- Ye Y, Ji J, Sun Z, et al. Recent advances in electrochemical biosensors for antioxidant analysis in foodstuff. Trends Analyt Chem. 2020;122:115718. doi: 10.1016/J.TRAC.2019.115718
- Bougadi ET, Kalogianni DP. Paper-based DNA biosensor for food authenticity testing. Food Chem. 2020;322:126758. doi: 10.1016/J.FOODCHEM.2020.126758
- Li L, Zhang M, Chen W. Gold nanoparticle-based colorimetric and electrochemical sensors for the detection of illegal food additives. J Food Drug Anal. 2020;28(4):641. doi: 10.38212/2224-6614.3114
- Dey A, Sarkar SK. Fabrication and application of TiO2 based thin film capacitive biosensor towards fruit freshness detection on silicon substrate. Silicon. 2021;13(9):3031–3037. doi: 10.1007/s12633-020-00638-4
- Lorenzo JM, Munekata PE, Muchenje V, et al. Biosensors applied to quantification of ethanol in beverages. In: Grumezescu AM, Holban AM, editors. Engineering tools in the beverage industry. Vol. 3. United Kingdom: The Science of Beverages; 2019. pp. 447–468. doi:10.1016/B978-0-12-815258-4.00015-9
- Xiong X, Tan Y, Mubango E, et al. Rapid freshness and survival monitoring biosensors of fish: progress, challenge, and future perspective. Trends Food Sci Technol. 2022;129:61–73. doi: 10.1016/J.TIFS.2022.08.011
- Aquino A, Conte-Junior CA. A systematic review of food allergy: nanobiosensor and food allergen detection. Biosensors 2020. 2020;10(12):194. doi: 10.3390/BIOS10120194
- Zhou X, Pullman M, Xu Z. The impact of food supply chain traceability on sustainability performance. Oper Manag Res. 2021;15(1–2):93–115. doi: 10.1007/S12063-021-00189-W/TABLES/19
- Majdinasab M, Mishra RK, Tang X, et al. Detection of antibiotics in food: new achievements in the development of biosensors. Trends Analyt Chem. 2020;127:115883. doi: 10.1016/J.TRAC.2020.115883
- Nnachi RC, Sui N, Ke B, et al. Biosensors for rapid detection of bacterial pathogens in water, food and environment. Environ Int. 2022;166:107357. doi: 10.1016/J.ENVINT.2022.107357
- Zhou Q, Tang D. Recent advances in photoelectrochemical biosensors for analysis of mycotoxins in food. Trends Analyt Chem. 2020;124:115814. doi: 10.1016/J.TRAC.2020.115814
- Bhavadharini B, Kavimughil M, Malini B, et al. Recent advances in biosensors for detection of chemical contaminants in food—a review. Food Anal Methods. 2022;15(6):1545–1564. doi: 10.1007/S12161-021-02213-Y
- Kadam US, Hong JC. Advances in aptameric biosensors designed to detect toxic contaminants from food, water, human fluids, and the environment. Trends Environ Anal Chem. 2022;36:e00184. doi: 10.1016/J.TEAC.2022.E00184
- Kundu M, Krishnan P, Kotnala RK, et al. Recent developments in biosensors to combat agricultural challenges and their future prospects. Trends Food Sci Technol. 2019;88:157–178. doi: 10.1016/J.TIFS.2019.03.024
- Mondal R, Dam P, Chakraborty J, et al. Potential of nanobiosensor in sustainable agriculture: the state-of-art. Heliyon. 2022;8(12):e12207. doi: 10.1016/J.HELIYON.2022.E12207
- Sellappan L, Manoharan S, Sanmugam A, et al. Role of nanobiosensors and biosensors for plant virus detection. Nanosensors For Smart Agri. 2022;493–506. doi: 10.1016/B978-0-12-824554-5.00004-5
