Abstract
Purpose
To investigate the role of clinical parameters and immunohistochemical (IHC) biomarkers in their feasibility to predict the effect of neo-adjuvant chemotherapy (NAC) in patients with muscle-invasive urothelial bladder cancer (MIBC).
Materials and methods
The first 76 consecutive patients with MIBC treated with NAC and radical cystectomy in two University hospitals in Finland between 2008 and 2013 were chosen for this study. After excluding patients with non-urothelial cancer, less than two cycles of chemotherapy, no tissue material for IHC analysis or non-muscle-invasive bladder cancer in re-review, 59 patients were included in the final analysis. A tissue microarray block was constructed from the transurethral resection samples and IHC stainings of Ki-67, p53, Her-2 and EGFR were made. The correlations between histological features in transurethral resection samples and immune-histochemical stainings were calculated. The associations of clinicopathological parameters and IHC stainings with NAC response were evaluated. Factors affecting survival were estimated.
Results
The complete response rate after NAC was 44%. A higher number of chemotherapy cycles was associated with better response to neo-adjuvant chemotherapy. No response to neo-adjuvant chemotherapy and female gender was associated with decreased cancer-specific survival. The IHC stainings used failed to show an association with neo-adjuvant chemotherapy response and overall or cancer specific survival.
Conclusions
Patients who do not respond to neo-adjuvant chemotherapy do significantly worse than responders. This study could not find clinical tools to distinguish responders from non-responders. Further studies preferably with larger cohorts addressing this issue are warranted to improve the selection of patients for neo-adjuvant chemotherapy.
Introduction
Muscle invasive bladder cancer (MIBC) is a disease with poor prognosis. Among patients with MIBC treated with radical cystectomy (RC) and pelvic lymphadenectomy alone, the 5-year overall survival (OS) is between 25 and 77%, decreasing with more advanced stage [Citation1,Citation2]. Platinum-based combination neo-adjuvant chemotherapy (NAC) prior to RC improves the absolute survival of patients at 5 years by 5–7% as compared to those receiving cystectomy alone [Citation3,Citation4]. The rationale to use NAC is to eradicate micro-metastasis and to downstage the primary tumor. Down-staging to non-muscle invasive disease and especially complete pathological response (pT0) is associated with improved overall survival (OS) [Citation5]. However, in the case of chemo-resistant tumors, NAC predisposes patients to possible adverse effects and increases the risk of cancer progression prior to surgery.
To date, there are no clinically validated methods to interrogate the chemo-sensitivity of MIBC prior to treatment. It has been shown that a low neutrophil-to-lymphocyte ratio is associated with better cancer-specific survival (CSS) and OS in patients receiving NAC prior to radical cystectomy [Citation6]. In addition, certain genetic alterations (e.g., ERBB2 missense mutations and p53 status) are associated with improved NAC response [Citation7].
In our study we describe the first 76 consecutive patients that were treated in Helsinki and Turku University Hospitals with NAC prior to open RC with bilateral pelvic lymphadenectomy.
Our objective was to investigate whether any pretreatment clinical variables and/or well established immune-histochemical (IHC) markers, namely p53, Ki-67, ERBB2 (Her-2) and EGFR, were associated with NAC response, OS or CSS. These markers were chosen as they are widely used in clinical practice of cancer diagnosis and treatment.
Materials and methods
Patients
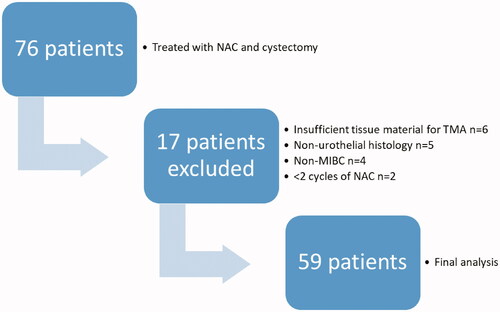
Altogether the first 76 consecutive patients who underwent NAC prior to open RC for MIBC were included retrospectively in the study. Patients were treated in Helsinki University Hospital (2010–2013) and Turku University Hospital (2008–2013). After excluding patients with non-urothelial cancer (four small cell carcinomas, one squamous cell carcinoma), two patients with less than two cycles of NAC, four patients without muscle invasive carcinoma in the re-evaluation of the uropathologists (T.M. and P.T.) and six patients with insufficient tissue material for tissue microarray (TMA), there were n = 59 patients eligible for the final analysis of both clinical data and IHC stainings (). The study has been approved by the ethics committee of Helsinki University Hospital (169/13/03/02/2013).
