ABSTRACT
Extracellular vesicles (EVs) are lipid-bilayer-enclosed vesicles that contain proteins, lipids and nucleic acids. EVs produced by cells from healthy tissues circulate in the blood and body fluids, and can be taken up by unrelated cells. As they have the capacity to transfer cargo proteins, lipids and nucleic acids (mostly mRNAs and miRNAs) between different cells in the body, EVs are emerging as mediators of intercellular communication that could modulate cell behavior, tissue homeostasis and regulation of physiological functions. EV-mediated cell-cell communications are also proposed to play a role in disease, for example, cancer, where they could contribute to transfer of traits required for tumor progression and metastasis. However, direct evidence for EV-mediated mRNA transfer to individual cells and for its biological consequences in vivo has been missing until recently. Recent studies have reported elegant experiments using genetic tracing with the Cre recombinase system and intravital imaging that visualize and quantify functional transfer of mRNA mediated by EVs in the context of cancer and metastasis.
Tumors are sites of intense exchange of information between genetically and phenotypically heterogeneous cancer cells intermingled with diverse non-cancer cell types that, along with extracellular matrix, comprise the tumor microenvironment.Citation1 In breast cancers, tumor cells signal to stromal cells, such as fibroblasts and macrophages, which in turn remodel the microenvironment to secrete factors promoting growth, invasion, and metastasis programs within cancer cells, and activating aberrant angiogenesis.Citation2,3
In the past few years, extracellular vesicles (EVs) emerged as important vectors conveying signals and transferring biomolecules from producing to recipient cells.Citation4,5 EV is a generic term designating a plethora of cell-originating membrane-enclosed structures with diverse and still evolving nomenclature.Citation6 They can be distinguished by their cell and tissue of origin, size and underlying biogenesis mechanisms.Citation7 Exosomes correspond to 50–100-nm vesicles generated intracellularly by inward budding from the limiting membrane of late endosomal/lysosomal compartments to produce multivesicular bodies (MVBs) and released upon MVB fusion with the plasma membrane.Citation7 Microvesicles are larger, more heteregeneous in size structures (200 nm to a few micrometers in diameter) shed from the plasma membrane of normal and tumor cells.Citation4 Because they consist of a small volume of cytosol enclosed in a cell membrane, EVs encapsulate a broad range of cellular molecules including bioactive proteins and nucleic acids, which remain protected from the external enzymatic degradation. Depending on yet unclear targeting, capture and/or fusion mechanisms with target cells, EVs have a potential to act as modulators of intercellular communication and to alter cell behavior and fate.Citation4
Early works detected the presence of mRNAs in microvesicles and exosomes produced by in vitro cultured cell lines and primary cells and reported horizontal transfer of cargo mRNAs and miRNAs in target cells with functional biological consequences.Citation8,9 Although these represented significant advances in understanding the biology of EVs, the high EV concentrations used in these studies made it difficult to appraise actual relevance of such transfer in vivo, and the direct evidence for EV-mediated mRNA transfer to individual cells and its biological consequences in vivo has been lacking. This critical gap was recently addressed by the Momma's group (Edinger Institute, Frankfurt Am Main, Germany), who used genetic tracing system based on Cre recombinase to demonstrate functional EV-mediated transfer of mRNAs in vivo. In this set-up, Cre was specifically expressed in haematopoietic cells and GFP expression in individual cells reported for Cre protein activity upon deletion of a floxed stop codon between LacZ and GFP transgenes. This system revealed recombined non-haematopoietic LacZ/GFP-positive cells in non-lymphoid tissues, including neurons in the brain.Citation10 Additional experiments established that Cre activity was transferred via Cre mRNA (and not protein as association of Cre protein with EVs could not be detected using sensitive antibody-based methods), contained in exosomes and larger microvesicles recovered from haematopoietic cells. Systemic inflammatory conditions increased number of LacZ/GFP-positive neurons in the brain, and targeted neurons showed altered miRNAs profiles as compared to those in non-recombined counterparts.Citation10 Altogether, these data were suggestive of secreted EVs by peripheral immune cells delivering cargo Cre mRNA in individual neurons with physiologically relevant output.
