Abstract
Background
Contrasting findings have been reported regarding a possible constitutive expression of inducible nitric oxide synthase (iNOS) in a normal mammalian bladder. The current study was designed to further investigate such putative iNOS expression.
Materials and methods
The experiments were conducted with paraffin-embedded archival material from the urinary bladder of 6 normal, male Sprague–Dawley rats. In addition, two normal female mice (C57BL/6) were sacrificed and the urinary bladders were harvested. The occurrence of iNOS mRNA was examined by the RNAScope in situ hybridization method. Protein expression of iNOS and 3-nitrotyrosine (the latter used as an indicator of oxidative stress) was investigated by immunohistochemistry.
Results
No expression of iNOS mRNA was observed in the bladder tissue. iNOS protein and 3-nitrotyrosine were strongly expressed in the urothelium. iNOS was also expressed perinuclearly in the detrusor.
Conclusions
Although the RNAScope methodology could not demonstrate mRNA for iNOS in the normal urinary bladder, the results by immunohistochemistry strongly suggest the occurrence of iNOS in particular, in the urothelium. Positive reactivity for 3-nitrotyrosine may indicate ongoing oxidative stress of the urothelium. The finding of perinuclear iNOS immunoreactivity could suggest an intracrine signaling function by iNOS to the nucleus.
Introduction
Originally, large amounts of nitric oxide (NO), as produced by the action of the inducible isoform of NO synthase (iNOS), were ascribed a protective function for the host, by destroying pathogens and malignant cells [Citation1]. Up-regulation of iNOS after exposure to lipopolysaccharide has been confirmed on the mRNA and protein levels [Citation2,Citation3]. However, the role of iNOS in physiology/pathophysiology appears to be far more complex than initially assumed. Thus, iNOS was demonstrated in a number of cancer cell lines [Citation4,Citation5] Intriguingly, in the normal murine gut mucosa, Hoffman et al. demonstrated seemingly constitutive expression of iNOS in the ileum, but not jejunum or colon [Citation6]. Constitutive iNOS expression has been demonstrated also in non-diseased human airway epithelium [Citation6], myenteric plexus of uninflamed rat colon [Citation7,Citation8], uninflamed human colon mucosa [Citation9] and healthy porcine myocardium [Citation10].
With particular respect to the urinary bladder, findings regarding the constitutive expression of iNOS in the uninflamed mammalian bladder are far from uniform. Johansson et al. reported that while iNOS immunoreactivity could be elicited in rat urothelium by three days of partial urethral obstruction, untreated control bladders did not express such immunoreactivity [Citation11]. Moreover, in human bladder cancer, iNOS immunoreactivity was demonstrated in tumor areas but not in non-cancerous control bladder tissues [Citation11,Citation12]. Conversely, in normal human bladder, Renström-Koskela noted mRNA expression, but not protein expression, for iNOS [Citation13]. To the best of our knowledge, only one study has been published reporting iNOS mRNA expression in normal rat urinary bladder [Citation14]. Moreover, iNOS protein has been demonstrated in at least two studies of such tissue, with predominantly urothelial localization [Citation14,Citation15]. Thus, the question of whether, or not, iNOS may be constitutively expressed in the normal mammalian urinary bladder so far remains to be answered. Applying in situ hybridization and immunohistochemistry on archival material from acutely harvested rat urinary bladder, the aim of the present study was to investigate whether we could confirm a constitutive expression of iNOS in non-diseased bladder tissue. For species comparison, two female mice were sacrificed, and the urinary bladders were harvested. Moreover, in the rat bladder material, the expression of 3-nitrotyrosine, a biomarker of oxidative stress, was also investigated.