- Bhardwaj A, Sharma N, Sharma V, et al. Smart Food Packaging Systems. Trends Environ Anal Chem. 2022;235–260. doi: 10.1007/978-981-19-1746-2_8
- Chen Y, Yang Y, Wang Y, et al. Development of an Escherichia coli-based electrochemical biosensor for mycotoxin toxicity detection. Bioelectrochemistry. 2020;133:107453. doi: 10.1016/J.BIOELECHEM.2019.107453
- Salgado PR, di Giorgio L, Musso YS, et al. Recent developments in smart food packaging focused on biobased and biodegradable polymers. Front Sustain Food Syst. 2021;5:125. doi: 10.3389/FSUFS.2021.630393/BIBTEX
- Rodrigues C, Souza VGL, Coelhoso I, et al. Bio-based sensors for smart food packaging—Current applications and future trends. Sensors 2021. 2021;21(6):2148. doi: 10.3390/S21062148
- Madhusudan P, Chellukuri N, Shivakumar N. Smart packaging of food for the 21st century–a review with futuristic trends, their feasibility and economics. Mater Today Proc. 2018;5(10):21018–21022. doi: 10.1016/J.MATPR.2018.06.494
- Zheng L, Cai G, Wang S, et al. A microfluidic colorimetric biosensor for rapid detection of Escherichia coli O157:H7 using gold nanoparticle aggregation and smart phone imaging. Biosens Bioelectron. 2018;124–125:143–149. doi: 10.1016/J.BIOS.2018.10.006
- Chaudhary V, Bangar SP, Thaku N, et al. Recent advancements in smart biogenic packaging: reshaping the future of the food packaging industry. Polymers. 2022;14(4):829. doi: 10.3390/POLYM14040829
- el Bilali H, Callenius C, Strassner C, et al. Food and nutrition security and sustainability transitions in food systems. Food And Energy Security. 2018;8(2):e00154. doi: 10.1002/FES3.154
- FAO. Sustainable food systems concept and framework. Rome: FAO; 2018.
- Cole MB, Augustin MA, Robertson MJ, et al. The science of food security. Npj Sci Food. 2018;2(1):1–8. doi: https://doi.org/10.1038/s41538-018-0021-9
- Yasmin J, Ahmed MR, Cho B-K. Biosensors and their applications in food safety: a review. J Biosyst Eng. 2016;41(3):240–254. doi: 10.5307/JBE.2016.41.3.240
- Huang C, Zhao J, Lu R, et al. A phage-based magnetic relaxation switching biosensor using bioorthogonal reaction signal amplification for salmonella detection in foods. Food Chem. 2022;400:134035. doi: 10.1016/J.FOODCHEM.2022.134035
- Wang Y, Li H, Zhou J, et al. An antifouling polydopamine-based fluorescent aptasensor for determination of arginine kinase. Food Sci Hum Wellness. 2022;12(3):737–744. doi: 10.1016/J.FSHW.2022.09.007
- Man Y, Ban M, Li A, et al. A microfluidic colorimetric biosensor for in-field detection of salmonella in fresh-cut vegetables using thiolated polystyrene microspheres, hose-based microvalve and smartphone imaging APP. Food Chem. 2021;354:129578. doi: 10.1016/J.FOODCHEM.2021.129578
- Bacchu MS, Ali MR, Das S, et al. A DNA functionalized advanced electrochemical biosensor for identification of the foodborne pathogen Salmonella enterica serovar typhi in real samples. Anal Chim Acta. 2022;1192:339332. doi: 10.1016/J.ACA.2021.339332
