Abstract
Objective
The prevalence of behavior impairment (27.38%) in the Chinese amyotrophic lateral sclerosis (ALS) cohort is lower. We hypothesize that the screening scales used among studies might not be appropriate to diagnose behavioral disorders in ALS patients. So, we urgently need to find a behavior scale with a high detection rate designed specifically for ALS. This study aims to verify the Chinese translation of the Beaumont Behavioral Inventory (BBI) as an effective assessment in a Chinese ALS cohort.
Methods
Ninety-eighty ALS patients and ninety-three healthy controls were included in this cross-sectional study. All participants took emotional state, overall cognitive, sleep quality and gastroenteric function, and behavioral evaluation.
Results
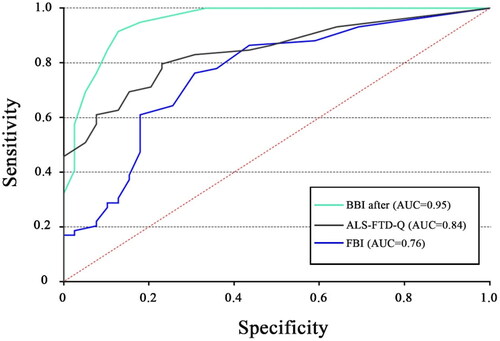
The BBI scores showed a strong association with the amyotrophic lateral sclerosis-Frontotemporal Dementia-Questionnaire (ALS-FTD-Q) (rs = 0.71, p < 0.001) as well as a moderate correlation with the Frontal Behavioral Inventory (FBI) (rs = 0.55, p < 0.001). High internal consistency was demonstrated in patients using BBI-after items (Cronbach’s a = 0.89). When tested against clinical diagnoses, the optimal cutoff of total BBI score was identified at 5.5 (AUC = 0.95; SE = 0.02; 95% CI [0.91, 0.99]), the BBI reached optimal sensitivity and specificity values (91.5% and 87.2%). The BBI turned out to be more precise than the FBI (AUC = 0.76; SE = 0.05; 95% CI [0.66, 0.86]) and the ALS-FTD-Q (AUC = 0.84; SE = 0.04; 95% CI [0.77, 0.92]).
Conclusion
The Chinese version of BBI is a quicker and more efficient instrument for assessing behavioral impairment in the ALS population in China.
1. Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative illness marked by the relentless loss of motor function and is thought to be part of a clinical and neuropathological continuum with frontotemporal dementia (FTD) (Citation1). Cognitive and behavioral impairment are now recognized as frequent neuropsychological manifestations of the ALS frontotemporal spectrum (Citation2, Citation3). Prior to the development of evident motor weakness in ALS patients, behavioral symptoms may appear independently of cognitive alterations and have an impact on the patients’ survival (Citation4–7). Besides, recent studies have shown that behavioral disorders (such as apathy or disinhibition) negatively affect physical and psychological health of caregivers (Citation7). ALS patients with behavioral symptoms may not fully appreciate the impact of risk-taking and may not cooperate with interventions and evaluation processes. Therefore, it is worthwhile to early recognize and intervent behavioral symptoms in ALS.
In Europe, the proportion of ALS with behavior impairment is up to 40% (Citation8, Citation9). However, the prevalence of behavior impairment (27.38%) in the Chinese ALS cohort is lower (Citation10). The following are some possible explanations for this difference. It might be related to different ethnic backgrounds. Abnormal amplification of C9orf72 which is associated with behavior impairment is common in Europe but the abnormal rate is low in East Asian population (Citation11). Although SOD1 gene mutation is common in China, it has not been found to be associated with impaired ALS behavior (Citation12). Secondly, clinicians don’t pay enough attention to the behavioral disorders of ALS patients, lack of awareness of early identification which might result in a low detection rate of behavioral disorders. Furthermore, the screening scales used among studies might not appropriate to diagnose behavioral disorders in ALS patients. So, we urgently need to find a behavior scale with a high detection rate and designed specifically for ALS.