Materials and methods
The investigation was approved by the Ethical Committee of the University of Gothenburg. The study was undertaken with paraffin-embedded archive material (urinary bladders) from 6 healthy, inbred, male Sprague–Dawley rats (∼300 g b.w.). Moreover, for the current study, two mature, normal, female mice of the C57BL/6 strain were deeply anesthetized with Isofluran and were killed by cervical dislocation and were bled. Then, the urinary bladder was removed from each mouse and was incubated in 4% phosphate-buffered formaldehyde solution for 24 h, and was then dehydrated in alcohol and embedded in paraffin. From the various blocks, sections of 4 µm thickness were cut for ensuing in situ hybridization or immunohistochemistry.
In situ hybridization
In situ detection of iNOS transcripts was carried out on paraffin-embedded tissue sections using the RNAScope 2.5 HD assay – RED (Advanced Cell Diagnostics, Inc., Hayward, CA, USA). Sections were pretreated using the standard protocol (Formalin-Fixed Paraffin-Embedded (FFPE) Sample Preparation and Pretreatment) followed by hybridization and detection under normal conditions according to the manufacturer’s instructions for the manual assay (RNAScope 2.5 HD Detection Reagent – RED). An RNAScope probe for iNOS, a standard negative DapB (a bacterial gene) and a positive polR2A control probes were used (Advanced Cell Diagnostics, Inc). Slides were counterstained with hematoxylin (Histolab, Gothenburg, Sweden). The slides were scanned using an automatic brightfield scanner, Pannoramic 250 (3DHISTECH Ltd., Hungary) with a 40× objective. A positive reaction was denoted by a red dot.
Immunohistochemistry
This part of the investigation was undertaken with the MACH1 Universal HRP-Polymer Detection Kit (Biocare Medical, Concord, CA, USA). All procedures were undertaken at room temperature unless otherwise specified. After deparaffinization and rehydration, the sections were immersed in 10 mM citrate buffer (pH 6), placed in a microwave oven at medium power for 2 × 5 min, for antigen retrieval. Next, endogenous peroxidase was blocked for 5 min, either by 3% hydrogen peroxide in methanol, or Peroxidazed 1 (Biocare Medical). Unspecific protein binding was then blocked by Background Sniper (Biocare Medical) for 15 min. The slides were thereupon incubated overnight at 4 °C in a moist chamber with either of the following primary antibodies: Rabbit polyclonal anti-iNOS (sc-649, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), diluted 1:100 to 1:800 in Da Vinci Green Diluent (Biocare Medical); rabbit polyclonal anti-iNOS (bs-2072R, Nordic Biosite, Täby, Sweden), diluted 1:400 to 1:1600 in Da Vinci Green Diluent (Biocare Medical); mouse monoclonal anti-nitrotyrosine (MAB5404, formerly from Millipore, currently from Sigma-Aldrich, Stockholm, Sweden), diluted 1:500 to 1:2000 in Da Vinci Green Diluent (Biocare Medical). After the incubation with the respective antibody, the following procedures were undertaken (all incubations were performed in a moist chamber): For the mouse antibody, MACH 1 Mouse Probe (Biocare Medical) was added and incubated for 15 min. and after rinsing, the MACH1 Universal HRP-Polymer (Biocare Medical) was applied and was incubated for another 15 min. For the rabbit antibodies, the MACH1 Universal HRP-Polymer (Biocare Medical) was added and the slides were incubated for 30 min. Staining was then performed using DAB solution (Biocare Medical). The sections were then counterstained with Mayer’s hematoxylin (Histolab). After drying in an oven at 60 °C for 15–20 min, the sections were finally mounted with Pertex (Histolab) and were then photographed under a light microscope. Positive immunoreactivity was manifested by brown staining. Negative controls were performed either by excluding the primary antibody and incubating the tissues with Da Vinci Green Diluent (Biocare Medical) instead (resulting in no immunoreactivity) or applying the anti-iNOS antibody (sc-649; Santa Cruz) after this had been pre-absorbed with its immunogenic peptide (‘blocking peptide’; sc-649P, Santa Cruz; 10 times the concentration of the primary antibody), resulting in a marked attenuation of immunoreactivity.