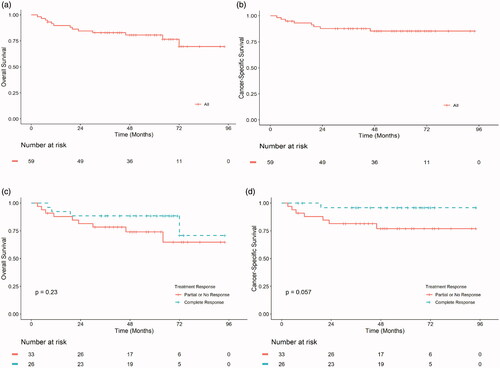
Figure 2. OS (a and c) and CSS (b and d) in months from TURBT of urothelial MIBC patients treated with NAC and radical cystectomy in Helsinki and Turku University Hospitals between 2008 and 2013 (n = 59). The whole patient cohort (a and b), according to treatment response (c and d). TURBT, Transurethral resection of bladder tumour; MIBC, Muscle invasive bladder cancer; NAC, Neo-adjuvant chemotherapy; Complete response, pT0N0; Partial response, < pT2N0; No response, ≥ pT2 and/or N+.
Diagnosis, treatment and follow-up
The diagnosis of MIBC was made by performing (Transurethral resection of bladder tumor, TURBT). RC in males included the removal of urinary bladder, prostate and seminal vesicles. In females the uterus, ovaries, fallopian tubes and the anterior part of the vaginal wall was removed together with the bladder. Bilateral pelvic lymphadenectomy was performed according to institution standards. Included with every patient’s lymphadenectomy were at least areas of obturator fossae, external iliac vessels medial to genitofemoral nerve and internal iliac vessels up to iliac crossing.
Complete pathological response to treatment was defined as no detection of cancer in the cystectomy specimen (T0) and no detection of metastasis in the lymph nodes (N0). Partial response was defined as detection of non-muscle invasive cancer in the bladder (Ta, T1 or Tis) and no lymph node metastasis. Patients having muscle invasive (T2) or locally advanced (T3–T4) cancer or lymph node metastasis were defined as non-responders.
After cystectomy, follow-up was according to institutional guidelines, most commonly at 3 months postoperatively, semiannually for 2 years and annually thereafter. FU visits included physical examination, laboratory tests and cross-sectional imaging.
Construction of tissue microarrays (TMA)
Two uropathologists (TM, PT) reviewed the TURBT and cystectomy slides and selected corresponding areas in the TURBT slides for tissue microarray (TMA) construction. TMA was constructed by punching three 1.0 mm cores from each of the selected TURBT block presenting BC.
Immunohistochemistry and marker scoring
For the detection of protein expression, TMA slides were first pretreated for antigen retrieval in Ventana Ultra CC1 for 64 min at 98 °C (Ki-67, p53 and c-erbB2) or Target retrieval solution (Agilent DAKO, Glostrup, Denmark) for 24 min at 98 °C (EGFR). The antibody clones, dilutions and incubation times are shown in Appendix Table A. The detection kits with chromogens were Ventana Ultraview DAB Detection kit (Ki-67, p53, c-erbB2) and Dako REAL EnVision Detection System (EGFR), and the reactions were conducted in Ventana Benchmark ULTRA or LabVision Autostainer, respectively.
For the scoring of p53 IHC, TMA cores were considered positive if >10% of all cells were stained positive, and negative if ≤10% of all cells were stained positive as described by Shariat et al. [Citation8]. Ki-67 scores were calculated by counting the number of positively stained cells out of 500 cancer cells. The received values were then dichotomized by mean value (62.8). Her-2 and EGFR scoring was done by comparing no staining with any staining.
The highest count of positively stained cells in any of the three punches was used in the statistical analysis.