Currently, some behavioral questionnaires such as the Frontal Behavioral Inventory (FBI), Amyotrophic lateral sclerosis-Frontotemporal Dementia-Questionnaire (ALS-FTD-Q), Edinburgh scale have been used to evaluate behavior in ALS (Citation10, Citation13, Citation14). And the rate of behavioral changes in ALS was relatively low by these scales in China. The Beaumont Behavioral Inventory (BBI) is a 41-item, self-explanatory/proxy-report questionnaire, specifically designed for ALS patients (Citation15). In ALS patients, BBI demonstrated great sensitivity and specificity, avoiding the confounding effects of motor impairment and dysarthria on behavior. In addition, it has been validated in Italian and is recommended as a preferred tool by a 2022 Lancet Neurol review (Citation16, Citation17). So we want to translate Beaumont Behavioral Inventory into Chinese and then estimate whether its sensitivity and specificity are better than FBI or ALS-FTD-Q in Chinese cohort.
The goals of this study are to confirm the Chinese translation of the BBI as a useful tool for measuring behavioral dysfunction in a Chinese cohort of people with amyotrophic lateral sclerosis and to examine the prevalence and characteristics of behavioral dysfunction at a cross-sectional level using the BBI.
2. Methods
2.1. Participants
One hundred and fifty ALS patients were recruited in the department of Neurology, Second Hospital of Hebei Medical University, China. All patients who met the updated El Escorial criteria for potential, probable, or definite ALS and showed clinical and electrophysiological evidence of mixed upper and lower motor neuron involvement were included (Citation18). Finally, 98 ALS patients consented to participate in our study, after excluding patients who were uncooperative or refused. We interviewed all of the patients’ primary caregivers about any behavioral changes. Other neurological, mental, or medical problems associated with cognitive or behavioral abnormalities are exclusion criteria. Exclusion criteria for controls were the same as those for patients.
The ALS patients with behavioral impairment (ALS-bi) in this study were classified according the diagnostic criteria proposed by Strong MJ et al. that required the identification of apathy with or without other behavioral changes or the presence of at least two non-overlapping supportive diagnostic features from the Rascovsky criteria. An expert neurologist and a neuropsychologist diagnose ALS-bi following this clinical criteria (Citation1).
The Second Hospital of Hebei Medical University’s Research Ethics Committee gave its approval to this study. According to the Declaration of Helsinki, all patients were enrolled after receiving informed written consent from them or their legal guardians, and controls gave their own informed written consent. Ethics committees gave their approval to the consent procedure.
2.2. Materials
We followed the recommended guidelines to translate. First, two neurologists with knowledge of both the Chinese language and the theoretical underpinnings of behavior disorder translated the original BBI into Chinese. Second, two separate specialists who had never read the original English edition translated the Chinese versions back into English. A team of experienced neuropsychologists and translators worked together to revise and resolve any discrepancies between the translated and back-translated versions, also taking into account semantic differences.
Caregivers were given the BBI, a 41-item behavioral questionnaire, and asked to score behavioral changes in relation to two separate time periods: “in the last ten years” and “since the onset of MND.” Each symptom in BBI is grated on a scale from 0 to 3 (0 for no change, 1 for mild, 2 for moderate, and 3 for severe)(Citation15).
FBI is also a questionnaire completed by caregivers, a total of 24 items, mainly divided into two main types of behavior. The first group mainly consists of some negative behavior or several abnormal behavior; The second group is associated with disinhibition programs that often lead to excessive or abnormal behavior (Citation19).
ALS-FTD-Q is a caregiver survey that analyzes alterations in aberrant behavior in ALS patients and prevents response biases brought on by physical impairment. The cutoff value of this scale distinguishes between mild and severe behavioral dysfunction. The ALS-FTD-Q score (≥22) is considered mild behavior disorder(ALSbi) while the ALS-FTD-Q score (≥29) is considered severe behavior disorder (bvFTD)(Citation14).
The revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R) is used to document disease severity. The cognition was tested using the Mini-Mental Status Examination (MMSE). The severity of mental state was evaluated using Zung’s Self-rating Anxiety Scale (SAS) and Zung’s Self-rating Depression Scale (SDS)(Citation7, Citation20). The Pittsburgh Sleep Quality Index (PSQI), a self-rated questionnaire with seven components, was used to measure the quality of sleep (Citation21). Gastrointestinal symptoms were evaluated with Knowles-Eccersley-Scott Symptom Constipation Score (KESS)(Citation22).