Many studies have provided correlative evidence between the presence of EVs’ in the tumor milieu and in body fluids from cancer patients and tumor progression parameters such as cell survival, proangiogenic, immunosuppressive or prometastatic programs.Citation11-13 Two recent studies addressed the issue of EV-based intratumoral RNA transfer and consequences during tumor progression. In the first study, Momma and colleagues expressed Cre recombinase in Tu-2449 glioma and LLC2 Lewis lung carcinoma cell lines and transplanted Cre-positive tumor cells into recipient mice with a stop-floxed β-galactosidase reporter transgene. Recombination events could be observed at the tumor site mostly targeting myeloid-derived suppressor cells (MDSCs).Citation14 In the lung carcinoma model, recombination was associated with altered miRNA repertoire in MDSCs and could be correlated with activation of their immunosuppressive functions with potential consequences for tumor progression.Citation14
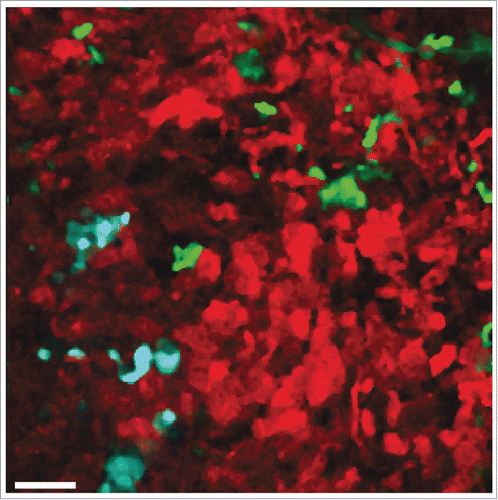
Independently, Jacco van Rheenen's group at Hubrecht Institute (Utrecht, The Netherlands) also used the Cre recombinase system to demonstrate functional transfer of mRNAs mediated by EVs produced by tumor cells. Like in the study by Ridder and colleague, the authors detected Cre-encoding RNA, but not Cre protein in EVs secreted by Cre-expressing tumor cells as the unique source of Cre-mediated recombination events in recipient cells.Citation15 This work, however, has gone a step ahead thanks to state-of-the-art intravital imaging to follow simultaneously the fate of individual Cre- and CFP-expressing tumor cells and tumor reporter cells engineered with a double-color detector system switching from DsRed to GFP expression after recombination. Using mixtures of congenic Cre-expressor and Cre-reporter human breast cancer cells injected into immunodeficient NOD.scid.Ilgr2−/− mice, the authors observed the recombined GFP-positive cells in the experimental tumors. The emergence of recombined GFP-positive reporter cells was observed across a variety of human and mouse tumor grafts in vivo (Fig. 1). Moreover, reporter activation was observed in heterogeneous cell mixtures, when the Cre-expressor and Cre-reporter cells were derived from different patients or even different species. Most significantly, when tracked for several hours, the originally non-motile tumor cells that received Cre-containing EVs from the adjacent more aggressive tumor cells, or even from a distant aggressive tumor implanted on the opposite flank of the mouse, migrated more efficiently and were more metastatic than their non-Cre-recombined counterparts. On a correlative basis, in the absence of data elucidating the nature of the pro-metastatic activity (protein, mRNA, miRNA,…), the authors reported some enrichment of pro-migratory mRNA species associated with EVs prepared from invasive breast tumor cells.
While the performance of the Cre-mediated reporter in these experiments produced convincing and reproducible evidence of EV-based cell-cell communication in cancer, it also revealed that functional EV-mediated transfer is a rare event. In vitro experiments suggest that the prevalence of recombined cells can be substantially increased by raising the expressor-to-reporter cell ratio by 100-fold. Yet even under these conditions, the numbers of recombined cells ranged between a fraction of percent to a few percent points, making it difficult to envision how an aggressive minor clone may confer malignant properties to its less aggressive neigbours. However, EV-mediated communication may be a powerful player in mediating normal tissue communication with individual tumor cells, as may occur during metastatic cell seeding process.
Indeed, the authors present data using mouse B16 melanoma tumors, showing normal-tumor cell-cell communication. The Cre-based evidence of EV-mediated transfer was more robust when tested in the tumor-to-normal transfer configuration, indicating that the role of EVs in intercellular communication may be exacerbated under pathological conditions such as cancer,Citation5 but reciprocal activation of the tumoral Cre reporter in the tissues of Cre-expressing hosts was also observed. Anti-metastatic effect of EV-mediated transfer from normal tissue is an attractive explanation for the poorly understood nature of cancer cell dormancy and metastatic inefficiency in general.
Several issues will have to be addressed in the future regarding the function of EVs in gain of traits that are required for cells to metastasize. Several investigators set the needs to establish guidelines for better standardized biochemical, biophysical, and clinically adaptable methods to define and characterize EVs from any biological samples given their potential as circulating biomarkers and platforms for personalized therapy.Citation4,5,16 At the mechanistic level, specific efforts will be required to identify molecular details of pathway(s) contributing to release of exosomes and microvesicles by tumor cells in vivo including Rab27 and ARF6 GTPase pathways.Citation17–19 Another burning question will be to decipher potential mechanisms responsible for targeting and capture of EVs by tumor cells as well as possible mechanisms controlling EV enrichment of active biomolecules such as mRNAs or miRNAs with pro-metastatic potential, if any. Yet, reports under scrutiny here can be regarded as important milestones as they provide first demonstrations of promotion of metastasis mediated by EVs secreted and captured in vivo, in the absence of any ex-vivo isolation/concentration step of EVs.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors wish to thank Drs A. Zomer and J. van Rheenen (Hubrecht Institute, Utrecht, The Netherlands) for kindly providing the image used for .