Statistical analysis
All of the statistical analyses were performed with SPSS v.22 (IBM, Chicago, IL). The correlations between histological features in TURBT samples and IHC stainings were calculated by Pearson Chi-square or Fisher’s exact test where less than five units were available. The associations of clinicopathological parameters and IHC marker status with NAC response were evaluated by binary logistic regression. Univariate Cox proportional hazards regression analysis was used to estimate the factors affecting OS and CSS.
Results
The demographics of patient cohort are described in . Most of the patients were male (83%) and the median age was 65 years. Both Helsinki and Turku treated an equal number of patients. Almost three out of four (73%) of the patients had a high grade (WHO grade 3) urothelial carcinoma in the initial diagnosis made with TURBT, but only 27% had lympho-vascular invasion (LVI). Ten patients (17%) had primarily concurrent carcinoma in situ (data not shown). Gemcitabine and cisplatin (GC) was the chosen chemotherapy regime for 95% of the patients, and half (49%) received two or three cycles and the remainder had four or more cycles. During the median follow-up time of 49 months, eight (14%) patients succumbed to metastatic disease while five patients (8%) died due to other causes.
Table 1. Urothelial MIBC patients treated with NAC and radical cystectomy in Helsinki and Turku University Hospitals between 2008 and 2013 (n = 59).
The positively stained cancer cells in the TMAs by individual IHC markers are shown in . Almost half (47%) of the patients had over 10% staining for p53. Her2 staining was positive in 72% of the patients (any staining intensity), with intermediate staining intensity being the most prevalent (38%). EGFR staining, on the other hand, was positive in only 24% of the patients.
Table 2. Immuno-histochemical markers selected to stain the cancer samples from TURBT sections and their distribution according to expression.
The correlation between IHC status and cancer grade or LVI are shown in . Higher cancer grade (WHO 3) was correlated with 10% or less expression of p53 (p = 0.001). Staining results from other biomarkers were not correlated with grade or LVI.
Table 3. Correlation between marker status with both WHO Grade and Lympho-vascular invasion (LVI) from TURBT sections.
The associations of clinical variables and marker status with chemotherapy response are described in . Initially patients with complete response were compared to partial and non-responders. Then the comparison was made by grouping complete responders together with partial responders and comparing them to non-responders. A higher number of chemotherapy cycles was associated statistically significantly with the complete and partial response group (p = 0.014; OR = 4.044, 95% CI = 1.321–12.377). Other statistically significant associations were not observed.
Table 4. Binary logistic regression analysis evaluating associations of variables with chemotherapy response in cystectomy specimen.
Presented in are the factors affecting OS and CSS in a univariate Cox proportional hazard regression model. Female gender was associated significantly with decreased CSS (p = 0.011). There was also a trend toward association with OS, but this was not statistically significant (p = 0.072). MIBC (≥T2) found in cystectomy was also associated with poorer CSS compared to ≤ T1 (p = 0.035). As expected, positive lymph nodes (N+) were associated both with poorer CSS (p = 0.011) and OS (p = 0.004). None of the investigated biomarkers were associated with CSS or OS.
Table 5. Univariate Cox regression analysis (OS and CSS) evaluating association of variables for survival.
Discussion
We demonstrate here that NAC induced down-staging of non-MIBC or a complete response is associated with improved CSS. We also observed that a higher number of chemotherapy cycles was associated with better response to NAC treatment. However, none of the tested immune-histochemical markers were associated with NAC response or survival.