- Dehghani Z, Mohammadnejad J, Hosseini M, et al. Whole cell FRET immunosensor based on graphene oxide and graphene dot for Campylobacter jejuni detection. Food Chem. 2019;309:125690. doi: 10.1016/J.FOODCHEM.2019.125690
- Cheng X, Liu W, Wang Z, et al. Improved triple-module fluorescent biosensor for the rapid and ultrasensitive detection of campylobacter jejuni in livestock and dairy. Food Control. 2022;137:108905. doi: 10.1016/J.FOODCONT.2022.108905
- Sun D, Fan T, Liu F, et al. A microfluidic chemiluminescence biosensor based on multiple signal amplification for rapid and sensitive detection of E. coli O157:H7. Biosens Bioelectron. 2022a;212:114390. doi: 10.1016/J.BIOS.2022.114390
- Zhou Y, Li Z, Huang J, et al. Development of a phage-based electrochemical biosensor for detection of Escherichia coli O157: H7 GXEC-N07. Bioelectrochemistry. 2022b;150:108345. doi: 10.1016/J.BIOELECHEM.2022.108345
- Pandit C, Alajangi HK, Singh J, et al. Development of magnetic nanoparticle assisted aptamer-quantum dot based biosensor for the detection of Escherichia coli in water samples. Sci Total Environ. 2022;831:154857. doi: 10.1016/J.SCITOTENV.2022.154857
- Sobhan A, Lee J, Park MK, et al. Rapid detection of Yersinia enterocolitica using a single–walled carbon nanotube-based biosensor for kimchi product. LWT. 2019;108:48–54. doi: 10.1016/J.LWT.2019.03.037
- Wei W, Lin H, Hao T, et al. DNA walker-mediated biosensor for target-triggered triple-mode detection of Vibrio parahaemolyticus. Biosens Bioelectron. 2021;186:113305. doi: 10.1016/J.BIOS.2021.113305
- Ali MR, Bacchu MS, Das S, et al. Label free flexible electrochemical DNA biosensor for selective detection of shigella flexneri in real food samples. Talanta. 2022;253:123909. doi: 10.1016/J.TALANTA.2022.123909
- Ouyang Q, Zhang M, Yang Y, et al. Mesoporous silica-modified upconversion biosensor coupled with real-time ion release properties for ultrasensitive detection of staphylococcus aureus in meat. Food Control. 2022;145:109444. doi: 10.1016/J.FOODCONT.2022.109444
- Ali MR, Bacchu MS, Setu MAA, et al. Development of an advanced DNA biosensor for pathogenic vibrio cholerae detection in real sample. Biosens Bioelectron. 2021;188:113338. doi: 10.1016/J.BIOS.2021.113338
- Nasrin F, Khoris IM, Chowdhury AD, et al. Impedimetric biosensor of norovirus with low variance using simple bioconjugation on conductive polymer-au nanocomposite. Sensors and Actuat B Chem. 2022;369:132390. doi: 10.1016/J.SNB.2022.132390
- Cho CH, Park TJ, Park JP. Affinity peptide-based electrochemical biosensor for the highly sensitive detection of bovine rotavirus. Biotechnol Bioproc E. 2022;27(4):607–614. doi: 10.1007/s12257-022-0044-6
- Chaudhari PP, Chau LK, Tseng YT, et al. A fiber optic nanoplasmonic biosensor for the sensitive detection of ampicillin and its analogs. Mikrochim Acta. 2020;187(7):1–11. doi: 10.1007/s00604-020-04381-w
- Yuan R, Yan Z, Shaga A, et al. Design and fabrication of an electrochemical sensing platform based on a porous organic polymer for ultrasensitive ampicillin detection. Sensors and Actuat B Chem. 2020;327:128949. doi: 10.1016/J.SNB.2020.128949