Results
RNAScope and immunohistochemical investigations of iNOS expression
The bladder specimens were stained with either the DapB mRNA probe (negative control) or the iNOS mRNA probe, either treatment resulting in no reactivity in the tissues. Conversely, the polR2A mRNA probe, used as a positive control for the methodology, generated a clear-cut positive reaction in the urothelial cells and also, to a markedly lower intensity, in the remaining parts of the bladder (not shown). However, it was observed that the intensity of the expression of the positive control was notably weaker in our specimens as compared to the illustration shown in the product sheet from the manufacturer of the RNAScope.
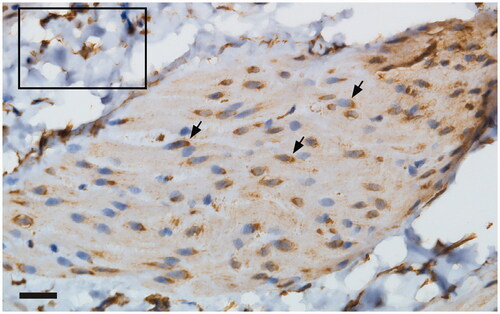
Both anti-iNOS antibodies elicited intense, seemingly cytoplasmic immunoreactivity in the urothelium (, showing the immunoreactivity as elicited by the Santa Cruz antibody). In addition, the Santa Cruz antibody produced weak immunoreactivity in the lamina propria and detrusor (). The immunoreactivity was markedly attenuated when this antibody had been pre-absorbed with its immunogenic peptide (‘blocking peptide’; ). Interestingly, a clear-cut perinuclear immunoreactivity was noted in the detrusor after staining with the Santa Cruz antibody ().
Figure 1. Representative images of the protein expression of iNOS or nitrotyrosine in normal urinary bladder in rat. (A) The bladder specimen was stained without primary antibody against iNOS (negative control; Neg), resulting in no immunoreactivity. (B) Application of the Santa Cruz anti-iNOS antibody to the rat bladder specimen demonstrated immunoreactivity in the urothelium (U) and to a lesser extent also in the lamina propria (Lp) and the detrusor muscle (D). (C) Application of the Santa Cruz anti-iNOS antibody to the bladder specimen, after incubation of the antibody with its immunogenic peptide (‘blocking peptide’) markedly attenuated the immunoreactivity. (D) Application of the anti-nitrotyrosine antibody to the bladder specimen demonstrated immunoreactivity in the urothelium (U) and leaving the lamina propria (Lp) essentially unstained. Scale bars: 50 µm.
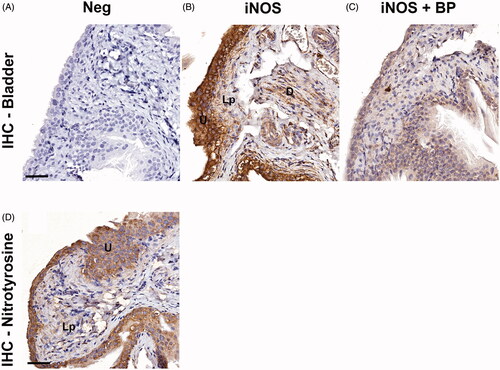
Figure 2. Application of the anti-iNOS antibody from Santa Cruz elicited a perinuclear immunoreactivity in the detrusor (arrowhead). Inlet: immunoreactivity noted in the lamina propria of the urinary bladder. Scale bar: 20 µm.
In addition, the anti-iNOS antibody from Nordic Biosite was applied to sections from two murine urinary bladders. This resulted in identical immunoreactivity to that obtained by this antibody when applied to rat urinary bladder (not shown).
Immunohistochemical investigation of 3-nitrotyrosine expression
3-nitrotyrosine as an indicator of nitrogen-related stress was strongly expressed in the urothelium ().