The tumor protein 53 (p53), the so-called gatekeeper of the cell cycle has been demonstrated to correlate with bladder cancer grade and advanced stage when overexpressed (presumably due to a mutation) in cancer cells [Citation9]. Only a few studies have examined the role of p53 and its value to predict NAC response or survival in MIBC patients after cystectomy. In a prospective study of Plimack et al. [Citation10] with accelerated Methotrexate, Vinblastine, Doxorubicin (=Adriamycin) and Cisplatin (MVAC) as neo-adjuvant treatment, p53 status, determined by DNA sequencing, failed to select patients with complete response in cystectomy. On the other hand, p53 protein overexpression (suggestive of TP53 mutation) was shown to have an independent predictive role for survival in a multivariate analysis by Sarkis et al. [Citation11] after MVAC as NAC and cystectomy. They used a cut-off of ≥20% in positively stained cells determining altered p53 staining in IHC. In contrast, Maluf et al. [Citation12] demonstrated that altered p53 status was associated with improved long-term survival (p = 0.05), but not with pathologic down-staging after NAC in their retrospective study. The patient cohort studied was relatively small (n = 59) and the cut-off of ≥20% was similarly used. It is worth mentioning the prospective randomized study of Stadler et al. [Citation13], where 272 T1/T2 bladder cancer patients with p53 overexpression (cut-off ≥10%) were randomly assigned for MVAC adjuvant treatment or surveillance after cystectomy. The authors found no difference between the groups in the recurrence-rates or survival. Based on these results, to date p53 cannot be regarded as an (established) predictive marker for treatment response or for survival in NAC treated MIBC.
Previously, a high percentage of Ki-67 (a proliferation marker) positive cells has been shown to correlate with high stage and poor differentiation in bladder cancer [Citation14]. In one study a high Ki-67 percentage was associated with higher stage, higher grade, lympho-vascular invasion and metastasis to lymph nodes. Among patients (n = 91) not receiving neo-adjuvant or adjuvant chemotherapy, a high Ki-67 percentage was also an independent predictor of both disease recurrence and cancer-specific mortality [Citation15]. According to the literature only one published paper addresses the association of Ki-67 expression in NAC treated MIBC patients. In the sub-study of the SWOG8710 trial where patients were randomized prospectively to NAC and cystectomy versus cystectomy alone in locally advanced bladder cancer, a high Ki-67 percentage was related to better progression-free survival (p = 0.063) [Citation16]. The median survival of patients with higher Ki-67 expression was 73 months, compared to 38 months for those with lower expression, but such difference failed to reach statistical significance (p = 0.25). Although the evidence is quite weak, it suggests that a high percentage of Ki-67 in NAC treated patients leads to better outcome after cystectomy, while cystectomy only patients do worse.
Human Epidermal Growth Factor Receptor-2 (Her-2 also erbB2) is an oncogenic growth factor receptor and overexpression of EGFR has been reported in 12–53% of bladder cancers and more frequently in MIBC [Citation17–19]. Studies have shown that, in MIBC Her-2, overexpression is associated with worse CSS [Citation20,Citation21]. Groenendijk et al. [Citation7] showed that MIBC with Her-2 missense mutations characterize a subgroup of MIBC patients with excellent response to NAC. A total of n = 9 of 38 complete responders had Her-2 missense mutations, while none of the 33 non-responders had any (p = 0.003). Her-2 overexpression was identified in both responders and non-responders, and there was no association with response to NAC (p = 0.52). Using immunohistochemistry, Kramer et al. [Citation22] demonstrated that overexpression of EGFR correlates with higher grade and stage and strong EGFR expression was associated with poorer survival in univariate, but not multivariate analysis. To our knowledge, this is the first study report of EGFR expression and NAC response in MIBC and we could not see any predictive value with EGFR immunohistochemistry.
In our study 61% of MIBC patients were down-staged to either non-MIBC (17%) or pT0N0 (44%). This was associated with a significantly higher number of NAC cycles (2–3 versus 4–6, p = 0.014). Peyton et al. [Citation23] described a complete response rate of 41% in patients treated with dose dense MVAC (on average 3.3 cycles) and 25% in patients treated with GC (on average 3.7 cycles), respectively. In an international retrospective study cohort consisting of 146 MIBC patients treated with GC, the complete response rate was 31% in patients who received four or more cycles of NAC [Citation24]. Taken together, these results suggest that four or more cycles of GC NAC favor likelihood complete response.
In our study we observed that women had poorer survival than men in MIBC, a finding in line with previously reported results. For CSS this difference was statistically significant (p = 0.011). Similarly, a large Swedish study by Radkiewicz et al. [Citation25] showed that MIBC in women leads to excess mortality when most confounding factors are taken into account.