- Xiu Y, Luo R, Han B, et al. Construction of Co@C hybrid nanostructure: electrochemical biosensor for detection of penicillin sodium in milk. Food Anal Methods. 2019;13(3):617–628. doi: 10.1007/S12161-019-01677-3/METRICS
- Tian L, Zhang Y, Wang L, et al. Ratiometric Dual Signal-Enhancing-Based Electrochemical Biosensor for Ultrasensitive Kanamycin Detection. ACS Appl Mater Interfaces. 2020;12(47):52713–52720. doi: 10.1021/ACSAMI.0C15898/SUPPL_FILE/AM0C15898_SI_001.PDF
- Akbarzadeh S, Khajehsharifi H, Hajihosseini S. Detection of oxytetracycline using an electrochemical label-free aptamer-based biosensor. Biosensors 2022. 2022;12(7):468. doi: 10.3390/BIOS12070468
- Jampasa S, Pummoree J, Siangproh W, et al. “Signal-on” electrochemical biosensor based on a competitive immunoassay format for the sensitive determination of oxytetracycline. Sensors and Actuat B Chem. 2020;320:128389. doi: 10.1016/J.SNB.2020.128389
- Mohammad-Razdari A, Ghasemi-Varnamkhasti M, Rostami S, et al. Development of an electrochemical biosensor for impedimetric detection of tetracycline in milk. J Food Sci Technol. 2020;57(12):4697–4706. doi: 10.1007/S13197-020-04506-2/METRICS
- Yan X, Wang Y, Kou Q, et al. A novel aptasensor based on Fe3O4/Au/g-C3N4 for sensitive detection of sulfameter in food matrices. Sensors and Actuat B Chem. 2021;353:131148. doi: 10.1016/J.SNB.2021.131148
- Zhang Y, Zhao C, Bi H, et al. A cell-free paper-based biosensor dependent on allosteric transcription factors (aTfs) for on-site detection of harmful metals Hg2+ and Pb2+ in water. J Hazard Mater. 2022;438:129499. doi: 10.1016/J.JHAZMAT.2022.129499
- Singh AK, Dhiman TK, Lakshmi VSGB, et al. Dimanganese trioxide (Mn2O3) based label-free electrochemical biosensor for detection of Aflatoxin-B1. Bioelectrochemistry. 2020;137:107684. doi: 10.1016/J.BIOELECHEM.2020.107684
- Wu Z, Sun DW, Pu H, et al. A novel fluorescence biosensor based on CRISPR/Cas12a integrated MXenes for detecting aflatoxin B1. Talanta. 2022;252:123773. doi: 10.1016/J.TALANTA.2022.123773
- Wei L, Wang Z, Chen Y. Optical biosensor for ochratoxin a detection in grains using an enzyme-mediated click reaction and polystyrene nanoparticles. J Agric Food Chem. 2022;70(46):14798–14804. doi: 10.1021/ACS.JAFC.2C05137/SUPPL_FILE/JF2C05137_SI_001.PDF
- Lu L, Yuan W, Xiong Q, et al. One-step grain pretreatment for ochratoxin A detection based on bipolar electrode-electrochemiluminescence biosensor. Anal Chim Acta. 2020;1141:83–90. doi: 10.1016/J.ACA.2020.10.035
- Li Y, Peng Z, Li Y, et al. An aptamer-array-based sample-to-answer biosensor for ochratoxin a detection via fluorescence resonance energy transfer. Chemosensors 2021. 2021;9(11):309. doi: 10.3390/CHEMOSENSORS9110309
- Yang C, Abbas F, Rhouati A, et al. Design of a quencher-free fluorescent aptasensor for ochratoxin a detection in red wine based on the guanine-quenching ability. Biosensors (Basel). 2022;12(5):297. doi: 10.3390/BIOS12050297
- Ouyang Q, Wang L, Ahmad W, et al. A highly sensitive detection of carbendazim pesticide in food based on the upconversion-MnO2 luminescent resonance energy transfer biosensor. Food Chem. 2021;349:129157. doi: 10.1016/J.FOODCHEM.2021.129157