2.3. Statistical analyses
A normal distribution analysis was performed using the Kolmogorov-Smirnov test. Given that the variables were not normally distributed, intergroup comparisons were carried out using the Mann-Whitney U test. Kruskal-wallis test was used to compare BBI after score between multiple group comparison. To compare proportions, the Chi square test was applied. The correlation coefficients for the BBI score and clinical parameters (education, age, ALSFRS-R, bulbar involvement, and length of disease) were calculated using the Spearman correlation analysis. We then use a partial correlation analysis to analyze the correlation coefficient of the BBI and ALSFRS-R scores normalized by z-score when corrected for disease duration. When all variables were not normally distributed and there was overdispersion, negative binomial generalized linear models were used instead of linear models.
A Cronbach’s alpha test was used to evaluate internal Consistency. In order to investigate behavioral clustering and pinpoint the key elements of measuring instruments, principal component analysis (PCA) was performed.
Receiver-Operating Characteristic analyses were done to evaluate the diagnostic accuracy of various behavioral assessment tools, as well as to examine the sensitivity and specificity of the BBI by making appropriate comparisons. To determine the ideal cutoff, the Youden index was calculated (Citation23). Statistical analysis was performed using SPSS 22.0 statistical software and SPSSAU, a web-based data science algorithm platform tool.
3. Results
3.1. Comparison between patients and controls
We identified no significant differences in age, gender, years of education, sleep quality, gastrointestinal condition between ALS patients and controls. However, MMSE was considerably lower in ALS patients compared to controls (z = −2.2, p = 0.03), whereas SAS and SDS were significantly higher in ALS patients (z = −5.37, p < 0.001; z = −6.12, p < 0.001) [].
Table 1. Demographic and clinical characteristics of participants.
3.2. Internal consistency and factor structure
High internal consistency was demonstrated in patients using BBI-after items (Cronbach’s a = 0.89). The PCA was conducted with the Kaiser–Meyer–Olkin measure =0.66 and Bartlett’s test of sphericity showing sufficient correlations between items(p < 0.01) except items 16, 17, 19, and 26. The components (obsessive counting, excessively store food in mouth, hyperorality, see/hears things/people) least frequently occur in Chinese ALS cohort.
3.3. Convergent validity
The BBI and the FBI showed a moderately positive association (rs =0.55, p < 0.001). Additionally, there were high significant correlations between the BBI and ALS-FTD-Q. High significant correlations were also observed between the BBI and ALS-FTD-Q (rs =0.71, p < 0.001). Our results indicated high convergent validity.
3.4. Correlation analysis
In healthy controls, no relationships were observed between age, gender, years of education, MMSE, PSQI, and BBI score (p > 0.05). Despite the fact that p < 0.05 in this case, r < 0.3 implied that there was no link between SAS and BBI (rs = 0.29, p = 0.004). There are very slight relationships between KESS, SDS, and BBI (rs = 0.34, p = 0.001; rs = 0.3, p = 0.003). O test was used to test the dispersion of variables(o = 1.25, p < 0.001). So, the negative binomial regression model was used to analyze the influencing factors. The model was constructed with BBI score as the dependent variable and PSOI, KESS, SAS, SDS and MMSE as the independent variables. We found PSQI, KESS, SAS, SDS, and MMSE all had no influence on BBI (p > 0.05) [].
Table 2. Summary of negative binomial regression analysis results in healthy participants (n = 93).