Discussion
In the current study, conducted on normal rat urinary bladder, mRNA for iNOS could not be detected using the RNAScope methodology. Conversely, urothelial expression of iNOS protein was evident as demonstrated by two different, polyclonal, rabbit antibodies. The Santa Cruz antibody had been raised against the human C-terminus while the Nordic Biosite one had been raised against the human N-terminus. The immunoreactivity elicited by the Santa Cruz antibody was attenuated by the preabsorption of the antibody with its immunogenic peptide, thus demonstrating the specificity of this antibody (a strength of the study). The two antibodies differed with respect to staining other parts of the bladder; the Santa Cruz antibody elicited immunoreactivity also in the lamina propria and the detrusor, albeit to a lower intensity than in the urothelium. Moreover, in the detrusor, the iNOS immunoreactivity was clearly located perinuclearly. The demonstration of iNOS in the nuclear compartment in some tissues is well known, strongly suggesting a role for NO in nuclear signaling [Citation16,Citation17]. Hence, as the two antibodies used in the current study bind to different epitopes, it is not to be expected that they should provide identical results. We, moreover, showed that our finding of iNOS expression in rat urothelium could be extended also to the murine urinary bladder.
The product of peroxynitrite-mediated nitration of tyrosine residues is 3-nitrotyrosine. Peroxynitrite, in turn, results from a reaction between superoxide and NO. It was previously suggested that 3-nitrotyrosine expression might function as a marker of the activation of iNOS [Citation18]. More recently, this view has been somewhat challenged. Thus, the current opinion states that 3-nitrotyrosine serves as an indicator of oxidative stress [Citation19,Citation20], which does not exclude that the expression of 3-nitrotyrosine marks the presence of active iNOS. There are few, if any, previous reports of 3-nitrotyrosine expression in normal rat urothelium, the presence of which naturally could be due to other factors within the bladder wall than the production of NO as catalyzed by iNOS. The physiological or pathophysiological significance of protein nitration in a healthy rat bladder can only be speculative; we suggest a protective function for the maintaining of urothelial homeostasis.
It is puzzling that we were unable to demonstrate iNOS mRNA with the RNAscope methodology (a clear limitation of our study), since, in our opinion, the protein expression of iNOS appears convincing. It should, however, be noted that the positive control for the RNAscope was markedly weak when compared to the illustration in the product sheet (as already mentioned). Therefore, we suspect that the RNAscope method may have been unsatisfactory in the current investigation. Possible explanations for this discrepancy include the non-optimal concentration of the iNOS probe utilized in the RNAscope experiments and/or instability of the mRNA in the paraffin-embedded tissue. Furthermore, contrasting findings have been reported with respect to constitutive iNOS expression in healthy rat bladder (see Introduction for references). Unfortunately, so far, we can offer no explanation as to the reason(s) for these conflicting data.
Conclusion
In the current study, conducted with healthy urinary bladder tissue from mature, male Sprague–Dawley rats, we have demonstrated the urothelial immunohistochemical expression of iNOS. This result could be obtained also in the acutely harvested murine urinary bladder. We were unable to corroborate our findings on the mRNA level. iNOS protein expression was noted to a far lesser extent in the lamina propria and the detrusor components of the rat bladder, respectively. Interestingly, in the detrusor, a perinuclear iNOS immunoreactivity could be seen, a finding possibly suggesting intracrine signaling by iNOS to the nucleus.
Acknowledgments
Thanks are due to Profs. Kjell Johansson and Stefan Lange for valuable support with the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43(2):109–142.
- Mulsch A, Schray-Utz B, Mordvintcev PI, et al. Diethyldithiocarbamate inhibits induction of macrophage NO synthase. FEBS Lett. 1993;321(2–3):215–218.