- Peng L, Zhu J, Yang B, et al. A green photocatalytic-biosensor for colorimetric detection of pesticide (carbaryl) based on inhibition of acetylcholinesterase. Talanta. 2022;246:123525. doi: 10.1016/J.TALANTA.2022.123525
- Nunes EW, Silva MKL, Rascón J, et al. Acetylcholinesterase biosensor based on functionalized renewable carbon platform for detection of carbaryl in food. Biosensors (Basel). 2022;12(7):486. doi: 10.3390/BIOS12070486
- Qi S, Sun A, Dong X, et al. High-stable perovskite nanocrystal fluorescent probe-based aptasensor for ultrasensitive detection of peanut allergen Ara h1. Sensors and Actuat B Chem. 2022;379:133232. doi: 10.1016/J.SNB.2022.133232
- Jiang H, Guo Q, Zhang C, et al. Microfluidic origami nano-aptasensor for peanut allergen Ara h1 detection. Food Chem. 2021;365:130511. doi: 10.1016/J.FOODCHEM.2021.130511
- Wang Y, Li L, Li H, et al. A fluorometric sandwich biosensor based on rationally imprinted magnetic particles and aptamer modified carbon dots for the detection of tropomyosin in seafood products. Food Control. 2021;132:108552. doi: 10.1016/J.FOODCONT.2021.108552
- Li R, Zhang Y, Zhao J, et al. Quantum-dot-based sandwich lateral flow immunoassay for the rapid detection of shrimp major allergen tropomyosin. J Food Compost Anal. 2022;114:104776. doi: 10.1016/J.JFCA.2022.104776
- Shin JH, Park TJ, Hyun MS, et al. A phage virus-based electrochemical biosensor for highly sensitive detection of ovomucoid. Food Chem. 2022;378:132061. doi: 10.1016/J.FOODCHEM.2022.132061
- Wang J, Li H, Li C, et al. EIS biosensor based on a novel myoviridae bacteriophage SEP37 for rapid and specific detection of Salmonella in food matrixes. Food Res Int. 2022;158:111479. doi: 10.1016/J.FOODRES.2022.111479
- Yang J, Zhang Y, Lu Y. A fluorescence detection method for the determination of β-lactoglobulin in foods. Anal Methods. 2022;14(19):1872–1879. doi: https://doi.org/10.1039/D2AY00158F
- Yang J, Zhang Y, Wu L, et al. A coffee-ring effect-based paper sensor chip for the determination of beta-lactoglobulin in foods via a smartphone. Sensors and Actuat B Chem. 2022;374:132807. doi: 10.1016/J.SNB.2022.132807
- Kim Y, Choi H, Shin WH, et al. Development of colorimetric whole-cell biosensor for detection of heavy metals in environment for public health. Int J Environ Res Public Health 2021. 2021;18(23):12721. doi: 10.3390/IJERPH182312721
- Zhai H, Wang Y, Yin J, et al. Electrochemiluminescence biosensor for determination of lead(II) ions using signal amplification by Au@SiO2 and tripropylamine-endonuclease assisted cycling process. Microchim Acta. 2022;189(9):1–11. doi: 10.1007/S00604-022-05429-9/METRICS
- Sciuto EL, Petralia S, van der Meer JR, et al. Miniaturized electrochemical biosensor based on whole-cell for heavy metal ions detection in water. Biotech & Bioengineering. 2020;118(4):1456–1465. doi: 10.1002/BIT.27646
- Lukyanenko KA, Denisov IA, Sorokin VV, et al. Handheld enzymatic luminescent biosensor for rapid detection of heavy metals in water samples. Chemosensors. 2019;7(1):16. doi: 10.3390/CHEMOSENSORS7010016