Patients who did not have a response to NAC (≥pT2) had an over 5-fold risk of dying from MIBC compared to responders. There was, however, no significant difference in OS between the groups. The patients with negative pN-status had better CSS (p = 0.011) and OS (p = 0.004) compared to pN + patients. Sonpavde et al. [Citation26] observed similar results in their substudy of SWOG-S8710. Interestingly Bhindi et al. [Citation27] showed in their retrospective registry study that patients with muscle invasive or advanced residual tumor after NAC had worse CSS and OS than the patients with similar postoperative residual tumor but no NAC prior to cystectomy. This suggests that today there are only a few treatment options for MIBC found to be chemoresistant after NAC and cystectomy, and the prognosis of such patients is particularly poor.
Promising results of NAC treatment in MIBC patients has been previously shown by molecular subtyping of tumors. For example, Seiler et al. [Citation28] demonstrated that basal tumors responded well to NAC when OS was used as an endpoint.
The weaknesses of our study is the retrospective nature and the small patient cohort. Benefits include detailed real world clinical follow-up data from two academic institutes.
In conclusion, the detection of protein expression by IHC of Ki67, HER2, EGFR, and p53 (as detected by IHC) is unable to stratify patients for NAC treatment before cystectomy. Bladder cancer specific and genetic DNA level studies are needed to advise MIBC patients with cisplatin resistant cancers and urologists treating them to optimize the treatment.
| Abbreviations | ||
| MIBC | = | Muscle invasive bladder cancer |
| NAC | = | Neo-adjuvant chemotherapy |
| RC | = | Radical cystectomy |
| TURBT | = | Transurethral resection of bladder tumor |
| OS | = | Overall survival |
| CSS | = | Cancer specific survival |
| TMA | = | Tissue microarray |
| GC | = | Gemsitabine Cisplatin |
| MVAC | = | Methotrexate Vinblastine Doxorubicin (Adriamycin) Cisplatin |
Acknowledgements
We would like to acknowledge Jenni Niinimäki for help in TMA preparation and slide cutting for IHC.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19(3):666–675.
- Russell B, Sherif A, Haggstrom C, et al. Neoadjuvant chemotherapy for muscle invasive bladder cancer: a nationwide investigation on survival, scand. J. Urol. 2019;53(4):206–212.
- Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and Meta-Analysis. Lancet. 2003;361:1927–1934.
- Winquist E, Kirchner TS, Segal R, et al. Neoadjuvant chemotherapy for transitional cell carcinoma of the bladder: a systematic review and Meta-Analysis. J Urol. 2004;171(2 Pt 1):561–569.
- Rosenblatt R, Sherif A, Rintala E, et al. Pathologic downstaging is a surrogate marker for efficacy and increased survival following neoadjuvant chemotherapy and radical cystectomy for Muscle-Invasive urothelial bladder cancer. Eur Urol. 2012;61(6):1229–1238.
- Buisan O, Orsola A, Areal J, et al. Low pretreatment neutrophil-to-Lymphocyte ratio predicts for good outcomes in patients receiving neoadjuvant chemotherapy before radical cystectomy for muscle invasive bladder cancer. Clin Genitourin Cancer. 2017;15(1):145–151.e2.
- Groenendijk FH, de Jong J, Fransen van de Putte EE, et al. ERBB2 mutations characterize a subgroup of Muscle-Invasive bladder cancers with excellent response to neoadjuvant chemotherapy. Eur Urol. 2016;69(3):384–388.
- Shariat SF, Tokunaga H, Zhou J, et al. p53, p21, pRB, and p16 expression predict clinical outcome in cystectomy with bladder cancer. J Clin Oncol. 2004;22(6):1014–1024.
- Esrig D, Elmajian D, Groshen S, et al. Accumulation of nuclear p53 and tumor progression in bladder cancer. N Engl J Med. 1994;331(19):1259–1264.
- Plimack ER, Hoffman-Censits JH, Viterbo R, et al. Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for Muscle-Invasive bladder cancer: results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol. 2014;32(18):1895–1901.
- Sarkis AS, Bajorin DF, Reuter VE, et al. Prognostic value of p53 nuclear overexpression in patients with invasive bladder cancer treated with neoadjuvant MVAC. J Clin Oncol. 1995;13(6):1384–1390.