Figure 1. In vivo visualization of intratumoral EV-mediated transfer of Cre recombinase (seeCitation15). Multiphoton microscopy image of a tumor containing a mixture of human breast cancer-derived Cre-expressing T47D cells (cyan) and Cre-reporter-expressing MDA-MB-231 cells (red and green). MDA-MB-231 cells that took up Cre-containing EVs released by T47D cells switch from DsRed to GFP expression. Scale bar represents 50 µm.
Additional information
Funding
References
- Polyak K, Kalluri R. The role of the microenvironment in mammary gland development and cancer. Cold Spring Harbor Perspectives Biol 2010; 2:a003244; http://dx.doi.org/10.1101/cshperspect.a003244
- Boudreau A, van't Veer LJ, Bissell MJ. An “elite hacker:” breast tumors exploit the normal microenvironment program to instruct their progression and biological diversity. Cell Adh Migr 2012; 6:236-48; PMID:22863741; http://dx.doi.org/10.4161/cam.20880
- Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer 2009; 9:239-52; PMID:19279573; http://dx.doi.org/10.1038/nrc2618
- D'Souza-Schorey C, Clancy JW. Tumor-derived microvesicles: shedding light on novel microenvironment modulators and prospective cancer biomarkers. Genes Dev 2012; 26:1287-99; PMID:22713869; http://dx.doi.org/10.1101/gad.192351.112
- Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 2015; 4:27066; PMID:25979354
- Gould SJ, Raposo G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles 2013; 2:20389; PMID:24009890; http://dx.doi.org/10.3402/jev.v2i0.20389
- Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 2014; 30:255-89; PMID:25288114; http://dx.doi.org/10.1146/annurev-cellbio-101512-122326
- Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006; 20:847-56; PMID:16453000; http://dx.doi.org/10.1038/sj.leu.2404132
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9:654-9; PMID:17486113; http://dx.doi.org/10.1038/ncb1596
- Ridder K, Keller S, Dams M, Rupp AK, Schlaudraff J, Del Turco D, Starmann J, Macas J, Karpova D, Devraj K, et al. Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biol 2014; 12:e1001874; PMID:24893313; http://dx.doi.org/10.1371/journal.pbio.1001874
- Hood JL, San RS, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res 2011; 71:3792-801; PMID:21478294; http://dx.doi.org/10.1158/0008-5472.CAN-10-4455
- Bobrie A, Thery C. Unraveling the physiological functions of exosome secretion by tumors. Oncoimmunology 2013; 2:e22565
- Martins VR, Dias MS, Hainaut P. Tumor-cell-derived microvesicles as carriers of molecular information in cancer. Curr Opin Oncol 2013; 25:66-75; PMID:23165142; http://dx.doi.org/10.1097/CCO.0b013e32835b7c81
- Ridder K, Sevko A, Heide J, Dams M, Rupp AK, Macas J, Starmann J, Tjwa M, Plate KH, Sültmann H, et al. Extracellular vesicle-mediated transfer of functional RNA in the tumor microenvironment. Oncoimmunology 2015; 4:e1008371; PMID:26155418; http://dx.doi.org/10.1080/2162402X.2015.1008371
- Zomer A, Maynard C, Verweij FJ, Kamermans A, Schafer R, Beerling E, Schiffelers RM, de Wit E, Berenguer J, Ellenbroek SI, et al. In Vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell 2015; 161:1046-57; PMID:26000481; http://dx.doi.org/10.1016/j.cell.2015.04.042
- Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015; 523:177-82; PMID:26106858; http://dx.doi.org/10.1038/nature14581
- Bobrie A, Krumeich S, Reyal F, Recchi C, Moita LF, Seabra MC, Ostrowski M, Théry C. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res 2012; 72:4920-30; PMID:22865453; http://dx.doi.org/10.1158/0008-5472.CAN-12-0925
- Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 2010; 12:19-30; sup pp 1-13; http://dx.doi.org/10.1038/ncb2000
- Muralidharan-Chari V, Clancy J, Plou C, Romao M, Chavrier P, Raposo G, D'Souza-Schorey C. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr Biol 2009; 19:1875-85; PMID:19896381; http://dx.doi.org/10.1016/j.cub.2009.09.059