- Wei Q, Zhang P, Liu T, et al. A fluorescence biosensor based on single-stranded DNA and carbon quantum dots for acrylamide detection. Food Chem. 2021;356:129668. doi: 10.1016/J.FOODCHEM.2021.129668
- Wang L, He Y, Wu Z. Design of a blockchain-enabled traceability system framework for food supply chains. Foods. 2022;11(5):744. doi: 10.3390/foods11050744
- Mustafa F, Othman A, Andreescu S. Cerium oxide-based hypoxanthine biosensor for fish spoilage monitoring. Sensors and Actuat B Chem. 2021;332:129435. doi: 10.1016/J.SNB.2021.129435
- Yazdanparast S, Benvidi A, Abbasi S, et al. Enzyme-based ultrasensitive electrochemical biosensor using poly(l-aspartic acid)/MWCNT bio-nanocomposite for xanthine detection: a meat freshness marker. Microchem J. 2019;149:104000. doi: 10.1016/J.MICROC.2019.104000
- Zheng S, Yang Q, Yang H, et al. An ultrasensitive and specific ratiometric electrochemical biosensor based on SRCA-CRISPR/Cas12a system for detection of salmonella in food. Food Control. 2022;146:109528. doi: 10.1016/J.FOODCONT.2022.109528
- Dong Q, Yue X, Li S, et al. A novel rapid detection method for salmonella based on NMR macromolecular Gd biosensor. LWT. 2022;171:114138. doi: 10.1016/J.LWT.2022.114138
- Sun D, Fan T, Liu F, et al. A microfluidic chemiluminescence biosensor based on multiple signal amplification for rapid and sensitive detection of E. coli O157:H7. Biosens Bioelectron. 2022b;212:114390. doi: 10.1016/J.BIOS.2022.114390
- Zhou Y, Li Z, Huang J, et al. Development of a phage-based electrochemical biosensor for detection of Escherichia coli O157: H7 GXEC-N07. Bioelectrochemistry. 2022a;150:108345. doi: 10.1016/J.BIOELECHEM.2022.108345
- Ramić D, Klančnik A, Možina SS, et al. Elucidation of the AI-2 communication system in the food-borne pathogen campylobacter jejuni by whole-cell-based biosensor quantification. Biosens Bioelectron. 2022;212:114439. doi: 10.1016/J.BIOS.2022.114439
- Vizzini P, Manzano M, Farre C, et al. Highly sensitive detection of campylobacter spp. In chicken meat using a silica nanoparticle enhanced dot blot DNA biosensor. Biosens Bioelectron. 2020;171:112689. doi: 10.1016/J.BIOS.2020.112689
- Guo J, Liu D, Yang Z, et al. A photoelectrochemical biosensor for rapid and ultrasensitive norovirus detection. Bioelectrochemistry. 2020;136:107591. doi: 10.1016/J.BIOELECHEM.2020.107591
- Baek SH, Kim MW, Park CY, et al. Development of a rapid and sensitive electrochemical biosensor for detection of human norovirus via novel specific binding peptides. Biosens Bioelectron. 2018;123:223–229. doi: 10.1016/J.BIOS.2018.08.064
- Jiang H, Sun Z, Zhang C, et al. 3D-architectured aptasensor for ultrasensitive electrochemical detection of norovirus based on phosphorene-gold nanocomposites. Sensors And Actuat B Chem. 2021;354:131232. doi: 10.1016/J.SNB.2021.131232
- Shkembi X, Svobodova M, Skouridou V, et al. Aptasensors for mycotoxin detection: a review. Anal Biochem. 2022;644:114156. doi: 10.1016/J.AB.2021.114156
- Jia Y, Zhao S, Li D, et al. Portable chemiluminescence optical fiber aptamer-based biosensors for analysis of multiple mycotoxins. Food Control. 2022;144:109361.