In ALS patients, there was no relationship between age, gender, years of education, MMSE, disease duration, bulbar involvement and BBI after score (p > 0.05). Despite the fact that p < 0.05 in this case, r < 0.3 implied that there was no link between SAS and BBI (rs = 0.25, p = 0.01). Slight or moderate correlations were noted between PSQI, KESS, SAS, ALSFRS-R and BBI score (rs = 0.37, p < 0.001; rs = 0.35, p < 0.001; rs = 0.393, p < 0.001; rs = −0.54, p < 0.001); We then performed a partial correlation analysis of the BBI and ALSFRS-R scores with disease duration as a control. We still find a moderate negative correlation between BBI values and ALSFRS (r= −0. 459, p < 0.001). O test was used to conduct the over discrete test (o = 74.56, p < 0.001. So, the negative binomial regression model was used to analyze the influencing factors. The model was constructed with BBI score as the dependent variable and PSOI, KESS, SAS, SDS, MMSE, disease duration, bulbar involvement, ALSFRS-R as the independent variables. ALSFRS-R has a significant negative effect on BBI after (z = −2.949, p = 0.003). However, PSQI, KESS, SAS, SDS, MMSE, bulbar involvement and disease duration did not affect BBI after [].
Table 3. Summary of negative binomial regression analysis results in ALS patients(n = 98).
3.5. Identification of cutoffs
When tested against clinical diagnoses, the optimal cutoff of total BBI score was identified at 5.5 (AUC =0.95; SE =0.02; 95% CI [0.91, 0.99]), the BBI reached optimal sensitivity and specificity values (91.5 and 87.2%). With regard to identifying behavioral dysfunctions in ALS patients, the BBI (AUC =0.95) outperformed the FBI (AUC =0.76; SE =0.05; 95% CI [0.66, 0.86]) and the ALS-FTD-Q (AUC =0.84; SE =0.04; 95% CI [0.77, 0.92]) [].
Figure 1. ROC curve for BBI-after scores, ALS-FTD-Q, FBI as tested against clinical diagnoses of behavioral impairment in ALS patients. ROC: receiver-operating characteristic; BBI: Beaumont behavioral inventory; ALS-FTD-Q: amyotrophic lateral sclerosis-frontotemporal dementia-questionnaire; FBI: frontal behavioral inventory.
3.6. Prevalence of behavioral change
Young-onset ALS is characterized by an onset age of less than 45 years. The forms were classified whether the bulbar is involved. A higher education group was defined as more than 12 years of education. During the initial visit, the ALS Functional Rating Scale-Revised (ALSFRS-R) scale was used to gauge the severity of the illness (mild >36; 36 ≥medium > 24; severe ≤24). No differences were noted when patients were grouped by age, involved form, or educational status(p > 0.05). Interestingly, BBI score was different between the three severity levels of the disease (X2 =79.57; p < 0.001). Bonferroni corrected post hoc test found BBI score in the severe dysfunction group is significantly higher than medium dysfunctional group(p < 0.001) while BBI score in medium dysfunction group is significantly higher than slight dysfunctional group (p < 0.001).
The most frequently described new-onset behavior in ALS was increased irritability (52%), followed by emotional lability (33.7%), lost interest (29.6%), more withdraw (27.6%), and new bizarre belief (27.6%). The Lowest frequency of behavioral items were obsessive counting (3.4%), storing food in mouth(3.4%), hyperoralty(3.1%), see/hear thing/people (3.1%) [].
Table 4. Total ALS cohort’s behavioral change on BBI (n = 98).
4. Discussion
Our research validated the Chinese translation of the BBI in a Chinese ALS cohort and found that this scale can be widely used in clinics to accurately assess, psychometric quantify, and classify the behavioral changes in ALS patients. The constructive and convergent validity of FBI and ALS-FTD-Q have been evaluated and shown to have good reliability in ALS patients, but they do not have explicit cutoff values. So, when we compared BBI to clinical diagnosis, 5.5 was shown to be the best cutoffs. Only integers are produced by the BBI, hence a cutoff of 6 is suggested. By referring to this value, all but five of the fifty-nine ALSbi patients had BBI scores above 6, compared to five of 39 of patients who had no behavioral impairment. This revealed that the sensitivity and specificity for ALSbi were 91.5% and 87.2%, respectively, for this cutoff.