- Maluf FC, Cordon-Cardo C, Verbel DA, et al. Assessing interactions between mdm-2, p53, and bcl-2 as prognostic variables in Muscle-Invasive bladder cancer treated with Neo-Adjuvant chemotherapy followed by locoregional surgical treatment. Ann Oncol. 2006;17(11):1677–1686.
- Stadler WM, Lerner SP, Groshen S, et al. Phase III study of molecularly targeted adjuvant therapy in locally advanced urothelial cancer of the bladder based on p53 status. J Clin Oncol. 2011;29(25):3443–3449.
- Mellon K, Neal DE, Robinson MC, et al. Cell cycling in bladder carcinoma determined by monoclonal antibody Ki67. Br J Urol. 1990;66(3):281–285.
- Margulis V, Shariat SF, Ashfaq R, et al. Ki-67 is an independent predictor of bladder cancer outcome in patients treated with radical cystectomy for Organ-Confined disease. Clin Cancer Res. 2006;12(24):7369–7373.
- Grossman HB, Tangen CM, Cordon-Cardo C, et al. Evaluation of Ki67, p53 and angiogenesis in patients enrolled in a randomized study of neoadjuvant chemotherapy with or without cystectomy: a southwest oncology group study. Oncol Rep. 2006;16:807–810.
- King CR, Kraus MH, Aaronson SA. Amplification of a novel V-erbB-related gene in a human mammary carcinoma. Science. 1985;229(4717):974–976.
- Chow NH, Liu HS, Yang HB, et al. Expression patterns of erbB receptor family in normal urothelium and transitional cell carcinoma. an immunohistochemical study. Virchows Arch. 1997;430(6):461–466.
- Chaux A, Cohen JS, Schultz L, et al. High epidermal growth factor receptor immunohistochemical expression in urothelial carcinoma of the bladder is not associated with EGFR mutations in exons 19 and 21: a study using Formalin-Fixed, Paraffin-Embedded archival tissues. Hum Pathol. 2012;43(10):1590–1595.
- Kolla SB, Seth A, Singh MK, et al. Prognostic significance of Her2/neu overexpression in patients with muscle invasive urinary bladder cancer treated with radical cystectomy. Int Urol Nephrol. 2008;40(2):321–327.
- Krüger S, Weitsch G, Büttner H, et al. Overexpression of C-erbB-2 oncoprotein in Muscle-Invasive bladder carcinoma: relationship with gene amplification, clinicopathological parameters and prognostic outcome. Int J Oncol. 2002;21(5):981–987.
- Kramer C, Klasmeyer K, Bojar H, et al. Heparin-Binding epidermal growth Factor-Like growth factor isoforms and epidermal growth factor receptor/ErbB1 expression in bladder cancer and their relation to clinical outcome. Cancer. 2007;109(10):2016–2024.
- Peyton CC, Tang D, Reich RR, et al. Downstaging and survival outcomes associated with neoadjuvant chemotherapy regimens among patients treated with cystectomy for Muscle-Invasive bladder cancer. JAMA Oncol. 2018;4(11):1535–1542.
- Galsky MD, Pal SK, Chowdhury S, et al. Comparative effectiveness of gemcitabine plus cisplatin versus methotrexate, vinblastine, doxorubicin, plus cisplatin as neoadjuvant therapy for Muscle-Invasive bladder cancer. Cancer. 2015;121(15):2586–2593.
- Radkiewicz C, Edgren G, Johansson ALV, et al. Sex differences in urothelial bladder cancer survival. Clin Genitourin Cancer. 2020;18(1):26–34.e6.
- Sonpavde G, Goldman BH, Speights VO, et al. Quality of pathologic response and surgery correlate with survival for patients with completely resected bladder cancer after neoadjuvant chemotherapy. Cancer. 2009;115(18):4104–4109.
- Bhindi B, Frank I, Mason RJ, et al. Oncologic outcomes for patients with residual cancer at cystectomy following neoadjuvant chemotherapy: a pathologic Stage-Matched analysis. Eur.Urol. 2017;72(5):660–664.
- Seiler R, Ashab HAD, Erho N, et al. Impact of molecular subtypes in Muscle-Invasive bladder cancer on predicting response and survival after neoadjuvant chemotherapy. Eur Urol. 2017;72(4):544–554.