- Zangheri M, di Nardo F, Calabria D, et al. Smartphone biosensor for point-of-need chemiluminescence detection of ochratoxin a in wine and coffee. Anal Chim Acta. 2021;1163:338515. doi: 10.1016/J.ACA.2021.338515
- Zhu W, Li L, Zhou Z, et al. A colorimetric biosensor for simultaneous ochratoxin a and aflatoxins B1 detection in agricultural products. Food Chem. 2020;319:126544. doi: 10.1016/J.FOODCHEM.2020.126544
- Li X, Jia M, Yu L, et al. An ultrasensitive label-free biosensor based on aptamer functionalized two-dimensional photonic crystal for kanamycin detection in milk. Food Chem. 2022;402:134239. doi: 10.1016/J.FOODCHEM.2022.134239
- Tian L, Zhang J, Fan H, et al. High efficient electrochemical biosensor based on exonuclease-III-assisted dual-recycling amplification for ultrasensitive detection of kanamycin. Anal Biochem. 2023;663:115028. doi: 10.1016/J.AB.2022.115028
- Tang Y, Huang X, Wang X, et al. G-quadruplex DNAzyme as peroxidase mimetic in a colorimetric biosensor for ultrasensitive and selective detection of trace tetracyclines in foods. Food Chem. 2021;366:130560. doi: 10.1016/J.FOODCHEM.2021.130560
- Ahmad F, Zhu D, Sun J. Environmental fate of tetracycline antibiotics: degradation pathway mechanisms, challenges, and perspectives. Environmental Sciences Europe. 2021;33(1):64. doi: 10.1186/s12302-021-00505-y
- Xu D, Shen Z, Wang G, et al. Dual-catalytic colorimetric biosensor based on double-active Fe@Co-N stellate porous carbon and DNAzyme for simultaneous detection of tetracycline antibiotics. Sensors And Actuat B Chem. 2022;376:133024. doi: 10.1016/J.SNB.2022.133024
- Yadav AK, Verma D, Lakshmi GBVS, et al. Fabrication of label-free and ultrasensitive electrochemical immunosensor based on molybdenum disulfide nanoparticles modified disposable ITO: an analytical platform for antibiotic detection in food samples. Food Chem. 2021;363:130245. doi: 10.1016/J.FOODCHEM.2021.130245
- Li M, He B. Ultrasensitive sandwich-type electrochemical biosensor based on octahedral gold nanoparticles modified poly (ethylenimine) functionalized graphitic carbon nitride nanosheets for the determination of sulfamethazine. Sensors and Actuat B Chem. 2021;329:129158. doi: 10.1016/J.SNB.2020.129158
- Fatunsin OT, Oyeyiola AO, Moshood MO, et al. Dietary risk assessment of organophosphate and carbamate pesticide residues in commonly eaten food crops. Sci African. 2020;8:e00442. doi: 10.1016/j.sciaf.2020.e00442
- Mishra A, Kumar J, Melo JS, et al. Progressive development in biosensors for detection of dichlorvos pesticide: a review. J Environ Chem Eng. 2021;9(2):105067. doi: 10.1016/J.JECE.2021.105067
- Jain U, Saxena K, Hooda V, et al. Emerging vistas on pesticides detection based on electrochemical biosensors – An update. Food Chem. 2021;371:131126. doi: 10.1016/J.FOODCHEM.2021.131126
- Karadurmus L, Kaya SI, Ozkan SA. Recent advances of enzyme biosensors for pesticide detection in foods. J Food Meas Charact. 2021;15(5):4582–4595. doi: 10.1007/s11694-021-01032-3
- Umar AM, Aisami A. Acetylcholinesterase enzyme (AChE) as a biosensor and biomarker for pesticides: a mini review. Bull Environ Sci And Sustain Manag (E-ISSN 2716-5353). 2020;4(1):7–12. doi: 10.54987/BESSM.V4I1.526
- Fallatah A, Kuperus N, Almomtan M, et al. Sensitive biosensor based on shape-controlled ZnO nanostructures grown on flexible porous substrate for pesticide detection. Sensors. 2022;22(9):3522. doi: 10.3390/S22093522
- Xie X, Zhou B, Zhang Y, et al. A multi-residue electrochemical biosensor based on graphene/chitosan/parathion for sensitive organophosphorus pesticides detection. Chem Phys Lett. 2021;767:138355. doi: 10.1016/J.CPLETT.2021.138355
- Liu X, Cheng H, Zhao Y, et al. Portable electrochemical biosensor based on laser-induced graphene and MnO2 switch-bridged DNA signal amplification for sensitive detection of pesticide. Biosens Bioelectron. 2021;199:113906. doi: 10.1016/J.BIOS.2021.113906