According to our validation study, the BBI has good construct validity and reliability in ALS patients. In addition, the BBI had superior sensitivity and specificity when compared to FBI and ALS-FTD, and it was sensitive to behavioral alterations that occurred after the onset of the disease. Although age, gender, education, SDS, MMSE, disease duration, and bulbar involvement were not connected to the BBI scores, they did exhibit a moderate correlation with the FBI and a strong correlation with the ALS-FTD-Q. Sleep quality, gastrointestinal symptoms, anxiety, and disease severity were moderately associated with BBI. Further negative binomial generalized linear models showed that the severity of disease dysfunction was closely related to BBI. In the original study, BBI scores were not related to ALSFRS-R measures (Citation15). In contrast, Stage 4 ALS patients reported much more behavioral change in comparison to Stage 1 ALS patients in a large cross-sectional study, where BBI scores were significantly different between King’s stages (Citation9). Another cross-sectional study from Southwest China suggests that the ALSFRS-R, which uses FBI as a behavioral instrument, may be related to how severe behavioral alterations are in ALS patients (Citation13). Therefore, it is crucial to give targeted therapies and early behavioral change recognition. The frontal behavioral changes, however, were not observed to worsen ALS survival in either of the cross-sectional trials (Citation9, Citation13). Additionally, our findings imply that behavioral impairment may worsen over time in Chinese ALS patients, however, this is unlikely to be a significant factor in the development of overall behavioral impairment. Future longitudinal studies are needed to investigate whether behavioral disorders are associated with disease progression and survival in a larger Chinese cohort.
The previous study showed that BBI was constructed by five specific subdomains of behavioral impairment have been identified, comprising disinhibited behaviors, irregularity of reward/impulse control, dysexecutive behaviors, cognitive rigidity and neuropsychiatric features (Citation9). However, in our study BBI yielded a clear single-factor structure when despited items 16, 17, 19, and 26. (obsessive counting, excessively store food in mouth, hyperorality, see/hears things/people). It can be hypothesized that this difference may be attributed to the high inter-individual variability and sparse prevalence in Asia, where common genetic variants of ALS are distinct from those in Europe. Currently, more than 30 genes have been linked to the onset of ALS, with chromosome 9 open reading frame 72 (C9orf72), TAR DNA-binding protein 43 (TDP43), fused in sarcoma (FUS), and superoxide dismutase 1 (SOD1) being the most prevalent genetic variations (Citation24). TDP43, FUS, C9orf72 have been shown to cause behavioral changes in ALS (Citation25). However, ALS showed significant genetic heterogeneity between Asian and European populations. C9orf72 genetic variations increasingly associated with psychiatric manifestations(hallucinations and delusions, obsessive- compulsive disorder, et al.,) were rare in the Asian population (Citation26–28). Besides, SOD1 that is the most common gene responsible for ALS in Asia have not been shown to be associated with behavioral disorders. Therefore, the structure of behavior disorder expression in Asians may be slightly different from that in Europeans.
Our data found that irritability is a frequent finding in ALS (52%), followed by emotional lability (33.7%), lost interest (29.6%), more withdrawal (27.6%), and new bizarre belief (27.6%). The clinical features distribution of behavioral impairment in ALS is roughly the same trend as before (Citation9), but the sample size in this cohort is small and should be further expanded in the future. In clinical consultation, we need to pay more attention to these behavioral symptoms with high incidence, so as to detect the signs of abnormal behavior in ALS patients as early as possible.
We found emotional state(anxiety and depression), overall cognitive level, sleep quality, and gastroenteric function (autonomic nervous system) would not influence behavioral impairment in ALS patients. Previous studies have found similar results, which are consistent with the findings of this study (Citation7, Citation29–31).
Our study showed no association was found between bulbar involvement and behavioral impairment, which was in contrast with previous studies (Citation13, Citation32). However, Lazzolino etal., also found no association between bulbar signs and the behavioral impairment using the Italian version of BBI (Citation16). We guess that some items of previous tools which is used to evaluate behavior impairment may be influenced by the presence of bulbar symptoms. BBI was a disease-specific instrument that was designed to avoid the influence of dysarthria on behavior. It might be a more accurate tool and provide dependable information. Or perhaps our patients’ bulbar symptoms were not severe enough to indicate their link to behavioral impairment. We might need further follow-up or a larger sample size for research.