- Sato K, Miyakawa K, Takeya M, et al. Immunohistochemical expression of inducible nitric oxide synthase (iNOS) in reversible endotoxic shock studied by a novel monoclonal antibody against rat iNOS. J Leukoc Biol. 1995;57(1):36–44.
- Jenkins DC, Charles IG, Baylis SA, et al. Human colon cancer cell lines show a diverse pattern of nitric oxide synthase gene expression and nitric oxide generation. Br J Cancer. 1994;70(5):847–849.
- Ambs S, Merriam WG, Bennett WP, et al. Frequent nitric oxide synthase-2 expression in human colon adenomas: implication for tumor angiogenesis and colon cancer progression. Cancer Research. 1998;58:334–341.
- Guo FH, De Raeve HR, Rice TW, et al. Continuous nitric oxide synthesis by inducible nitric oxide synthase in normal human airway epithelium in vivo. Proc Natl Acad Sci USA. 1995;92(17):7809–7813.
- Miampamba M, Sharkey KA. Temporal distribution of neuronal and inducible nitric oxide synthase and nitrotyrosine during colitis in rats. Neurogastroenterol Motil. 1999;11(3):193–206.
- Borjesson L, Delbro DS. Neurogenic and non-neurogenic mechanisms in response of rat distal colon muscle to dextran sulphate sodium treatment. Auton Neurosci. 2003;107(2):74–80.
- Roberts PJ, Riley GP, Morgan K, et al. The physiological expression of inducible nitric oxide synthase (iNOS) in the human colon. J Clin Pathol. 2001;54(4):293–297.
- Paslawska U, Kiczak L, Bania J, et al. Inducible NO synthase is constitutively expressed in porcine myocardium and its level decreases along with tachycardia-induced heart failure. Cardiovasc Pathol. 2016;25(1):3–11.
- Johansson R, Pandita RK, Poljakovic M, et al. Activity and expression of nitric oxide synthase in the hypertrophied rat bladder and the effect of nitric oxide on bladder smooth muscle growth. J Urol. 2002;168(6):2689–2694.
- Swana HS, Smith SD, Perrotta PL, et al. Inducible nitric oxide synthase with transitional cell carcinoma of the bladder. J Urol. 1999;161(2):630–634.
- Koskela LR, Thiel T, Ehren I, et al. Localization and expression of inducible nitric oxide synthase in biopsies from patients with interstitial cystitis. J Urol. 2008;180(2):737–741. Epub 2008/06/17.
- Yuan X, Wu S, Lin T, et al. Role of nitric oxide synthase in bladder pathologic remodeling and dysfunction resulting from partial outlet obstruction. Urology. 2011;77(4):1008.e1–1008.e8.
- Gu D, Huang J, Yin Y, et al. Long-term ketamine abuse induces cystitis in rats by impairing the bladder epithelial barrier. Mol Biol Rep. 2014;41(11):7313–7322.
- Giordano A, Tonello C, Bulbarelli A, et al. Evidence for a functional nitric oxide synthase system in brown adipocyte nucleus. FEBS Lett. 2002;514(2–3):135–140. Epub 2002/04/12.
- Provost C, Choufani F, Avedanian L, et al. Nitric oxide and reactive oxygen species in the nucleus revisited. Can J Physiol Pharmacol. 2010;88(3):296–304. Epub 2010/04/16.
- Miller MJ, Thompson JH, Zhang XJ, et al. Role of inducible nitric oxide synthase expression and peroxynitrite formation in guinea pig ileitis. Gastroenterology. 1995;109(5):1475–1483. Epub 1995/11/01.
- Ferrer-Sueta G, Campolo N, Trujillo M, et al. Biochemistry of peroxynitrite and protein tyrosine nitration. Chem Rev. 2018;118(3):1338–1408. Epub 2018/02/06.
- Bartesaghi S, Radi R. Fundamentals on the biochemistry of peroxynitrite and protein tyrosine nitration. Redox Biol. 2018;14:618–625. Epub 2017/11/21.