- Rafaqat S, Raqba AN, Hussain A. Validating role of different enzymes (laccases and catalases) based voltammetric biosensors in detection of pesticide and dye. Materials Chemistry & Physics. 2022;290:126545. doi: 10.1016/J.MATCHEMPHYS.2022.126545
- Gamella M, Laza A, Parrón-Ballesteros J, et al. First PCR-free electrochemical bioplatform for the detection of mustard sin a 1 protein as a potential “hidden” food allergen. Bioelectrochemistry. 2022;150:108357. doi: 10.1016/J.BIOELECHEM.2022.108357
- Chinnappan R, Rahamn AA, AlZabn R, et al. Aptameric biosensor for the sensitive detection of major shrimp allergen, tropomyosin. Food Chem. 2020;314:126133. doi: 10.1016/J.FOODCHEM.2019.126133
- Jeong JY, Kim SO, Bang S, et al. Adaptive biosensing platform using immune cell-based nanovesicles for food allergen detection. Biosens Bioelectron. 2022;222:114914. doi: 10.1016/j.bios.2022.114914
- Zhang Y, Lin T, Shen Y, et al. A high-performance self-supporting electrochemical biosensor to detect aflatoxin B1. Biosensors (Basel). 2022;12(10):897. doi: 10.3390/bios12100897
- Pohanka M. Copper and copper nanoparticles toxicity and their impact on basic functions in the body. Sci Citation Index Expanded and In J Citation Rep/Sci Edition Bratisl Med J. 2019;120(6):397–409. 120(6. doi: 10.4149/BLL_2019_065
- Žunar B, Mosrin C, Bénédetti H, et al. Re-engineering of CUP1 promoter and Cup2/Ace1 transactivator to convert Saccharomyces cerevisiae into a whole-cell eukaryotic biosensor capable of detecting 10 nM of bioavailable copper. Biosens Bioelectron. 2022;214:114502. doi: 10.1016/J.BIOS.2022.114502
- Vasconcelos H, Matias A, Mendes J, et al. Compact biosensor system for the quantification of hydrogen peroxide in milk. Talanta. 2022;253:124062. doi: 10.1016/J.TALANTA.2022.124062
- Manjunatha JG. A novel voltammetric method for the enhanced detection of the food additive tartrazine using an electrochemical sensor. Heliyon. 2018;4(11):e00986. doi: 10.1016/J.HELIYON.2018.E00986
- Hao S, Sun X, Zhang H, et al. Recent development of biofuel cell based self-powered biosensors. J Mat Chem B. 2020;8(16):3393–3407. doi: 10.1039/C9TB02428J
- Wan Y, Wang H, Zhang L, et al. Highly stable acetylcholinesterase electrochemical biosensor based on polymerized ionic liquids microgel for pesticides detection. Mikrochim Acta. 2022;189(8):300. doi: 10.1007/s00604-022-05383-6
- Zheng H, Liu M, Yan Z, et al. Highly selective and stable glucose biosensor based on incorporation of platinum nanoparticles into polyaniline-montmorillonite hybrid composites. Microchem J. 2020;152:104266. doi: 10.1016/j.microc.2019.104266
- Sonu, Chaudhary V. A paradigm of internet-of-nano-things inspired intelligent plant pathogen-diagnostic biosensors. ECS Sensors Plus. 2022;1(3):031401. doi: 10.1149/2754-2726/AC92ED
- Huang CW, Lin C, Nguyen MK, et al. A review of biosensor for environmental monitoring: principle, application, and corresponding achievement of sustainable development goals. Bioengineered. 2023;14(1):58–80. doi: 10.1080/21655979.2022.2095089
- Antonacci A, Scognamiglio V. Biotechnological advances in the design of algae-based biosensors. Trends Biotechnol. 2020;38(3):334–347. doi: 10.1016/j.tibtech.2019.10.005
- Abdelbasset WK, Savina SV, Mavaluru D, et al. Smartphone based aptasensors as intelligent biodevice for food contamination detection in food and soil samples: recent advances. Talanta. 2023;252:123769. doi: 10.1016/J.TALANTA.2022.123769
- Xie Y, Lu L, Gao F, et al. Integration of artificial intelligence, blockchain, and wearable technology for chronic disease management: a new paradigm in smart healthcare. Curr Med Sci. 2021;41(6):1123–1133. doi: 10.1007/s11596-021-2485-0