There are some limitations. First, ALS patients were recruited from a single center in China and the distribution of behavioral disorders in ALS may be fluctuant in different regions. Second, the frequency and characteristics of behavioral changes were only examined at the baseline in our cross-sectional investigation. Third, the frontal system behavior scale (FrSBe) was not valid in China, so we can’t use it as the gold standard for detecting behavioral changes. Fourth, since this study focused primarily on behavioral abnormalities in ALS, we performed a simple overall cognitive function screening using MMSE, which is simple and more widely used in China. In a follow-up study of the relationship between behavioral disorders and cognitive dysfunction in Chinese ALS patients, we will use a more detailed cognitive function scale such as ECAS. Besides, our patient was not genetically tested, because genetic testing is not routinely performed in clinical practice currently and was not a key part of this study.
The Chinese version of BBI is a more effective and rapid tool to screen behavioral impairments in ALS patients. Timely identification of behavioral disorders and early intervention can improve the quality of life of ALS patients and reduce the burden on caregivers. In addition, it can assist in identifying the effects of behavioral modifications on the development and prognosis of ALS in China.
Ethical sapproval
This study was approved by the Research Ethics Committee of Second Hospital of Hebei Medical University.
Authors’ contribution
The content of the manuscript has not been published or submitted for publication elsewhere.
Acknowledgements
The authors thank the ALS patients, their caregivers, and the healthy controls for their participations in this study. We would like to thank Professor Orla Hardiman for her help to obtain the authorization from Professor Marwa Elamin and Professor Niall Pender. The authors also thank Professor Marwa Elamin for providing guidance during the translation.
Disclosure statement
The authors declare no competing financial interests.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request from any qualified investigator, maintaining anonymization of the patients.
Additional information
Funding
Reference
- Strong MJ, Abrahams S, Goldstein LH, Woolley S, Mclaughlin P, Snowden J, et al. Amyotrophic lateral sclerosis - frontotemporal spectrum disorder (ALS-FTSD): revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:153–74.
- Bersano E, Sarnelli MF, Solara V, Iazzolino B, Peotta L, De Marchi F, et al. Decline of cognitive and behavioral functions in amyotrophic lateral sclerosis: a longitudinal study. Amyotroph Lateral Scler Frontotemporal Degener. 2020;21:373–9.
- Mehl T, Jordan B, Zierz S. “Patients with amyotrophic lateral sclerosis (ALS) are usually nice persons”-How physicians experienced in ALS see the personality characteristics of their patients. Brain Behav. 2017;7:e00599.
- Longinetti E, Mariosa D, Larsson H, Ye W, Ingre C, Almqvist C, et al. Neurodegenerative and psychiatric diseases among families with amyotrophic lateral sclerosis. Neurology 2017;89:578–85.
- Turner MR, Goldacre R, Talbot K, Goldacre MJ. Psychiatric disorders prior to amyotrophic lateral sclerosis. Ann Neurol. 2016;80:935–8.
- Mioshi E, Caga J, Lillo P, Hsieh S, Ramsey E, Devenney E, et al. Neuropsychiatric changes precede classic motor symptoms in ALS and do not affect survival. Neurology 2014;82:149–55.
- Nguyen C, Caga J, Mahoney CJ, Kiernan MC, Huynh W. Behavioural changes predict poorer survival in amyotrophic lateral sclerosis. Brain Cogn. 2021;150:105710.
- Abrahams S, Newton J, Niven E, Foley J, Bak TH. Screening for cognition and behaviour changes in ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:9–14.
- Burke T, Pinto-Grau M, Lonergan K, Bede P, O'Sullivan M, Heverin M, et al. A cross-sectional population-based investigation into behavioral change in amyotrophic lateral sclerosis: subphenotypes, staging, cognitive predictors, and survival. Ann Clin Transl Neurol. 2017;4:305–17.
- Ye S, Ji Y, Li C, He J, Liu X, Fan D. The Edinburgh cognitive and behavioural ALS Screen in a Chinese amyotrophic lateral sclerosis population. PLOS One. 2016;11:e0155496.
- Chio A, Moglia C, Canosa A, Manera U, Vasta R, Brunetti M, et al. Cognitive impairment across ALS clinical stages in a population-based cohort. Neurology 2019;93:e984–e994.
- Finsterer J, Burgunder JM. Recent progress in the genetics of motor neuron disease. Eur J Med Genet. 2014;57:103–12.
- Wei Q, Chen X, Zheng Z, Huang R, Guo X, Cao B, et al. Frontal lobe function and behavioral changes in amyotrophic lateral sclerosis: a study from Southwest China. J Neurol. 2014;261:2393–400.
- Raaphorst J, Beeldman E, Schmand B, Berkhout J, Linssen WH, van den Berg LH, et al. The ALS-FTD-Q: a new screening tool for behavioral disturbances in ALS. Neurology 2012;79:1377–83.
- Elamin M, Pinto-Grau M, Burke T, Bede P, Rooney J, O'Sullivan M, et al. Identifying behavioural changes in ALS: validation of the Beaumont behavioural inventory (BBI). Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:68–73.
- Iazzolino B, Pain D, Laura P, Aiello EN, Gallucci M, Radici A, et al. Italian adaptation of the Beaumont behavioral inventory (BBI): psychometric properties and clinical usability. Amyotroph Lateral Scler Frontotemporal Degener. 2022;23:81–6.
- Goutman SA, Hardiman O, Al-Chalabi A, Chió A, Savelieff MG, Kiernan MC, et al. Recent advances in the diagnosis and prognosis of amyotrophic lateral sclerosis. Lancet Neurol. 2022;21:480–93.
- Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–9.
- Kertesz A, Davidson W, Fox H. Frontal behavioral inventory: diagnostic criteria for frontal lobe dementia. Can J Neurol Sci. 1997;24:29–36.
- Shi Y, Liao Y, Zhou Y, Liu H, Lei Y, Luo L. Anxiety, depression, and related factors in hemodialysis patients during the lockdown period of COVID- 19 in China: a multicenter study. Psychol Health Med. 2023;28:1513–19.
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213.
- Namazov A, Kathurusinghe S, Mehdi E, Merlot B, Prosszer M, Tuech JJ, et al. Evolution of bowel complaints after Laparoscopic endometriosis surgery: a 1497 women comparative study. J Minim Invasive Gynecol. 2022;29:499–506.
- Fluss R, Faraggi D, Reiser B. Estimation of the Youden index and its associated cutoff point. Biom J. 2005;47:458–72.
- Li HF, Wu ZY. Genotype-phenotype correlations of amyotrophic lateral sclerosis. Transl Neurodegener. 2016;5:3.
- Giordana MT, Ferrero P, Grifoni S, Pellerino A, Naldi A, Montuschi A. Dementia and cognitive impairment in amyotrophic lateral sclerosis: a review. Neurol Sci. 2011;32:9–16.
- van der Ende EL, Jackson JL, White A, Seelaar H, van Blitterswijk M, Van Swieten JC. Unravelling the clinical spectrum and the role of repeat length in C9ORF72 repeat expansions. J Neurol Neurosurg Psychiatry. 2021;92:502–9.
- Roggenbuck J, Quick A, Kolb SJ. Genetic testing and genetic counseling for amyotrophic lateral sclerosis: an update for clinicians. Genet Med. 2017;19:267–74.
- Gossink F, Dols A, Stek ML, Scheltens P, Nijmeijer B, Cohn Hokke P, et al. Early life involvement in C9orf72 repeat expansion carriers. J Neurol Neurosurg Psychiatry. 2022;93:93–100.
- Zucchi E, Ticozzi N, Mandrioli J. Psychiatric symptoms in amyotrophic lateral sclerosis: beyond a motor neuron disorder. Front Neurosci. 2019;13:175.
- Piccione EA, Sletten DM, Staff NP, Low PA. Autonomic system and amyotrophic lateral sclerosis. Muscle Nerve. 2015;51:676–9.
- Fang T, Jozsa F, Al-Chalabi A. Nonmotor symptoms in amyotrophic lateral sclerosis: a systematic review. Int Rev Neurobiol. 2017;134:1409–41.
- Chio A, Vignola A, Mastro E, Giudici AD, Iazzolino B, Calvo A, et al. Neurobehavioral symptoms in ALS are negatively related to caregivers’ burden and quality of life. Eur J Neurol. 2010;17:1298–303.

